Abstract
We describe a murine model of early pregnancy failure induced by systemic activation of the CD40 immune costimulatory pathway. Although fetal loss involved an NK cell intermediate, it was not due to lymphocyte-mediated destruction of the fetus and placenta. Rather, pregnancy failure resulted from impaired progesterone synthesis by the corpus luteum of the ovary, an endocrine defect in turn associated with ovarian resistance to the gonadotropic effects of prolactin. Pregnancy failure also required the proinflammatory cytokine TNF-α and correlated with the luteal induction of the prolactin receptor signaling inhibitors suppressor of cytokine signaling 1 (Socs1) and Socs3. Such links between immune activation and reproductive endocrine dysfunction may be relevant to pregnancy loss and other clinical disorders of reproduction.
Introduction
The mechanisms underlying immune-mediated pregnancy failure remain largely unknown (1). In women with recurrent pregnancy loss, increased numbers and cellular activation of peripheral blood CD56+ NK cells have suggested an important role for the innate immune system (2–6). Since NK cells produce the signature Th1 cytokine IFN-γ, NK cell activation during pregnancy might explain the apparent Th1 bias also associated with recurrent pregnancy loss (7, 8). These associations, however, have not led to a clear understanding of the ultimate cause of fetal demise. Data from rodent studies have suggested that the major mechanism of immune-mediated pregnancy failure is immune activation at the maternal-fetal interface. Activation of decidual immune cells with attendant inflammatory cytokine production or activation of complement via the binding of anti-phospholipid antibodies is thought to directly damage the fetus and placenta or lead to derangements in decidual or placental hemostasis (1, 9–11).
The possibility that immune activation might cause pregnancy failure by inhibiting the reproductive endocrine system has been largely unexplored, despite indications that immune processes regulate reproductive endocrine function in nonpregnant mammals. Inflammatory cytokines are thought to inhibit gonadotropin production at the level of the hypothalamus and pituitary in cases of chronic or acute illness (12), and to inhibit progesterone synthesis by the corpus luteum of the ovary and promote luteal regression as part of the normal ovulatory cycle (13). During early gestation in rodents, ovarian progesterone production is driven primarily by pituitary-derived prolactin binding to its Janus kinase 2/signal transducer and activator of transcription 5–coupled (JAK2/STAT5–coupled) receptor expressed by corpus luteal cells (14). The binding of progesterone to its nuclear receptor, in turn, is thought to maintain decidual viability and inhibit myometrial contractility (15, 16). In addition, there is strong evidence that luteinizing hormone (LH) produced by the pituitary is also critical for maintaining luteal function in rats early in pregnancy (17), and this is likely also to be the case in mice (18). Thus, the reproductive endocrine system provides multiple potential points for inhibition by the immune system.
We describe a new link between the immune system and the reproductive endocrine system that is regulated by the TNF receptor superfamily member CD40, an immune costimulatory molecule whose expression on dendritic cells is critical for the initiation of cell-mediated immune responses (19, 20). Forced ligation of CD40 with agonistic anti-CD40 antibodies caused a systemic inflammatory response that efficiently induced embryo resorption in mice early in gestation. However, pregnancy failure occurred not through immune cell activation at the maternal-fetal interface, but rather through decreased progesterone synthesis by the corpus luteum. Luteal insufficiency was induced largely by activated NK cells, required the actions of the proinflammatory cytokine TNF-α, and was associated with impaired prolactin receptor signaling. Furthermore, luteal insufficiency correlated with the induction of suppressor of cytokine signaling 1 (Socs1) and Socs3, two members of the Socs family of proteins that provide critical feedback inhibition of JAK/STAT signaling at the immediate postreceptor level (21–23). The gestational effects of CD40 ligation delineate an in vivo pathway whereby activation of the innate immune system with associated inflammatory cytokine production leads to pregnancy failure through inhibition of the reproductive endocrine system.
Results
Systemic CD40 ligation in mice causes early pregnancy failure preceded by decreased serum progesterone concentrations.
To determine the gestational effects of systemic CD40 ligation, we injected the agonistic anti-CD40 mAb FGK45 (24) at various times during pregnancy. Four daily injections of FGK45 from embryonic day 4 (E4) to E7 dramatically reduced the percentage of pregnant mice per total mated mice to nearly 0% in females of all strains tested, with control pregnancy rates after treatment with polyclonal rat IgG varying between 40% and 100% depending on the background strain (Table 1). The effect of FGK45 injection occurred in both syngeneic and allogeneic mating combinations and was restricted to the immediate postimplantation period, since similar daily injections on E8–11 did not reduce pregnancy rates or litter sizes. FGK45 injection also did not inhibit pregnancy in CD40–/– females (25) mated to wild-type males, ruling out the possibility that its abortifacient effect was nonspecific or due to embryonic or placental ligation of CD40 (data not shown). Rather, pregnancy failure required maternal CD40 expression.
Table 1.
Systemic CD40 ligation causes murine pregnancy failure in the postimplantation period
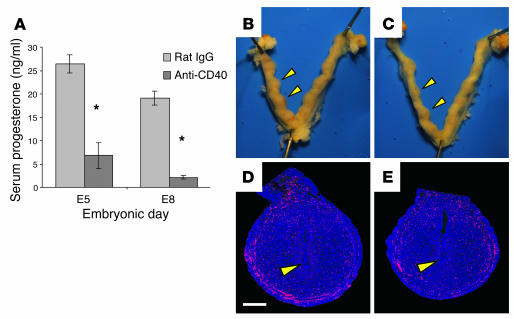
Although CD40 is largely considered an immune stimulatory molecule, the timing of events leading to embryo loss suggested that the ultimate cause of pregnancy failure following CD40 ligation was endocrinological rather than immunological. Using B6CBAF1 females to take advantage of their high natural pregnancy rate (Table 1), we found that a single FGK45 injection on E4 dramatically reduced serum progesterone levels within 24 hours (Figure 1A), yet implantation sites from these mice showed no evidence of a pathological leukocyte infiltration that might be expected with a tissue-rejection response. Rather, compared with controls (Figure 1, B and D), implantation sites from FGK45-treated mice were only mildly reduced in size at E5 (Figure 1C) and contained implanted embryos with the typical clearing of CD45+ leukocytes (red) from the early primary maternal decidua (Figure 1E). By E8, however, after daily FGK45 injections on E4–7, all implantation sites had been completely resorbed (see below), and serum progesterone levels remained low (Figure 1A). Since progesterone is the sole ovarian hormone required for the maintenance of postimplantation pregnancy (26), its reduced concentration in serum prior to overt embryo resorption suggested that CD40 ligation–induced abortion involved an inhibition of the hypothalamic-pituitary-gonadal axis. This possibility would also be consistent with the observation that pregnancy failure occurred in an all-or-nothing fashion, with the normal-sized litters of those few females that maintained pregnancy after E4–7 FGK45 treatment (Table 1) showing no evidence of piecemeal embryonic resorption.
