Abstract
Objective:
The effect of 10% sodium ascorbate, 10% grape seed extract, and 10% pine bark extract on the shear bond strength of composite resin to bleached enamel was evaluated.
Materials and Methods:
Ninety recently extracted human premolars were divided into six groups of 15 teeth each. Except Group I (negative control), the labial enamel surface of all specimens in the other groups were bleached with 37.5% hydrogen peroxide. After bleaching, Group II specimens were stored in artificial saliva for 3weeks before composite bonding. Immediately following bleaching; Groups III, IV, and V specimens were treated with antioxidants 10% sodium ascorbate, 10% grape seed extract, and 10% pine bark extract, respectively, for 10 min and bonded with composite resin. In Group VI (positive control), the composite bonding was done immediately after bleaching. All specimens were stored in deionized water for 24 h at 37C before shear bond strength testing. The data obtained were tabulated and statistically analyzed using analysis of variance (ANOVA) and Duncan's multiple range test.
Results:
The unbleached teeth showed the highest shear bond strength followed by the bleached teeth treated with the antioxidant 10% pine bark extract.
Conclusion:
Within the limitations of this study, it was observed that the use of antioxidants effectively reversed the compromised bond strength of bleached enamel. Among the antioxidants, 10% pine bark extract application after bleaching showed better bond strength.
Keywords: Antioxidant, bleaching, grape seed extract, oligomeric proanthocyanidin, pine bark extract, shear bond strength, sodium ascorbate
INTRODUCTION
Increased awareness and concern about esthetics has resulted in the popularity of the more conservative technique of tooth whitening with bleaching. Bleaching agents like carbamide peroxide and hydrogen peroxide are used to bleach the teeth that are discolored as a result of extrinsic or intrinsic staining.[1]
Studies have shown that the shear bond strength of composite resin bonded to the tooth surface immediately after bleaching was significantly lower than that on unbleached tooth surface due to the presence of residual oxygen layer. Removal of this residual oxygen layer was found to increase the shear bond strength of composite resin to bleached enamel. The general approach of overcoming this post bleaching compromised bond strength was to delay the bonding procedure by a period varying from 24 h to 3 weeks.[2,3]
Several methods have been proposed to reverse the compromised bond strength following bleaching such as subjecting the bleached enamel to alcohol treatment before the restoration, removing the outermost layer of enamel, and the use of organic solvent containing adhesives.[4] Others include exposing the bleached enamel specimens to water, saline solution, or artificial saliva.[5] Antioxidants like 10% sodium ascorbate have also been used to reverse the reduced bond strength of bleached enamel.[6,7,8]
The interest in natural antioxidants of plant origin has greatly increased in recent years. Oligomericproanthocyanidin complexes present in natural antioxidants like grape seed extract and pine bark extract have free radical scavenging activity.[9] The aim of this in vitro study was to evaluate and compare the effects of oligomeric proanthocyanidin on the bond strength of composite resins to bleached enamel. The hypothesis of this study was that the use of an antioxidant reversed the reduced bond strength of composite resins to bleached enamel.
MATERIALS AND METHODS
Preparation of solutions
Ten grams of sodium ascorbate powder (SD Fine Chem Limited, Mumbai, India) was dissolved in 100ml of distilled water to make 10% sodium ascorbate solution, and 10% solutions of grape seed extract and pine bark extract were obtained from Aver Dynam Herbals Private Limited, India.
