Abstract
Aim:
The aim of the study is to evaluate and compare the antimicrobial efficacy of two new materials MTA Plus and Biodentine with ProRoot MTA using tube dilution method.
Materials and Methods:
The materials used were ProRoot MTA (Dentsply), MTA Plus (compounded by Prevest Denpro, Jammu, India for Avalon Biomed Inc, USA) and a calcium silicate based material Biodentine (Septodont, Saint-Maur-des-Fosses, France). Doubling dilutions of the material were prepared in Sabouraud's dextrose broth (SDB) and Brain Heart Infusion (BHI) broth for Candida albicans and Enterococcus faecalis, respectively. The minimal concentration at which inhibition of microorganism occurred was measured and noted as minimal inhibitory concentration (MIC) of the material.
Results:
There was no statistically significant difference between the materials against C. albicans. Biodentine was statistically significant than MTA Plus against E. faecalis (P-value-0.022). ProRoot MTA was statistically significant at different time intervals against E. faecalis (P-value-0.001).
Conclusion:
ProRoot MTA and Biodentine proved to have antimicrobial property. MTA Plusproved as a good antifungal agent.
Keywords: Antimicrobial efficacy, minimal inhibitory concentration, root-end filling
INTRODUCTION
Microorganisms play a key role in the development of pulpal and periapical diseases. Pulpal diseases leading to inflammation of periapical tissues show the presence of bacteria in the root canal system. Teeth with pulpal or periapical pathology have a complex microbial flora consisting of cocci, rods, spirochetes, and fungi.[1] Studies have shown that there is a difference between the microbial flora of root canal in cases of primary endodontic infection and in cases of reinfection.[2] The microorganisms in retreatment cases possess greater resistance to intracanal medicaments. Also, it is found that microbes not only grow in planktonic cells or in aggregates but also forms biofilms.[3] It was in 1894 when WD Miller first published his observations on microflora. Since then studies have shown that the endodontic environment is selective and it supports the specific microorganisms to grow.[4]
Later, Fabricius et al., in 1982 showed that strict anaerobes succeed over facultative anaerobes in root canal due to changes in root canal ecology.[5] Studies have also shown that in post treatment diseased teeth, gram positive microorganisms predominate.[6,7] Some of these microorganisms have the capability to survive in harsh and nutrient limited condition in root canal filled teeth. Teeth with persistent disease showed a high prevalence of Enterococci ranging from 29 to 77%.[8,9] Enterococcus faecalis can withstand high pH of intracanal dressings like calcium hydroxide, and is hence found in higher concentration in reinfection cases.[10]
The other common organism which persists in post treatment apical pathology is Candida albicans.[9] These resemble Enterococci in some characteristics. Both these organisms can survive as monoinfection and invade dentinal tubules.[11] Hence, amongst the 24 million endodontic treatment performed on an annual basis, 5.5% procedures involve endodontic surgery and perforation repair.[12]
When endodontic surgeries are performed at the apex, it involves placement of the material at apical end of the root to seal the root canal from the periapex. Historically, a plethora of materials have been used from amalgam, zinc oxide eugenol, composite resins, and glass ionomer cements.[13] Unfortunately these materials failed to satisfy the ideal requirements of root-end filling material.[14] Later in 1990s, mineral trioxide aggregate (MTA) was introduced as a root-end filling material. Till date it is being used as a material of choice for root-end filling. MTA has wide applications in operative dentistry and endodontics. MTA has the disadvantages of long setting time and poor handling properties.[15,16]
Recently, two new materials have been introduced into market, namely, MTA like material MTA Plus (compounded by Prevest Denpro, Jammu, India for Avalon Biomed, Inc USA) and a calcium silicate based material Biodentine (Septodont, Saint-Maur-des-Fosses, France). Manufacturers claim that these materials can be used as a root-end filling material.
Thus, the aim of the study is to evaluate and compare the antimicrobial efficacy of ProRoot MTA, MTA Plus, and Biodentine against the E. faecalis and C. albicans.