Figure 1.
Decreased serum progesterone concentrations following systemic CD40 ligation precedes overt embryo resorption. Mice either were treated with control rat IgG or agonistic anti-CD40 antibodies (FGK45) once on E4 and sacrificed 24 hours later on E5, or were treated daily on E4–7 and sacrificed on E8. (A) Serum progesterone concentrations. Data represent mean ± SEM of six mice per group. Progesterone concentrations in FGK45-treated mice were significantly reduced compared with those in control mice. *P < 0.001. (B–E) Implantation sites on E5 from mice treated with rat IgG (B and D) or FGK45 (C and E). (B and C) Whole-mount preparations of uteri, with arrowheads indicating two implantation sites. (D and E) Paraffin sections of implantation sites stained with anti-CD45 antibodies to visualize all leukocytes (red), and counterstained with DAPI to visualize all cell nuclei (blue). The mesometrial pole of each implantation site is oriented toward the top of the panel. A recently implanted embryo can be seen in the center of each section (arrowhead). Immunostaining is representative of two to three mice per group, encompassing a total of 15–20 implantation sites in each group. Scale bar: 0.5 mm.
Pregnancy failure following CD40 ligation is due to luteal insufficiency associated with ovarian prolactin resistance and defective prolactin receptor signaling.
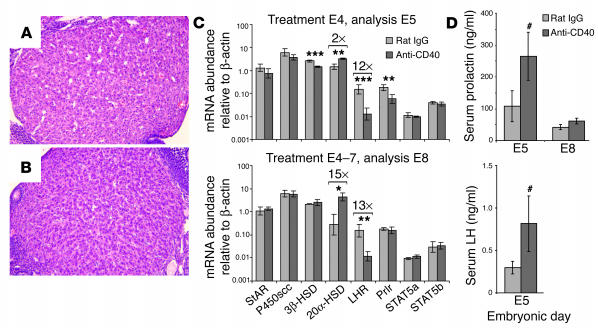
We next sought to understand why ovarian function was impaired following CD40 ligation. Compared with controls (Figure 2A), there was little microscopic evidence that CD40 ligation caused the histological regression of corpora lutea (i.e., structural luteolysis), since these structures were still clearly present on E8 following daily FGK45 injections on E4–7 and only showed a mild and variable increase in luteal cellularity (Figure 2B). Furthermore, quantitative real-time RT-PCR analysis of ovarian RNA from FGK45-treated mice (Figure 2C) revealed no significant change on either E5 or E8 in the levels of mRNA encoding the steroidogenic enzymes steroidogenic acute regulatory protein (StAR) and P450 side chain cleavage enzyme (P450scc), and only a transient twofold decrease on E5 alone in the mRNA level of 3β-hydroxysteroid dehydrogenase (3β-HSD).
Figure 2.
Postreceptor prolactin signaling defects and luteal insufficiency following systemic CD40 ligation. (A and B) Histological appearance of corpora lutea from mice on E8 following daily E4–7 injections of rat IgG (A) or anti-CD40 antibodies (FGK45) (B). Paraffin sections stained with H&E are shown. (C) Whole-ovary mRNA expression levels determined by real-time RT-PCR. Ovaries were collected from mice that either had been treated with control rat IgG or anti-CD40 antibodies (FGK45) once on E4 and sacrificed 24 hours later on E5 (n = 4 mice per group; upper panel), or had been treated daily on E4–7 and sacrificed on E8 (n = 3 mice per group; lower panel). Data represent mean ± SD. On both E5 and E8, 20α-HSD and LHR mRNA levels were increased and decreased, respectively (fold changes are indicated by brackets), whereas 3β-HSD and prolactin receptor (Prlr) mRNA levels were decreased on E5 only. *P < 0.02; **P < 0.005; ***P < 0.001. (D) Serum prolactin and LH concentrations. Prolactin and LH concentrations in FGK45-treated mice on E5 were significantly elevated compared with those in control mice (#P < 0.05; data represent mean ± SEM of 12 mice per group), but there was no change in prolactin concentrations on E8 (n = 6 mice per group). The limit of detection for LH was 0.2 ng/ml.
On the other hand, CD40 ligation induced dramatic changes in the mRNA expression levels of the luteinizing hormone receptor (LHR) and 20α-HSD, the two major transcriptional targets of prolactin receptor signaling in corpus luteal cells (14, 27) (Figure 2C). Normally, prolactin receptor signaling via JAK2/STAT5 induces the transcription of LHR and represses the transcription of 20α-HSD (14). However, a single FGK45 injection on E4 led to a 12-fold decrease in the abundance of LHR mRNA and a twofold increase in 20α-HSD mRNA within 24 hours, which progressed after daily FGK45 injections on E4–7 to a 13-fold decrease and a 15-fold increase in mRNA transcript levels, respectively, on E8. Thus, CD40 ligation led to a functional luteal insufficiency associated with defective prolactin receptor target-gene expression. Since 20α-HSD catalyzes the intraovarian catabolism of newly synthesized progesterone (14), its derepression in FGK45-treated mice likely contributed to the decrease in serum progesterone concentrations.
Further molecular and hormonal analyses suggested that the defect in prolactin receptor target-gene expression was due to inhibition at the postreceptor level. Thus, serum levels of prolactin were actually modestly elevated from those of controls 24 hours after a single FGK45 injection on E4, consistent with a loss of feedback inhibition by progesterone (13), and unimpaired pituitary function was also suggested by the modest elevation in serum LH levels on E5 (Figure 2D). On E8, after daily E4–7 FGK45 injection, serum prolactin levels were similar to those of controls (Figure 2D), with the overall increase in prolactin levels at this later time point consistent with the known pattern of pituitary prolactin secretion during early mouse gestation (28). Furthermore, we observed only a transient threefold decrease in prolactin receptor mRNA expression on E5 alone (due to proportionate decreases in mRNAs for the long and short isoforms; data not shown) and normal expression levels on E8, with unaltered mRNA expression of STAT5a and STAT5b on both E5 and E8 (Figure 2C). Collectively, these data implied that the deregulation of prolactin target genes was due to the presence of a dominant inhibitor of STAT5 signaling, a possibility we explore further below.
