Abstract
Introduction:
The main objective of a root canal sealer is to provide a fluid tight seal. The purpose of this systematic meta-analysis was to determine the relative toxicity of commonly used root canal sealers like zinc oxide eugenol, calcium hydroxide, and resin-based sealers.
Materials and Methods:
An online search was conducted in peer-reviewed journals listed in PubMed, Cochrane, EBSCO, and IndMed databases between 2000 and 2012). Statistical analysis was carried out by using analysis of variance (ANOVA) followed by post-hoc comparison by Bonferroni method. The comparison between toxicity at 24 h and between 3 and 7 days was done by using paired t-test for each sealer.
Results:
At 24 h, the relative biotoxicity of the three sealers reported was insignificant (P- value 0.29), but the difference in toxicity was found significant (P < 0.001) after 3 days.
Conclusion:
Calcium hydroxide sealer and zinc oxide eugenol were found to be significantly biotoxic as compared to resin-based sealers after 3 days.
Keywords: Biocompatibility, cytotoxicity, meta-analysis, root canal sealers
INTRODUCTION
Endodontic treatment aims to eliminate infection of the root canal and to completely fill the root canal space in three-dimension, in order to prevent apical and coronal penetration of liquids and microorganisms. Most root canals are filled with gutta-percha points in combination with an endodontic sealer which are essential components of root canal obturation to establish a fluid-tight seal. The main function of a sealer is to fill the spaces between the core material and the walls of root canal and between the gutta-percha cones, in an attempt to form a coherent mass of obturating material without voids. The sealer is expected to fill irregularities and minor discrepancies between the filling and canal walls, accessory canals, and multiple foramina. By its germicidal action, it is also expected to destroy the remaining bacteria left after cleaning and shaping of the root canal. Although all efforts are concentrated to confine the sealer within the root canal space, some extrusion inadvertently occurs during obturation procedure. When the sealer cement comes in contact with soft and hard tissues apically, it can cause persistent inflammation of periradicular tissues and may result in delayed wound healing manifesting as pain, tenderness, and swelling of the affected area; hence, biocompatibility of sealers is an important issue in selecting the right type of sealer for different types of endodontic cases.
All contemporary endodontic sealers are known to have some toxic properties, but meta-analysis on their toxicity has not been reported; hence, the present study was undertaken to determine relative toxicity of the most commonly used root canal sealers like calcium hydroxide, zinc-oxide eugenol, and resin-based sealers.
MATERIALS AND METHODS
The commonly used sealers were classified into following three groups:
Group 1: Zinc oxide eugenol based sealers, for example, Pulp Canal Sealer, Tubli-Seal.
Group 2: Calcium hydroxide based sealers, for example, Apexit, Vitapex.
Group 3: Resin-based sealers, for example, AH26, AH Plus, Diaket.
A comprehensive search was initiated to identify studies on sealer biotoxicity published in English language, on PubMed, Cochrane, EBSCO, and IndMed databases between 2000 and 2012. The keywords used in search queries on MESH were “Biotoxicity”, “root canal sealers”, and “biocompatibility”. The reference section of each of these articles was also analyzed to determine the articles which pertain to our search criteria.
Inclusion criteria
All in vitro and in vivo (animal) experimental studies (original research) between 2000 and 2012 were included.
Only those studies comparing minimum of two of the three groups of sealers, that is, resin, calcium hydroxide, and zinc oxide eugenol.
The studies should compare cytotoxicity/biotoxicity/biocompatibility of sealers at day 1 (24h) and between 3 and 7 days.
Only those studies were finally selected in which systematic analysis and statistical tests were applied.
