Abstract
Under normal circumstances, the respiratory tract maintains immune tolerance in the face of constant antigen provocation. Using a murine model of tolerance induced by repeated exposure to a low dose of aerosolized antigen, we show an important contribution by CD4+ T cells in the establishment and maintenance of tolerance. The CD4+ T cells expressed both cell surface and soluble TGF-β and inhibited the development of an allergic phenotype when adoptively transferred to naive recipient mice. While cells expressing cell surface TGF-β were detectable in mice with inflammation, albeit at a lower frequency compared with that in tolerized mice, only those from tolerized mice expressed FOXP3. Blockade of TGF-β in vitro and in vivo interfered with immunosuppression. Although cells that expressed TGF-β on the cell surface (TGF-β+), as well as the ones that did not (TGF-β–), secreted equivalent levels of soluble TGF-β, only the former were able to blunt the development of an allergic phenotype in mice. Strikingly, separation of the TGF-β+ cells from the rest of the cells allowed the TGF-β– cells to proliferate in response to antigen. We propose a model of antigen-induced tolerance that involves cell-cell contact with regulatory CD4+ T cells that coexpress membrane-bound TGF-β and FOXP3.
Introduction
Recent studies that have focused on relationships between Th2-dominated responses in asthma and childhood exposures suggest that tolerogenic mechanisms probably play an important role in the establishment of the early immune repertoire that inhibits the development of inappropriate responses to antigens (1, 2). This early education of the immune system is not only important for the regulation of Th2-type responses but also of Th1-mediated responses, a deficiency of which could explain the increased incidence of both allergic and autoimmune diseases in recent years.
To understand the mechanism of antigen unresponsiveness in the respiratory tract, models have been developed in mice involving repeated delivery of antigen to the respiratory tract (3–5). Similar to the induction of oral tolerance by a low or high dose of antigen (6–8), instillation of a low dose of antigen by inhalation or of a high dose of antigen by intranasal delivery into the respiratory tract has been associated with antigen unresponsiveness upon subsequent challenge by the same antigen (3–5). High-dose intranasal antigen instillation has been shown to be dependent on IL-10 production by DCs, and these DCs in turn were shown to induce IL-10 production in CD4+ T cells in vitro (5, 9). In the case of tolerance induced by inhalation of a low dose of antigen, adoptive transfer of γδ T cells and CD8+ T cells from OVA-tolerized mice suppressed Th2-dependent IgE responses, and in vitro challenge of the γδ cells resulted in production of high levels of IFN-γ (3). In a subsequent use of this model, mice deficient in γδ or CD8+ T cells or IFN-γ were still capable of mounting aerosolized antigen (Ag) unresponsiveness (4). While these observations do not rule out a role for γδ cells, CD8+ T cells, or IFN-γ in the induction of tolerance when they are present in WT animals, they do show that cell type(s) and/or mediators other than γδ cells, CD8+ T cells, or IFN-γ can induce antigen-induced tolerance in the respiratory tract.
In the last 5–10 years, there has been an intense focus on the role of CD4+ T regulatory cells (Treg’s) in controlling immune responses in virtually every field of immunology. Initially defined in the 1970s by Gershon and Kondo (10) and subsequently identified as CD4+CD25+ T cells by virtue of their ability to suppress day 3 thymectomy-induced polyautoimmune syndrome (11, 12), other types of Treg’s have also been identified in both in vitro and in vivo studies. The Treg’s are either naturally occurring, such as CD4+CD25+ cells (12) or are adaptive, being induced in response to specific tolerogenic stimuli. The adaptive Treg’s include CD4+CD25+ cells, Tr1 cells that owe their suppressive effects to IL-10 secretion (13), and Th3 cells that confer immunosuppressive effects by TGF-β secretion (7). For the naturally occurring CD4+CD25+ cells, cell-cell contact-dependent mechanisms have been proposed, while for the adaptive Treg’s cytokine-dependent mechanisms involving cell surface TGF-β expression (CD4+CD25+), as well as cell contact–independent mechanisms through soluble IL-10 and TGF-β, have been demonstrated (14). Many laboratories are currently engaged in the characterization of the development of these cells, their relationships to each other, and their relevance in health and disease. Here we show that repeated inhalation of low-dose inhaled antigen induces tolerogenic mechanisms that involve CD4+ T cells that express both cell surface–associated and soluble TGF-β. These cells have a poor proliferative response to antigen, yet have potent inhibitory effects on Th2-type cells from mice immunized for airway inflammation. In vivo inhibition of TGF-β interfered with the induction of tolerance. Our studies highlight immunosuppressive functions of CD4+CD25+ cells that express TGF-β on the cell surface.
Results
Initial exposure to low dose of Ag by inhalation induces tolerance in mice.
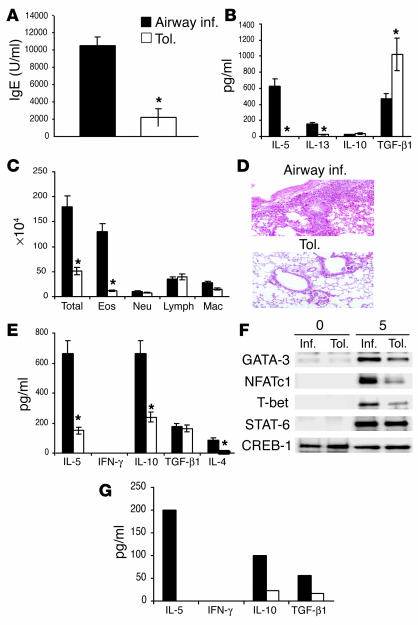
In this study, we tolerized mice by exposure to a low dose of Ag (OVA) based on a previously described model (3). The mice were evaluated for tolerance after two intraperitoneal immunizations with OVA and alum followed by additional exposure to aerosolized OVA. The results of these experiments show that repeated Ag inhalation downregulates the ability to mount Th2-type responses to Ag as evidenced by a reduction in Ag-specific IgE responses, minimal cellular infiltration in the lung parenchyma and also in the airway lumen, and reduction in airway cytokine levels as assayed in the bronchoalveolar lavage (BAL) fluid (Figure 1, A–D). By contrast, the control mice, which were initially exposed to aerosolized PBS, displayed increased cellular infiltration in the lung parenchyma and in the airways, increased mucus plugging of the airways, and high levels of OVA-specific IgE in the sera derived from their blood in response to intraperitoneal OVA and alum plus aerosol challenge (Figure1, A–D). The level of serum IgE from mice immunized for airway inflammation varied between 8,000 and 10,000 arbitrary units in different experiments, and it was at approximately 25–30% of this level in the sera from tolerized animals. In contrast to the expected Th2-specific cytokines that were detected, such as IL-5 and IL-13, we did not detect much of IL-10 in the BAL fluid from either group of mice. Interestingly, the level of TGF-β recovered in the BAL fluid of tolerized mice was almost twice the level of that detected in the BAL fluid of control mice (Figure 1B). The two groups of mice have been referred to as “inflammation” and “tolerance” groups in this article.
Figure 1.
