Abstract
The objective of this study was describe the impact of sildenafil on echocardiographic measures of myocardial performance in children and young adults with a functional single-ventricle physiology late after Fontan surgery. A double-blind, placebo-controlled, crossover trial was conducted in children and young adults after the Fontan operation at a single pediatric center. Subjects were randomized to receive placebo or sildenafil (20 mg tid) for 6 weeks. After a 6-week washout period, subjects were crossed for an additional 6 weeks. Each subject underwent an echocardiogram at the start and finish of each phase. A total of 27 subjects completed study testing at a mean age of 14.9 years and a mean time from Fontan surgery of 11.3 years. After sildenafil, subjects demonstrated improvement in their myocardial performance index (MPI; −0.051; 95% CI −0.095, −0.0077; p 0.02) and in the product of the velocity time integral (VTI) of the dominant outflow tract and the heart rate (HR; 110 cm × bpm; 95% CI 7.5, 220; p = 0.04). Measures of diastolic performance, including inflow velocities, myocardial velocities, and the ratio of blood pool velocity to myocardial velocity during passive inflow, did not change. In this cohort, there were significant improvements in both the MPI and the product of the VTI × HR after 6 weeks of treatment with sildenafil. These findings suggest that sildenafil may be a useful therapy to improve or maintain ventricular performance in select patients after the Fontan operation.
Keywords: Fontan procedure, Echocardiography, Physiology, Trials, Sildenafil
Introduction
The Fontan operation is the final surgery in the strategy of staged palliation for children born with congenital heart defects resulting in functional single-ventricle physiology [7, 13]. Despite improved short- and medium-term outcomes, long-term morbidity and mortality continue to pose a challenge [10, 20, 24]. Systolic and diastolic ventricular performance are crucial determinants of cardiac output and are therefore intimately related to late morbidity and mortality [4, 6, 12]. An intervention that improves ventricular performance could have tremendous value in this population and might improve long-term outcomes.
In recent years, sildenafil has emerged as a potential therapeutic agent for adults with heart failure. Abnormal expression of phosphodiesterase E5 (PDE5) has been documented in the myocardium of hypertrophied right ventricles and in the maladaptive remodeling process of stressed left ventricles [16, 17, 19, 26]. Inhibition of PDE5 has demonstrated a positive effect on cardiac performance and on reversal of the maladaptive remodeling process [2, 9, 16, 22]. Abnormal ventricular performance is a well-described feature of single-ventricle physiology after Fontan palliation [1, 25]. Although there are no data demonstrating abnormal expression of PDE5 in this specific scenario, it seems likely that the same mechanistic features would be present in the failing single ventricle as in the failing ventricle in the more traditional adult heart failure patient.
The myocardial performance index (MPI) is a geometry-independent measure of systolic and diastolic function obtained by indexing the sum of isovolumetric contraction and relaxation time to ejection time [23]. This measure has been used extensively in many forms of heart disease, congenital and acquired, and is a useful measure to describe overall ventricular performance [3, 5, 15, 18, 21, 25]. In the single-ventricle population, there are many reports describing the status of the MPI after the Fontan operation, but there are few clinical trials of medical therapies in survivors of Fontan surgery and no studies to date evaluating the potential impact of sildenafil on ventricular performance. In this study, we report the echocardiographic results of a phase-II clinical trial of oral sildenafil given after the Fontan operation. This study was designed to evaluate efficacy of sildenafil in children and young adults late after Fontan surgery (Sildenafil After Fontan Operation trial; clinicaltrials.gov identifier: NCT00507819). Our primary objective was to determine if oral sildenafil improves ventricular performance as measured by the MPI compared with placebo during a 6-week period.
Methods
Study Design
This study was a randomized, double-blind, placebo-controlled, crossover trial of oral sildenafil (20 mg tid) administration conducted in children and young adults after the Fontan operation. After a baseline screening assessment, eligible subjects were randomized to start with a 6-week course of either placebo or sildenafil (phase 1). Next, after a 6-week washout period of no drug or placebo, subjects switched treatments for an additional 6 weeks (phase 2); each subject thereby acted as their own control. Subjects underwent echocardiographic evaluation at the beginning and end of each phase yielding a total of four assessments. Placebo capsules were identical in appearance to sildenafil capsules and were taken according to the same schedule (tid). The study was approved by the Institutional Review Board for the Protection of Human Subjects at The Children’s Hospital of Philadelphia.