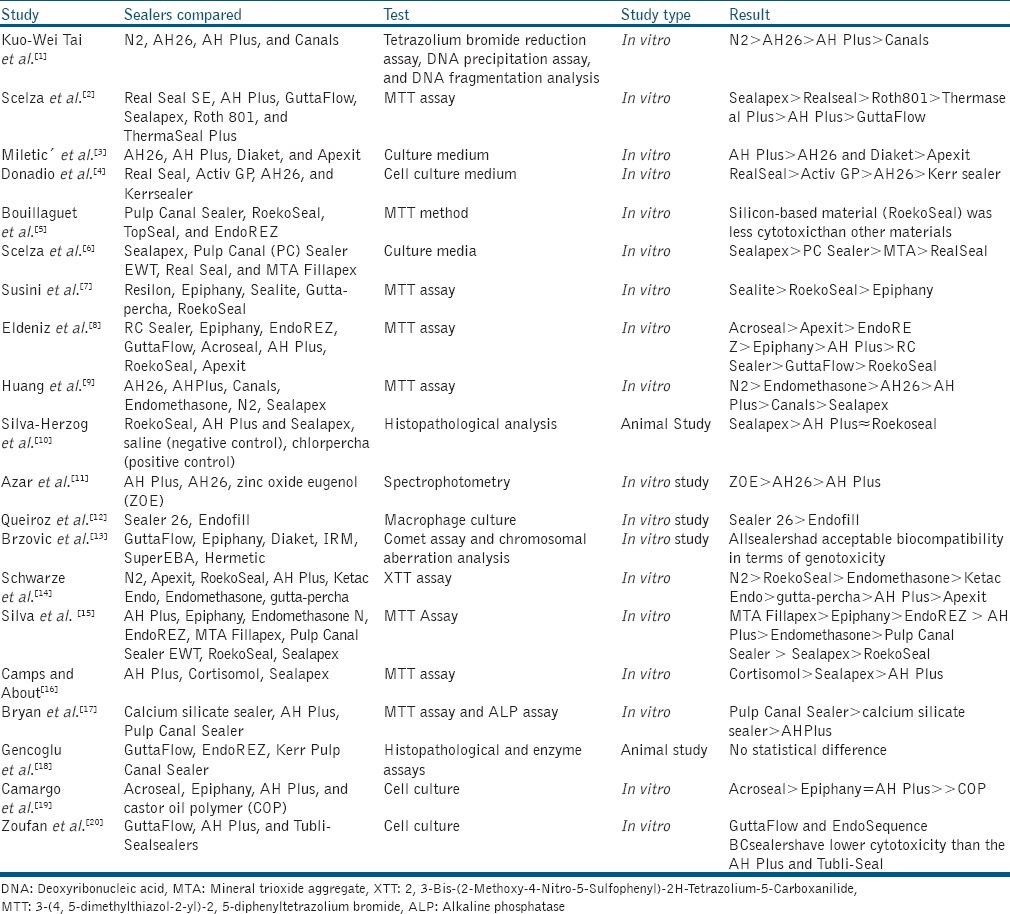
The one that matched was placed on the final list. Using this method; 58 articles were found in the search, out of which 31 article abstracts, that is, six animal studies and 25 in vitro studies were reviewed by two endodontists from which only 20 studies (18 in vitro and two animal studies) met the inclusion criteria and were finally selected for statistical analysis [Table 1].
Table 1.
Research articles selected for final statistical meta-analysis
Statistical analysis was carried out using analysis of variance (ANOVA) followed by post-hoc comparison by Bonferroni method. The comparison between 24 h and after 3rd day was done by using paired t-test for each sealer. The quantitative data was compared using Student's t-test and paired data by using paired t-test. Since the data was skewed, log transformation was also applied. The software used was STATA version 12. The level of significance was set at 0.05.
RESULTS
At 24 h, the relative biotoxicity of the three sealers reported was insignificant (P- value 0.29), but the difference in toxicity was found significant (P < 0.001) after 3 days. Calcium hydroxide and zinc oxide eugenol sealers were found to be significantly biotoxic as compared to resin-based sealers after 3 days. It was also observed that toxicity of calcium hydroxide was slightly more than zinc oxide eugenol after 3 days, although the results were statistically insignificant. The meta-analysis also revealed the heterogeneity of various studies and their results included.