Repeated antigen exposure induces tolerance in the respiratory tract. Mice were exposed to aerosolized PBS alone (airway inflammation [inf.]; black bars) or 1% OVA in PBS (tolerance, [tol.]; white bars) daily for 20 minutes each day for 10 consecutive days (days 0–10). Both groups were then immunized intraperitoneally with 10 ∝g OVA and 1 mg alum on days 21 and 27. Treated mice were challenged by exposure to aerosolized 1% OVA in PBS for 20 minutes each day for 7 days from day 34 through day 42 (A–D, G) or used for spleen CD4 T cell isolation on day 34 (E and F). In the case of mice challenged by inhaled OVA, on day 43 BAL was performed, serum was collected, and lungs were removed for histological evaluation. Shown is a representative experiment of five, with three to six animals per group in each experiment. (A) OVA-specific serum IgE concentration was measured by ELISA. Shown is the mean plus or minus SD; *P < 0.05 versus mice with airway inflammation. (B) Cytokine profile in the BAL fluid of tolerized mice compared with mice with airway inflammation as measured by ELISA. Shown is mean plus or minus SD with three mice per group (*P < 0.05). (C) Mean plus or minus SD of total and differential cell (eosinophils, Eos; neutrophils, Neu; lymphocytes, Lymph; and macrophages, Mac) counts in the airways of tolerized mice and mice with airway inflammation as recovered by BAL (*P < 0.05). (D) H&E staining demonstrating the absence of airway inflammation in the lungs of tolerized mice. Magnification ∞10. (E) Six animals were used per group and subjected to the inflammation or tolerance protocol, immunized with OVA and alum, and spleens isolated. Spleens from two animals were pooled in each group (three pools per group), and CD4+ T cells were isolated and stimulated with OVA and APCs in vitro for 5 days. Shown are the cytokine profiles in the supernatants as determined by ELISA (*P < 0.05). (F) Nuclear factor expression in nuclear fractions of the CD4+ T cells after 5-day culture as determined by Western blot analysis using specific Ab’s. CREB-1 expression is shown as a marker for protein loading. (G) Lung-draining lymph nodes (LNs) were harvested from mice on day 37 after initial exposure to OVA or PBS, immunization with OVA/alum, and 3 days of challenge with inhaled OVA. LNs were pooled from three mice, cells recovered from the LNs were cultured with OVA and APCs for 3 days, and cytokines were measured in the culture supernatants by ELISA.
In the next series of experiments, mice were first exposed for 10 consecutive days to aerosolized PBS or OVA and were subsequently immunized twice with OVA/alum. Splenic CD4 T cells were isolated 24 hours after the second immunization and stimulated in vitro with OVA and T cell–depleted, mitomycin C–treated APCs. An assay of cytokines in culture supernatants showed lower Th2-type cytokine production from cells isolated from tolerized mice (Figure 1E). The level of IL-10 produced by cells from mice with airway inflammation was more than threefold higher than that produced by cells from tolerized mice. TGF-β, which is produced by both Th2 cells and regulatory cells (Th3 and CD4+CD25+ cells), was detected at equivalent levels in both groups (Figure 1E). We also analyzed the activation of multiple transcription factors that we and others have previously associated with T cell differentiation (15–17). The CD4 T cells from the tolerized group showed lower expression of the Th2-specific transcription factor GATA-3 and also of nuclear factor of activated T cells-c1 (NFATc1), both known to be crucial for differentiation along the Th2 lineage (15–17) (Figure 1F). The diminished Th2 response was not due to accentuated Th1 skewing since neither T-bet expression nor IFN-γ production was higher in the cells from the tolerized group. Signal transducer and activator of transcription-6 (STAT-6) expression was the same in both groups. Although most effector cells exit the LN and traffic to the lung tissue after repeated inhaled Ag challenge, we were able to detect cytokine expression by residual LN CD4+ T cells. Overall, the cytokine secretion profile of the cells from the two groups resembled that of the splenic CD4+ T cells (Figure1G). These data allowed us to conclude that repeated exposure of mice to inhaled Ag inhibits the potential to develop Ag-induced Th2-type events in the periphery.
Active immunosuppression by CD4+ T cells in tolerized mice.
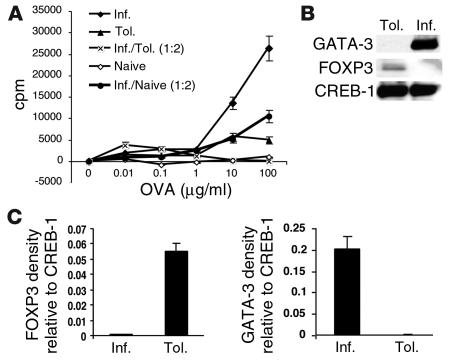
Our next objective was to determine whether active immunosuppression by CD4 T cells contributed to Ag-induced tolerance in the respiratory tract. The proliferative capacity of splenic CD4 T cells from both groups was first analyzed. CD4+ T cells from the tolerized group responded poorly to Ag, unlike cells from mice immunized for inflammation (Figure 2A). When cells from the two groups were mixed in a 1:2 ratio (inflammation/tolerance), the CD4 T cells from the inflammation group were inhibited from proliferating and resembled the phenotype of cells from naive mice. The inhibition observed in the mixed culture was not due to a simple reduction in cell numbers from the inflammation group, since mixing these cells with CD4+ T cells from naive mice did not cause the drastic inhibition, and the reduced proliferation was in proportion to the reduced numbers of cells from the inflammation group. The diminished ability of the cells from tolerized mice to proliferate was also reflected in lower IL-2 levels in the culture supernatant (data not shown). The cells from the two groups of mice were compared for the expression of Th2 versus regulatory cell markers. As shown in Figure 2B, the Th2-specific marker GATA-3 (18–20) was prominently expressed in cells from the inflammation group, while the expression of FOXP3, a transcription factor expressed by CD4+CD25+ regulatory cells (21), was upregulated in cells from the tolerance group. These results showed that the net immune response generated in inflammation was one that promoted GATA-3 expression and therefore an allergic phenotype, while the response in tolerance was induction of FOXP3 expression and absence of GATA-3 upregulation.
Figure 2.
Suppressive function of CD4+ T cells from tolerized mice. (A) Mice were exposed to PBS (airway inflammation) or 1% OVA (tolerance) and subsequently immunized with OVA and alum on days 21 and 27 after the initiation of OVA/PBS exposure. Splenic CD4+ T cells isolated from mice on day 34 were stimulated in vitro with different concentrations of OVA (0.01–100 ∝g/ml) and mitomycin C–treated, T cell–depleted APCs at equivalent cell numbers (105 cells each per well). After 72 hours of incubation, small samples of culture supernatants were removed for cytokine determination, and the remaining cells were pulsed for measurement of [3H]-thymidine incorporation. *P < 0.025 compared with proliferation of cells from mice immunized for inflammation. The proliferative response of CD4+ T cells from naive mice (open diamonds) is shown as a negative control. An additional control used was a mixture of cells from the inflammation group and from naive mice used in a 1:2 ratio. Each data point represents the mean plus or minus SEM of triplicate wells. Shown is a representative experiment of three. (B) As described above, CD4+ T cells isolated after day 34 were subjected to two rounds of stimulation with OVA and APCs in vitro, and nuclear extracts were prepared. Expression of GATA-3 and FOXP3 was assessed in the nuclear extracts (7–10 ∝g of total protein) by Western blotting techniques. CREB-1 expression is shown as a marker for protein loading. (C) Shown is an average densitometric reading of FOXP3 and GATA-3 expression relative to that of CREB-1 from two independent experiments.
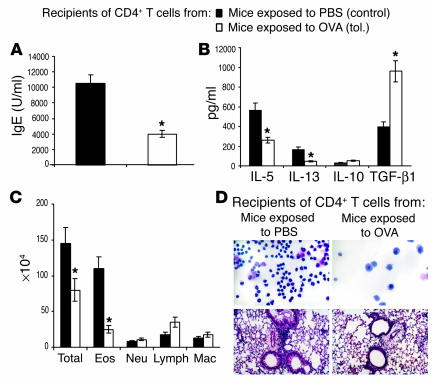
To test the suppressive function of the CD4 T cells from tolerized animals in vivo, the cells were isolated from the spleens of tolerized animals on day 21 after the initiation of the OVA exposure for 10 days and were adoptively transferred to naive animals. The recipients were immunized with OVA/alum and challenged with aerosolized OVA. As shown in Figure 3, A–D, all features of an allergic response, including OVA-specific IgE in the recipients’ sera, cytokine levels in BAL fluid, and cellular infiltration in the lung tissue and airways, were diminished in the recipient mice that received CD4+ T cells from tolerized animals compared with those that received cells from control animals (exposed to PBS only). Interestingly, TGF-β was again detected at higher levels in the BAL fluid of mice that received cells from tolerized animals. IL-10 levels were low in the BAL fluid from both groups. These data suggested that a mechanism of active immunosuppression by CD4+ T cells, possibly producing TGF-β, was responsible for the development of tolerance to inhaled Ag.