Inclusion and Exclusion Criteria
This study was performed as part of a broader investigation evaluating the impact of sildenafil on measures of heart function and exercise performance after Fontan surgery [8]. Children and young adults age ≥8 years with single-ventricle congenital heart disease who met the physical requirements for exercise stress testing were screened for participation. Informed consent and assent were obtained as appropriate before enrollment. To exclusively study the effects of sildenafil on the physiology of the Fontan circulation, recruitment was intentionally aimed at a relatively healthy cohort of outpatients without significant additional complications. Subjects with implantable pacemakers, residual cardiac lesions (coarctation of the aorta, severe ventricular dysfunction, severe atrioventricular valvar regurgitation, Fontan baffle or conduit obstruction, single-lung Fontan connection), severe renal or hepatic dysfunction, or a history of sildenafil use in the 6 months before study enrollment were excluded from the study.
Echocardiographic Assessment
Echocardiograms were performed by one of two echocardiographic sonographers with specific training for this protocol. A Phillips IE33 (Phillips, Andover, MA) machine was used for image acquisition. Appropriate transducers were selected based on subject body habitus. Images were transferred to a hospital server for digital storage. Measurements were performed using Syngo Dynamics software version 5 (Siemens, Ann Arbor, MI) blinded to subject demographic information and to study phase.
Echocardiographic images were obtained according to the standards of the American Society of Echocardiography [14]. Early diastolic inflow velocity (E wave) and late diastolic inflow velocity (A wave) were measured by pulse wave Doppler at the tips of the atrioventricular (AV) valve leaflets in an apical view. Pulsed Doppler tissue velocities were recorded at the intersection of the ventricular free wall with the AV annulus and, when applicable, at the septal annular junction, also from an apical view, to ensure an optimal angle of interrogation. The systolic (S′), early diastolic (E′), and late diastolic (A′) velocities were recorded at each myocardial site. The velocity time integral (VTI) for the dominant semilunar valve was calculated from apical imaging with anterior angulation and rotation to optimize the view of the ventricular outflow tract. The VTI was calculated using pulse wave Doppler interrogation of the outflow tract at the level of the semilunar valve. Each Doppler variable was measured three times, and the mean value was recorded for analysis. Heart rate (HR) was recorded from the electrocardiogram tracing at the time of image acquisition. For analysis, the MPI was regarded as an index of global ventricular performance; the VTI × HR product was regarded as a surrogate of cardiac output; and the myocardial and inflow velocities, including the ratio of early diastolic inflow velocity to early diastolic tissue velocity (E:E′), were regarded as indices of diastolic performance. Baseline ventricular performance was determined at the pre-phase 1 echocardiogram.
Power Calculation
This study was part of a broader investigation evaluating the effect of sildenafil on heart function and exercise capacity. The power calculation was generated from the exercise component of the trial for which a sample size of 28 was calculated based on differences in maximal oxygen consumption. Given a sample size of 28 subjects, there was 80% power to detect a mean decrease in MPI of 0.05 based on a two-sided crossover analysis of variance with a type-I error rate of 5% assuming that the SD of the change was 0.05.