Summary statistics at 24h and between 3 and 7 days (n = 20) for zinc oxide eugenol, calcium hydroxide, and resin revealed the values for means as 49.05, 64.10, and 63.35 and 51.00, 80.45, and 75.00; standard deviation as 30.45, 21.05, and 22.21 and 18.67, 14.78, and 16.63; and median as 41.50, 64.00, and 63.50 and 48.00, 84.00, and 77.50 with range as 5-95, 23-92, and 17-94 and 21-87, 39-95, and 32-92, respectively.
Comparison of biotoxicity (log values) of three sealers at 24 h and between 3 and 7 days shows that there is no significant difference at 24 h between the three sealers (P- value 0.29). Average log value of zinc oxide eugenol sealer at 24 h is 4.07 ± 0.44, calcium hydroxide is 4.09 ± 0.39, and resin-based sealers is 3.66 ± 0.77.
When compared between 3rd and 7th day, statistically significant difference was observed between the three sealers with P-value < 0.001 and the Bonferroni correction showed that resin sealers had significant lower values [Table 2].
Table 2.
Biotoxicity of three sealers at 24 h and between 3 and 7days
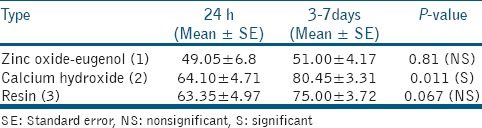
Based on paired data, no significant difference was observed in zinc oxide eugenol and resin-based sealers with P = 0.81 and 0.067, respectively. The significant increase of 16.35 (25%) was observed after 3 days in calcium hydroxide sealer (P = 0.01). Based on meta-analysis, the random pooled estimates were 49, 64, and 63% for zinc oxide eugenol, calcium hydroxide, and resin-based sealers, respectively. The pooled estimate was found to be 0.56 with 95% confidence interval (CI) as 0.55-0.58. Heterogeneity was observed to be 99%.
DISCUSSION
Root canal treatment involves a thorough cleaning and shaping, followed by three-dimensional obturation of the root canal system. Presently, root canals are obturated with a core material in combination with an endodontic sealer.
There are a variety of sealers to choose from, and the clinician must be careful to evaluate all characteristics of a sealer before selecting. It should be tacky when mixed to provide good adhesion between it and the canal wall, and when set a fluid tight seal should be obtained, while also having ample setting time for the clinician to make necessary adjustments to the filling material. The particles of powder should be very fine so that they can mix easily with the liquid and ideally should not shrink upon setting, although all currently available sealers shrink slightly upon setting.
Although during routine endodontic therapy, it is desired that the endodontic sealers remain inside the root canal, they may inadvertently be pushed beyond the apical constriction sometimes. In fact, they remain in intimate contact with the surrounding soft and hard tissues for an extended period of time.
Hence it is of utmost importance that they have an acceptable biocompatibility, that is, they should be nontoxic, nonmutagenic, and noncarcinogenic. All sealers exhibit toxicity when freshly mixed; however, their toxicity is greatly reduced on setting. Intense neutrophil infiltration is seen in response to all sealers during the initial period. Breakdown products from the sealers may have an adverse effect on the proliferative capability of periradicular cell populations. It has been observed by Kolokouris et al.,[21] that the intensity of the reaction diminishes by 60th day and the reduction progresses through to 120th day. As a result, adequate precaution should be taken not to extrude sealer in to the periapical tissue during obturation.