MATERIALS AND METHODS
The materials evaluated for antimicrobial efficacy were ProRoot MTA (Dentsply), MTA Plus (compounded by Prevest Denpro, Jammu, India for Avalon Biomed, Inc USA), and Biodentine (Septodont, Saint-Maur-des-Fosses, France).
Two hundred milligrams of the material was dissolved in 20 ml of sterile Brain Heart Infusion (BHI) broth. This solution now contained 10 mg of material in 1 ml of broth and is called master dilution. Nine test tubes were arranged in a row. Further dilutions were prepared from tube no. 2 to 6 such that subsequent test tubes had half the concentration of material than the previous. Hence, tube no. 6 had the concentration of 0.156 mg/ml.
Ten microliter of standardized E. faecalis was added to tube no. 1-6 and tube no. 8. Tube no. 7 was used to check for sterility of the material. Tube no. 8 and 9 were used as positive and negative control, respectively. The tubes were then incubated at 35°C. Subcultures from each of the tubes were made on Mac Conkey's agar and read at the end of 24, 48, and 72 h of incubation. Ten different strains of E. faecalis were used for evaluation of antibacterial efficacy of the materials in a similar manner.
For antifungal efficacy, similar methodology was followed. Sabouraud's dextrose broth (SDB) and Sabouraud's dextrose agar (SDA) was used for dilutions and culture, respectively. Ten different strains of C. albicans were used for evaluation of antifungal efficacy of the materials.
The results were tabulated and statistically analyzed using one-way analysis of variance (ANOVA) and Scheffe's post-hoc tests using Statistical Package for Social Sciences (SPSS) 20#.
RESULTS
Results of antimicrobial efficacy were evaluated between different time intervals andbetween different materials using one-way ANOVA followed by Scheffe's post-hoc test.
Antibacterial efficacy
There was a statistically significant difference between the materials at all time intervals (24, 48, and 72 h) with P-value 0.019, 0.000, and 0.043, respectively [Table 1]. Scheffe's post-hoc test revealed that there was statistically significant difference between Biodentine and MTA Plus at 24 and 48 h with P-value of 0.022 and 0.002, respectively. Biodentine showed statistically significant difference with ProRootMTA at 48 h with P-value 0.000.
Table 1.
One-way ANOVA test between three materials at different time intervals
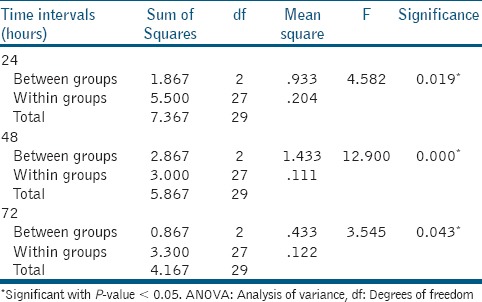
ProRootMTA showed statistically significant difference at different time intervals with P-value 0.001 [Table 2]. Scheffe's post-hoc tests revealed there was statistically significant difference in antibacterial efficacy of ProRoot MTA between 24 and 48 h (P-value 0.04) and between 24 and 72 h (P-value 0.004). There was statistically no significant difference for Biodentine and MTA Plus at different time intervals.
Table 2.
One-way ANOVA test for materials between different time intervals
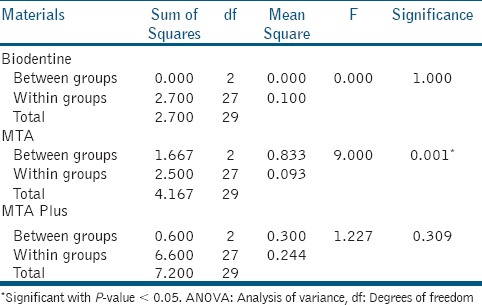
Antifungal efficacy
There was no statistically significant difference between MTA Plus, Biodentine, and MTA at all time intervals (24, 48, and 72 h).