Statistical Analysis
Baseline and demographic characteristics were summarized using means and SDs for continuous variables and percentages for categorical variables. Echocardiographic outcomes were summarized across treatment phases (before and after placebo and before and after sildenafil) using means and SDs. Because each outcome exhibited a symmetric distribution, a linear mixed-effects model was used to estimate the difference in the average post-phase outcome between sildenafil and placebo, adjusted for pre-phase values, study phase (phase 1 or phase 2), and treatment sequence (placebo → sildenafil, or sildenafil → placebo) [11]. Subject-specific random intercepts were used to account for the correlation due to repeated measurements. Subgroup analyses were specified a priori by ventricular morphology (single right ventricle vs. single left or mixed ventricular morphology) and by baseline serum brain natriuretic peptide (BNP) as a biochemical marker of heart failure (≥100 pg/ml vs. <100 mg/ml). A test of interaction was performed to assess whether the size of the treatment effect differed by patient subgroup (e.g., BNP ≥100 pg/ml vs. <100 mg/ml). Because the objective of the subgroup analysis was to explore the possible selective effect of sildenafil on a subpopulation, within-subgroup statistical testing for treatment effect was conducted even in the absence of a significant interaction. For all analyses, p<0.05 was considered suggestive of statistical significance. All analyses were completed using R 2.12 (R Development Core Team, Vienna, Austria).
Results
Of the 28 study participants, 27 were able to complete at least 1 study phase. One subject withdrew related to discomfort associated with exercise testing. The demographic and baseline characteristics are listed in Table 1. Of those who participated in the study, two thirds were male, and 25 were white. Ventricle morphology was diverse: 54% of subjects were classified as having single right ventricles, and single left-ventricular morphology and mixed ventricular morphology accounted for 46% of subjects. Trivial ventricular dysfunction or normal ventricular function was present at baseline in 86% of subjects, and 68% of subjects had at least mild AV valve regurgitation. The mean baseline body mass index (BMI) was in the normal range, and the mean baseline serum BNP was 110 pg/ml.
Table 1.
Demographic characteristics for 28 subjects at screening
| Age (year) | 14.9 (5.1) |
| No. (%) male | 18 (64) |
| No. (%) race | |
| White | 25 (89) |
| Black | 2 (7) |
| Multiracial | 1 (4) |
| Time (y) since Fontan (%) | 11.3 (3.8) |
| No. (%) ventricular morphology | |
| Single right ventricle | 15 (54) |
| Single left ventricle | 8 (29) |
| Two ventricles | 5 (18) |
| No. (%) ventricular dysfunction | |
| None | 19 (68) |
| Trivial | 5 (18) |
| Mild | 4 (14) |
| Moderate | 0 (0) |
| Severe | 0 (0) |
| No. (%) AV valve regurgitation | |
| None | 2 (7) |
| Trivial | 7 (25) |
| Mild | 16 (57) |
| Moderate | 3 (11) |
| Severe | 0 (0) |
| BMI (kg/m2) | 19.7 (3.9) |
| Serum BNP (pg/ml) | 110 (75) |
Data presented as mean (SD) unless otherwise noted as n (%)
Summary statistics for all echocardiographic outcomes are listed in Table 2. Although no differences were detected in the VTI or HR alone, the VTI × HR product increased significantly (Fig. 1), suggesting an increase in cardiac output after the sildenafil phase. Similarly, MPI was significantly decreased (Fig. 2), indicating improved global ventricular performance. Although E velocity increased after the sildenafil phase, this difference did not reach statistical significance. No difference was detected in A velocity, and no significant differences were observed in Doppler tissue measurements of E′ and S′ velocities from the ventricular free wall. The E:E′ ratio, a marker of diastolic performance, was likewise unchanged.
Table 2.
