Abstract
Objective:
We aimed to investigate the relationship between the horizontal tumor diameter and prognosis.
Material and Methods:
Patients’ records were analyzed retrospectively. Patient data, including age, gender, vertical penetration, anatomic location, differentiation of the tumor, tumor node metastasis (TNM) stage, survival rate, and disease-free survival, were analyzed to find out if there was any correlation with horizontal tumor diameter.
Results:
A total of 439 colorectal cancer patients were enrolled. Patients were stratified into two groups according to the horizontal tumor diameter (≤4.5 cm vs. >4.5 cm). Poorly differentiated tumors were significantly larger than other differentiation groups (p=0.003). The horizontal diameter increased with increase in T-stage (p<0.001). Similarly, the number of positive lymph nodes increased significantly as the size of the horizontal tumor diameter increased (p<0.001). The relationship between TNM staging and the horizontal diameter of tumors in both groups was examined, and it was found that the progression of tumor stage was accompanied by increased horizontal diameter (p<0.001). It was also found that the horizontal tumor diameter was not correlated with local recurrence (p=0.063). However, distant metastasis was higher in patients with a tumor larger than 4.5 cm (p=0.02). Although the disease-free survival was shorter in patients with a horizontal tumor diameter more than 4.5 cm, the difference was not statistically significant.
Conclusion:
There is a significant relation between horizontal diameter of the tumor and depth of the tumor, lymph node involvement, overall survival, and distant metastasis. Horizontal diameter of the tumor can possibly be used as a prognostic factor in colorectal cancer patients.
Keywords: Colon, rectum, tumor, horizontal diameter, prognosis
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer among men and women in developed countries (1, 2). The preferred management of non-metastatic colon cancer is removal of the tumor and surrounding lymph nodes. Post-surgical treatment is closely related to the tumor node metastasis (TNM) staging system (3, 4).
Depth of tumor penetration (T), regional lymph node involvement (N), and distant metastasis (M) are major parameters predicting the prognosis in CRC patients. The literature data show that tumor staging may be more accurate and the prognosis may be more favorable as the number of harvested lymph nodes increases (5–7).
Several studies show that vertical penetration of the tumor on the bowel wall is related to the number of positive lymph nodes and a poorer prognosis. The relationship between horizontal tumor diameter and prognosis is still controversial (8–10). Few studies in gastric and colon cancer indicate that the horizontal extension of the tumor could be an important prognostic factor (2, 11, 12).
In this study, we aimed to investigate the relationship between horizontal tumor diameter and prognosis, as well as other well-known prognostic factors of CRC.
MATERIAL AND METHODS
A total of 486 colorectal cancer patients who were treated in our surgical clinic between 1991 and 2012 were enrolled. Data were obtained from a CRC database and the medical records of the patients. Clinical information and follow-up data were obtained from hospital charts and electronic records. Patients who received neo-adjuvant therapy or underwent palliative resection, had a pathological diagnosis other than adenocarcinoma, and patients with inflammatory bowel disease were excluded (n=47). The remaining 439 patients were included in our retrospective analysis.
Adjuvant chemotherapy was given according to the lymph node involvement. Patients with node-negative tumors did not receive chemotherapy. Patients showing poor prognostic indicators, such as vascular invasion, perineural invasion, and preoperative high levels of CEA, received 5-fluorouracil-based chemotherapy, regardless of their nodal status.
Patient data, including age, gender, vertical penetration, anatomic location, and differentiation of the tumor; TNM stage, survival rate, and disease-free survival were analyzed to find out if there was any correlation with the horizontal tumor diameter. Tumors located from the cecum to the splenic flexure were defined as right-sided cancers, and tumors located from the splenic flexure to the sigmoid colon were defined as left-sided cancers. Tumors originating from the recto-sigmoid junction or rectum were defined as rectal cancers. Patients were staged using the 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Horizontal tumor diameters were determined from formalin-fixed cancer specimens based on the archived pathology report. We used receiver-operating characteristic (ROC) curves for the determination of the appropriate cut-off value of the tumor size, which affects long-term survival in all stages. Different cut-off levels were examined according to the ROC curve analysis to find out the best cut-off level to correlate the survival, disease-free survival, and the other prognostic parameters mentioned above.