Zinc oxide eugenol sealers have a history of successful use in root canal obturation for over 100 years. It gets resorbed if extruded into the periapical tissue. It has prolonged setting time, shrinkage on setting, high solubility, and can stain the tooth structure. The advantage of zinc oxide eugenol sealer is its antimicrobial activity and popularity among clinicians, especially when used with thermoplasticized obturation technique.[22] But eugenol is found to leak from zinc oxide eugenol sealers, which is known to induce toxic effect and decrease the transmission in nerve cells. The effect is persistent even after setting of the material. Zinc oxide eugenol sealer with para-formaldehyde is both cytotoxic and mutagenic. Localized inflammation with zinc oxide eugenol sealers has been seen, both in soft tissue and in the bone.[23]
Calcium hydroxide sealers exhibit antimicrobial activity and have osteogenic-cementogenic potential. The rationale for the addition of calcium hydroxide to root canal sealers is from observations of bases and liners containing the material and their antibacterial and tissue regenerating ability, exerted via the leaching of calcium and hydroxyl ions to surrounding tissues.[24] Solubility is required for release of calcium hydroxide and sustained activity, hence it is not consistent with the purpose of an ideal sealer. Soares et al.,[25] observed that canals overfilled with calcium hydroxide sealers caused chronic inflammatory reaction in periapical tissues in dog's teeth. Sealapex is primarily made of calcium hydroxide and has been shown to be cytotoxic in various studies, which probably resulted from components/additives such as polymethylene methyl salicylate resin and isobutyl salicylate present in Sealapex.[3] Another possible explanation for the cytotoxicity of Sealapex may come from the calcium hydroxide itself, which produces high pH (Leonardo et al., 2000[26]).
Resin sealers also have a long history of use. It provides adhesion and does not contain eugenol. AH26 is a slow setting epoxy resin that was found to release formaldehyde when setting. AH Plus is a modified formulation of AH26 in which formaldehyde is not released. The sealing abilities of AH26 and AH Plus are comparable. EndoREZ is a methacrylate resin-based sealer with hydrophilic properties. When used with gutta-percha cones, the dual cure EndoREZ sealer bonds to both the canal walls and the core material.
Epiphany and RealSeal are other resin-based sealers introduced for use with a new core material. Advocates of these sealers propose that they bond to the canal wall and to the core material to create a “monobloc”. But toxicity of Epiphany might be explained by the presence of unpolymerized hydrophilic monomers (such as hydroxyethyl methacrylate (HEMA)), which show diffusabilty into the surrounding tissues and exhibit toxicity. Consequently, extrusion of a methacrylate resin-based sealer through the periapical foramen would create an uncured surface layer for extended time periods.[27] This might alter the toxicity profile of resin-based sealers because the incompletely polymerized monomers present in the exposed sealer are more toxic.
The meta-analysis of various studies revealed toxic potential of unset endodontic sealers, which may result in a localized inflammation and influence the healing of an apical periodontitis. Maximum toxicity was seen for calcium hydroxide-based sealers. These results disagree with the previous popular notion that called calcium hydroxide-based sealers as ‘biological sealers’.[28]
The use of so-called ‘biological’ sealers based on calcium hydroxide has been proposed for the permanent obturation of the root canal system. However, it exhibited increasing toxicity when set to confirm the results of previous studies that reported considerable leakage of cytotoxic substances from the disintegrating sealer (Gerosa et al., 1995,[29] Leonardo et al., 2000[26]). This instability in an aqueous environment might enhance the release of substances from set Sealapex.
In a study done by Scelza et al., (2012),[2] Sealapex was strongly cytotoxic at days 1, 7, and 14. In another animal study,[11] the severity of the inflammation produced by the Ca (OH)2 sealer, Sealapex, increased at the 3rd day of evaluation, with no resolution until the 14th day. Inflammatory cells were observed at the 7th day and foreign-body multinucleated giant cell reaction occurred after 14 days. The spectrophotometric data revealed a correlation with the histopathological findings.[11] Using Sealapex, Leonardo et al., (2007)[30] also observed an intense inflammatory infiltrate in dogs, suggesting that the alterations in the original formulation might have affected negatively the tissue compatibility of this material.
In case of zinc oxide eugenol-based sealers, moderate to severe cytotoxicity was observed. It was suggested by Lindqvist and Otteskog (1981)[31] that the cytotoxicity of zinc oxide eugenol root canal sealers was attributable to free eugenol liberated from the set material. A previous study has shown that these sealers can release formaldehyde after setting (Leonardo et al., 1999).[32] The combined effects of eugenol and formaldehyde might explain why N2 and Endomethasone were highly toxic. Apart from formaldehyde and eugenol, N2 also contains a variety of aromatic oils that are cytotoxic.