Summary statistics for echocardiographic measurements at each treatment phase
| Summary statistics by study phase
|
Regression modeling results
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Before placebo | After placebo | Before sildenafil | After sildenafil | Coefficient | 95% CI | p | n | |
| Ventricular inflow | ||||||||
| E velocity (cm/s) | 83 (23) | 81 (22) | 80 (21) | 87 (20) | 5.0 | (−0.69, 11) | 0.08 | 27 |
| A velocity (cm/s) | 49 (15) | 47 (14) | 47 (16) | 48 (14) | 0.83 | (−2.0, 3.7) | 0.55 | 24 |
| Tissue Doppler | ||||||||
| S′ velocity (cm/s) | 6.7 (1.9) | 7.1 (1.9) | 6.9 (1.5) | 7.3 (1.8) | 0.24 | (−0.26, 0.73) | 0.33 | 23 |
| E′ velocity (cm/s) | 11.1 (4.5) | 11.6 (4.0) | 10.8 (4.0) | 11.8 (4.2) | 0.43 | (−0.69, 1.6) | 0.43 | 23 |
| A′ velocity (cm/s) | 4.4 (1.4) | 5.0 (2.1) | 4.9 (2.4) | 4.6 (1.9) | −0.090 | (−0.66, 0.48) | 0.75 | 21 |
| Diastolic performance | ||||||||
| E velocity/A velocity | 1.8 (0.74) | 1.9 (0.72) | 1.9 (0.71) | 2.0 (0.74) | 0.038 | (−0.12, 0.20) | 0.63 | 24 |
| E velocity/E′ velocity | 8.4 (3.6) | 7.8 (3.5) | 8.8 (6.4) | 8.3 (3.7) | 0.26 | (−0.95, 1.5) | 0.66 | 23 |
| Systolic performance | ||||||||
| VTI (cm) | 21.0 (5.5) | 21.0 (5.9) | 21.4 (5.0) | 21.9 (6.0) | 1.2 | (−0.12, 2.5) | 0.07 | 27 |
| Heart rate (bpm) | 69.7 (14) | 71.3 (13) | 70.7 (17) | 73.0 (15) | 1.9 | (−2.6, 6.4) | 0.40 | 26 |
| VTI × HR (cm × bpm) | 1442 (350) | 1451 (310) | 1466 (290) | 1562 (420) | 110 | (7.5, 220) | 0.04 | 26 |
| Global ventricular performance | ||||||||
| MPI | 0.37 (0.11) | 0.38 (0.13) | 0.42 (0.14) | 0.35 (0.10) | −0.051 | (−0.095, -0.0077) | 0.02 | 26 |
Data are presented as mean (SD), and regression modeling results for echocardiographic measurements are presented as coefficient, 95% CI, and p value. Each regression coefficient corresponds to the difference in the average post-phase outcome between sildenafil and placebo adjusted for pre-phase values, study period, and treatment sequence
n number of subjects with a complete measurement series
Fig. 1.
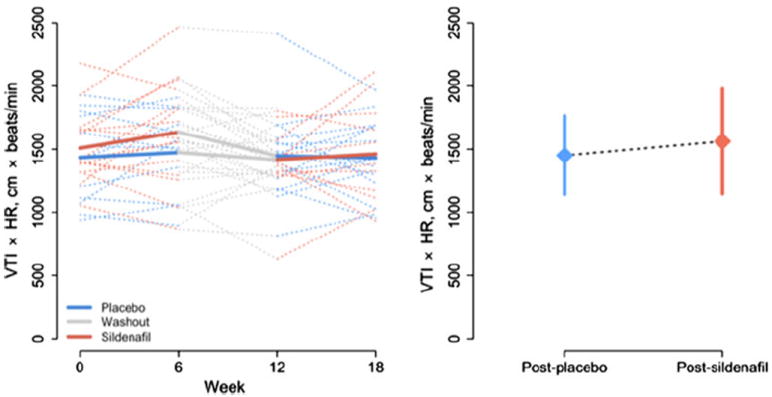
Product of VTI and HR. (Left panel) Observed subjectspecific profiles (dotted lines) and average trend for each treatment group (solid lines) during the study period. (Right panel) Mean ± SD after placebo and after sildenafil
Fig. 2.