Statistical Analysis
All statistical analyses were performed using Statistical Package for the Social Sciences 16.0 (SPSS Inc., Chicago, IL, USA). Student’s t-test and chi-square test were used in order to determine the distribution of demographic characteristics of patients and to make probability charts. The Kaplan-Meier method was used in order to calculate cumulative survival rates according to the horizontal tumor diameter, lymph node metastasis (N stage), and TNM staging. The difference in survival rates between groups was calculated by using the log-rank test. Cox regression analysis was performed in order to determine the prognostic factors that affected the survival time (p<0.05).
RESULTS
Demographic characteristics of the patients, histological characteristics of the tumors, and overall survival are summarized in Table 1.
Table 1.
Clinical and pathological characteristics of the patients
| n | % | |
|---|---|---|
| Gender | ||
| Female | 188 | 42.8 |
| Male | 251 | 57.2 |
| Age | ||
| Mean±S.E.M | 66.6±13.3 | |
| Female | 67.5±13.2 | |
| Male | 66.0±13.5 | |
| Tumor location | ||
| Right colon | 117 | 26.7 |
| Left colon | 187 | 42.6 |
| Rectum | 135 | 30.8 |
| Tumor differentiation | ||
| Well | 74 | 16.9 |
| Moderate | 333 | 75.9 |
| Poor | 28 | 6.4 |
| Undifferentiated | 4 | 0.9 |
| pT stage | ||
| 1 | 16 | 3.0 |
| 2 | 73 | 16.6 |
| 3 | 304 | 69.2 |
| 4 | 46 | 10.5 |
| pN stage | ||
| Median (range) | 10 (1–67) | |
| Mean±S.E.M | 12±0.4 | |
| <12 | 257 | 58.5 |
| ≥12 | 182 | 41.5 |
| 0 | 257 | 58.5 |
| 1 | 135 | 30.8 |
| 2 | 47 | 10.7 |
| TNM stage | ||
| I | 78 | 17.8 |
| II | 161 | 36.7 |
| III | 136 | 31 |
| IV | 64 | 14.6 |
| Follow-up | ||
| Alive | 210 | 56 |
| Dead | 165 | 44 |
| Overall survival (month) | ||
| Median (range) | 30.2 (0.1–237.1) | |
| Mean±S.E.M | 42.3 ± 2.2 | |
| Disease-free survival (month) | ||
| Median (range) | 22.6 (0–237.1) | |
| Mean±S.E.M | 38±2.2 |
S.E.M: standard error of the mean; pT: pathologic T stage; pN: pathologic N stage; LN: lymph node; TNM: tumor, node, metastasis
By using ROC curve analysis, we determined a cut-off value of 4.5 cm for the horizontal diameter to best discriminate between patients’ survival in the whole group. Based on the ROC curve analysis, patients were stratified into two groups according to the horizontal tumor diameter (≤4.5 cm vs. >4.5 cm). The relationship between prognostic pathological features and the horizontal tumor diameter was examined (Table 2). The horizontal tumor diameter was significantly lower in left-sided tumors of the colon compared to the right-sided tumors (p=0.004). Poorly differentiated tumors were significantly larger than others when we used a cut-off value of 4.5 cm (p=0.003).
Table 2.