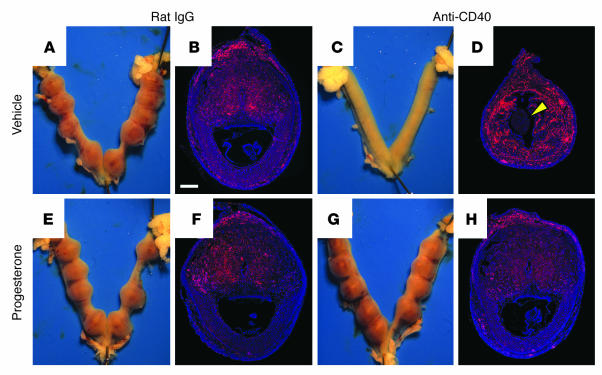
To directly evaluate whether luteal insufficiency was the ultimate cause of pregnancy failure, we treated pregnant mice with both FGK45 and progesterone. Strikingly, daily progesterone administration on E4–7, which led to serum concentrations of about 35 ng/ml 24 hours after the E7 injection, almost completely prevented abortions caused by daily E4–7 FGK45 injection (Table 2). Implantation sites at E8 from dually treated mice appeared completely normal both grossly (Figure 3G) and histologically (Figure 3H) and showed no evidence of an altered distribution of CD45+ leukocytes (red-stained cells) compared with those from control mice treated with either rat IgG plus progesterone (Figure 3, E and F) or rat IgG plus sesame seed oil vehicle (Figure 3, A and B). In contrast, E8 uteri of mice treated with anti-CD40 antibodies plus vehicle showed no evidence of implantation (Figure 3C), occasionally contained debris in the uterine lumen (Figure 3D, arrowhead), and by this end stage were infiltrated with CD45+ leukocytes scattered throughout the uterine stroma. Continued daily progesterone injection, up to E17, also maintained pregnancy in mice treated with FGK45 on E4–7 (six of six mice).
Table 2.
Rescue of CD40 ligation–induced pregnancy failure by exogenous progesterone or prolactin
Figure 3.
Rescue of CD40 ligation–induced pregnancy failure by exogenous progesterone. (A–H) Whole-mount uteri (A, C, E, and G) and implantation-site histology (B, D, F, and H) from mice on E8 following daily treatments on E4–7. Paraffin sections of implantation sites were stained with anti-CD45 antibodies to visualize all leukocytes (red), and counterstained with DAPI (blue). Only mice treated with FGK45 and sesame seed oil vehicle (C and D) showed abortion, leukocytic infiltration into the uterine stroma, and occasional cell debris in the uterine lumen (D, arrowhead). The CD45 staining intensity in these sections tended to be greater than that at viable implantation sites, because they do not contain the high numbers of CD45dim uterine NK cells that are present in the decidua. Immunostaining is representative of two mice per group, encompassing a total of about 12 implantation sites in each group. Scale bar: 0.5 mm.
In addition to progesterone, twice-daily coadministration of 100 μg ovine prolactin prevented CD40 ligation–induced abortion (Table 2), and implantation sites from these mice also showed no histological abnormalities (data not shown). Prolactin injections raised serum progesterone concentrations above those seen in mice treated with FGK45 alone; however, these levels still remained subphysiological (10.9 ± 1.5 ng/ml [mean ± SEM] on E8). Thus, although the corpora lutea of FGK45-treated mice were resistant to physiological or modestly elevated serum prolactin levels, this could be overcome by exogenous prolactin administration at high doses.
Induction of luteal insufficiency requires a lymphoid intermediate and TNF-αand is associated with increased ovarian Socs1 and Socs3 expression.
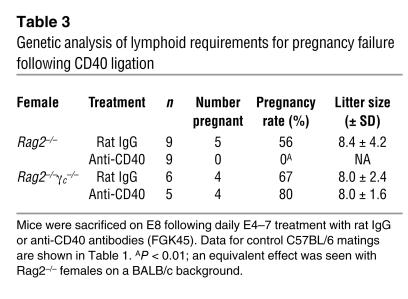
To understand how systemic CD40 ligation caused luteal insufficiency, we first used flow cytometry to determine that there was no significant CD40 staining of enzymatically dispersed E4 ovarian cell suspensions aside from a subset of CD45+ leukocytes (data not shown). This ruled out the possibility that FGK45 treatment induced abortion via direct ligation of CD40 on corpus luteal cells themselves. Furthermore, CD40 ligation induced abortion in Rag2–/– mice, which lack B cells, T cells, and NKT cells (29), yet had no effect on pregnancy in completely alymphoid Rag2–/–γc–/– mice, which additionally lack NK cells (30) (Table 3). Thus, a lymphocyte population was required for the abortifacient effects of CD40 ligation, and NK cells were sufficient to perform this function.
Table 3.
Genetic analysis of lymphoid requirements for pregnancy failure following CD40 ligation
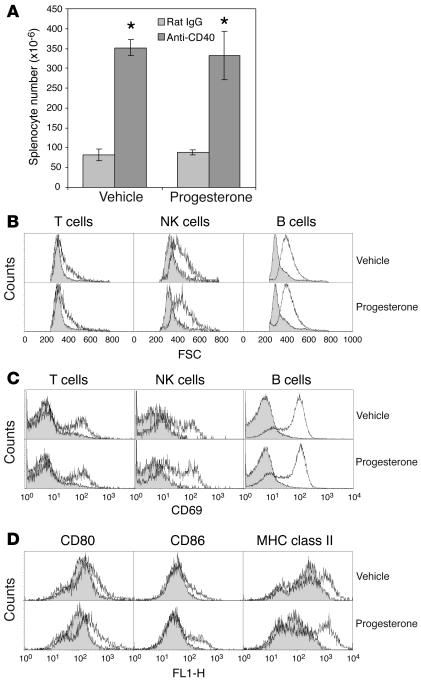
As expected, systemic CD40 ligation led to broad activation of lymphocytes and dendritic cells. Importantly, this activation was not affected by concurrent progesterone administration, which suggested that it lay entirely upstream of luteal insufficiency. Thus, we found the same degree of splenomegaly (Figure 4A), increased lymphocyte cell size (Figure 4B), modulated expression of the lymphoid activation markers CD69 (Figure 4B), CD25, CD44, CD45RB, and CD62L (data not shown), and upregulation of the costimulatory molecules CD80, CD86, MHC class II (Figure 4D), CD40, and CD54 (data not shown) on splenic CD11c+ dendritic cells. The splenic CD11b+CD11c– macrophage population expressed low levels of CD40 and showed only modest changes in surface marker expression after daily E4–7 FGK45 treatment (data not shown).
Figure 4.
Systemic immune activation by CD40 ligation is unaffected by progesterone administration. Mice were sacrificed on E8 after daily treatment on E4–7 with rat IgG or FGK45 plus either concurrent progesterone injection (which produced no abortion with either rat IgG or FGK45) or concurrent sesame seed oil vehicle injection (which produced abortion with FGK45 only). (A) Elevated splenocyte numbers following FGK45 treatment in both groups. *P < 0.005. Data represent mean ± SD for three to four mice per group. (B–D) Three-color flow cytometric analysis of splenocytes to determine cell size and surface activation marker expression. In all panels, shaded histograms show data from rat IgG–treated mice, while open histograms show data from FGK45-treated mice. Data represent three mice per group. (B and C) Lymphocyte activation. Increased forward scatter (FSC), indicating increased cell size (B), and increased CD69 expression, indicating cell activation (C), were seen in B cells, NK cells, and a fraction of T cells following FGK45 treatment in both groups. (D) Dendritic cell activation. Increased CD80, CD86, and MHC class II expression, indicating cell activation, was seen on splenic CD11c+ cells following FGK45 treatment in both groups.