Figure 3.
Inhibition of development of the allergic phenotype in mice that received CD4+ T cells from tolerized mice. On day 21 after the initial 10-day exposure to OVA/PBS, CD4+ T cells were purified from spleens of mice from both groups and 5 ∞ 105 cells were adoptively transferred into naive BALB/c mice that were then immunized with 10 ∝g of OVA and 1 mg of alum intraperitoneally (day 0). Recipients were boosted with OVA/alum 7 days after transfer (day 7) and were challenged by exposure to an aerosol of 1% OVA for 7 days. Twenty-four hours after the last exposure, mice were evaluated for (A) blood IgE levels, (B) cytokine levels, and (C) cell differentials in the BAL fluid. *P < 0.05 in all panels compared with data from inflammation group. (D) Cytospin preparations of cells in the BAL fluid are shown in the upper panels and lung tissue histology is shown in the lower panels. Results are representative of three independent experiments with three to five mice per group.
Suppressive activity of tolerogenic CD4+ T cells involves TGF-β function through cell-cell contact-dependent mechanisms, but not IL-10.
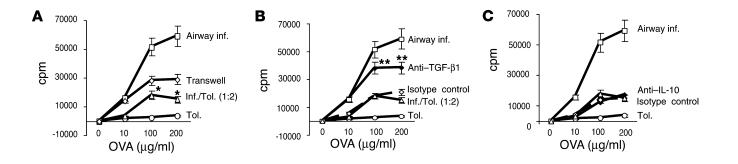
We next used cell proliferation experiments to determine the inhibitory function of CD4+ T cells from tolerized mice. We used the same experimental setup as shown in Figure 2. We investigated the proliferative response in each group individually as well as after mixing. In addition, we also separated the mixed cells using a transwell approach. Again, in mixed cultures we maintained the same number of total cells and the same ratio (1:2) of cells from the inflammation and tolerance groups. In the transwell experiments, cells from the inflammation group were plated in the wells and cells from tolerized mice on the insert, and thymidine incorporation in the former group was measured, since cells in the tolerance group were found not to proliferate (Figure 2A). As shown in Figure 4A, transwell separation of cells prevented inhibition by cells from the tolerized animals, and the level of thymidine incorporation simply reflected what would be expected given a third of the numbers of cells from the inflammation group. We also examined cell proliferation in the presence of neutralizing anti–TGF-β or anti–IL-10 Ab’s or their isotype controls, each added to the mixed cultures. Neutralization of TGF-β not only prevented inhibition, but also allowed cells to reach a greater proliferative potential (i.e., greater than that expected given the 1:2 ratio) (Figure 4B). This suggested some effect of soluble TGF-β in inhibition by tolerized cells since otherwise the level of proliferation should be the same as that observed by transwell separation. IL-10 neutralization, on the other hand, did not improve cell proliferation in mixed cultures (Figure 4C).
Figure 4.
Cell contact and TGF-β–dependent inhibition of proliferation by CD4+ T cells from tolerized mice. (A) Mice were first exposed to PBS (inflammation group) or 1% OVA (tolerance group) daily for 10 days and then were immunized with OVA/alum on days 21 and 27. Splenic CD4+ T cells isolated on day 34 were stimulated in vitro with different concentrations of OVA (10–200 ∝g/ml) and APCs at equivalent cell numbers (105 cells per well). Cells were mixed as described in the legend to Figure 2 or separated by transwell. In the transwell experiments, cells from the inflammation group were plated in the wells, and cells from tolerized mice on the insert and thymidine incorporation in the former group was measured. *P < 0.05 versus proliferation of cells in the inflammation group. (B) Chicken IgY anti–TGF-β1 (100 ng/ml) or isotype control (chicken IgY) was added to mixed cultures. **P < 0.05 of mixed cultures incubated with anti–TGF-β1 compared with mixed cultures incubated without Ab. (C) Anti–IL-10 (1 mg/ml) or isotype control was added to mixed cultures. All assays were incubated for 72 hours, after which the cells were pulsed for measurement of [3H]-thymidine incorporation. Each data point represents the mean plus or minus SEM of triplicate wells. Shown is a representative experiment of three experiments.
TGF-β–expressing CD4+ T cells in tolerized mice.
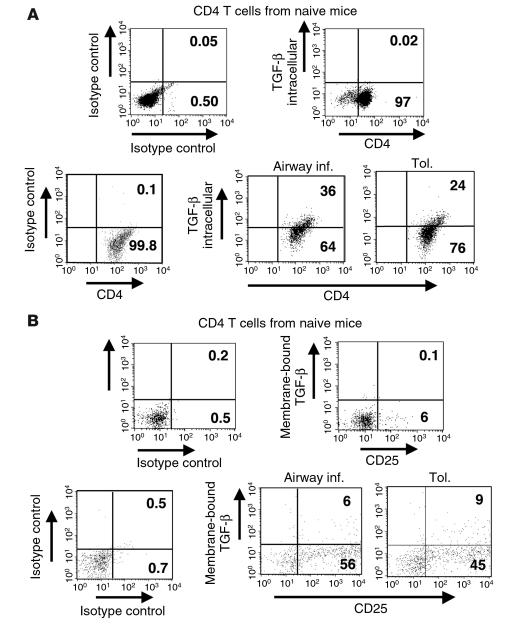
We next focused on the characteristics of the CD4 T cells from the tolerized mice, particularly their ability to produce TGF-β. Mice were exposed to aerosolized PBS or OVA and were then immunized with OVA/alum. CD4+ T cells were isolated from both groups, stimulated briefly with antigen in vitro, and analyzed for TGF-β expression by intracellular cytokine-staining techniques (Figure 5A). Although the data presented in Figures 2 and 4 show that CD4+ T cells from tolerized mice have poor proliferative potential compared with those from mice immunized for inflammation (as can be expected of Treg cells), the frequency of TGF-β–expressing cells was similar in both groups. These data suggested a role for TGF-β in the maintenance of the CD4+ T cells from tolerized mice and is in line with previous observations that showed the ability of TGF-β to generate and expand CD4+CD25+ T cells. We also detected cell surface–associated TGF-β expression in both groups, largely on CD25+ cells (Figure 5B). Thus, the CD4+ T cells isolated from the tolerized group expressed both CD25 and intracellular as well as extracellular TGF-β. Most importantly, TGF-β, but not IL-10, neutralization prevented the inhibitory phenotype of the CD4+ T cells from the tolerized animals (Figure 4).
Figure 5.
CD4+ T cells in tolerized mice express both soluble and membrane-bound TGF-β. On day 34, splenic CD4+ T cells from the two groups of mice (tolerance and airway inflammation), as well as cells from naive animals, were isolated and stimulated with OVA/APCs in vitro for 48 hours. The T cells were then examined for both (A) intracellular and (B) cell-surface expression of TGF-β by flow cytometry. Appropriate isotype controls are shown, and the percentages presented for the stained cells have had the indicated background isotype control levels subtracted. (A) T cells were stained for cell-surface CD4 expression, then were fixed, permeabilized, and stained for intracellular expression of TGF-β. The isotype for CD4 is shown for the naive animals. For the airway inflammation and tolerance conditions, the control is stained for CD4 and for the isotype for TGF-β. (B) T cells were double-stained for cell-surface expression of CD25 and TGF-β.
Inhibition of tolerance induction in the presence of neutralizing anti–TGF-β Ab.