Patient demographics, tumor characteristics, and survival between two groups according to horizontal diameter
| ≤4.5 cm (n=208) | >4.5 cm (n=231) | p | |
|---|---|---|---|
| Gender | |||
| Female | 92 | 96 | 0.32 |
| Male | 116 | 135 | |
| Age groups | |||
| Median (range) | 69 (21–92) | 68 (22–93) | 0.133 |
| Mean±S.E.M | 67.6±0.8 | 65.7±1 | |
| Tumor location | |||
| Right colon | 40 | 77 | 0.004 |
| Left colon | 98 | 89 | |
| Rectum | 70 | 65 | |
| Tumor Differentiation | |||
| Well | 43 | 31 | 0.003 |
| Moderate | 157 | 176 | |
| Poor | 7 | 21 | |
| Undifferentiated | 1 | 3 | |
| pT Stage | |||
| 1 | 13 | 3 | <0.001 |
| 2 | 46 | 27 | |
| 3 | 137 | 167 | |
| 4 | 12 | 34 | |
| pN Stage | |||
| 0 | 139 | 118 | <0.001 |
| 1 | 53 | 80 | |
| 2 | 16 | 33 | |
| TNM Stage | |||
| I | 53 | 25 | 0.001 |
| II | 74 | 87 | |
| III | 54 | 82 | |
| IV | 27 | 37 | |
| Local recurrence | |||
| Yes | 16 | 28 | 0.063 |
| No | 166 | 167 | |
| Distant metastasis | |||
| Yes | 44 | 66 | 0.02 |
| No | 122 | 110 | |
| Unknown | 42 | 55 | |
| Follow-up | |||
| Alive | 117 | 93 | 0.001 |
| Dead | 64 | 101 | |
| Missed | 27 | 27 | |
| Overall survival (month) | |||
| Median (range) | 31 (0.1–187.3) | 28.5 (0.1–237.1) | 0.014 |
| Mean±S.E.M | 47.9±3.3 | 37.2±2.8 | |
| Disease-free survival (month) | |||
| Median (range) | 25.8 (0.1–187.3) | 19.5 (0.1–237.1) | 0.06 |
| Mean±S.E.M | 42.3±3.3 | 33.9±3 | |
The depth of vertical tumor penetration was also analyzed, and it was found that the horizontal diameter increased with increase in T-stage (p<0.001). Similarly, the number of positive lymph nodes increased significantly as the size of the horizontal tumor diameter increased (p<0.001). The relationship between TNM staging and the horizontal diameter of tumors in both groups was examined, and it was found that the progression of tumor stage was accompanied by increased horizontal diameter (p<0.001). Cox regression analysis showed that TNM staging and horizontal diameter had a significant effect on survival (p<0.001, p=0.012, respectively).
It was also found that horizontal tumor diameter was not correlated with local recurrence (p=0.063). However, distant metastasis was higher in patients with a tumor larger than 4.5 cm (p=0.02). Patients with a horizontal tumor diameter less than 4.5 cm showed favorable survival (p=0.014) (Table 2). Although the disease-free survival was shorter in patients with a horizontal tumor diameter more than 4.5 cm, the difference was not statistically significant (p=0.06). Kaplan-Meier curves representing overall survival and disease-free survival for horizontal diameter of the tumor are shown in Figure 1 and Figure 2, respectively. The survival of patients whose tumors were >4.5 cm was significantly worse than patients whose tumors were ≤4.5 cm. According to these data, the survival rate decreased as tumor size increased.
Figure 1.
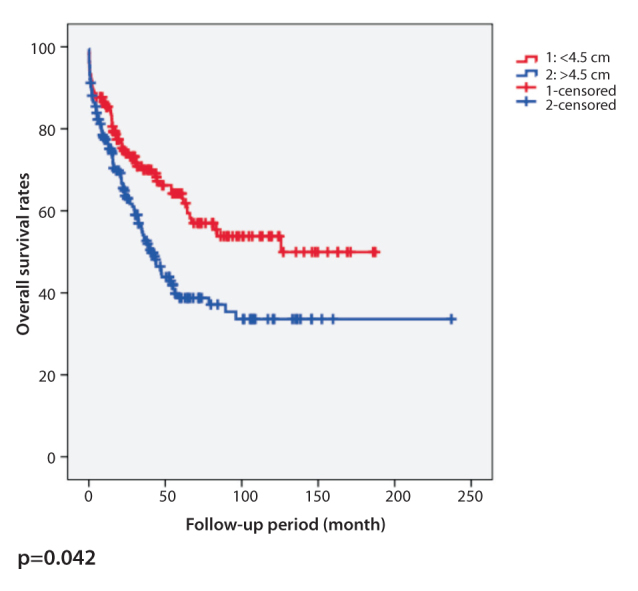
Kaplan-Meier curve of overall survival for horizontal diameter of colorectal tumors with a cut-off value of 4.5 cm
Figure 2.