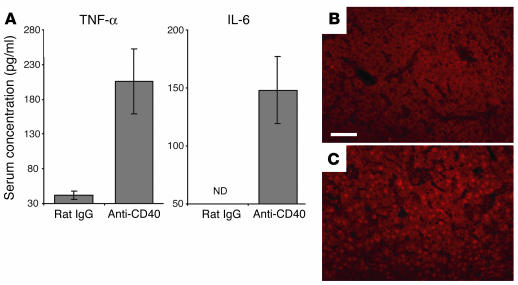
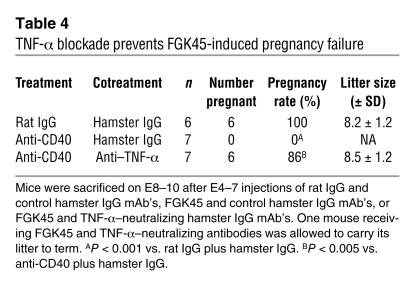
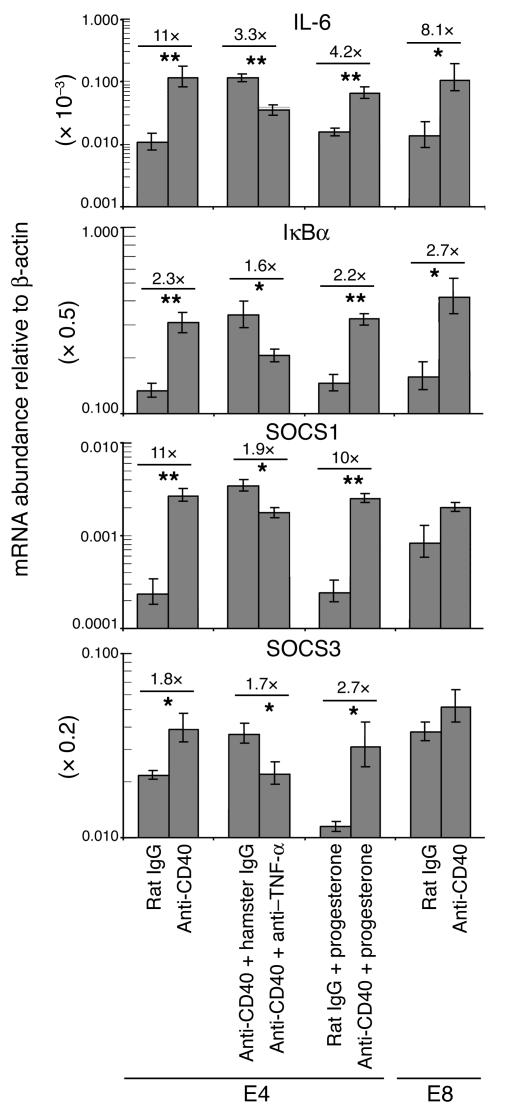
Since FGK45 injection did not cause a significant leukocytic infiltration into the ovaries (data not shown), luteal insufficiency following CD40 ligation was likely due to inflammatory cytokines produced by the immune system potentially acting at the systemic level. In contrast to unaltered serum levels of IL-1β, IL-2, IL-4, IL-10, IL-12, or IFN-γ, a single FGK45 injection on E4 induced a transient increase after 24 hours in the serum concentrations of TNF-α and its downstream target IL-6 (Figure 5A) (31). Indeed, TNF-α was critical for pregnancy failure, as its neutralization with a specific mAb prevented FGK45-induced abortion (Table 4). Furthermore, immunohistochemical and biochemical studies suggested that the cytokine was acting directly on corpus luteal cells. Thus, the p65 subunit of NF-κB, a transcriptional mediator of TNF receptor signaling present in the cytoplasm of unstimulated cells (Figure 5B), had assumed a nuclear distribution pattern in corpus luteal cells after FGK45 injection on E4 (Figure 5C). FGK45 injection also led to 11-fold and twofold respective increases in the ovarian abundances of transcripts for IL-6 and the additional NF-κB target IκBα (31), and these increases could largely be inhibited by administration of TNF-α–neutralizing antibodies (Figure 6). Collectively, these data show that TNF-α was required for FGK45-induced pregnancy failure and suggest that corpus luteal cells were functionally exposed to this cytokine during the course of FGK45-induced abortion. The ovary was also likely exposed to IL-6 as a secondary consequence of its systemic or local induction.
Figure 5.
Involvement of TNF-α in anti-CD40–induced pregnancy failure. (A) Elevated TNF-α and IL-6 serum concentrations 24 hours after treatment with anti-CD40 antibodies (FGK45). Data represent mean ± SEM of five mice per group. The limits of detection were 31.1 pg/ml TNF-α and 54.7 pg/ml IL-6. ND, not detectable. (B and C) NF-κB p65 distribution in E4 corpora lutea visualized by immunostaining. Whereas the p65 distribution was cytoplasmic in mice treated 24 hours earlier on E4 with control rat IgG (B), it assumed a nuclear distribution when mice were treated with FGK45 (C). Data represent immunostained ovaries from three mice per group. Scale bar: 50 μm.
Table 4.
TNF-α blockade prevents FGK45-induced pregnancy failure
Figure 6.
Ovarian mRNA expression of IL-6, IκBα, Socs1, and Socs3 upon systemic CD40 ligation. Whole-ovary mRNA expression levels were determined by real-time RT-PCR. Ovaries were collected from mice on E4 12 hours after treatment with rat IgG or FGK45 (n = 4 mice per group); with FGK45 plus control hamster IgG or FGK45 plus TNF-α–neutralizing mAb’s (n = 5 mice per group); or with rat IgG plus progesterone or FGK45 plus progesterone (n = 4 mice per group). Ovaries were collected on E8 12 hours after injection of rat IgG or FGK45 (n = 3 mice per group). Data represent mean ± SEM. On E4, IL-6, IκBα, Socs1, and Socs3 mRNA levels were increased following FGK45 injection (*P < 0.05; **P < 0.005). These increases were inhibited by TNF-α blockade but not prevented by concurrent progesterone treatment. On E8, FGK45 injection led to upregulated mRNA expression of IL-6 and IκBα, but not Socs1 or Socs3. For ease of graphical representation, transcript levels for IL-6, IκBα, and Socs3 were multiplied by 1,000, 2, and 5, respectively.
