Abstract
Background:
Achieving a good apical seal for root canals is known to be associated with good mineral trioxide aggregate (MTA) adaptation to dentin.
Aims:
This study aims to compare the marginal adaptation of MTA with root dentin between orthograde and retrograde application techniques using microcomputed tomography (micro-CT) analysis.
Settings and Design:
Fifty-two single-rooted human teeth were divided into four equal groups: (Group 1) Retrograde MTA (RMTA), (Group 2) Orthograde MTA (OMTA), (Group 3) Etched RMTA (ERMTA), and (Group 4) Etched OMTA (EOMTA).
Materials and Methods:
For Group 1, 3-mm retrograde cavities were prepared and filled with MTA. For Group 2, the apical 6 mm of the canals were filled with MTA and sealed with sealer cement and warm gutta-percha. In Groups 3 and 4, canals were treated the same as Groups 1 and 2, respectively, except that before placing the MTA, canals were irrigated with 17% ethylenediaminetetraacetic acid (EDTA). After 48 hours, all the teeth were analyzed using a micro-CT scanner.
Statistical Analysis:
Mean dentin-MTA contact and the mean length and width of each gap was analysed using one-way analysis of variance (ANOVA). Statistical significance was set at an α level of 5%.
Results:
No significant difference in gap volumes was observed in the dentin-MTA adaptation in both orthograde and retrograde application techniques. However, significant difference in the gap volumes was observed between RMTA and ERMTA (P = 0.045). Etching significantly improved the MTA-Dentin adaptation (P < 0.05). The type of application technique did not significantly improve the dentin-MTA adaptation, instead with the use of 17% EDTA, a significant improvement could be achieved.
Conclusion:
Within the limitations of the present study, it concludes that MTA adaptation to dentin tooth structure is not significantly different between an orthograde and retrograde approach. However, the use of EDTA significantly improved the MTA-Dentin adaptation.
Keywords: Acid etching, marginal adaptation, microcomputed tomography, mineral trioxide aggregate
INTRODUCTION
Periradicular surgery helps to create favorable conditions for healing by promoting regeneration of tissues.[1] The procedure includes surgical debridement of pathological periradicular tissue, root-end resection, preparation of a root-end cavity, and placement of a root-end filling material to seal the root canal.[2]
One of the most important objectives of endodontic surgery is to seal the apex with appropriate material.[3] Several materials including silver amalgam, gutta-percha, zinc oxide eugenol cements, glassionomer, composite resins, calcium hydroxide cements, Portland cement (PC), and mineral trioxide aggregate (MTA) are used as root-end filling materials.[4,5,6,7] An ideal root-end filing material should adhere to tooth tissue sealing the root-end and should prevent leakage of micro-organisms into the periradicular tissues. It should be biocompatable and stable. Also, the sealing ability of the material should not be affected by the presence of moisture.[8]
If an adequate apical seal is not achieved, healing may not occur.[9] An ideal healing response after periradicular surgery is evaluated by the reformation of the periodontal ligament; presence of fibrous tissue (scar) and inflammation without scar tissue.[10] Resection of the root-end during periradicular surgery exposes the dentin, which will be surrounded peripherally by cementum with a root canal system (s) located centrally in the root. The deposition of cementum is considered to be a desired healing response which also provides a double seal at the root-end.[11]
Most of the root-end filling materials currently available do not meet the standards of an “root-end” idealistic “root-filling material”.[10] However, MTA has been reported to be a superior root-end filling material compared to the rest root-end.[12,13,14] MTA prevents microleakage and is reported to be highly biocompatible that also favors regeneration of the dental tissues.[1]
Achieving a good apical seal for root canals is known to be associated with good MTA adaptation to dentin. Commonly used root canal irrigation regimen like ethylenediaminetetraacetic acid (EDTA) 0.5%, sodium hypochlorite (NaOCl) 2%, chlorhexidinegluconate (CHX), and normal saline affects the sealing ability of MTA.[15] Very few studies have compared the marginal adaptation of MTA with root dentin while using different application techniques. The purpose of this study was to compare the marginal adaptation of MTA to root dentin between orthograde and retrograde application techniques and also to assess the effect of 17% EDTA on the MTA-root dentin adaptation using microcomputed tomography (micro-CT) analysis.
MATERIALS AND METHODS
Fifty-two single-rooted intact human teeth, extracted for periodontal reasons, were used in present study. Teeth with significant apical curvatures, root fractures, root caries and/or root resorption, immature apices, deep-root concavities, and root canal treatment were excluded. Prior to initiation of the study, the teeth were stored at 4°C in 0.9% sodium chloride solution supplemented with 0.02% sodium azide to prevent bacterial growth.