The cytotoxicity of resin-based sealers may be related to the release of formaldehyde (Spångberg et al., 1993).[33] In addition, bisphenol A diglycidyl ether was identified as a mutagenic component of resin-based materials, which may also be cytotoxic.
These results also agree with previous reports (Arenholt-Bindslev and Hörsted-Bindslev 1989,[34] Gerosa et al., 1995,[29] Cohen et al., 2000,[35] Leonardo et al., 2000[26]) that all materials tested showed some cytotoxicity.
Cytotoxicity testing of freshly mixed sealers is relevant as they are placed into the root canal system in a freshly mixed and incompletely polymerized stage. Changes in cytotoxicity levels may be observed after diffusion of toxic components from the materials into the surrounding environment.[15] This could be attributed to the release of small amounts of toxic substances present in the sealers. Probably as a result of the diminished leaching of these toxic substances, cytotoxicity of the tested sealers diminished over a period of time.[16]
We also observed in our meta-analysis that different studies showed varied results and data were skewed. This might be because divergent results may arise from the diverse experimental conditions. There are reports of divergent responses and sensibilities for the XTT (2, 3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide) and MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assays on a cell-type-dependent basis (Scudiero et al., 1988).[36] It was also reported that these methods, in reality, do not evaluate the same enzyme systems, because evidence was found that MTT reduction is associated not only with mitochondria but also with the cytoplasm and with nonmitochondrial membranes including the endosome/lysosome compartment and the plasma membrane (Berridge et al., 2005).[37]
Camps et al., (2003)[16] state that ISO standards lead us to accept the cytotoxicity of endodontic sealers that were rated noncytotoxic when tested with the root-dipping technique. For example, Sealapex showed a high cytotoxicity ranging from 91 to 96% of cell death when evaluated with ISO standards, whereas a cytotoxicity ranging from 0 to 9% was observed when working with the other technique.[17] This discrepancy between the results could be even more pronounced if the ratio between the surface of the sample and the volume of culture medium increases.
Among the reviewed articles, exploration of P- values of the selected comparisons indicated that most of them had suggested that the commonly used root canal sealers show biological risks inducing potential tissue biotoxicity leading to damage and inflammatory response in the periapical area.[16] In cases where the sealer does not penetrate into the periradicular area directly, the elutable substances or the degradable products leach through the dentinal tubules, lateral and accessory canals, and the apical foramina.
CONCLUSION
From the reviewed articles, meta-analysis revealed:
At 24 h, the relative biotoxicity of the three sealers was insignificant (P- value 0.29), but toxicity was significantly more (P < 0.001) after 3days.
Calcium hydroxide sealer and zinc oxide eugenol were found to be significantly biotoxic as compared to resin-based sealers after 3days. It was also observed that toxicity of calcium hydroxide was slightly more than zinc oxide eugenol after 3days, although results were statistically insignificant.
The meta-analysis also revealed the heterogeneity of various studies and their results included.