Both TNF-α and IL-6 are known to transcriptionally induce multiple members of the Socs family of JAK/STAT signaling inhibitors (22, 23). While only assessed to date outside the context of pregnancy, Socs induction is thought to be the major mechanism of cross-talk inhibition between JAK/STAT signaling cascades of the immune and endocrine systems (21–23). By analogy, the ovarian exposures to TNF-α and IL-6 directly suggested Socs proteins as the dominant inhibitors of STAT5 signaling that we inferred to exist from our expression studies of ovarian steroidogenic enzymes and prolactin receptor signaling components (see above). Indeed, 12 hours after a single FGK45 injection on E4, ovarian Socs1 and Socs3 mRNA levels were increased 11-fold and twofold, respectively (Figure 6), while Socs2 mRNA levels remained unchanged (data not shown). Compared with mice treated with FGK45 plus control hamster IgG mAb’s, mice treated with FGK45 plus TNF-α–neutralizing antibodies showed partially inhibited Socs1 induction and largely inhibited Socs3 induction (Figure 6). Furthermore, Socs1 and Socs3 inducibility on E4 correlated with the E4–7 temporal window of sensitivity toward FGK45-induced abortion, since FGK45 injection on E8 did not significantly increase Socs1 or Socs3 mRNA expression (Figure 6). Base-line ovarian Socs1 and Socs3 expression was higher at this later point in gestation. The failure to induce Socs1 and Socs3 was likely due to altered ovarian responsiveness on E8, since the pattern of immune cell surface marker modulation following FGK45 injection was identical in the E8–11 and E4–7 periods (see above, and data not shown), and similar levels of IL-6 and IκBα mRNA induction were seen 12 hours after FGK45 injection on E8 versus E4 (Figure 6). Lastly, progesterone treatment did not affect the FGK45-induced ovarian upregulation of IL-6, IκBα, Socs1, or Socs3 expression (Figure 6), consistent with immune activation acting entirely upstream of luteal insufficiency. Together, these data suggest that the acute inductions of Socs1 and Socs3 are important mechanisms of deregulated luteal prolactin target-gene expression and decreased ovarian progesterone synthesis following systemic CD40 ligation.
Discussion
We describe a model of early pregnancy loss in mice that links the immune system with the reproductive endocrine system. In this model, forced ligation of CD40 with the agonistic anti-CD40 mAb FGK45 stimulates dendritic cells and initiates a systemic inflammatory response. This inflammatory response in turn induces an ovarian resistance toward the gonadotropic effects of pituitary-derived prolactin and thus ultimately causes pregnancy failure by inhibiting ovarian progesterone production. The link between immune activation and endocrine failure is provided at the cellular level by NK cells, which our genetic analysis had indicated were sufficient to mediate pregnancy loss in the absence of other lymphoid lineages, and at the molecular level by TNF-α, which was required for pregnancy loss likely through its direct effects on ovarian function. Indeed, TNF-α is produced upon the cellular activation of both NK cells and dendritic cells and is thought to play an important role as an amplifier of the reciprocal interactions between the two cell types (32). Consistent with our model, TNF-α has previously been shown to inhibit luteal steroidogenesis in vitro (13, 33).
Although our results strongly suggest this straightforward epistatic relationship between the immune and endocrine systems, it remains possible that more complex interactions are taking place in a manner consistent with a potential immunosuppressive role for progesterone during pregnancy (16, 34). However, we consider this to be very unlikely, because we found that concurrent treatment of mice with pharmacological doses of progesterone did not alter the systemic immune activation or the ovarian upregulation of IL-6, IκBα, Socs1, and Socs3 following CD40 ligation, even though it prevented pregnancy failure. Furthermore, exogenous prolactin also rescued FGK45-induced pregnancy failure, yet only raised serum progesterone concentrations to subphysiological levels and had no effect on FGK45-induced lymphoid activation (data not shown). Lastly, the progesterone antagonist RU486 is able to induce abortion in Rag2–/–γc–/– mice (A. Erlebacher and L.H. Glimcher, unpublished observations), which demonstrates that the lymphoid system is not required for pregnancy failure following endocrine withdrawal.
The pathway described here also stands in contrast to the local processes at the maternal-fetal interface that are currently thought to underlie other examples of immune-mediated pregnancy loss in rodents (1). For example, systemically injected anti-phospholipid antibodies, LPS, or inflammatory cytokines such as TNF-α or IFN-γ are thought to induce abortion or modulate embryo resorption rates through localized actions within implantation sites with direct damage to the fetus and placenta or deranged placental and decidual hemostasis emerging as possible common pathogenic mechanisms (1, 9–11). Although many of these agents would be expected to invoke inflammatory cytokine cascades similar to those produced by CD40 ligation, some have been shown to be effective throughout gestation, with their actions ultimately resistant to progesterone injection (10, 35). Thus, it is possible that some agents cause pregnancy failure by two independent but superimposed mechanisms: systemic inhibition of ovarian function, and local induction of inflammatory processes within implantation sites. In support of this idea, we have found that concurrent progesterone administration grossly prevented the complete fetal resorption and decidual sloughing caused by LPS injection early in gestation, although implantation sites showed large areas of hemorrhage (A. Erlebacher and L.H. Glimcher, unpublished observations).
Molecular mechanisms of luteal insufficiency.
Ovarian mRNA expression studies suggested that the luteal insufficiency following CD40 ligation was likely due to a number of quantitative changes in gene expression, including upregulated 20α-HSD expression on E5 and particularly E8, leading to increased intraovarian progesterone catabolism, and downregulated 3β-HSD expression on E5, leading to decreased progesterone synthesis. Furthermore, downregulated LHR expression on both E5 and E8 might lead to decreased posttranslational activation of StAR (33, 36), and decreased prolactin or LH signaling in either corpus luteal cells or ovarian endothelial cells might cause additional developmental or metabolic defects that secondarily impair progesterone production (14).
While decreased LHR mRNA expression might be due in part to a direct effect of TNF-α as described previously (37), the constellation of LHR downregulation and 20α-HSD upregulation suggested a more general defect in the ovarian prolactin response. Since serum prolactin levels were unimpaired and ovarian prolactin receptor and STAT5 mRNA levels were largely maintained, the defect likely involved dominant inhibition of postreceptor signal transduction. We suggest that this inhibitory function can be ascribed at least in part to the cytokine-inducible JAK/STAT signaling inhibitors Socs1 and Socs3. In prior studies, both Socs1 and Socs3 have been shown to inhibit prolactin receptor signaling via STAT5 in vitro (38–40), and a genetic interaction between Socs1 and prolactin receptor signaling in vivo has been shown by the ability of Socs1 heterozygosity to rescue the lactation defect seen in Prlr+/– mice (41). This result also emphasizes how final STAT5 signal strength can be profoundly influenced by as little as presumably twofold changes in the relative levels of Socs proteins and signaling components. Lastly, luteal induction of Socs1 and in particular Socs3 following prostaglandin F-2α administration in rats has recently been postulated to explain the luteolytic effects of this prostaglandin and its ability to inhibit prolactin signaling (42). In our system, both Socs1 and Socs3 were acutely upregulated in the ovaries following systemic CD40 ligation, and this upregulation could be partially (Socs1) or largely (Socs3) inhibited by concomitant TNF-α blockade, a treatment that ultimately prevented pregnancy failure. The inducibility of these factors following CD40 ligation also temporally correlated with the postimplantation window of sensitivity toward FGK45-induced pregnancy failure.