Specimen preparation
Ninety recently extracted human premolars of comparable sizes and from the same age group were used in this study. The teeth which were free of caries, cracks, wear lesions, or developmental enamel defects were selected using a stereomicroscope (Magnus MS 13/MS24, Olympus India Pvt Ltd, New Delhi). The teeth were stored in a disinfectant solution of 0.1% thymol (NICE Chemical Laboratory Supplies Limited, India) prior to the study. All teeth were decoronated at the cementoenameljunction using water cooled diamond disc in a hard tissue sectioning machine (Labcut Extec Corp. Enfield, USA) and the decoronated surface was sealed with self-cureacrylic resin. All the specimens were then embedded in self-cure acrylic resin molds such that the labial surfaces faced upwards. The samples were kept in deionized water (NICE Chemical Laboratory Supplies Limited, India) until the resin was completely cured. The labial surfaces of teeth were polished with 600 grit silicon carbide paper (Moyco Precision Abrasives, Montgomeryville, PA, USA) for 60 s to create smooth, flat surfaces for treatment and bonding.[10]
The specimens were randomly assigned into six groups of 15 teeth each [Table 1]. All specimens except Group I (negative control) were bleached with 37.5% hydrogen peroxide, HP gel - Pola Office Plus (SDI Limited, Bayswater, Victoria 3153, Australia) with three applications of eight min each. Following bleaching, the specimens were thoroughly rinsed with deionized water for 1 min.
Table 1.
The distribution of specimens and study groups
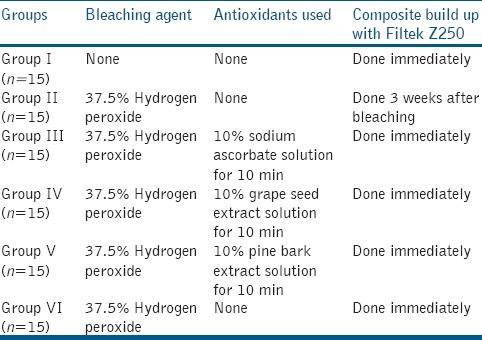
In Group I (negative control), the unbleached teeth were bonded with composite. In Group II, bleaching was followed by storage in 250 ml of artificial saliva solution (Wet Mouth, ICPA Health Products, India) at 37°C for 3 weeks. Bonding with composite was done 3weeks after bleaching.
In Groups III, IV, and V, bleaching was followed by treatment with antioxidants 10% sodium ascorbate solution, 10% grape seed extract solution, and 10% pine bark extract solution respectively for 10 min. This was followed by rinsing with deionized water for 30 s and then bonded with composite. In Group VI (positive control), bonding with composite was done immediately following bleaching.
For composite bonding, labial enamel surface of all specimens were etched with 37% phosphoric acid (3M ESPE Dental Products) for 15 s, rinsed off with deionized water for 20 s, blot dried, and bonded with Adper Single bond (3M ESPE Dental Products, St Paul, MN, USA). A plastic mold of 5 ml height and 3 ml diameter was used for composite build up (Filtek Z 250- 3M ESPE, Dental products) on the labial surface. Composite resin was build up in increments of 2 ml or less with each increment being light-cured for 40 s using a quartz tungsten halogen light curing unit- Spectrum 800 (Dentsply Caulk, Milford, DE, USA).
All the specimens were stored in deionized water for 24 h at 37°C prior to shear bond strength testing.
Shear bond strength testing
Each specimen was placed in between the jigs of the universal testing machine (Model 3345; Instron Corp, Canton, Mass, USA) and a pointed shearing rod was placed on the composite resin/tooth interface and was subjected to static loading at a rate of 0.5 mm/min until fracture occurred. The machine was interfaced with a computer through which the operation was controlled and shear bond strength was calculated.
Statistical analysis
The values obtained were statistically analyzed using computer software Statistical Package for Social Sciences (SPSS) version 16.0. One-way analysis of variance (ANOVA) followed by Duncan's multiple range test was used to analyze the data. Significance was established at P < 0.05 level.
RESULTS
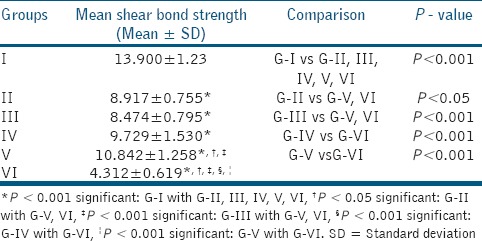
Highest mean shear bond strength value was observed in Group I, which was statistically significant compared to other groups (P < 0.001). The mean shear bond strength value of Group II was statistically significant with that of Group V (P < 0.05) and Group VI (P < 0.05), but not with that of Groups III and IV (P > 0.05). The mean shear bond strength value of Group III was statistically significant compared to Group V (P < 0.001) and Group VI (P < 0.001), but not with that of Group IV (P > 0.05). The mean shear bond strength value of Group IV was statistically significant when compared to that of Group VI (P < 0.001). The mean shear bond strength values of Groups IV and V were not statistically significant (P > 0.05). Group VI showed the least mean shear bond strength values compared to all other groups and was statistically significant (P < 0.001) [Table 2].