Exploration of P – values of the selected comparisons indicated that most of them had suggested that the commonly used root canal sealers exhibited some degree of cytotoxicity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tai KW, Huang FM, Huang MS, Chang YC. Assessment of the genotoxicity of resin and zinc-oxide eugenol-based root canal sealers using an in vitro mammalian test system. J Biomed Mater Res. 2002;59:73–7. doi: 10.1002/jbm.1218. [DOI] [PubMed] [Google Scholar]
- 2.Scelza MZ, Coil J, Alves GG. Effect of time of extraction on the biocompatibility of endodontic sealers with primary human fibroblasts. Braz Oral Res. 2012;26:424–30. doi: 10.1590/s1806-83242012000500008. [DOI] [PubMed] [Google Scholar]
- 3.Miletic´ I, Anic´ I, Karlovic´ Z, Marsan T, Pezelj-Ribaric´ S, Osmak M. Cytotoxic effects of the four root filling materials. Endod Dent Traumatol. 2000;16:287–90. doi: 10.1034/j.1600-9657.2000.016006287.x. [DOI] [PubMed] [Google Scholar]
- 4.Donadio M, Jiang J, He J, Wang YH, Safavi KE, Zhu Q. Cytotoxicity evaluation of Activ GP and Resilon sealers in vitro. Oral Surgery, Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e74–8. doi: 10.1016/j.tripleo.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 5.Bouillaguet S, Wataha JC, Lockwood PE, Galgano C, Golay A, Krejci I. Cytotoxicity and sealing properties of four classes of endodontic sealers evaluated by succinic dehydrogenase activity and confocal laser scanning microscopy. Eur J Oral Sci. 2004;112:182–7. doi: 10.1111/j.1600-0722.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 6.Scelza MZ, Linhares AB, da Silva LE, Granjeiro JM, Alves GG. A multiparametric assay to compare the cytotoxicity of endodontic sealers with primary human osteoblasts. Int Endod J. 2012;45:12–8. doi: 10.1111/j.1365-2591.2011.01941.x. [DOI] [PubMed] [Google Scholar]
- 7.Susini G, About I, Tran-Hung L, Camps J. Cytotoxicity of Epiphany and Resilon with a root model. Int Endod J. 2006;39:940–4. doi: 10.1111/j.1365-2591.2006.01167.x. [DOI] [PubMed] [Google Scholar]
- 8.Eldeniz AU, Mustafa K, Ørstavik D, Dahl JE. Cytotoxicity of new resin-, calcium hydroxide-, and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007;40:329–37. doi: 10.1111/j.1365-2591.2007.01211.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang FM, Tai KW, Chou MY, Chang YC. Cytotoxicity of resin-, zinc oxide–eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J. 2002;35:153–8. doi: 10.1046/j.1365-2591.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 10.Silva-Herzog D, Ramírez T, Mora J, Pozos AJ, Silva LA, Silva RA, et al. Preliminary study of the inflammatory response to subcutaneous implantation of three root canal sealers. Int Endod J. 2011;44:440–6. doi: 10.1111/j.1365-2591.2011.01849.x. [DOI] [PubMed] [Google Scholar]
- 11.Azar NG, Heidari M, Bahrami ZS, Shokri F. In vitro cytotoxicity of a new epoxy resin root canal sealer. J Endod. 2000;26:462–5. doi: 10.1097/00004770-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Queiroz CE, Soares JA, Leonardo Rde T, Carlos IZ, Dinelli W. Evaluation of cytotoxicity of two endodontic cements in a macrophage culture. J Appl Oral Sci. 2005;13:237–42. doi: 10.1590/s1678-77572005000300007. [DOI] [PubMed] [Google Scholar]
- 13.Brzovic V, Miletic I, Zeljezic D, Mladinic M, Kasuba V, Ramic S, et al. In vitro genotoxicity of root canal sealers. Int Endod J. 2009;42:253–63. doi: 10.1111/j.1365-2591.2008.01510.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwarze T, Leyhausen G, Geurtsen W. Long-term cytocompatibility of various endodontic sealers using a new root canal model. J Endod. 2002;28:749–53. doi: 10.1097/00004770-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Silva EJ, Santos CC, Zaia AA. Long-term cytotoxic effects of contemporary root canal sealers. J Appl Oral Sci. 2013;21:43–7. doi: 10.1590/1678-7757201302304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camps J, About I. Cytotoxicity testing of endodontic sealers: A new method. J Endod. 2003;29:583–6. doi: 10.1097/00004770-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Bryan TE, Khechen K, Brackett MG, Messer RL, El-Awady A, Primus CM, et al. In vitro osteogenic potential of an experimental calcium silicate-based root canal sealer. J Endod. 