It should be emphasized, however, that the cellular and molecular mechanisms of immune-mediated luteal insufficiency are likely to be overlapping and manifold. TNF-α is a product of immune cell populations besides NK cells and dendritic cells, and the actions of TNF-α might be indirectly mediated, for example through the induction of prostaglandin F-2α, a luteolytic agent also induced by other inflammatory cytokines (13, 33). Furthermore, inflammatory cytokines besides TNF-α are able to inhibit luteal steroidogenesis in vivo and in vitro (e.g., IL-1 and IFN-γ) (13, 33, 43), and many cytokines are able to induce Socs protein expression (22, 23). Thus, it is likely that multiple inflammatory pathways induced by a variety of stimuli might lead to luteal insufficiency and endocrine failure in the absence of TNF-α, or contribute to luteal insufficiency in the presence of TNF-α. Indeed, the only partial ability of TNF-α blockade to reverse Socs1 upregulation points to a role for other cytokines in luteal insufficiency even in our system. Further experiments will be necessary to define the precise roles of Socs proteins in the immune regulation of ovarian function.
Since prolactin is not thought to be a major stimulus for progesterone production in humans, it is not immediately clear how mechanisms of suppressed prolactin receptor signaling would be directly relevant to disorders of human reproduction. Progesterone production by the corpus luteum early in human pregnancy (up to about week 8) is driven by human chorionic gonadotropin (hCG) produced by the placenta, while later in gestation the placenta itself becomes the primary source of progesterone, likely stimulated by hCG acting in an autocrine fashion (16). Furthermore, hCG and LH bind to a shared G protein–coupled receptor, and so these hormones have been thought to signal largely through cAMP second messengers. However, there has been increasing evidence that signaling via G protein–coupled receptors also induces JAK/STAT activation (42), and a recent report has shown that the rat LHR couples to JAK2/STAT1 and JAK2/STAT5 in the ovaries (44). Thus, we might expect that hCG/LH signaling in the corpus luteum or placenta would be sensitive to SOCS inhibition, and this might explain the in vitro inhibitory effects of TNF-α and other inflammatory cytokines on LH- and hCG-stimulated luteal steroidogenesis that have been previously noted in many species (13). Intriguingly, ovarian resistance towards endogenous LH or exogenous hCG has been associated clinically with luteal insufficiency in humans (45).
Thus, it may be worth considering the possibility that immune activation contributes to human infertility and in particular occult or recurrent pregnancy loss through the negative effects on the reproductive endocrine system that we describe here. For example, activation of the CD40 pathway might occur through CD40L upregulation by either activated T cells or activated NK cells in response to infection or to potentially transformed or overinvasive trophoblasts at the maternal-fetal interface. Interestingly, complement C4b-binding protein (C4BP), produced in part by activated monocytes, has recently been shown to be another activating ligand for CD40 (46). This protein, which also regulates the complement and coagulation cascades, might provide a nonlymphoid stimulus for CD40 activation at sites of inflammation. Although we do not know whether NK cell activation or TNF-α production alone is sufficient to mediate the full effect of CD40 ligation, we would expect these intermediates to cause some level of endocrine dysfunction as well.
Methods
Mice, antibody treatments, and hormone treatments.
CD40–/– mice were the gift of R. Geha (Harvard Medical School). C57BL/6 (B6), BALB/c, B6CBAF1, and B6129F1 mice (the latter being a strain-matched control for CD40–/– mice) were from The Jackson Laboratory (Bar Harbor, Maine, USA). Rag2–/– mice (on a B6 background) and Rag2–/–γc–/– mice (on a mixed C57BL/6 ∞ C57BL/10 background) were from Taconic (Germantown, New York, USA). Males were mated with individual females no more than once per week; B6CBAF1 females and B6 males were used in all experiments except when noted. The day of the copulation plug was counted as E0. All antibodies were given via intraperitoneal injection at the dose of 100 μg in 0.1 ml PBS. The FGK45 rat hybridoma cell line was the gift of A. Groenewegen (Basel Institute for Immunology, Basel, Switzerland); antibodies were prepared from hybridoma supernatants by protein G chromatography either in house or by BioExpress Cell Culture Services (West Lebanon, New Hampshire, USA). Polyclonal rat IgG antibodies were purchased from ICN Biomedicals Inc. (Aurora, Ohio, USA). Mice received FGK45 or rat IgG antibodies between 0830 and 1200 hours. For experiments evaluating the role of TNF-α, mice were additionally injected 2 hours later with TN3-19.12, a hamster IgG mAb that neutralizes murine TNF-α (47), or 100 μg PIP, an irrelevant hamster IgG mAb that recognizes glutathione-S-transferase. Both of these antibodies were the gifts of R. Schreiber (Washington University, St. Louis, Missouri, USA). Progesterone (Sigma-Aldrich, St. Louis, Missouri, USA) was dissolved in sesame seed oil (Sigma-Aldrich), and 2 mg/0.1 ml was injected subcutaneously at the time of concurrent antibody injections. Ovine prolactin (NIDDK-oPRL-21, produced by the National Hormone and Peptide Program, Torrance, California, USA) was dissolved in alkaline saline, and 100 μg/0.1 ml was injected subcutaneously twice a day between 0800–0830 hours and 1600–1630 hours. All mouse experiments were approved by Harvard Medical School’s Standing Committee on Animals.
Serum hormone and cytokine measurements.
Serum was collected by retro-orbital eye bleeds. Progesterone concentrations were measured by enzyme-linked immunoassay (ALPCO Diagnostics, Windham, New Hampshire, USA), prolactin and LH concentrations were measured by RIA in the laboratory of the National Hormone and Peptide Program, and cytokines were measured by the SearchLight assay (Pierce Biotechnology Inc., Rockford, Illinois, USA).
Flow cytometry.
Splenocyte forward scatter and cell surface marker expression was determined by flow cytometry using a BD Biosciences FACSCalibur and CellQuest software (San Diego, California, USA). The following antibodies were purchased from BD Biosciences: CyChrome-conjugated anti–TCR-β (clone H57-597), FITC–anti-NK1.1 (PK136), phycoerythrin–anti-B220 (RA3-6B2), phycoerythrin–anti-CD69 (H1.2F3), allophycocyanin–anti-CD11c (HL3), phycoerythrin–anti-CD11b (M1/70), FITC–anti-CD80 (16-10A1), FITC–anti-CD86 (GL1), FITC–anti–MHC class II (AF6-120.1), and FITC–anti-CD40 (HM40-3). Following an initial gating on all lymphoid cells based on their forward and side scatter characteristics, T cells were identified as the TCR-β+NK1.1– population, NK cells were identified as the TCR-β–NK1.1+ population, and B cells were identified as the TCR-β–NK1.1– population. True B220+ B cells comprised 70–80% of this latter population in rat IgG–treated mice, and 85–95% of this latter population in FGK45-treated mice. Dendritic cells were identified and gated by virtue of their expression of CD11c, with an unused fluorescence channel employed to gate away autofluorescent cells.