Table 2.
Comparison of mean shear bond strength (Mpa) values between thedifferent groups
DISCUSSION
All the three antioxidants were capable of reversing the reduced bond strength following bleaching which confirmed our study hypothesis.
Human premolars recently extracted for orthodontic purposes were selected for this study. Following extraction they were stored in a disinfectant solution of 0.1% thymol solution until use to prevent cross infection.[5] The decoronated tooth surface was sealed with acrylic resin to avoid the penetration of the embedding medium or the bleaching solution into the pulp chamber.[8] The embedded specimens in acrylic resin molds were kept in deionized water until the resin was completely cured in order to avoid the thermal effects generated by the resin curing process.[1] Bonded specimens were stored in deionized water for 24 h at 37°C before shear bond strength testing to artificially age the composite resins. Artificial aging simulates the chemical and physical oral environments, producing in a relatively short period, degradation similar to that which a composite resin would undergo in its clinical life.[11]
Peroxide containing bleaching agents remove tooth discolorations through oxidation. Hydrogen peroxide, a low molecular weight substance, decomposes into oxygen and perhydroxyl free radicals. The latter is associated with high permeability and high diffusibility in the tooth structure. Perhydroxyl radicals attack the long chained, dark colored macromolecules of pigments and spilt them into smaller, less colored and more diffusible molecules which are removed from the structure producing the bleaching effect.[12] Free radicals released from the hydrogen peroxide permeate into the enamel surface through interprismatic regions and may react not only with the pigmented organic molecules, but also with the organic enamel. The removal of organic material can result in morphological alterations and surface irregularities. Thus, despite the favorable results achieved with the bleaching agents, several studies have also reported surface alterations, decreased microhardness, and reduced bond strength of composite resin to enamel following bleaching.[13]
In this study, 37.5% hydrogen peroxide, HP gel (Pola Office Plus, SDI) was used for three applications of 8 min each. The reason for the gel to be refreshed is due to the fast degradation of hydrogen peroxide since significant amount of active ingredients are available only for the first 15-20 minutes. In order to sustain the same degree of bleaching obtained in the first 15 min, three applications of 8 min each were used for bleaching. Moreover, studies have shown that hydrogen peroxide used for tooth whitening has a pH around 7 immediately after application; but when single application of longer time periods is used, the pH of the gel gets decreased to approximately 5, which may increase tooth sensitivity.[14]
The compromised bond strength following bleaching is due to the fact that the bleaching agent leaves behind aresidual oxygen layer which interferes with the resin infiltration into etched enamel and inhibits the polymerization of resin.[15,16] During bleaching with hydrogen peroxide, peroxide apatite is formed as a result of the substitution of hydrogen radicals by peroxide ions. The structural changes caused by the incorporation of peroxide ions are eliminated upon storage for 2-3 weeks as the peroxide ions decomposes and the substituted hydroxyl radicals reenter the apatite lattice.[17]
Studies have suggested post bleaching waiting periods of varying durations to reverse the compromised bond strengths. In the first 2 weeks after bleaching, the bond strength of composite resins to enamel was found to be low. However, after 3 weeks, thebond strength values reversed to that of the unbleached teeth.[18]
Despite the favorable results achieved with the bleaching agents, studies have reported surface alterations and decreased microhardness of enamel and reduced bond strength of composite resin to enamel following bleaching.[10]