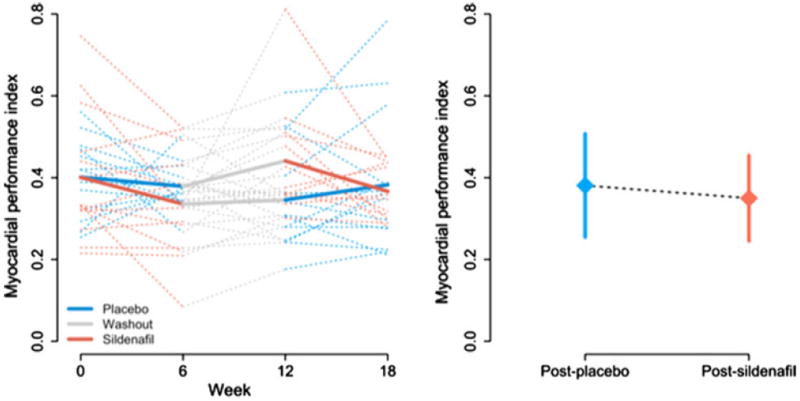
MPI. (Left panel) Observed subject-specific profiles (dotted lines) and average trend for each treatment group (solid lines) during the study period. (Right panel) Mean ± SD after placebo and after sildenafil
Subgroup analysis by ventricular morphology (Table 3) showed no significant differences between subjects with single right-ventricular morphology and those with single left or mixed morphology. Neither group, evaluated on its own, demonstrated a treatment effect size that reached statistical significance. However, although subgroup analysis by serum BNP level (Table 4) demonstrated no significant differences between subgroups, the withinsubgroup effect on MPI and on the product of VTI × HR was more pronounced in those with a serum BNP level >100 pg/ml. No significant changes were appreciated in any of the measures of ventricular inflow, Doppler tissue velocities, or diastolic performance.
Table 3.
Regression modeling results for echocardiographic measurements stratified by ventricular morphology
| Echocardiographic measurements | Single right ventricle
|
Single left ventricle or mixed ventricular morphology
|
p* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | n | Coefficient | 95% CI | p | n | ||
| Ventricular inflow | |||||||||
| E velocity (cm/s) | 5.0 | (−2.7, 13) | 0.19 | 15 | 5.0 | (−3.8, 14) | 0.25 | 12 | 0.99 |
| A velocity (cm/s) | –1.4 | (−5.1, 2.3) | 0.45 | 14 | 3.6 | (−0.59, 7.7) | 0.09 | 11 | 0.09 |
| Tissue Doppler | |||||||||
| S′ velocity (cm/s) | 0.20 | (−0.52, 0.91) | 0.57 | 13 | 0.28 | (−0.49, 1.1) | 0.46 | 12 | 0.87 |
| E′ velocity (cm/s) | 0.41 | (−1.1, 2.0) | 0.58 | 13 | 0.45 | (−1.3, 2.2) | 0.59 | 12 | 0.97 |
| A′ velocity (cm/s) | –0.10 | (−0.91, 0.70) | 0.79 | 12 | –0.074 | (−0.95, 0.81) | 0.86 | 11 | 0.96 |
| Diastolic performance | |||||||||
| E velocity/A velocity | 0.076 | (−0.15, 0.31) | 0.49 | 14 | –0.0094 | (−0.27, 0.25) | 0.94 | 11 | 0.62 |
| E velocity/E′ velocity | 0.29 | (−1.4, 2.0) | 0.73 | 13 | 0.22 | (−1.6, 2.0) | 0.80 | 12 | 0.96 |
| Systolic performance | |||||||||
| VTI × HR (cm × bpm) | 38 | (−93, 170) | 0.56 | 15 | 220 | (62, 380) | 0.01 | 11 | 0.08 |
| VTI (cm) | 0.63 | (−1.2, 2.4) | 0.48 | 15 | 1.8 | (−0.15, 3.8) | 0.07 | 12 | 0.38 |
| Heart rate (bpm) | 1.3 | (−4.7, 7.3) | 0.66 | 15 | 2.7 | (−4.6, 10) | 0.45 | 11 | 0.76 |
| Global ventricular performance | |||||||||
| MPI | −0.043 | (−0.10, 0.016) | 0.15 | 14 | −0.057 | (−0.12, 0.0081) | 0.08 | 12 | 0.73 |
Data are presented as coefficient, 95% CI, and p value. Each regression coefficient corresponds to the difference in the average post-phase outcome between sildenafil and placebo adjusted for pre-phase values, study period, and treatment sequence
p-value from test of interaction evaluating whether the treatment difference is equal in the two morphology strata
n number of subjects with a complete measurement series
Table 4.