2010;36:1163–9. doi: 10.1016/j.joen.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Gencoglu N, Sener G, Omurtag GZ, Tozan A, Uslu B, Arbak S, et al. Comparision of biocompatibility and cytotoxicity of two new root canal sealers. Acta Histochem. 2010;112:567–75. doi: 10.1016/j.acthis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Camargo CH, Camargo SE, Valera MC, Hiller KA, Schmalz G, Schweikl H. The induction of cytotoxicity, oxidative stress, and genotoxicity by root canal sealers in mammalian cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:952–60. doi: 10.1016/j.tripleo.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Zoufan K, Jiang J, Komabayashi T, Wang YH, Safavi KE, Zhu Q. Cytotoxicity evaluation of Gutta flow and endo sequence BC sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:657–61. doi: 10.1016/j.tripleo.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Kolokouris I, Economides N, Beltes P, Vlemmas I. In vivo comparison of the biocompatibility of two root canal sealers implanted into the subcutaneous connective tissue of rats. J Endod. 1998;24:82–5. doi: 10.1016/S0099-2399(98)80082-4. [DOI] [PubMed] [Google Scholar]
- 22.Gutmann JL, Rakusin H. Perspectives on root canal obturation with thermoplasticized injectable gutta-percha. Int Endod J. 1987;20:261–70. doi: 10.1111/j.1365-2591.1987.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 23.Yesiloy C, Koren LZ, Morse DR, Kobayashi C. A comparative tissue toxicity evaluation of established and newer root-canal sealers. Oral Surg Oral Med Oral Pathol. 1988;65:459–67. doi: 10.1016/0030-4220(88)90361-1. [DOI] [PubMed] [Google Scholar]
- 24.Zander HA. Reaction of pulp to calcium hydroxide. J Dent Res. 1939;18:373–9. [Google Scholar]
- 25.Soares I, Goldberg F, Massone EJ, Soares IM. Periapical tissue response to two calcium hydroxide-containing endodontic sealers. J Endod. 1990;16:166–9. doi: 10.1016/S0099-2399(06)81964-3. [DOI] [PubMed] [Google Scholar]
- 26.Leonardo MR, da Silva LA, Tanomaru Filho M, Bonifácio KC, Ito IY. In vitro evaluation of antimicrobial activity of sealers and pastes used in endodontics. J Endod. 2000;26:391–4. doi: 10.1097/00004770-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Bouillaguet S, Wataha JC, Tay FR, Brackett MG, Lockwood PE. Initial in vitro biological response to contemporary endodontic sealers. J Endod. 2006;32:989–92. doi: 10.1016/j.joen.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.JOE Editorial Board. Uses of calcium hydroxide: An online study guide. J Endod. 2008;34:e87–92. doi: 10.1016/j.joen.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Gerosa R, Menegazzi G, Borin M, Cavalleri G. Cytotoxicity evaluation of six root canal sealers. J Endod. 1995;21:446–8. doi: 10.1016/S0099-2399(06)81525-6. [DOI] [PubMed] [Google Scholar]
- 30.Osorio RM, Hefti A, Vertucci FJ, Shawley AL. Cytotoxicity of endodontic materials. J Endod. 1998;24:91–6. doi: 10.1016/S0099-2399(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 31.Lindqvist L, Otteskog P. Eugenol: Liberation from dental materials and effect on human diploid fibroblast cells. Scand J Dent Res. 1980;88:552–6. doi: 10.1111/j.1600-0722.1980.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 32.Leonardo MR, Bezerra da Silva LA, Filho MT, Santana da Silva R. Release of formaldehyde by 4 endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:221–5. doi: 10.1016/s1079-2104(99)70119-8. [DOI] [PubMed] [Google Scholar]
- 33.Spångberg LS, Barbosa SV, Lavigne GD. AH 26 releases formaldehyde. J Endod. 1993;19:596–8. doi: 10.1016/S0099-2399(06)80272-4. [DOI] [PubMed] [Google Scholar]
- 34.Arenholt-Bindslev D, Hörsted-Bindslev P. A simple model for evaluating relative toxicity of root filling materials in cultures of human oral fibroblasts. Endod Dent Traumatol. 1989;5:219–26. doi: 10.1111/j.1600-9657.1989.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 35.Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. An in vitro study of the cytotoxicity of two root canal sealers. J Endod. 2000;26:228–9. doi: 10.1097/00004770-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–33. [PubMed] [Google Scholar]
- 37.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–52. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]