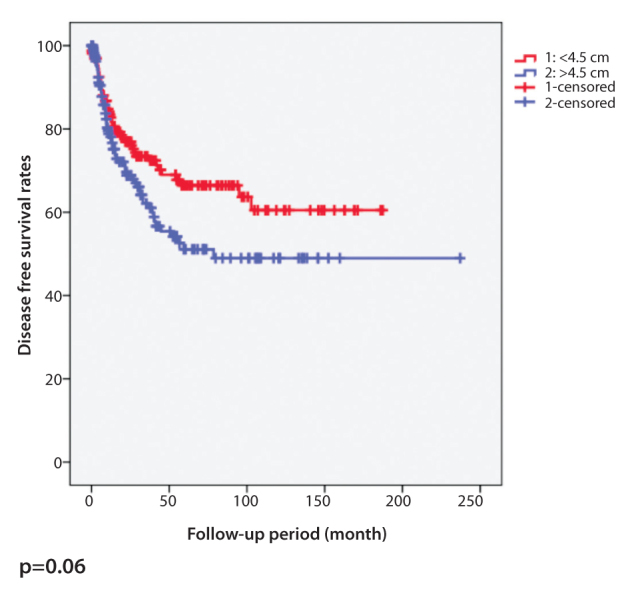
Kaplan-Meier curve of disease-free survival for horizontal diameter of colorectal tumors with a cut-off value of 4.5 cm
DISCUSSION
TNM staging is the best prognostic parameter for patients with CRC (13). The average survival rate according to the stage of the tumor is 93% for stage 1, 78% for stage 2, 69% for stage 3, and 8% for stage 4, respectively (14). There are several studies indicating that tumor size is associated with the depth of invasion, lymph node metastasis, and survival in patients with gastric carcinoma (12, 15–17). However, the studies about the relationship between horizontal diameter of the tumor and prognosis in patients with CRC are limited and the results are controversial (18, 19). Kornprat et al. (2) identified 4.5 cm as the optimal cut-off value in a group of patients using ROC curve analysis, as we did, and they concluded that tumor size is the most important prognostic parameter for patients with CRC. Patients were evaluated according to this cut-off value, and the authors demonstrated that there was a significant relationship between tumor size and anatomic location of the tumor, high T and N classification, International Union against Cancer (UICC) stage, and tumor grade.
Right-sided tumors mostly do not cause any signs or symptoms in the early stages, and they are generally diagnosed when they attain a large size. These tumors are diagnosed in the early stages with the technological improvement and common use of colonoscopy, but the problem is still going on. Several studies emphasized that larger tumors are more frequent in the right colon (2, 20, 21). We also found that proximal tumor cancers tended to have a larger diameter.
We investigated the relationship between tumor differentiation and horizontal diameter of the tumor, and we found that poor differentiation was associated with larger tumor size. Although Tekuchi et al. (22) and Kornprat et al. (2) also highlighted the same relationship, similar to our study; Matsuda et al. (23), Crozier et al. (18), and Bjerkest et al. (20) did not report such a relationship.
Wolmark et al. (19) compared tumor size with depth of penetration, and their results indicated that tumor size was smaller in Duke’s stage C1 than stage C2. Such a relationship was shown in other studies that compared different-sized tumor groups by using TNM staging (18). Adachi et al. (12) and Saito et al. (17) showed a similar relationship in patients with gastric cancer. We also found that larger tumors penetrated deeper.