The reduced bond strength in Group VI when compared to Group I may be due to the residual oxygen layer left behind following the bleaching process which could have interfered with the resin infiltration into etched enamel and inhibited the polymerization of resin.[15,16] Group II specimens showed significantly higher bond strength than that of Group VI. These findings were in accordance with the findings of other studies which states that delaying the bonding procedures following bleaching could reverse the compromised bond strength as a result of the removal of residual oxygen.[2,3] Group III specimens showed comparable values as that of Group II, which supports the fact that the use of antioxidants immediately following bleaching could neutralize the residual oxygen and reverse the reduced bond strength.[6,7,8] Group III specimens could not reverse the bond strength as much as that of Groups IV and V which could be attributed to the fact that oligomericproanthocyanidins present in grape seed extract and pine bark extract are powerful antioxidants, far more potent than the common antioxidants like vitamins C, E, and beta-carotene.[19] Group V specimens showed a higher mean bond strength value than that of Group IV specimens and was not statistically significant. The difference in the antioxidant activity of pine bark extract and grape seed extract could probably be attributed to their different phenolic compositions.[9]
Sodium ascorbate is a derivative of ascorbic acid with a neutral pH. It has been reported that sodium ascorbate is a potent antioxidant capable of quenching the reactive free radicals. It neutralizes the effect on the residual oxygen layer, allows free radical polymerization of resin base materials to proceed without premature termination by restoring the altered redox potential of the oxidized bonding substrate, thus reversing the compromised bonding.[15] So in this study, 10% solution of sodium ascorbate was used.
Studies have shown that the inclusion of peroxide ions may be reversed by the use of antioxidants. An antioxidant solution of 10% sodium ascorbate applied on the bleached enamel surface for 10 min effectively reversed the reduced bond strength.[20,21] However, SEM images have demonstrated an etched appearance on enamel surfaces after ascorbic acid usage in bleached enamel specimens with the ascorbic acid causing super etching of the already bleached surface.[22]
The utilization of plant extracts as a viable alternative to chemical and synthetic antioxidants have been encouraging.[23] Hence in this study, emphasis was placed on the use of oligomericproanthocyanidins as antioxidants immediately following the bleaching procedure to reverse the compromised bond strength of composite resins to bleached enamel.
Studies have shown that the application of 5% grape seed extract as an antioxidant for 10 min reversed the compromised bond strength.[24] Application of 5% pine bark extract as an antioxidant for 10 min yielded almost the same bond strength as that of 10% sodium ascorbate.[17] In this study, 10% solutions of grape seed extract and pine bark extract were applied for 10 min in order to evaluate and compare the effect of natural antioxidants on the reversal of compromised bond strength of composite resins to bleached enamel.
Oligomericproanthocyanidins are a class of polyphenolic bioflavonoids found in fruits and vegetables and are present in grape seed extract, pine bark extract, cranberries, lemon tree bark, hazel nut tree leaves, etc. They have free radical scavenging and antioxidant activity. They also have antibacterial, antiviral, anti-inflammatory, antiallergic, anticarcinogenic, and vasodilatory actions.[25]
Grape seed extract contains oligomericproanthocyanidins and free flavanol monomers. Proanthocyanidins in the form of monomeric phenoliccompounds such as catechin, epicatechin, andepicatechin-3-0-gallate, and in dimeric, trimeric, and tetramericprocyanidin forms are present.[23]
Pine bark extract contains phenolic compounds broadly divided into monomers (catechin, epicatechin, and taxifolin) and condensed flavonoids (oligomeric to polymeric proanthocyanidins).[26]
Flavonoids can perform scavenging action on free radicals like superoxide, hydroxyl, and 1,1-dipheny l-2-picrylhydrazyl (DPPH) and have metal chelating properties. The presence of the functional group OH in the structure and its position on the ring of the flavonoid molecule determines its antioxidant capacity.[23] The antioxidant properties of grape seed extract and pine bark extract could be due to the flavonoids present.
According to the material safety analyses, the level of health hazard for sodium ascorbate is higher than that of oligomeric proanthocyanidins. Moreover, sodium ascorbate has been found to be mutagenic for mammalian somatic cells, while oligomeric proanthocyanidins have no mutagenic effect when their material safety data's were examined.[23] Natural antioxidants used in this study were capable of reversing the compromised bond strength of composite resins to bleached enamel. So this could be used to avoid the waiting period before bonding to bleached enamel which makes it clinically significant. Even though studies on grape seed extract showing its capability of reversing bond strength to bleached enamel has been done, it has not been compared with pine bark extract in this aspect.