Regression modeling results for echocardiographic measurements, stratified by serum BNP
| Echocardiographic measurements | BNP ≥100 pg/ml
|
BNP <100 pg/ml
|
p* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | n | Coefficient | 95% CI | p | n | ||
| Ventricular inflow | |||||||||
| E velocity (cm/s) | 5.6 | (−2.9, 14) | 0.19 | 12 | 4.7 | (−3.4, 13) | 0.24 | 15 | 0.88 |
| A velocity (cm/s) | 0.63 | (−4.2, 5.4) | 0.79 | 10 | 0.88 | (−2.9, 4.7) | 0.64 | 15 | 0.93 |
| Tissue Doppler | |||||||||
| S′ velocity (cm/s) | 0.54 | (−0.20, 1.3) | 0.14 | 11 | −0.084 | (−0.80, 0.64) | 0.81 | 14 | 0.25 |
| E′ velocity (cm/s) | 0.21 | (−1.6, 2.1) | 0.82 | 11 | 0.27 | (−1.4, 1.9) | 0.73 | 14 | 0.96 |
| A′ velocity (cm/s) | 0.032 | (−0.96, 1.0) | 0.95 | 9 | –0.35 | (−1.2, 0.50) | 0.40 | 14 | 0.56 |
| Diastolic performance | |||||||||
| E velocity/A velocity | −0.0047 | (−0.28, 0.27) | 0.97 | 10 | 0.07 | (−0.14, 0.28) | 0.50 | 15 | 0.67 |
| E velocity/E′ velocity | 0.82 | (−1.2, 2.9) | 0.42 | 11 | 0.48 | (−1.4, 2.4) | 0.60 | 14 | 0.82 |
| Systolic performance | |||||||||
| VTI × HR (cm × bpm) | 190 | (31, 340) | 0.02 | 11 | 58 | (−78, 190) | 0.38 | 15 | 0.21 |
| VTI (cm) | 1.3 | (−0.67, 3.2) | 0.19 | 12 | 1.0 | (−0.77, 2.8) | 0.25 | 15 | 0.87 |
| Heart rate (bpm) | 4.2 | (−2.7, 11) | 0.22 | 11 | 0.33 | (−5.6, 6.3) | 0.91 | 15 | 0.39 |
| Global ventricular performance | |||||||||
| MPI | −0.076 | (−0.15, -0.0043) | 0.04 | 11 | −0.034 | (−0.091, 0.023) | 0.23 | 15 | 0.35 |
Data are presented as coefficient, 95% CI, and p value. Each regression coefficient corresponds to the difference in the average post-phase outcome between sildenafil and placebo adjusted for pre-phase values, study period, and treatment sequence
p-value from test of interaction evaluating whether the treatment difference is equal in the two BNP strata
n number of subjects with a complete measurement series
Discussion
This study is the first randomized clinical trial to evaluate the impact of sildenafil on echocardiographic measures of ventricular performance in children and young adults after the Fontan operation. The crossover design allows each subject to serve as their own internal control, thereby decreasing the possibility of confounding based on the heterogeneity of indices of ventricular performance in this population. Baseline assessment of systolic function and global performance demonstrate that this is a relatively healthy cohort of children with single-ventricle physiology. In this setting, we demonstrate a beneficial effect of sildenafil on the product of VTI × HR, a surrogate of cardiac output, and on MPI, a marker of global ventricular performance. No change was noted in blood pool or myocardial velocities, and no change was observed in the E:E′ ratio, suggesting that these markers of diastolic performance were not specifically affected.
Our study is the first to demonstrate a benefit of sildenafil on echocardiographic parameters in children and young adults with single-ventricle physiology and is consistent (1) with studies from the heart failure literature suggesting that sildenafil has a positive effect on ventricular hypertrophy and remodeling as well as (2) with a recent article demonstrating improved echocardiographic indices of ventricular performance after sildenafil treatment [9, 16, 19, 22]. Although the mechanism behind the improvement in ventricular performance has not been completely elucidated, recent studies in mice and in humans suggest that inhibition of PDE5 may be important in avoiding the maladaptive myocardial response associated with myocardial stressors. PDE5 expression is increased in mice after exposure of the left ventricle to a pressure load [16], and expression is increased in the myocardium of hypertrophied right ventricles in humans [17]. In addition, investigators have shown that left-ventricular hypertrophy associated with a pressure load is reversible after PDE5 inhibition with sildenafil [26]. These studies demonstrate the following: that production of PDE5 is upregulated in both right and left ventricles in the heart failure state; that this upregulation is associated with a maladaptive hypertrophic response; and that inhibition of PDE5 seems to reverse the maladaptive myocardial remodeling process.