The teeth were divided into four equal groups (n = 13) according to the proposed root-end filling procedures:
Retrograde MTA (RMTA),
Orthograde MTA (OMTA),
Etched RMTA (ERMTA), and
Etched OMTA (EOMTA).
Root canal preparation
The extracted teeth were placed inside a mandible model in a mannequin to mimic clinical situation. Access cavity was performed in each tooth with a # 2 size round tungsten bur (Brassler, Savannah, GA) mounted on a high-speed handpiece (Dentsply, York, PA). Sterile saline was used as coolant. Canal patency was achieved by passing a size # 10 K-file (Dentsply-Maillefer, Ballaigues, Switzerland) in the root canal until its tip was visible at the apical foramen. Root canals were instrumented using stainless steel K-files # 10, 15, 20 (Dentsply-Maillefer, Ballaigues, Switzerland) and a commercial preparation containing EDTA (Glyde, Dentsply-Maillefer, Ballaigues, Switzerland) to the working length, established 1 mm from the apical foramen. Canal shaping was achieved using ProTaper rotary nickel-titanium files (Dentsply-Maillefer, Tulsa, OK) to F2 size (8% taper, 20/100 tip diameter). Root canals were irrigated between each instrument with 2 ml of 5.25% sodium hypochlorite (Ogna Muggiò, Milan, Italy) and dried. Following this, the teeth were divided into the four groups and treated separately as follows:
Retrograde root-end placement of MTA (Group 1 and 3)
For Groups 1 and 3, the roots were obturated using warm vertical condensation of F2 calibrated gutta-percha points (Dentsply-Maillefer, Tulsa, OK) and AH26 sealer (Dentsply-DeTrey, Konstanz, Germany).[16] After setting of the sealer, teeth were kept in artificial saliva and aerobic environment. Seventy-two hours later, root-end preparations and retrograde fillings were performed by a single operator under a surgical microscope (Global, St. Louis, MO, USA) at× 8 magnification. The teeth were resected apically 3 mm from the root apex at a 900 angle to the long axis of the root using a surgical microscope (Global, St. Louis, MO, USA) at × 8 magnification. The section was made with a tungsten carbide straight fissure bur mounted on contra-angle, high-speed handpiece (Kavo Dentale, Biberach, Germany) and with constant water irrigation to avoid overheating. Feather-like back and forth motions were applied with a slight coronal pressure using p5 ultrasonic (Spartan, MO, USA) and water-cooling. The same power was used in all cases throughout the experiment. A new retro-tip (KiS tips Spartan, MO, USA) was used for each root preparation.
For Group 1 (RMTA), p5 ultrasonic diamond tip (Spartan, Fenton, MO, USA) was used to prepare 3-mm retrograde cavities, which were filled with MTA carried with the microapical placement (MAP) system. Root-ends were wrapped with wet gauze to facilitate MTA setting. In Group 3 (ERMTA), canals were treated the same as Group1 except that canals were irrigated with 17% EDTA for 60 seconds before obturation and placing the MTA.
Orthograde root-end placement of MTA (Groups 2 and 4)
The apical resection was carried out using the same technique as mentioned earlier. For Group 2 (OMTA), the canals were dried with paper points, and the apical 6 mm of the canals was filled with MTA using MAP system, Roydent system. A wet cotton pellet was placed into the canal to facilitate MTA setting and cavity was temporarily closed with Cavit (3M, ESPE, Seefeld, Germany). After 24 hours, MTA setting was confirmed following removal of cotton pellets and canals were obturated using (Kerr Tubli-Seal EWT, Romulus, MI, USA) sealer cement and warm gutta-percha. In Group 4 (EOMTA), canals were treated the same of Group 2 except that before placing the MTA canals were irrigated with 17% EDTA for 60 seconds [Figure 1]. The crowns of the teeth were then sectioned with a slow-speed diamond saw (Isomet, Buehler, Lake Bluff, Il, USA) under water coolant.
Figure 1.