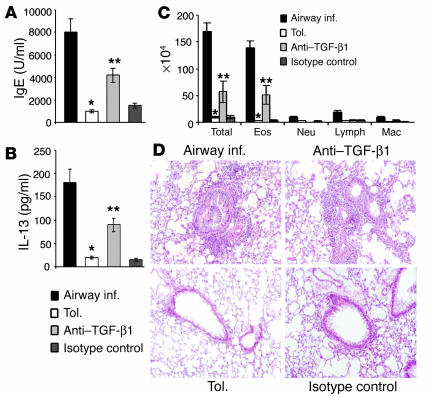
The detection of TGF-β in the BAL fluid from tolerized animals and in CD4+ T cells derived from them, combined with the ability of neutralizing anti–TGF-β to abolish the inhibitory potential of CD4+ T cells from tolerized animals, prompted us to investigate the consequence of TGF-β neutralization on tolerance induction in vivo. Neutralizing anti–TGF-β Ab or its isotype control was introduced intraperitoneally into mice at three different time points. Controls included mice immunized and challenged for airway inflammation and mice subjected to the tolerance model. Figure 6, A and B, show that anti–TGF-β1, but not the isotype control, caused increases in serum IgE and BAL cytokine levels, albeit not increased to the same levels as seen in the inflammation group. BAL fluid cell differentials and histology of lung tissue presented evidence of eosinophilic airway inflammation in anti–TGF-β–treated mice (Figure 6C), and histological evaluation revealed inflammation around bronchovascular bundles (Figure 6D). The mice that received the isotype control showed a phenotype similar to that observed in tolerized mice. It was not surprising to us that the degree of eosinophil infiltration in the Ab-treated mice was not equivalent to that seen in the control mice with airway inflammation. This was because if, indeed, cell surface TGF-β1 is an important contributor to tolerance induction, the Ab may not be able to completely access and thereby neutralize all membrane-bound cytokines, particularly on cells in contact with target cells. The partial reversal of tolerance by anti–TGF-β1 Ab, however, allowed us to conclude that TGF-β was involved in tolerance development in our model.
Figure 6.
Administration of anti–TGF-β1 interferes with tolerance development in vivo. Chicken IgY anti–TGF-β (50 ∝g/mouse) or matching isotype control was administered intraperitoneally into naive BALB/c mice at three time points: 1 hour prior to primary exposure to OVA, on day 5 of exposure, and 1 hour prior to first OVA/ alum immunization on day 21. On day 43, BAL was performed, serum was collected, and lungs were removed for histological evaluation. Shown is a representative experiment of two experiments, with three animals per group in each experiment. (A) IgE levels in blood and (B) cytokine (IL-13) levels in BAL fluid of animals in each group. Shown are mean plus or minus SD with three mice per group (*P < 0.05 versus levels in inflammation group; **P < 0.05 versus animals treated with isotype control). (C) Differential cell counts in BAL fluid. *P < 0.05 compared with inflammation group; **P < 0.05 compared with tolerized group. (D) Lung tissue histology. The grade of inflammation was +5 in all animals immunized for airway inflammation, less than +1 in tolerized animals and in animals that received the isotype control, and between +2 and +4 in mice that received anti–TGF-β1 Ab.
Purification of cell surface TGF-β–expressing cells.
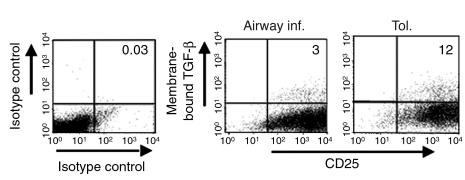
Our next goal was to specifically determine the suppressive function of cells that expressed TGF-β1 on the cell surface. CD4 T cells isolated from both groups were subjected to two rounds of stimulations with OVA and APCs in vitro and analyzed for CD25 expression and membrane-bound TGF-β. The level of background staining increased somewhat for CD25 in the FL4 channel compared with T cells in short-term cultures (see Figure 5B), but after subtraction of this background, the cells were still greater than 90% positive for CD25 (Figure 7) and were essentially 100% positive for CD4 (data not shown). Interestingly, the CD4 T cells in the tolerance group all shifted to a high level of CD25 expression, while those in the inflammation group included cells with an intermediate level of CD25 expression (cells just to the right of the cursor in the FL4 channel, mean fluorescence intensity approximately 100; Figure 7). While the cells in the tolerance group did not proliferate appreciably, they remained viable. Furthermore, the frequency of membrane-bound TGF-β–expressing cells in the tolerance group was consistently three- to fourfold higher than that detected in the inflammation group (Figure 7). Thus, the cells derived from the tolerized animals remained viable and displayed sustained cell-surface TGF-β expression.
Figure 7.
Stable cell surface TGF-β expression is enhanced on CD4+ T cells from tolerized mice relative to those with airway inflammation. Splenic CD4+ T cells were isolated from mice on day 34 after initial exposure and immunization with OVA/alum, subjected to two rounds of in vitro stimulation with OVA/APCs, and then examined for cell surface expression of TGF-β and CD25 by flow cytometry. Before restimulation in vitro, dead cells were removed by density gradient centrifugation using lymphocyte separation medium. Quadrant locations on dot plots were determined using appropriate isotype control Ab’s as shown in the left-hand panel. The indicated level of background for the isotype control staining was subtracted from positively staining samples to arrive at the percentages shown in the upper-right quadrants of the right-hand panels.
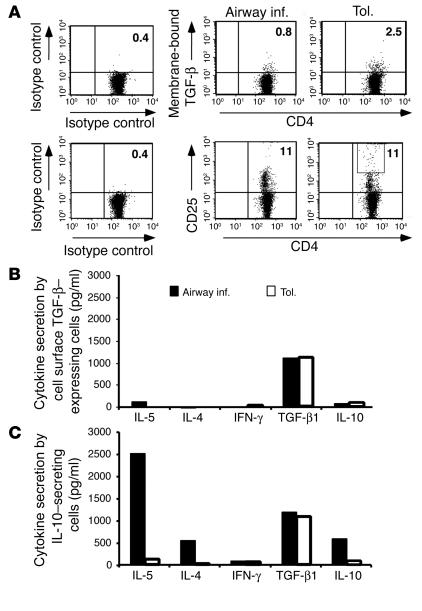
Having confirmed that the CD4 T cells derived from the tolerized animals maintained expression of cell surface TGF-β1, we decided to exploit this feature of the cells to enrich this population. We were also curious whether, in a similar fashion, IL-10 enrichment would allow us to detect a population, albeit small, of cells that displayed a typical Th2 cytokine-secretion profile in the tolerance group. The distinction between the two cell types that we planned to enrich was that, in the case of the TGF-β–expressing cells, we planned to use an anti–TGF-β1 Ab without cell permeabilization, which would cause Ab binding to cells that externally expressed the cytokine. For the enrichment of IL-10–producing cells, on the other hand, a bifunctional Ab was used that would capture all cells that had the ability to secrete IL-10; in this case, the technique called MACS IL-10 Secretion Assay was used to enrich cells that produced soluble IL-10 (see Methods). Cells were isolated from both the inflammation and tolerance groups of mice. Figure 8A shows cell surface TGF-β expression on freshly isolated CD4+ T cells from both groups. The frequency of cell surface TGF-β–expressing cells was threefold more in tolerized mice, and furthermore we saw a greater proportion of CD25hi cells in this group compared with that in the inflammation group (Figure 7). The cells were subjected to two rounds of stimulation with OVA and APCs in vitro, and the two cytokine-expressing (cell surface TGF-β–expressing and IL-10–secreting) populations were isolated. Equal numbers of cells from the two populations in each group (inflammation and tolerance) were restimulated for 72 hours, and the culture supernatants were analyzed for cytokine production. The cytokine secretion profile shown in Figure 8B shows that the cell surface TGF-β–expressing cells in both groups produced only TGF-β and low or undetectable levels of IL-10 or other cytokines. It should be noted, however, that the frequency of the cells expressing cell surface TGF-β was higher in the tolerance group (Figures 7 and 8A). The IL-10–secreting cells with a typical Th2-type profile of cytokine secretion could be easily enriched in the inflammation group (Figure 8B, lower panel). The IL-10–secreting cells producing low levels of IL-10, which were enriched from the tolerance group, again displayed a predominantly TGF-β–secretion profile. Thus, irrespective of whether cell surface TGF-β expression or IL-10 secretion was used to enrich cells from the tolerized animals, the cells that were recovered were essentially the same population displaying a high TGF-β and low IL-10 profile. Cells devoid of cell surface TGF-β (TGF-β– cells) from the tolerized group showed the same profile of cytokine secretion as the IL-10–secreting cells captured using the IL-10 secretion assay, with TGF-β being the dominant secreted cytokine (data not shown). These experiments clearly showed that cells secreting Th2-type cytokines were clearly the dominant population in the inflammation group, while a TGF-β–expressing population that expressed low levels of IL-10 was essentially the exclusive population in the tolerance group whose frequency increased upon antigen stimulation. Taken together, these results showed that appropriate Th2-skewing conditions can overcome normal homeostatic mechanisms, and, conversely, appropriate tolerizing protocols can induce immunoregulatory mechanisms.