DISCUSSION
The most common method for assessing the antimicrobial activity of root-end filling materials is agar diffusion test. However, this method has disadvantages like lack of standardization of inoculums density, adequate culture medium, agar viscosity, etc.[17,18] In this study, tube dilution method was used to evaluate the antimicrobial efficacy of the materials. It is an efficient method for evaluating as there is a direct contact between the microorganisms and experimental materials which allows a more realistic interaction.[19] Various dilutions of the material are prepared and the concentration at which the material was able to inhibit the growth of microorganism was recorded as the minimal inhibitory concentration (MIC) of the material.
The results of antibacterial efficacy against E. faecalis showed that ProRoot MTA and Biodentine inhibited the growth of majority of strains of E. faecalis; whereas, MTA Plus was not that effective. The MIC at which ProRoot MTA and Biodentine were effective in inhibiting E. faecalis is 5 mg/ml. Whereas, MTA Plus inhibited a few strains at 10 mg/ml. For antifungal efficacy, there was no statistically significant difference between ProRoot MTA, MTA Plus, and Biodentine. The MIC at which ProRoot MTA, MTA Plus, and Biodentine were effective in inhibiting C. albicans is 2.5 mg/ml.
The antimicrobial efficacy of ProRoot MTA is due to release of calcium ions and hydroxyl ions which results in increase in pH.[20,21,22] This release of calcium is when calcium silicate gets hydrated and calcium hydroxide is released as a byproduct. Also, the dissociation of calcium hydroxide leads to release of calcium ions. The antimicrobial effect of ProRoot MTA against E. faecalis and C. albicans obtained in this study is similar to other studies.[23]
MTA Plus is available in the powder form which is sealed in a desiccant container. It consists of tricalcium silicate, dicalcium silicate, bismuth oxide calcium sulfate, and silica. It can be mixed with either water or an antiwashout gel. On hydration of MTA Plus, strong peak of calcium hydroxide was elicited. A study has shown increased calcium ion release over time. The release of calcium ions and subsequent increase in pH leading to alkaline nature of the material is significant only after 7 and 14 days.[24] This could be the reason why MTA Plus did not prove to have a good antibacterial property. But it proved to have good antifungal property.
A calcium silicate-based material Biodentine was introduced in 2010 by Gilles and Olivier. Biodentine showed higher release of free calcium ions when compared with ProRoot MTA, and hence higher alkalinizing capacity. The high release of calcium in Biodentine is attributed to presence of calcium silicate and calcium chloride.[25] Biodentine proved to have a better antibacterial and antifungal property.
CONCLUSION
Within the limitations of the study, ProRoot MTA and Biodentine proved to have antifungal and antibacterial property. This study shows that MTA plus was a good antifungal agent, but not an efficient antibacterial. The antibacterial activity of MTA Plus will probably be good at a higher concentration.
ACKNOWLEDGEMENT
The Rajiv Gandhi University of Health Sciences for funding (research grant no: RGUHS/R&D/Research Grants/D06/2012-13); Dr Carolyn Primus for the material MTA Plus; Department of Microbiology, SDM College of Medical Sciences for assistance and continued support.
Footnotes
Source of Support: The project was funded by Rajiv Gandhi University Health Sciences, Bangalore, Karnataka. The grant no is RGUHS/R&D/Research Grants/D06/2012-13
Conflict of Interest: None declared.