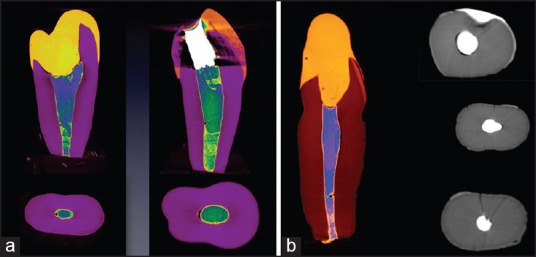
(a) Micro computed tomography photos of RMTA and OMTA analyzing the contact between dentin and MTA. (b) Micro computed tomography photos of Group I (RMTA) analyzing the contact between dentin and MTA
Microcomputed tomography
After 48 hours, all teeth (n = 52) were scanned using and three-dimensional micro-CT images were constructed using an ex vivo micro-CT scanner (model 1172; Skyscan, Kontich, Belgium). This was done to analyze the gap volume present between the root canal obturation, apical filling material, and root dentin. During the scanning process, each tooth was wrapped in parafilm (West Chester, PA, USA) to prevent desiccation. Specimens were scanned at 110 kV and 96 μA with a resolution of 37.4 μm using a 1-mm thick aluminium filter and 54% beam hardening reduction. The region of interest (ROI) was specified as an annular area of 1.7 mm diameter surrounding the tooth over a length of 3 mm.
Statistical analysis
All statistical analyses were carried out using SPSS 17.0 (Statistical Package for the Social Sciences for Windows; SPSS Inc., Chicago, IL, USA). Mean dentin-MTA contact and the mean length and width of each gap was analysed using one-way analysis of variance (ANOVA). Statistical significance was set at an α level of 5%.
RESULTS
No significant difference was observed in the dentin-MTA adaptation for orthograde and retrograde MTA application technique. The reconstructed longitudinal micro-CT images showed gap volumes of 0.224 ± 0.174 mm3 and 0.266 ± 0.233 mm3 for RMTA and OMTA, respectively [Figure 1; Table 1]. No significant difference in gap volumes between RMTA and OMTA were observed.
Table 1.
Mean dentin-MTA contact and Gap volume between the RMTA/OMTA group and between ERMTA/EOMTA group
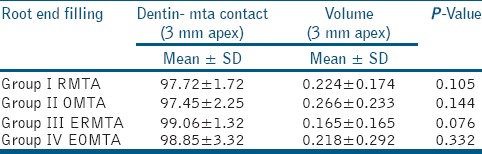
Dentin-MTA adaptation for orthograde and retrograde MTA after the use of 17% EDTA were also not significantly different between the two groups [Table 1]. Gap volumes of 0.165 ± 0.165 mm3 for ERMTA and 0.218 ± 0.292 mm3 for EOMTA [Figure 2; Table 1] were observed. No significant differences in the gap volumes were observed between ERMTA and EOMTA.
Figure 2.
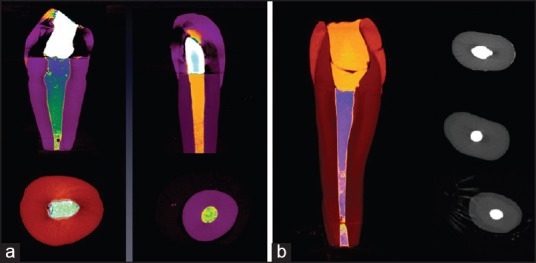
(a) Micro CT photos of group ERMTA and EOMTA analyzing the contact between dentin and MTA. (b) Micro computed tomography photos of Group III (ERMTA) analyzing the contact between dentin and MTA
On comparing the RMTA with ERMTA, it was observed that the MTA dentin adaptation was significantly better in the teeth which were irrigated with 17% EDTA prior to retrograde filling [Table 2]. Significant differences in the gap volumes was observed between RMTA (0.224 ± 0.174 mm3) and ERMTA (0.165 ± 0.165 mm3) (P = 0.045) [Table 2].
Table 2.
Dentin-MTA contact for RMTA/ERMTA and OMTA/EOMTA (retrograde and orthograde with and without 17% EDTA)
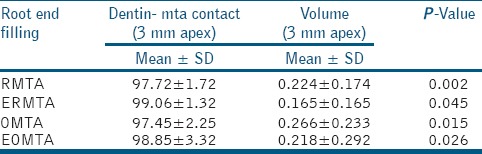
Similarly for orthograde technique, EOMTA showed better MTA root dentin adaptation when compared to OMTA (P = 0.015) [Table 2]. The micro-CT images showed gap volumes of 0.266 ± 0.233 mm3 and 0.218 ± 0.292 mm3 for OMTA and EOMTA, respectively. Significant difference in gap volumes between OMTA and EOMTA was observed (P = 0.026) [Table 2].
DISCUSSION
Conflicting results have been reported regarding the correlation between marginal adaptation and sealing ability of root-end filling materials. This study compared the marginal adaptation of MTA with root dentin between orthograde and retrograde application techniques using micro-CT analysis. Micro-CT helps in the identification of the voids and gap volume between root-end filling material and tooth surface.[17]
No significant difference in the adaptation of MTA to the root dentin surface was observed between orthograde and retrograde application techniques. This was in consensus to the findings of Andelin et al.[18] However, Hachmeister et al.,[19] reported that MTA placed in an orthograde manner in teeth with open apices shows more bacterial leakage compared to those placed in retrograde manner.