Figure 8.
Expression of membrane-bound TGF-β on freshly isolated cells from both groups of mice and their similar cytokine secretion profile. (A) Cell surface TGF-β and CD25 expression on freshly isolated CD4+ T cells from inflammation and tolerance groups. The boxed area denotes cells expressing high levels of CD25. (B) CD4+ T cells from the two groups’ cells were cultured with OVA/APCs for two rounds of stimulation (maintaining equal numbers of cells during restimulation). Cells expressing cell surface TGF-β were isolated using PE-labeled anti–TGF-β Ab, anti-PE microbeads, and separation on magnetic columns. Equal numbers of positively selected cells were restimulated with OVA/APCs for 72 hours, and the indicated cytokines in the culture supernatants were measured by ELISA. Cells expressing IL-10 were isolated using the MACS IL-10 secretion assay, stimulated, and assessed for cytokine production as described above. All data are representative of two independent experiments.
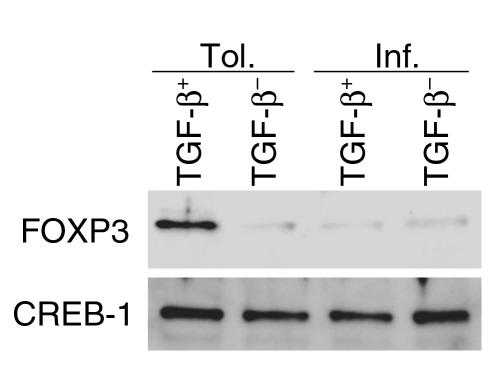
Selective expression of FOXP3 in TGF-β+ cells from tolerized mice.
Since cells expressing membrane-bound TGF-β+ could be isolated from both groups of mice (tolerance and inflammation) and both produced soluble TGF-β, we investigated the status of FOXP3 expression in TGF-β+ cells isolated from both groups. Cells were isolated from the two groups, fractionated into TGF-β+ and TGF-β– cells as described above, and equal numbers of cells were stimulated for 5 days in vitro with OVA and APCs. As shown in Figure 9, FOXP3 expression was detectable only in TGF-β+ cells from the tolerized mice. We have also confirmed this finding by RT-PCR techniques (data not shown).
Figure 9.
Selective FOXP3 expression in cells expressing membrane-bound TGF-β from tolerized mice. TGF-β+ and TGF-β– cells were isolated from both groups of mice, and 4 ∞ 106 cells of each type from each group were maintained in culture for 5 days with Ag and APCs. FOXP3 expression was investigated in the nuclear extracts of the cells (20 mg total protein) and was readily detectable only in the TGF-β+ cells from tolerized mice. CREB-1 expression was used as a loading control.
Specific immunosuppressive functions of cells from tolerized mice that express membrane-bound TGF-β.
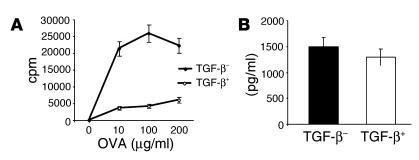
The same protocol of mice tolerization was used (10 consecutive days of exposure to OVA by inhalation). Ten days after the last exposure, TGF-β+ and TGF-β– cells were enriched from splenic CD4 T cells. A fraction of the purified cells was examined in a cell-proliferation assay, and the soluble TGF-β produced by the two populations was also measured. Strikingly, removal of the TGF-β+ population derepressed the proliferative potential of the rest of the TGF-β– cells in response to antigen (Figure 10A). Both populations secreted equivalent levels of soluble TGF-β (Figure 10B).
Figure 10.
Cell surface TGF-β is key to the immunosuppressive properties of CD4+ T cells from tolerized mice. Mice were exposed to 1% OVA (tolerance) for 10 days, and spleens were harvested on day 21. CD4+ T cells were prepared by negative selection, and cells expressing TGF-β on the cell surface (TGF-β+) were separated from those devoid of cell surface TGF-β (TGF-β–). The purity of the two populations was assessed by FACS analysis (approximately 75% enrichment of TGF-β+ cells; not shown). (A) Proliferative potential of TGF-β– cells in the absence of TGF-β+ cells. Equal numbers of the two populations were tested for cell proliferation, and culture supernatants were assayed for soluble TGF-β. (B) Production of soluble TGF-β by the two populations. For each group, an average of the concentrations detected with the different doses of OVA is shown.
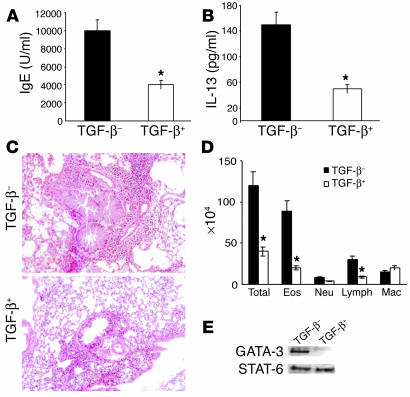
To determine the specific effect of membrane-bound TGF-β on the development of allergic airway inflammation, the remainder of the cells (105 cells of either type) were adoptively transferred to naive mice. To minimize any changes in their properties, these cells were not restimulated in vitro before adoptive transfer. The mice were then immunized with OVA/alum twice and were challenged with aerosolized OVA to induce allergic pulmonary inflammation. A control group of mice was immunized twice with OVA and alum and challenged with OVA by inhalation (not shown). Essentially, the allergic phenotype of the control mice were very similar to those that received the TGF-β– cells (for comparison, please refer to Figures 1 and 3); therefore, for simplicity, data derived from only the TGF-β+ and TGF-β– recipients are shown. As shown in Figure 11A, the mice that received TGF-β+ cells displayed significantly lower levels of serum IgE compared with those that received TGF-β– cells (compare with data presented in Figures 1 and 3). Similarly, the level of IL-13 in the BAL fluid of TGF-β+ recipients was lower than that detected in the BAL fluid of TGF-β– recipients (Figure 11B). While the TGF-β+ recipients displayed a low degree of inflammation in the tissue and airways, the mice that received TGF-β– cells showed massive pulmonary inflammation: narrowing of airway lumen due to the presence of mucus-plugged goblet cells and dramatic peribronchiolar and perivascular cellular infiltration (Figure 11, C and D).
Figure 11.
Adoptive transfer of TGF-β–expressing cells from tolerized mice into naive mice significantly attenuates the development of airway inflammation in the recipient mice. Cells (105) from each group were adoptively transferred into naive BALB/c mice, which were immunized intraperitoneally at the same time with OVA/alum (day 0). The recipients were boosted with OVA/alum 7 days after transfer (day 7) and were challenged by exposure to aerosol of 1% OVA for 7 days, from day 14 to 21. Control mice were immunized with OVA/alum and challenged with aerosolized OVA (not shown). Twenty-four hours after the last OVA challenge, mice were processed for (A) IgE levels in blood, (B) cytokine (IL-13) levels in BAL fluid, and (C and D) pulmonary inflammation. Lung infiltrates were graded as +5 in all TGF-β− cell transfers or in control OVA/OVA immunized mice (not shown) and were between +1 and +2 in mice that received TGF-β+ cells. There were three mice per group, and the results are representative of two independent experiments. *P < 0.05 versus animals that received TGF-β− cells. (E) In separate experiments, after adoptive transfer of TGF-β+ or TGF-β− cells and immunization with OVA/alum, splenic CD4+ T cells were isolated by positive selection from two recipients in each group on day 14. Cells were stimulated with OVA/APCs for 5 days in vitro. Nuclear extracts were prepared and subjected to Western blot analysis for GATA-3 and STAT-6.