REFERENCES
- 1.Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995;11:6–9. doi: 10.1111/j.1600-9657.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 2.Sundqvist G, Figdor D. Life as an endodontic pathogen. Etiological difference between untreated and root filled canals. Endod Topics. 2003;6:3–28. [Google Scholar]
- 3.Nair PN. On the causes of persistent apical periodontitis a review. Int End J. 2006;39:249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller WD. An introduction to the study of the bacteriopathology of the dental pulp. Dent Cosmos. 1894;36:505–27. [Google Scholar]
- 5.Fabricius L, Dahlen G, Ohman AE, Moller AJ. Predominant indigenous oral bacteria isolated from infected root canals after varied times of closure. Scand J Dent Res. 1982;90:134–44. doi: 10.1111/j.1600-0722.1982.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 6.Molender A, Reit C, Dahlen G, Kvist T. Microbiological status of root filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 7.Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative retreatment. Oral Surg Oral Med Oral pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 8.Peciuliene V, Balciuniene I, Haapasalo M, Eriksen HM. Isolation of enterococcus faecalis in previously root filled canals in a Lithuanian population. J Endod. 2000;26:593–5. doi: 10.1097/00004770-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–34. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 10.Hancock HH, III, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a north american population. Oral Surg Oral Med Oral pathol Oral Radiol Endod. 2001;91:579–86. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 11.Waltimo TM, Sen BH, Meurman JH, Orstavik D, Haapasalo MP. Yeasts in apical periodontitis. Crit Rev Oral Biol Med. 2003;14:128–37. doi: 10.1177/154411130301400206. [DOI] [PubMed] [Google Scholar]
- 12.Nash KD, Brown LJ, Hicks ML. Private practicing endodontists: Production of endodontic services and implications for workforce policy. J Endod. 2002;28:699–705. doi: 10.1097/00004770-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Chong BS. Managing endodontic failures in practice. In: Chong BS, editor. 1st ed. Vol. 23. Chicago: Quintessence Publishing Co; ltd; 2004. p. 12. [Google Scholar]
- 14.Johnson BR. Considerations in the selection of a root end filling material. Oral Surg Oral Med Oral pathol Oral Radiol Endod. 1999;87:398–404. doi: 10.1016/s1079-2104(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 15.Torabinejad M, Hong CU, Mc Donald F, Pitt Ford TR. Physical and chemical properties of a new root end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 16.Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root end filling material. J Endod. 2005;31:665–8. doi: 10.1097/01.don.0000157993.89164.be. [DOI] [PubMed] [Google Scholar]
- 17.Al-Nazhan S, Al-Judai A. Evaluation of antifungal activity of mineral trioxide aggregate. J Endod. 2003;29:826–7. doi: 10.1097/00004770-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Sipert CR, Hussne RP, Nishiyama CK, Torres SA. In vitro antimicrobial activity of Fill Canal, Sealapex, Mineral Trioxide Aggregate, Portland cement and EndoRez. Int Endod J. 2005;38:539–43. doi: 10.1111/j.1365-2591.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi Z, Modaresi J, Yazdizadeh M. Evaluation of the antifungal effects of mineral trioxide aggregate materials. Aust Endod J. 2006;32:120–2. doi: 10.1111/j.1747-4477.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- 20.Odabas ME, Cinar C, Akca G, Araz I, Ulusu T, Yucel H. Short-termantimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dent Traumatol. 2011;27:189–94. doi: 10.1111/j.1600-9657.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 21.Andelin WE, Shabahang S, Wright K, Torabinejad M. Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. J Endod. 2003;29:646–50. doi: 10.1097/00004770-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Accorinte Mde L, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E, Jr, et al. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp capping agent in human teeth. J Endod. 2008;34:1–6. doi: 10.1016/j.joen.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Pires de-Souza Fde C, Moraes PC, Garcia Lda F, Aguilar FG, Watanabe E. Evaluation of pH, calcium ion release and antimicrobial activity of a new calcium aluminate cement. Braz Oral Res. 2013;27:324–30. doi: 10.1590/s1806-83242013000400006. [DOI] [PubMed] [Google Scholar]
- 24.Formosa LM, Mallia B, Camilleri J. Mineral trioxide aggregate with anti-washout gelproperties and microstructure. Dent Mater. 2013;29:294–306. doi: 10.1016/j.dental.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Gandolfi MG, Siboni F, Polimeni A, Bossu FR, Riccitiello F, Rengo S, et al. In vitro screening of the apatite-forming ability, biointeractivity and physical properties of a tri calcium silicate material for endodontics and restorative dentistry. Dent J. 2013;1:41–60. [Google Scholar]