Sen et al.,[20] in his review, stated that the presence of a smear layer formed during root canal preparation tend to have an adverse effect on dentine bonding as well as the penetration of the irrigating solution and the sealers to the dentinal tubules. In a study by Timpawat et al.,[21] the authors have reported that that the absence of the smear layer resulted in increased apical microleakage than in the presence of smear layer. Chelating agents have been recommended for chemical and mechanical debridement during root canal therapy for the removal of smear layer. Yildirim et al., in his research evaluated the effect of smear layer on the sealing ability of MTA and reported that the amount of microleakage increases with the removal of smear layer.[22] Whether the presence of the smear layer within root canals is beneficial or not still remains as a controversial issue.[23]
The MTA-Dentin adaptation was significantly improved when 17% EDTA was applied, irrespective of the technique of application (orthograde or retrograde). Di Lenarda et al.,[24] reported that 15% EDTA was able to open the dentinal tubules after 3 minutes of irrigation. This will allow the obturation material to penetrate the tubules therefore improving the adaptation.[20] Nakashima et al.,[25] reported that the contact angle between endodontic sealer solution and dentin increased when 15% EDTA was applied. However, Yan et al.,[26] reported that the bond strength between MTA and dentine was reduced by the use of Glyde Prep gel with EDTA in comparison with other materials like NaOCl and CHX.
In another study, it was observed that irrigation with NaOCl and EDTA increased the microleakage in teeth sealed with MTA apical sealant when compared to irrigation with NaOCl alone.[27] Placing MTA in an orthograde or retrograde manner and then resecting the set material does not necessarily disturb the apical seal of MTA and does not have any effect on apical tissue regeneration Based on previous studies,[28,29] the resection of set MTA does not seem to affect its sealing ability. This is in concensus with the present study.
CONCLUSION
Within the limitations of the present study, it concludes that MTA adaptation to dentine tooth structure is not significantly different between an orthograde and retrograde approach. However, the use of EDTA significantly improved the MTA-Dentin adaptation.
ACKNOWLEDGEMENT
The atuthors thank Engineer Abdullah Bugshan Research Chair for Growth Factors and Bone Regeneration, Saudi Arabia for supporting this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Al-Kahtani A, Shostad S, Schifferle R, Bhambhani S. In-vitro evaluation of microleakage of an orthograde apical plug of mineral trioxide aggregate in permanent teeth with simulated immature apices. J Endod. 2005;31:117–9. doi: 10.1097/01.don.0000136204.14140.81. [DOI] [PubMed] [Google Scholar]
- 2.Gondim E, Zaia AA, Gomes BP, Ferraz CC, Teixeira FB, Souza-Filho FJ. Investigation of the marginal adaptation of root-end filling materials in root-end cavities prepared with ultrasonic tips. Int Endod J. 2003;36:491–9. doi: 10.1046/j.1365-2591.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- 3.Porter ML, Bertó A, Primus CM, Watanabe I. Physical and chemical properties of new-generation endodontic materials. J Endod. 2010;36:524–8. doi: 10.1016/j.joen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Gundam S, Patil J, Venigalla B, Yadanaparti S, Maddu R, Gurram S. Comparison of marginal adaptation of mineral trioxide aggregate, glass ionomer cement and intermediate restorative material as root-end filling materials, using scanning electron microscope: An in vitro study. J Conserv Dent. 2014;17:566. doi: 10.4103/0972-0707.144606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent. 2008;11:141–3. doi: 10.4103/0972-0707.48834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funteas UR, Wallace JA, Fochtman FW. A comparative analysis of mineral trioxide aggregate and portland cement. Aust Endod J. 2003;29:43–4. doi: 10.1111/j.1747-4477.2003.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 7.Girish CS, Ponnappa K, Girish T, Ponappa M. Sealing ability of mineral trioxide aggregate, calcium phosphate and polymethylmethacrylate bone cements on root ends prepared using an Erbium: Yttriumaluminium garnet laser and ultrasonics evaluated by confocal laser scanning microscopy. J Conserv Dent. 2013;16:304–8. doi: 10.4103/0972-0707.114355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gartner AH, Dorn SO. Advances in endodontic surgery. Dent Clin North Am. 1992;36:357–78. [PubMed] [Google Scholar]