It is clear that the single-ventricle cohort is different from the cohort with heart failure but structurally normal hearts. Nevertheless, it is plausible to presume that the response to stress in those with one functional ventricle is governed by the same transcriptional mechanisms that result in an increase in PDE5 expression in those with two pumping chambers. Indeed, the improvements in echocardiographic indices found in our study suggest that inhibition of PDE5 may carry the same benefits in the single-ventricle population as it does in those with heart failure but structurally normal hearts.
We recognize that although we were able to demonstrate an improvement in VTI × HR and MPI in our cohort, the clinical significance of these findings was not determined. Whether these findings translate into a durable change in clinical condition remains unanswered. However, we know that many of the complications that increase mortality after Fontan surgery are related to a chronic state of altered myocardial performance. If inhibition of PDE5 with sildenafil is successful at stopping the maladaptive myocardial response to stress, it may be an effective treatment both for significant ventricular dysfunction and for long-term post-Fontan complications associated with altered ventricular performance, thereby allowing for a longer transplant-free survival.
Limitations
Although echocardiographic parameters of ventricular performance were improved after 6 weeks of sildenafil, the functional impact of these improvements and the durability of the changes beyond 6 weeks was not defined. Furthermore, neither the VTI nor the HR change reached statistical significant independently, thus making it difficult to discern the mechanism of improved cardiac output. Due to the small number of study participants, subgroup analyses were limited. Further investigation is needed to determine if physiological or morphological characteristics are associated with differential response to treatment with sildenafil.
Conclusion
Our study demonstrates that sildenafil improves short-term measures of global ventricular performance and cardiac output. An evaluation of sildenafil during a longer duration of time and in a larger cohort of subjects is needed to determine the following: if improved measures will be maintained beyond 6 weeks, if there is a morphology-specific effect, if long-term administration of sildenafil is safe, and if the changes in ventricular performance are associated with improvements in functional capacity and quality of life. However, for a disease in which no long-term medical management has been shown to be effective, this study provides an early indication that PDE5 inhibition might improve ventricular performance and help to avoid some of the complications seen with single-ventricle physiology late after Fontan surgery.
Acknowledgments
The authors thank Erin Davis, Anysia Fedec, the staff of the Echocardiography Laboratory, and the staff of the Investigational Pharmacy at The Children’s Hospital of Philadelphia for their assistance and contributions to this work. This study was supported by grants from the The Mark H. and Blanche M. Harrington Family Foundation and from Big Hearts to Little Hearts. D. J. G. received support from NIH T32 Grant no. HL07915.