However, this being an in vitro study, it cannot mimic the in vivo conditions. In the oral cavity, the interface between the restoration and the tooth is exposed to diverse forces that act simultaneously. During its life time, a restoration is subjected to cyclic loading; each load is insufficient to provoke failure, but in the long-term can possibly lead to marginal deterioration and loss of restoration. Therefore, fatigue testing of dental adhesives is expected to better predict their in vivo performance. Nevertheless, the more expensive and long-lasting clinical trials remain necessary to validate the laboratory observations.[27] Since the results of this study showed a high statistical significant value, it could reproduce clinical significance as well. Further clinical trials are needed to confirm these findings.
CONCLUSION
Within the limitations of this in vitro study, it was observed that among the different antioxidants used, 10% pine bark extract was superior in reversing the reduced bond strength of composite resin to bleached enamel.
ACKNOWLEDGEMENT
We are grateful to the staff of Department of Polymer Processing laboratory, Biomedical technology Wing, Sree Chitra Thirunal Institute of Medical Science and technology, Poojapura, Thiruvananthapuram for all their support in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Joiner A. The bleaching of teeth: A review of the literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Borges AB, Rodrigues JR, Borges AL, Marsilio AL. The influence of bleaching agent son enamel bond strength of a composite resin according to the storage time. Rev Odontol UNESP. 2007;36:77–83. [Google Scholar]
- 3.Tabatabaei MH, Arami S, Nojoumian A, Mirzaei M. Antioxidant effect on the shear bond strength of composite to bleached bovine dentin. Braz J Oral Sci. 2011;10:33–6. [Google Scholar]
- 4.Silva JM, Botta AC, Barcellos DC, Pagani C, Torres CR. Effect of antioxidant agents on bond strength of composite to bleached enamel with 38% hydrogen peroxide. Mater Res. 2011;14:235–8. [Google Scholar]
- 5.Metz MJ, Cochran MA, Matis BA, Gonzalez C, Platt JA, Pund MR. Clinical evaluation of 15% carbamide peroxide on the surface microhardness and shear bond strength of human enamel. Oper Dent. 2007;32:427–36. doi: 10.2341/06-142. [DOI] [PubMed] [Google Scholar]
- 6.Dabas D, Patil AC, Uppin VM. Evaluation of the effect of concentration and duration of application of sodium ascorbate hydrogel on the bond strength of composite resin to bleached enamel. J Conserv Dent. 2011;14:356–60. doi: 10.4103/0972-0707.87197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimyai S, Rahimi S, Lotfi M, Valizadeh H, Mohammadi N, Zareh EJ. Effect of two forms of sodium ascorbate on microleakage of composite restorations immediately after bleaching. J Dent. 2009;6:78–84. [Google Scholar]
- 8.Torres CR, Koga AF, Borges AB. The effects of anti-oxidant agents as neutralizers of bleaching agents on enamel bond strength. Braz J Oral Sci. 2006;5:971–6. [Google Scholar]
- 9.Wood JE, Senthilmohan ST, Peskin AV. Antioxidant activity of procyanidin — containing plant extracts at different pHs. Food Chem. 2002;77:155–61. [Google Scholar]
- 10.Nour El-din AK, Miller BH, Griggs JA, Wakefield C. Immediate bonding to bleached enamel. Oper Dent. 2006;31:106–14. doi: 10.2341/04-201. [DOI] [PubMed] [Google Scholar]
- 11.Melo MA, Moyses MR, Santos SG, Alcantara CE, Riberio JC. Effects of different surface treatments and accelerated aging on the bond strength of composite resin repairs. Braz Oral Res. 2011;25:485–91. doi: 10.1590/s1806-83242011000600003. [DOI] [PubMed] [Google Scholar]