In separate experiments, after cell transfer and immunization with OVA and alum twice (day of transfer and day 7), instead of aerosol challenge splenic CD4 T cells were isolated from the mice on day 14, stimulated with Ag and APCs in vitro, and nuclear extracts were prepared after 5 days of stimulation. Cells from the TGF-β+ recipients showed low or barely detectable levels of nuclear GATA-3 (Figure 11E). CD4 T cells from the TGF-β recipients were GATA-3+, and both groups showed evidence of STAT-6 translocation (Figure 11E). These experiments further confirmed the specific inhibitory properties of the CD4+ T cells expressing membrane-bound TGF-β, thereby demonstrating an important component of antigen-induced airway tolerance to be active immunosuppression by these cells.
Discussion
The development of an aberrant immune response to allergens is not the norm but is rather an atypical response in certain people called “atopics.” While it is now known that Th2 cells have an important role in the orchestration of the allergic response, the mechanisms that prevent the development of a Th2 response in the face of constant allergen provocation is just beginning to be investigated. For example, instillation of a high dose of antigen intranasally has been shown to induce a tolerizing response that involves IL-10 (5, 9). We have used a different model of antigen delivery, one that mimics natural antigen inhalation, a model first established by Holt and colleagues (3). In this model, we have found that an important contributor to the development of airway tolerance is a CD4+ T cell that expresses membrane-bound TGF-β and also produces soluble TGF-β.
CD4+CD25+ cells with immunosuppressive functions are now well described in the literature (14, 22). The mechanisms that underlie their regulatory properties are not well understood, however. For example, while one study suggested membrane-bound TGF-β to be an important component of the regulatory mechanisms of CD4+CD25+ cells (23), another study refuted this concept (24). Both studies, however, investigated the immunosuppressive functions of TGF-β– expressing CD4+CD25+ cells in vitro. To the best of our knowledge, no study has yet identified or studied the functional significance of these cells in an in vivo model. Our studies show potent immunosuppressive functions of CD4+ T cells expressing cell surface TGF-β, which are in agreement with the observations of Strober and colleagues (23). Recently, B cells expressing cell surface TGF-β1, induced by exposure to LPS, were shown to induce anergy in CD8+ T cells (25). It is important to note that in previous studies (23, 24), the Ab that was used to study the role of membrane-bound TGF-β in immunosuppression recognized all three isoforms: TGF-β1, TGF-β2, and TGF-β3. Because of the controversy about the role of cell surface TGF-β in immunosuppression, we compared this Ab with the chicken IgY anti–TGF-β that recognizes only TGF-β1 and that has been successfully used to block TGF-β effects in vivo in mycobacteria-induced suppressive mechanisms in the airways (26). In our studies, the chicken IgY anti–TGF-β1 was far superior in blocking the TGF-β effects. Most importantly, we have been able to use the cell surface TGF-β–expression feature of the cells to tease them out of the CD4 population and demonstrate their ability to severely limit Th2 responses in vivo. In transwell studies, we have found a role for cell-cell contact-dependent mechanisms in the inhibitory properties of the CD4+ T cells.
We have recently reported an important mechanism by which TGF-β blocks both Th1 and Th2 differentiation (27). We have shown that TGF-β prevents Ca2+ influx in CD4+ T cells, which is important for both Th1 and Th2 differentiation. The phosphorylation of the Tec kinase, Itk, which regulates Ca2+ mobilization and NFATc translocation (28–32), was impaired in the presence of TGF-β. The important point to note in this study, however, is that soluble TGF-β was unable to inhibit cytokine production by fully differentiated T cells (27). Our present study shows that cell contact-dependent mechanisms are important for the immunosuppressive ability of the CD4+ T cells from tolerized mice. This suggests that the expression of TGF-β on the cell surface may impart special immunosuppressive functions to the regulatory cells since the cells were able to inhibit proliferation of antigen-experienced CD4+ T cells from the inflammation group of mice. Several cytokines and growth factors, including TGF-α, stem cell factor, TNF-α, and lymphotoxin (LT), are known to be expressed in both membrane-bound and soluble forms (33–38). Interestingly, for all of these cytokines and growth factors, the membrane-bound form has been shown to have potent biological effects. For example, in the case of TNF-α, the membrane-bound form was shown to kill target cells by cell-cell contact (39). In the case of SCF and LT, membrane-bound and soluble forms were shown to possess distinct biological properties (40, 41). It is possible that sustained signaling by Treg-expressed membrane-bound TGF-β via the TGF-β receptor is important for inhibition of the activity of the target cells. The recent description of endocytic pathways that regulate TGF-β signaling and receptor turnover (42–44) may also have important ramifications in TGF-β–mediated immunosuppression. These studies show that endocytosis of TGF-β receptors may not only cause downregulation of ligand-induced receptor activation, but that endocytosis may also be required for propagation of receptor-induced intracellular signaling (42). It should be noted that TGF-β is known to induce both Smad-dependent and Smad-independent (p38 MAP kinase) signaling pathways, which mediate distinct biological effects of TGF-β (45). It is therefore possible that when the ligand is membrane bound, it provides more sustained signaling to the target cells, which may also be distinct from that provided by the soluble protein. This may be required for inhibiting the activities of antigen-experienced cells, especially Th2 cells, which are not as dependent on calcium signaling. Our ongoing studies are devoted to the identification of inhibitory pathways induced by TGF-β in target cells. Whether membrane-bound TGF-β activates distinct pathways in target cells remains to be determined.
We have demonstrated expression of FOXP3 in CD4 T cells from the tolerance group of mice but not in those from the inflammation group. Conversely, GATA-3, a marker of Th2 cells, was highly expressed in the latter but not in the former. In one of the recent studies that showed FOXP3 expression in CD4+CD25+ T cells (46, 47), FOXP3 overexpression did not cause soluble TGF-β secretion (47). In our studies, FOXP3 expression was detectable only in cells from tolerized mice expressing cell surface TGF-β. It appears that a Th2 response induced in the presence of a strong Th2-skewing agent such as alum prevents the potential of TGF-β+ cells by limiting FOXP3 expression. In tolerance induced by Ag alone, the Th2 response cannot be induced. Instead, the activated TGF-β+ cells expressing FOXP3 ensure blockade of Th2-induction. It will be interesting to determine the functional significance of selective FOXP3 expression in the TGF-β+ cells in tolerance.
In summary, our studies have unraveled an important role for membrane-bound TGF-β in airway tolerance induced by a low dose of inhaled antigen. It appears, therefore, that a low dose of antigen induces a different mechanism of tolerance in the respiratory tract. This probably stems from differential effects of low and high doses of antigen on APCs. It will be interesting to determine the effects of different doses of antigen on lung DCs. The antigen specificity of the response in our model has yet to be determined. Antigen specificity is a complex issue, and in studies of oral tolerance, bystander suppression has been invoked to explain broad immunosuppression (48). On the other hand, in transplantation tolerance, some degree of antigen specificity has been shown (49, 50). Regardless, our studies raise the exciting possibility that a cocktail of antigens may be used in tolerizing protocols in early life in predisposed individuals to control unwarranted immune responses in later life. At the same time, it will be important to determine the role of TGF-β–induced pathways in the early education of the immune system through host-pathogen interactions that regulate both Th1 and Th2 responses in later life.
Methods
Mice.
BALB/cByJ mice between 6 and 8 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). The mice were housed and used in a pathogen-free facility at the University of Pittsburgh School of Medicine in accordance with all applicable guidelines.
Ab and reagents.