Histology and immunofluorescence microscopy.
Tissue was fixed overnight at 4°C in 4% paraformaldehyde prior to routine paraffin embedding. For leukocyte visualization, sections were stained with biotin-conjugated antibodies against the common leukocyte antigen CD45 (clone 30-F11; BD Biosciences), using HRP-conjugated streptavidin as a secondary reagent (NEN Life Science Products Inc., Boston, Massachusetts, USA), biotin-tyramide amplification (NEN Life Science Products Inc.), and streptavidin–Alexa 594 (Molecular Probes Inc., Eugene, Oregon, USA) as the final fluorochrome. Sections were counterstained with DAPI (Sigma-Aldrich) and digitally photographed at ∞4 magnification. Composites were assembled in Adobe Photoshop (Adobe Systems Inc., San Jose, California, USA). NF-κB p65 was visualized with a goat primary antibody (C-20; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), a biotin-conjugated horse anti-goat secondary antibody (Vector Laboratories Inc., Burlingame, California, USA), and streptavidin–Alexa 594.
RNA analysis.
RNA was prepared from homogenized ovaries using the Trizol reagent (Invitrogen Corp., Carlsbad, California, USA). Real-time RT-PCR was performed as described previously (48). All reactions were run in duplicate using cDNA template synthesized from 10 ng RNA. Difference in threshold cycle (ΔCT) values were calculated relative to β-actin, and statistical analyses were performed on groups of raw ΔCT values. Expression levels relative to β-actin were calculated as 2–ΔCT, with error bars extrapolated from the SD of the ΔCT mean for each group. We used the following primer/probe sets, listed 5′ to 3′ in the order of forward primer, reverse primer, and (when used) probe: β-actin, GCTCTGGCTCCTAGCACCAT, GCCACCGATCCACACCGCGT, TCAAGATCATTGCTCCTCCTGAGCGC; StAR, TCACTTGGCTGCTCAGTATTGAC, TCTATCTGGGTCTGCGATAGGAC; P450scc, CGGTACTTGGGCTTTGGCT, AGCAGATTGATAAGGAGGATGGTC; 3β-HSD, CCAGGCAGACCATCCTAGATGT, AGATGAAGGCTGGCACACTTG; LHR, CGAGACGCTTTATTCTGCCAT, AGCATCTGGTTCTGGAGTACATTG; 20α-HSD, GCTGATATGTTTAAGGCTCACCCTAA, AGAGTCCAGCATCACACAAAAGATC; prolactin receptor, GGATGTGACTTACATTGTTGAACCA, TACCCACAGATATGTTTTTTTGTCTTTT; STAT5a, TGGTCCCTGAGTTCGTCAATG, GTTGAGGGCACACGACTGG; STAT5b, GCGCCACCTACATGGATCA, GACGGAGTCCGGGTTGG; IL-6, TTCAACCAAGAGGTAAAAGATTTACATAA, CACTCCTTCTGTGACTCCAGCTT; IκBα, AGATGCTACCCGAGAGCGAG, TCATCATAGGGCAGCTCATCC; Socs1, TGTGCCGCAGCATTAAGTG, GGCATCTCACCCTCCACAAC, CCGCCTGGGTCGGAGGGAGT; Socs3, TGCTGGCCAAAGAAATAACCA, GGTCACCCCTTGCCACTCT, CCCACTGCCCAGCCTAGGTGAGGA. All probes were dual-labeled with FAM and TAMRA. SYBR green was used for amplifications when a primer set was used without a probe. For all primer/probe sets, the approximate difference in amplification between RT and mock RT reactions was greater than 1,000-fold, except the SYBR green amplifications of β-actin (50-fold), STAT5a (100-fold), and STAT5b (50-fold).
Statistical analysis.
Pregnancy rates were compared by Fisher’s exact test; all other comparisons used Student’s t test.
Acknowledgments
We are grateful to A. Groenewegen, R. Schreiber, and R. Geha for valuable reagents and thank K. Price, L. Kangaloo, and C. McCall for technical and administrative assistance, J. Strauss, J. Boyson, M. Townsend, and members of the Glimcher laboratory for valuable discussions, and G. Lord and D. Schust for critical reading of the manuscript. This work was supported by NIH grants AI01650 and AI54370.
Footnotes
See the related Commentary beginning on page 15.
Nonstandard abbreviations used: C57BL/6 (B6); embryonic day (E); human chorionic gonadotropin (hCG); 3β-hydroxysteroid dehydrogenase (3β-HSD); 20α-hydroxysteroid dehydrogenase (20α-HSD); Janus kinase (JAK); luteinizing hormone (LH); LH receptor (LHR); P450 side chain cleavage enzyme (P450scc); signal transducer and activator of transcription (STAT); steroidogenic acute regulatory protein (StAR); suppressor of cytokine signaling (Socs).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Laird SM, et al. A review of immune cells and molecules in women with recurrent miscarriage. Hum. Reprod. Update. 2003;9:163–174. doi: 10.1093/humupd/dmg013. [DOI] [PubMed] [Google Scholar]
- 2.Aoki K, et al. Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet. 1995;345:1340–1342. doi: 10.1016/s0140-6736(95)92539-2. [DOI] [PubMed] [Google Scholar]
- 3.Coulam CB, Goodman C, Roussev RG, Thomason EJ, Beaman KD. Systemic CD56+ cells can predict pregnancy outcome. Am. J. Reprod. Immunol. 1995;33:40–46. doi: 10.1111/j.1600-0897.1995.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwak JY, et al. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am. J. Reprod. Immunol. 1995;34:93–99. doi: 10.1111/j.1600-0897.1995.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 5.Emmer PM, et al. Peripheral natural killer cytotoxicity and CD56+ CD16+ cells increase during early pregnancy in women with a history of recurrent spontaneous abortion. Hum. Reprod. 2000;15:1163–1169. doi: 10.1093/humrep/15.5.1163. [DOI] [PubMed] [Google Scholar]
- 6.Ntrivalas EI, et al. Status of peripheral blood natural killer cells in women with recurrent spontaneous abortions and infertility of unknown aetiology. Hum. Reprod. 2001;16:855–861. doi: 10.1093/humrep/16.5.855. [DOI] [PubMed] [Google Scholar]
- 7.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 8.Piccinni MP, et al. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 9.Gendron RL, Nestel FP, Lapp WS, Baines MG. Lipopolysaccharide-induced fetal resorption in mice is associated with the intrauterine production of tumour necrosis factor-alpha. J. Reprod. Fertil. 1990;90:395–402. doi: 10.1530/jrf.0.0900395. [DOI] [PubMed] [Google Scholar]
- 10.Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA. Cytokine-dependent abortion in CBA x DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase. J. Immunol. 1998;160:545–549. [PubMed] [Google Scholar]
- 11.Holers VM, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 2002;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivest S, Rivier C. The role of corticotropin-releasing factor and interleukin-1 in the regulation of neurons controlling reproductive functions. Endocr. Rev. 1995;16:177–199. doi: 10.1210/edrv-16-2-177. [DOI] [PubMed] [Google Scholar]
- 13.Davis JS, Rueda BR. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front. Biosci. 2002;7:d1949–d1978. doi: 10.2741/davis1. [DOI] [PubMed] [Google Scholar]
- 14.Risk, M., and Gibori, G. 2001. Mechanisms of luteal cell regulation by prolactin. In Prolactin. N.D. Horseman, editor. Kluwer Academic Publishers. Boston, Massachusetts, USA. 265–295.