As the presence of lymph node involvement is used in order to determine adjuvant therapy in CRC patients, the relationship between horizontal diameter of the tumor and lymph node invasion could be important. According to our data, we found that there was a significant statistical relationship between the size of the horizontal diameter and lymph node invasion; large tumors were associated with more positive lymph nodes. Although some studies in the literature support our data (2, 19, 24), other studies concluded that the size of the horizontal diameter is not an important factor in determining lymph node involvement (18, 20, 25).
The significant relationship that was found in our study between horizontal diameter of the tumor and increases in both penetration depth and number of positive lymph nodes was also seen in TNM staging. Li et al. (26) found that the diameter of the tumor was smaller than 3 cm in patients with Duke’s stage A or B, and it was greater than 3 cm in patients with Duke’s stage C or D. Another study demonstrated that tumor size was significantly associated with UICC stage and concluded that increasing tumor diameter was not associated with either depth of invasion or lymph node involvement (2, 18). Poritz et al. (14) investigated the effect of tumor size on metastatic disease, and it was interesting that tumor volume was smaller in patients with metastatic disease compared to patients without metastatic disease. In our study, we found that local recurrence after surgery was not related with tumor size, but presence of distant metastasis was associated with tumor size.
In the literature, there are studies that have investigated the relationship between tumor size and survival rate (2, 27, 28). When overall survival rates and 5-year survival rates are examined, it is generally proposed that large tumors have a poor prognosis. In our study, we found no significant difference between groups regarding disease-free survival rates. However, the group with tumor size smaller than 4.5 cm had a better prognosis regarding overall survival rates.
The main limitation of our study was the retrospective nature. Another limitation was that medical records were not well completed at the beginning of the study, and patients were lost at follow-up. But, we thought that this study was important, as there are a limited number of publications related to this topic in the literature.
CONCLUSION
We have evaluated our groups by comparing them with TNM staging system, an approved prognostic scoring system, in order to determine the prognostic value of tumor size, and we concluded that horizontal diameter of CRC can possibly be used as a prognostic factor, as in gastric cancers.
Footnotes
Ethics Committee Approval: Ethical approval is not required because of the retrospective design of the study.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept -A.Z.B., Y.Ö., İ.S., S.T.D., M.B., D.D., M.L.A.; Design - A.Z.B., Y.Ö., D.D., M.B., M.L.A., İ.S., S.T.D.; Supervision -İ.S., M.L.A., A.Z.B., S.T.D.; Funding - D.D., A.Z.B., Y.Ö., M.B.; Materials - D.D., S.T.D., Y.Ö.; Data Collection and/or Processing - Y.Ö., A.Z.B., M.B.; Analysis and/or Interpretation - İ.S., A.Z.B., Y.Ö.; Literature Review - M.B., S.T.D., A.Z.B., Y.Ö.; Writer - A.Z.B., Y.Ö., İ.S., D.D.; Critical Review - D.D., M.L.A., İ.S., M.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Elias E, Mukherji D, Faraj W, Khalife M, Dimassi H, Eloubeidi H, et al. Lymph-node ratio is an independent prognostic factor in patients with stage III colorectal cancer: a retrospective study from the Middle East. World J Surg Oncol. 2012;10:63. doi: 10.1186/1477-7819-10-63. http://dx.doi.org/10.1186/1477-7819-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Wieth M, et al. Value of tumour size as a prognostic variable in colorectal cancer: a critical reappraisal. Am J Clin Oncol. 2011;34:43–49. doi: 10.1097/COC.0b013e3181cae8dd. http://dx.doi.org/10.1097/COC.0b013e3181cae8dd. [DOI] [PubMed] [Google Scholar]