Polyclonal chicken IgY anti–TGF-β1 Ab, chicken IgY isotype control, mAb with specificity for TGF-β1, -β2, and -β3, mouse IgG1, monoclonal anti-mouse IL-10, rat IgG1, and recombinant TGF-β were from R&D Systems Inc. (Minneapolis, Minnesota, USA). Anti-CD3 and anti-CD28, peridinin chlorophyll-a protein-labeled (PerCP-labeled) anti-mouse CD4 (RM4-5), allophycocyanin-labeled anti-mouse CD25 (PC611), and allophycocyanin-labeled anti-mouse IL-10 (JES5-16E3) were from BD PharMingen (San Diego, California, USA). Recombinant IL-10 was from PeproTech Inc. (Rocky Hill, New Jersey, USA). R-phycoerythrin–labeled (R-PE–labeled) anti-human TGF-β (TB21) was from IQ Products (Groningen, The Netherlands), anti-FOXP3 Ab was from Novus Biologicals Inc. (Littleton, Colorado, USA), and murine anti–GATA-3, anti-NFATc1, anti–STAT-6, and anti–cAMP-responsive element binding-1 (anti–CREB-1) Ab were from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA).
Immunization of mice.
The protocol for tolerance induction and assessment consisted of a 42-day protocol involving a series of antigen exposures and challenges. Mice were first exposed to aerosolized PBS alone or 1% OVA (Sigma-Aldrich, St. Louis, Missouri, USA) in PBS (Life Technologies Inc., Gaithersburg, Maryland, USA) for 20 minutes each day for 10 days (days 0–10). Inhalation of aerosolized OVA was carried out as previously described (51, 52). Mice that received PBS are referred to as the inflammation group, while those that received OVA have been named the tolerance group. The concentration (1%) and duration (20 min/d) of OVA administration to the lung used provided a low dose of OVA that initiated tolerance. Consideration of prior published data allowed estimation of this dose based on two methods of calculation. One assumed that normal mouse breathing frequency (163 breaths/min) and tidal volume (0.15 ml) (53) resulted in 500 ml of air inhaled over 20 minutes for each mouse, which, when multiplied by the nebulization rate (4 ml/min), airflow rate (6,000 ml/min), OVA concentration (10 mg/ml), and assumed efficiency of lung deposition of 1%, yielded a value of 33 ∝g OVA per day. This efficiency value was based on a 10% efficiency demonstrated in the rat and guinea pig (54, 55), scaled down to 1% accounting for the body size difference between a typical 30-g mouse and 300-g rat. The other method simply relied on a previous study in mice (56) in which 1% OVA was estimated to result in inhalation of 80 ∝g OVA per day when administered for 60 minutes; the particle size in this study was in the range of 1.5 ∝m. Since the average particle size with our nebulizer is 5 ∝m and deposition efficiency in the airways decreases with increase in particle size, we calculated that less than 27 ∝g OVA per day would have been obtained over a 20-minute period, based on the fact that exposure time is the major determinant of solute dose accumulation in rodents (54, 55). Therefore, we estimate that our protocol resulted in a dose of less than 30 ∝g OVA per day per mouse. Both PBS (inflammation) and OVA (tolerance) groups were challenged with 10 ∝g of OVA and 1 mg of alum (Resorptar; Intergen Co., New York, New York, USA) intraperitoneally on days 21 and 27, followed by exposure to aerosol of 1% OVA in PBS for 20 minutes each day for 7 days from day 34 to 42.
BAL and lung histology.
BAL was performed on day 43 (24 hours after the last aerosol challenge) as described previously (51, 52). Cytokine levels were measured in BAL fluids using ELISA kits (R&D Systems Inc.). Cell differentials in the BAL fluid were assessed as described previously (51, 52). Where needed, a semiquantitative method was used to score lung infiltrates. A greater than three-cell deep infiltrate around bronchovascular bundles was a +5 grade infiltrate; +1 or lower signified a low degree of inflammation (57).
OVA-specific IgE assay.
ELISA assays to measure OVA-specific IgE were performed using Immunolon-2 microplates (Dynal Biotech Inc., Lake Success, New York, USA) coated with rat anti-mouse IgE (4 ∝g/ml; Southern Biotechnology Associates, Birmingham, Alabama, USA). The microplates were washed five times with wash buffer (0.01 M PBS with 0.05% Tween-20) (Bio-Rad Laboratories Inc., Hercules, California, USA) between each of the following assay incubations: (a) 200 ∝l of blocking buffer (0.01 M PBS with 0.05% Tween-20 and 1% BSA; Sigma-Aldrich) per well for 2 hours at room temperature; (b) serial dilutions of pooled sera obtained from centrifuged peripheral blood in blocking buffer overnight at 4°C; (c) biotinylated OVA (biotinylated using a Biotin-X-NHS kit from Calbiochem-Novabiochem Corp., San Diego, California, USA) diluted to 0.25 mg/ml in blocking buffer for 30 minutes at room temperature; (d) avidin-peroxidase (Sigma-Aldrich) in wash buffer (1:20,000 dilution) for 30 minutes at room temperature; and (e) peroxidase substrate for 30 minutes at room temperature. The colorimetric reaction was terminated with stop solution (1% SDS in 0.01 M PBS), and the plate was read at a wavelength of 405 nm using an ELISA plate reader. Serum IgE concentrations were calculated by comparison to a standard generated from pooled sera collected from hyperimmunized mice. Pooled normal mouse serum was used as negative control.
CD4+ T cell isolation from spleen and lung-draining LNs and in vitro stimulation.
CD4+ T cells were purified by positive selection using magnetic bead–purification technology employing anti–CD4-coupled magnetic beads according to the manufacturer’s recommendations (Miltenyi Biotec, Auburn, California, USA). Typically, cells were greater than 97% pure, as determined by FACS analysis. APCs similarly prepared by magnetic depletion of CD4 and CD8 cells were treated with mitomycin C (Sigma-Aldrich) prior to their use in assays. CD4+ T cells, together with APCs (1:2 ratio), were cultured with OVA (100 ∝g/ml) in Bruff’s medium supplemented with 5% FCS. Culture supernatants were collected and nuclear extracts were prepared as previously described (18). LNs were harvested from mice on day 37 after sequential primary antigen exposure, OVA/alum immunization, and 3 days of Ag challenge. Total cells recovered from LNs by physical disruption of the nodes were cultured with OVA (100 ∝g/ml) in Bruff’s medium supplemented with 5% FCS for 3 days. Culture supernatants were then collected for measurement of cytokine levels.
Cytokine assays.
Cytokine concentration was measured by ELISA using commercially available kits (R&D Systems Inc.). The cytokines monitored and the respective detection limits of the ELISA kits were as follows: IL-2 (3 pg/ml), IL-4 (2 pg/ml), IL-5 (7 pg/ml), IL-10 (4 pg/ml), IFN-γ (2 pg/ml), and TGF-β1 (7 pg/ml). For TGF-β1 estimations, the TGF-β1 levels in serum-supplemented Bruff’s medium were assessed (200–500 pg/ml) and deducted from the TGF-β1 level readings of culture supernatants.
Cell proliferation assays.
Cell proliferation assays were carried out by measurement of [3H]-thymidine (NEN Life Science Products Inc., Boston, Massachusetts, USA) incorporation. Briefly, cells were cultured in 96-well flat-bottom culture plates at 105 cells/well in a total volume of 200 ∝l/well Bruff’s medium plus 5% FCS. In cell-mixing experiments, the total number of cells in the culture was kept constant (105 total cells/well). The assays were incubated at 37°C for 3 days, after which [3H]-thymidine (1 ∝Ci/well; NEN Life Science Products Inc.) was added for an additional 16 hours of incubation. Incorporation of [3H]-thymidine was measured by harvesting the cells onto glass fiber filters (Wallac Oy, Turku, Finland), followed by liquid scintillation counting. Results are reported as the mean plus or minus SEM of triplicate wells with the background (the mean of triplicate unstimulated wells) subtracted. For experiments involving cell separation in transwells, Nunc tissue culture inserts, with 0.2-∝m Anopore membranes (Nalge Nunc International, Naperville, Illinois, USA) were used. CD4+ T cells from mice from the inflammation group were placed in the bottom chamber, while CD4+ T cells from tolerized mice were placed on the insert. Cells were stimulated with T cell–depleted APCs and different concentrations of OVA (10–200 ∝g/ml). After 72 hours, samples of culture supernatant were collected for determination of cytokine production by ELISA, the inserts were removed, and the cells were pulsed with 1 ∝Ci/well of [3H]-thymidine. Incorporation of [3H]-thymidine was determined as described above.