- 15.Deanesly R. Termination of early pregnancy in rats after ovariectomy is due to immediate collapse of the progesterone-dependent decidua. J. Reprod. Fertil. 1973;35:183–186. doi: 10.1530/jrf.0.0350183. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe, R.B. 1999. Neuroendocrine-metabolic regulation of pregnancy. In Reproductive endocrinology. S.S.C. Yen, R.B. Jaffe, and R.L. Barbieri, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 751–784.
- 17.Raj HG, Moudgal NR. Hormonal control of gestation in the intact rat. Endocrinology. 1970;86:874–889. doi: 10.1210/endo-86-4-874. [DOI] [PubMed] [Google Scholar]
- 18.Mednick DL, Barkley MS, Geschwind II. Regulation of progesterone secretion by LH and prolactin during the first half of pregnancy in the mouse. J. Reprod. Fertil. 1980;60:201–207. doi: 10.1530/jrf.0.0600201. [DOI] [PubMed] [Google Scholar]
- 19.Diehl L, et al. The role of CD40 in peripheral T cell tolerance and immunity. J. Mol. Med. 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- 20.van Kooten C, Banchereau J. CD40-CD40 ligand. J. Leukoc. Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 21.Auernhammer CJ, Melmed S. The central role of SOCS-3 in integrating the neuro-immunoendocrine interface. J. Clin. Invest. 2001;108:1735–1740. doi:10.1172/JCI200114662. doi: 10.1172/JCI14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat. Rev. Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- 23.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 24.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 25.Castigli E, et al. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein L, Forbes TR. Maintenance of pregnancy with subcutaneous pellets of progesterone in ovariectomized mice. Proc. Soc. Exp. Biol. Med. 1963;113:1043–1046. doi: 10.3181/00379727-113-28567. [DOI] [PubMed] [Google Scholar]
- 27.Stocco C, Callegari E, Gibori G. Opposite effect of prolactin and prostaglandin F-2α on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology. 2001;142:4158–4161. doi: 10.1210/endo.142.9.8493. [DOI] [PubMed] [Google Scholar]
- 28.Markoff E, Talamantes F. Serum placental lactogen in mice in relation to day of gestation and number of conceptuses. Biol. Reprod. 1981;24:846–851. doi: 10.1095/biolreprod24.4.846. [DOI] [PubMed] [Google Scholar]
- 29.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 30.Cao X, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 32.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 33.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000;80:1–29. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Stites DP, Siiteri PK. Steroids as immunosuppressants in pregnancy. Immunol. Rev. 1983;75:117–138. doi: 10.1111/j.1600-065x.1983.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 35.Gasic GJ, Gasic TB, Strauss JF. Abortifacient effects of Vibrio cholerae exo-enterotoxin and endotoxin in mice. J. Reprod. Fertil. 1975;45:315–322. doi: 10.1530/jrf.0.0450315. [DOI] [PubMed] [Google Scholar]
- 36.Arakane F, et al. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J. Biol. Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- 37.Chen YJ, Feng Q, Liu YX. Expression of the steroidogenic acute regulatory protein and luteinizing hormone receptor and their regulation by tumor necrosis factor alpha in rat corpora lutea. Biol. Reprod. 1999;60:419–427. doi: 10.1095/biolreprod60.2.419. [DOI] [PubMed] [Google Scholar]
- 38.Pezet A, Favre H, Kelly PA, Edery M. Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J. Biol. Chem. 1999;274:24497–24502. doi: 10.1074/jbc.274.35.24497. [DOI] [PubMed] [Google Scholar]
- 39.Dif F, Saunier E, Demeneix B, Kelly PA, Edery M. Cytokine-inducible SH2-containing protein suppresses PRL signaling by binding the PRL receptor. Endocrinology. 2001;142:5286–5293. doi: 10.1210/endo.142.12.8549. [DOI] [PubMed] [Google Scholar]
- 40.Tam SP, Lau P, Djiane J, Hilton DJ, Waters MJ. Tissue-specific induction of SOCS gene expression by PRL. Endocrinology. 2001;142:5015–5026. doi: 10.1210/endo.142.11.8466. [DOI] [PubMed] [Google Scholar]
- 41.Lindeman GJ, et al. SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev. 2001;15:1631–1636. doi: 10.1101/gad.880801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curlewis JD, et al. A prostaglandin F-2α analog induces suppressors of cytokine signaling-3 expression in the corpus luteum of the pregnant rat: a potential new mechanism in luteolysis. Endocrinology. 2002;143:3984–3993. doi: 10.1210/en.2002-220344. [DOI] [PubMed] [Google Scholar]
- 43.Liu YX, Chen YX, Shi FW, Feng Q. Studies on the role of plasminogen activators and plasminogen activator inhibitor type-1 in rat corpus luteum of pregnancy. Biol. Reprod. 1995;53:1131–1138. doi: 10.1095/biolreprod53.5.1131. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho CR, et al. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- 45.Hinney B, Henze C, Kuhn W, Wuttke W. The corpus luteum insufficiency: a multifactorial disease. J. Clin. Endocrinol. Metab. 1996;81:565–570. doi: 10.1210/jcem.81.2.8636268. [DOI] [PubMed] [Google Scholar]
- 46.Brodeur SR, et al. C4b-binding protein (C4BP) activates B cells through the CD40 receptor. Immunity. 2003;18:837–848. doi: 10.1016/s1074-7613(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 47.Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J. Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- 48.Erlebacher A, Lukens AK, Glimcher LH. Intrinsic susceptibility of mouse trophoblasts to natural killer cell-mediated attack in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16940–16945. doi: 10.1073/pnas.222652199. [DOI] [PMC free article] [PubMed] [Google Scholar]