- 12.Tezel H, Kemaloglu H. Susceptibility of enamel treated with bleaching agentsto mineral loss after cariogenic challenge. In: Ming-yu Li., editor. Contemporary Approach to Dental Caries. 1st ed. Shangai, China: In Tech publishing company; 2012. pp. 75–92. [Google Scholar]
- 13.Sasaki RT, Arcanjo AJ, Florio FM, Basting RT. Micromorphology and microhardness of enamel after treatment with home-use bleaching agents containing 10% carbamide peroxide and 7.5% hydrogen peroxide. J Appl Oral Sci. 2009;17:611–6. doi: 10.1590/S1678-77572009000600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis A, Tay LY, Herrera DR, Kossatz S, Loguercio AD. Clinical effects of prolonged application time of an in- office bleaching gel. Oper Dent. 2011;36:590–6. doi: 10.2341/10-173-C. [DOI] [PubMed] [Google Scholar]
- 15.Kimyai S, Valizadeh H. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent. 2006;31:496–9. doi: 10.2341/05-85. [DOI] [PubMed] [Google Scholar]
- 16.Khoroushi M, Feiz A, Khodamoradi R. Fracture resistance of endodontically-treated teeth: Effect of combination bleaching and an antioxidant. Oper Dent. 2010;35:530–7. doi: 10.2341/10-047-L. [DOI] [PubMed] [Google Scholar]
- 17.Aksakalli S, Ileri Z, Karacam N. Effect of pine bark extract on bond strength of brackets bonded to bleached human tooth enamel. Acta Odontol Scand. 2013;71:1555–9. doi: 10.3109/00016357.2013.776108. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira R, Nunes TV, Luiz BK, Garcia RN. Effects of catalase, 2% chlorhexidine gel and 1% sodium hypochlorite on the microtensile bondstrength of teeth bleached with 35% hydrogen peroxide. RSBO. 2011;8:266–70. [Google Scholar]
- 19.Leigh, Jacena M. Health benefits of grape seed proanthocyanidin extract (GSPE) Nutr Noteworthy. 2003;6:1–5. [Google Scholar]
- 20.Kaya AD, Turkun M, Arici M. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent. 2008;33:441–7. doi: 10.2341/07-115. [DOI] [PubMed] [Google Scholar]
- 21.Danesh-Sani SA, Esmaili M. Effect of 10% sodium ascorbate hydrogel and delayed bonding on shear bond strength of composite resin and resin- modified glass ionomer to bleached enamel. J Conserv Dent. 2011;14:241–6. doi: 10.4103/0972-0707.85799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muraguchi K, Shigenobu S, Suzuki S, Tanaka T. Improvement of bonding to bleached bovine tooth surfaces by ascorbic acid treatment. Dent Mater J. 2007;26:875–81. doi: 10.4012/dmj.26.875. [DOI] [PubMed] [Google Scholar]
- 23.Perumalla AV, Hettiarachchy NS. Green tea and grape seed extracts-Potential applications in food safety and quality. Food Res Int. 2011;44:827–39. [Google Scholar]
- 24.Vidhya S, Srinivasulu S, Sujatha M, Mahalaxmi S. Effect of grape seed extract on the bond strength of bleached enamel. Oper Dent. 2011;36:433–8. doi: 10.2341/10-228-L. [DOI] [PubMed] [Google Scholar]
- 25.Fine AM. Oligomeric proanthocyanidin complexes: History, structure and phytopharmaceutical applications. Altern Med Rev. 2000;5:144–51. [PubMed] [Google Scholar]
- 26.Kim SM, Kang SW, Jeon JS, Um BH. A comparison of pycnogenol and bark extracts from Pinus thunbergii and Pinus densiflora: Extractability, antioxidant activity and proanthocyanidin composition. J Med Plants Res. 2012;6:2839–49. [Google Scholar]
- 27.Lai SC, Mak YF, Cheung GS, Osorio R, Toledano M, Carvalho RM, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80:1919–24. doi: 10.1177/00220345010800101101. [DOI] [PubMed] [Google Scholar]