In some experiments, neutralizing Ab or relevant, matching isotype controls were added to the mixture of CD4+ T cells from the inflammation and tolerance groups as follows: anti–TGF-β1 at 50 ng/ml and 100 ng/ml (R&D Systems Inc.); isotype control: normal chicken IgY (R&D Systems Inc.) at 100 ng/ml (shown); monoclonal anti-mouse IL-10 Ab (R&D Systems Inc.) at 0.1 ∝g/ml and 1 ∝g/ml (shown); and rat IgG1 isotype control (R&D Systems Inc.) at 1 ∝g/ml. After 72 hours, samples of culture supernatant were collected for determination of cytokine production, and the cells were pulsed for measurement of [3H]-thymidine incorporation.
In vivo treatment with Ab.
Anti–TGF-β1 (chicken IgY; R&D Systems Inc.) or matching isotype control was administrated intraperitoneally into naive BALB/c mice at a dose of 50 ∝g per mouse 1 hour prior to primary exposure to antigen on day 5 of exposure and on day 21, 1 hour before the first OVA/ alum immunization. On day 43, mice were anesthetized, lungs were lavaged with PBS, blood was collected for serum preparation, and lungs were removed for histological evaluation.
Flow cytometry.
For cell surface staining, 0.2 ∞ 106 CD4+ T cells were incubated with PerCP-labeled anti-mouse CD4 (RM4-5), APC-labeled anti-mouse CD25 (PC611), or R-PE–labeled anti-human TGF-β (TB21) (IQ Products), or the appropriate isotype control. For intracellular cytokine staining, Fc receptors were first blocked with excess mouse Fc Block (2.4G2; BD Pharmingen); 106 CD4+ T cells were then stained with PerCP-labeled anti-mouse CD4 (RM4-5). The cells were then fixed and permeabilized using reagents from the Cytofix-Cytoperm Plus kit and the Golgi plug protocol, both from BD Pharmingen. For the staining of intracellular TGF-β1, PE-conjugated anti–TGF-β1 (IQ Products) was used. Samples were fixed in 2% paraformaldehyde following staining and were analyzed using a FACScalibur flow cytometer and CellQuest software (BD PharMingen). The frequency of positive cells was calculated by subtracting the value obtained with the respective isotype controls.
Enrichment of cells expressing membrane-bound TGF-β1 or secreting IL-10.
CD4 T cells were isolated from the inflammation and tolerance groups of mice and were either used immediately for cell fractionation or subjected to two to three rounds of stimulation in vitro using antigen and APCs. After in vitro incubation, CD4+ T cells were purified again by two rounds of negative selection using a CD4+ T cell isolation kit (MACS Miltenyi Biotec). The purified cells were stained with R-PE–conjugated anti-human TGF-β (TB21) (IQ Products), followed by addition of anti-PE Microbeads (Miltenyi Biotec), and were positively selected by multiple passes on a magnetic separation column (Miltenyi Biotec). Cell surface TGF-β1 expression was determined by flow cytometry. IL-10–secreting cells similarly were enriched using mouse IL-10 secretion assay according to the manufacturer’s recommendations (Miltenyi Biotec).
Statistical analyses.
Student’s unpaired two-tailed t test was used for all statistical analyses. Differences between groups were considered significant if P values were less than 0.05.
Acknowledgments
We thank P. Ray for helpful discussions and L. Glimcher for anti–T-bet. This research was supported by grants AI-48927, HL-60995, and P50-HL56389 from the NIH (to A. Ray).
Footnotes
Nonstandard abbreviations used: aerosolized antigen (Ag); bronchoalveolar lavage (BAL); cAMP-responsive element binding-1 (CREB-1); lymphotoxin (LT); nuclear factor of activated T cells-c1 (NFATc1); peridinin chlorophyll-a protein (PerCP); phycoerythrin (PE); signal transducer and activator of transcription-6 (STAT-6); T regulatory cell (Treg).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 2.Braun-Fahrlander C, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 3.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 4.Seymour BW, Gershwin LJ, Coffman RL. Aerosol-induced immunoglobulin (Ig)-E unresponsiveness to ovalbumin does not require CD8+ or T cell receptor (TCR)-gamma/delta+ T cells or interferon (IFN)-gamma in a murine model of allergen sensitization. J. Exp. Med. 1998;187:721–731. doi: 10.1084/jem.187.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 6.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc. Natl. Acad. Sci. U. S. A. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 8.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 9.Akbari O, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 10.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 11.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 13.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 14.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 15.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J. Clin. Invest. 1999;104:985–993. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- 17.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D-H, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 19.Zheng WP, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, O’Garra A, Arai KI, Arai N. Characterization of cis-regulatory elements and nuclear factors conferring Th2-specific expression of the IL-5 gene: a role for a GATA-binding protein. J. Immunol. 1998;160:2343–2352. [PubMed] [Google Scholar]
- 21.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 22.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccirillo CA, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parekh VV, et al. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 Ab, induce anergy in CD8+ T cells: role of TGF-beta 1. J. Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 26.Zuany-Amorim C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T cells. Nat. Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, et al. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J. Exp. Med. 2003;197:1689–1699. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowell DJ, et al. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity. 1999;11:399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer EM, et al. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer EM, Schwartzberg PL. Tec family kinases in lymphocyte signaling and function. Curr. Opin. Immunol. 2000;12:282–288. doi: 10.1016/s0952-7915(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 31.Bunnell SC, et al. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 32.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brachmann R, et al. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell. 1989;56:691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 34.Wong ST, et al. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989;56:495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]
- 35.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 36.Paul NL, Ruddle NH. Lymphotoxin. Annu. Rev. Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- 37.Browning JL, et al. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 38.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 39.Perez C, et al. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990;63:251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- 40.Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- 41.Sacca R, Cuff CA, Lesslauer W, Ruddle NH. Differential activities of secreted lymphotoxin-alpha3 and membrane lymphotoxin-alpha1beta2 in lymphotoxin-induced inflammation: critical role of TNF receptor 1 signaling. J. Immunol. 1998;160:485–491. [PubMed] [Google Scholar]
- 42.Penheiter SG, et al. Internalization-dependent and -independent requirements for transforming growth factor beta receptor signaling via the Smad pathway. Mol. Cell. Biol. 2002;22:4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 44.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 47.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 48.Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J. Exp. Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCullagh P. The significance of immune suppression in normal self tolerance. Immunol. Rev. 1996;149:127–153. doi: 10.1111/j.1600-065x.1996.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 50.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J. Exp. Med. 1999;189:877–882. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, et al. Essential role of nuclear factor kB in the induction of eosinophilia in allergic airway inflammation. J. Exp. Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang DH, et al. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 53.Altman, P.L., and Dittmer, D.S., editors. 1974. Biology Data Book. 2nd edition. Federation of American Societies for Experimental Biology. Bethesda, Maryland, USA. 1571–1744
- 54.Wong KL, Alarie Y. A method for repeated evaluation of pulmonary performance in unanesthetized, unrestrained guinea pigs and its application to detect effects of sulfuric acid mist inhalation. Toxicol. Appl. Pharmacol. 1982;63:72–90. doi: 10.1016/0041-008x(82)90028-x. [DOI] [PubMed] [Google Scholar]
- 55.Mitruka SN, et al. Aerosol cyclosporine prevents acute allograft rejection in experimental lung transplantation. J. Thorac. Cardiovasc. Surg. 1998;115:28–36. doi: 10.1016/s0022-5223(98)70439-8. [DOI] [PubMed] [Google Scholar]
- 56.Yiamouyiannis CA, et al. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am. J. Pathol. 1999;154:1911–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J. Exp. Med. 2002;195:51–57. doi: 10.1084/jem.20011732. [DOI] [PMC free article] [PubMed] [Google Scholar]