Contributor Information
David J. Goldberg, Email: goldbergda@email.chop.edu, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Pediatrics, The Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
Benjamin French, Department of Biostatistics and Epidemiology, The Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
Anita L. Szwast, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA Department of Pediatrics, The Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
Michael G. McBride, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA
Bradley S. Marino, Divisions of Cardiology and Critical Care Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
Nicole Mirarchi, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Brian D. Hanna, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA Department of Pediatrics, The Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
Gil Wernovsky, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Pediatrics, The Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
Stephen M. Paridon, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA Department of Pediatrics, The Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
Jack Rychik, Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Pediatrics, The Perelman School of Medicine, The University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behling A, Rohde LE, Colombo FC, Goldraich LA, Stein R, Clausell N. Effects of 5′-phosphodiesterase four-week long inhibition with sildenafil in patients with chronic heart failure: a double-blind, placebo-controlled clinical trial. J Card Fail. 2008;14:189–197. doi: 10.1016/j.cardfail.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Caldas MC, Meira ZA, Barbosa MM. Evaluation of 107 patients with sickle cell anemia through tissue Doppler and myocardial performance index. J Am Soc Echocardiogr. 2008;21:1163–1167. doi: 10.1016/j.echo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Canter CE, Shaddy RE, Bernstein D, Hsu DT, Chrisant MR, Kirklin JK, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;115:658–676. doi: 10.1161/CIRCULATIONAHA.106.180449. [DOI] [PubMed] [Google Scholar]
- 5.Cheung EW, Lam WW, Cheung SC, Cheung YF. Functional implications of the right ventricular myocardial performance index in patients after surgical repair of tetralogy of Fallot. Heart Vessels. 2008;23:112–117. doi: 10.1007/s00380-007-1016-7. [DOI] [PubMed] [Google Scholar]
- 6.Diller GP, Giardini A, Dimopoulos K, Gargiulo G, Muller J, Derrick G, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31(24):3073–3083. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]
- 7.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, et al. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123:1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5-inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry and clinical status in patients with stable systolic heart failure: results of a 1-year prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4(1):8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins PC, Chinnock RE, Jenkins KJ, Mahle WT, Mulla N, Sharkey AM, et al. Decreased exercise performance with age in children with hypoplastic left heart syndrome. J Pediatr. 2008;152:507–512. doi: 10.1016/j.jpeds.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Kenward MG, Roger JH. The use of baseline covariates in crossover studies. Biostatistics. 2010;11:1–17. doi: 10.1093/biostatistics/kxp046. [DOI] [PubMed] [Google Scholar]
- 12.Klimes K, Ovroutski S, Abdul-Khaliq H, Ewert P, Alexi-Meskishvili V, Kuehne T, et al. Exercise capacity reflects ventricular function in patients having the Fontan circulation. Cardiol Young. 2009;19(4):340–345. doi: 10.1017/S1047951109990424. [DOI] [PubMed] [Google Scholar]
- 13.Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66:613–621. [PubMed] [Google Scholar]
- 14.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–1430. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Morner S, Lindqvist P, Waldenstrom A, Kazzam E. Right ventricular dysfunction in hypertrophic cardiomyopathy as evidenced by the myocardial performance index. Int J Cardiol. 2008;124:57–63. doi: 10.1016/j.ijcard.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, et al. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 18.Patel N, Mills JF, Cheung MM. Use of the myocardial performance index to assess right ventricular function in infants with pulmonary hypertension. Pediatr Cardiol. 2009;30:133–137. doi: 10.1007/s00246-008-9285-1. [DOI] [PubMed] [Google Scholar]
- 19.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rychik J. Forty years of the Fontan operation: a failed strategy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:96–100. doi: 10.1053/j.pcsu.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar H, Siddique MA, Haque KM, Ahmed CM, Mahmood M, Bhattacharjee B, et al. Evaluation of left ventricular global function using Doppler myocardial performance index in patients with systemic hypertension. Mymensingh Med J. 2008;17(Suppl):S65–S71. [PubMed] [Google Scholar]
- 22.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 23.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function: a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–366. [PubMed] [Google Scholar]
- 24.Tweddell JS, Nersesian M, Mussatto KA, Nugent M, Simpson P, Mitchell ME, et al. Fontan palliation in the modern era: factors impacting mortality and morbidity. Ann Thorac Surg. 2009;88:1291–1299. doi: 10.1016/j.athoracsur.2009.05.076. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YQ, Sun K, Zhu SL, Wu LP, Chen GZ, Zhang ZF, et al. Doppler myocardial performance index in assessment of ventricular function in children with single ventricles. World J Pediatr. 2008;4:109–113. doi: 10.1007/s12519-008-0021-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Takimoto E, Hsu S, Lee DI, Nagayama T, Danner T, et al. Myocardial remodeling is controlled by myocyte-targeted gene regulation of phosphodiesterase type 5. J Am Coll Cardiol. 2010;56:2021–2030. doi: 10.1016/j.jacc.2010.08.612. [DOI] [PMC free article] [PubMed] [Google Scholar]