- 9.Shahi S, Yavari HR, Rahimi S, Eskandarinezhad M, Shakouei S, Unchi M. Comparison of the sealing ability of mineral trioxide aggregate and Portland cement used as root-end filling materials. J Oral Sci. 2011;53:517–22. doi: 10.2334/josnusd.53.517. [DOI] [PubMed] [Google Scholar]
- 10.Chong B, Pitt Ford T. Root-end filling materials: Rationale and tissue response. Endod Topics. 2005;11:114–30. [Google Scholar]
- 11.Regan JD, Gutmann JL, Witherspoon DE. Comparison of Diaket and MTA when used as root-end filling materials to support regeneration of the periradicular tissues. Int Endod J. 2002;35:840–7. doi: 10.1046/j.1365-2591.2002.00582.x. [DOI] [PubMed] [Google Scholar]
- 12.Saini D, Nadig G, Saini R. A comparative analysis of microleakage of three root end filling materials — an in vitro study. Arch Orofac Sci. 2008;3:43–7. [Google Scholar]
- 13.Schwartz RS, Mauger M, Clement DJ, Walker WA., 3rd Mineral trioxide aggregate: A new material for endodontics. J Am Dent Assoc. 1999;130:967–75. doi: 10.14219/jada.archive.1999.0337. [DOI] [PubMed] [Google Scholar]
- 14.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 15.Hamidi MR, Mesgarani A, Dindar E. Effect of different irrigation regimens on the evaluation of apical sealing ability of mineral trioxide aggregate. Caspian J Dent Res. 2012;1:22–6. [Google Scholar]
- 16.Al-Fouzan K, Al-Garawi Z, Al-Hezaimi K, Javed F, Al-Shalan T, Rotstein I. Effect of acid etching on marginal adaptation of mineral trioxide aggregate to apical dentin: Microcomputed tomography and scanning electron microscopy analysis. Int J Oral Sci. 2013;4:202–7. doi: 10.1038/ijos.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaslansky P, Fratzl P, Rack A, Wu MK, Wesselink PR, Shemesh H. Identification of root filling interfaces by microscopy and tomography methods. Int Endod J. 2011;44:395–401. doi: 10.1111/j.1365-2591.2010.01830.x. [DOI] [PubMed] [Google Scholar]
- 18.Andelin WE, Browning DF, Hsu GH, Roland DD, Torabinejad M. Microleakage of resected MTA. J Endod. 2002;28:573–4. doi: 10.1097/00004770-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Hachmeister DR, Schindler WG, Walker WA, 3rd, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28:386–90. doi: 10.1097/00004770-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Sen BH, Wesselink PR, Turkun M. The smear layer: A phenomenon in root canal therapy. Int Endod J. 1995;28:141–8. doi: 10.1111/j.1365-2591.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 21.Timpawat S, Vongsavan N, Messer HH. Effect of removal of the smear layer on apical microleakage. J Endod. 2001;27:351–3. doi: 10.1097/00004770-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Yildirim T, Oruçoğlu H, Çobankara FK. Long-term evaluation of the influence of smear layer on the apical sealing ability of MTA. J En dod Czonstkowsky M, Wilson EG, Holstein FA. The smear layer in endodontics. Dent Clin North Am. 1990;34:13–25. [Google Scholar]
- 23.Di Lenarda R, Cadenaro M, Sbaizero O. Effectiveness of 1 mol L-1 citric acid and 15% EDTA irrigation on smear layer removal. Int Endod J. 2000;33:46–52. doi: 10.1046/j.1365-2591.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima K, Terata R. Effect of pH modified EDTA solution to the properties of dentin. J Endod. 2005;31:47–9. doi: 10.1097/01.don.0000134205.05404.8e. [DOI] [PubMed] [Google Scholar]
- 25.Yan P, Peng B, Fan B, Fan M, Bian Z. The effects of sodium hypochlorite (5.25%), Chlorhexidine (2%), and Glyde File Prep on the bond strength of MTA-dentin. J Endod. 2006;32:58–60. doi: 10.1016/j.joen.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Yildirim T, Er K, Taşdemir T, Tahan E, Buruk K, Serper A. Effect of smear layer and root-end cavity thickness on apical sealing ability of MTA as a root-end filling material: A bacterial leakage study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e67–72. doi: 10.1016/j.tripleo.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Milani AS, Shakouie S, Borna Z, Sighari Deljavan A, Asghari Jafarabadi M, Pournaghi Azar F. Evaluating the effect of resection on the sealing ability of MTA and CEM cement. Iran Endod J. 2012;7:134–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Lamb EL, Loushine RJ, Weller RN, Kimbrough WF, Pashley DH. Effect of root resection on the apical sealing ability of mineral trioxide aggregate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:732–5. doi: 10.1067/moe.2003.98. [DOI] [PubMed] [Google Scholar]