- 3.De Ridder M, Vinh-Hung V, Van Nieuwenhove Y, Hoorens A, Sermeus A, Storme G. Prognostic value of the lymph node ratio in node positive colon cancer. Gut. 2006;55:1681. doi: 10.1136/gut.2006.104117. http://dx.doi.org/10.1136/gut.2006.104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki O, Sekishita Y, Shiono T, Ono K, Fujimori M, Kondo S. Number of lymph node metastases is better predictor of prognosis than level of lymph node metastasis in patients with node-positive colon cancer. J Am Coll Surg. 2006;202:732–736. doi: 10.1016/j.jamcollsurg.2006.02.007. http://dx.doi.org/10.1016/j.jamcollsurg.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. http://dx.doi.org/10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 6.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Virginia A. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. http://dx.doi.org/10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 7.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. http://dx.doi.org/10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 8.Park YJ, Park KJ, Park JG, Lee KU, Choe KJ, Kim JP. Prognostic factors in 2230 Korean colorectal cancer patients: analysis of consecutively operated cases. World J Surg. 1999;23:721–726. doi: 10.1007/pl00012376. http://dx.doi.org/10.1007/PL00012376. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23:1068–1072. doi: 10.1038/modpathol.2010.88. http://dx.doi.org/10.1038/modpathol.2010.88. [DOI] [PubMed] [Google Scholar]
- 10.Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–543. doi: 10.1007/s00535-004-1339-4. http://dx.doi.org/10.1007/s00535-004-1339-4. [DOI] [PubMed] [Google Scholar]
- 11.Yamamura Y, Nakajima T, Ohta K, NAshimoto A, Arai K, Hiratsuka M, et al. Determining prognostic factors for gastric cancer using the regression tree method. Gastric Cancer. 2002;5:201–207. doi: 10.1007/s101200200035. http://dx.doi.org/10.1007/s101200200035. [DOI] [PubMed] [Google Scholar]
- 12.Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Tumour size as a simple prognostic indicator for gastric carcinoma. Ann Surg Oncol. 1997;4:137–140. doi: 10.1007/BF02303796. http://dx.doi.org/10.1007/BF02303796. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Sobin LH. A worldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC. J Surg Oncol. 2009;99:269–272. doi: 10.1002/jso.21237. http://dx.doi.org/10.1002/jso.21237. [DOI] [PubMed] [Google Scholar]
- 14.Poritz LS, Sehgal R, Hartnett K, Berg A, Koltun WA. Tumour volume and percent positive lymph nodes as a predictor of 5-year survival in colorectal cancer. Surgery. 2011;150:649–655. doi: 10.1016/j.surg.2011.07.049. http://dx.doi.org/10.1016/j.surg.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 15.Shiraishi N, Sato K, Yasuda K, Inomata M, Kitano S. Multivariate prognostic study on large gastric cancer. J Surg Oncol. 2007;96:14–18. doi: 10.1002/jso.20631. http://dx.doi.org/10.1002/jso.20631. [DOI] [PubMed] [Google Scholar]
- 16.Jun KH, Jung H, Baek JM, Chin HM, Park WB. Does tumour size have an impact on gastric cancer? A single institute experience. Langenbecks Arch Surg. 2009;394:631–635. doi: 10.1007/s00423-008-0417-0. http://dx.doi.org/10.1007/s00423-008-0417-0. [DOI] [PubMed] [Google Scholar]
- 17.Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Oro S, et al. Macroscopic tumour size as a simple prognostic indicator in patients with gastric cancer. Am J Surg. 2006;192:296–300. doi: 10.1016/j.amjsurg.2006.03.004. http://dx.doi.org/10.1016/j.amjsurg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Crozier JE, McMillan DC, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. Tumor size is associated with the systemic inflammatory response but not survival in patients with primary operable colorectal cancer. J Gastroenterol Hepatol. 2007;22:2288–2291. doi: 10.1111/j.1440-1746.2006.04792.x. http://dx.doi.org/10.1111/j.1440-1746.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolmark N, Fisher ER, Wieand HS, Fisher B. The relationship of depth of penetration and tumour size to the number of positive nodes in Dukes C colorectal cancer. Cancer. 1984;53:2707–2712. doi: 10.1002/1097-0142(19840615)53:12<2707::aid-cncr2820531225>3.0.co;2-r. http://dx.doi.org/10.1002/1097-0142(19840615)53:12<2707::AID-CNCR2820531225>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Bjerkeset T, Morild I, Mørk S, Søreide O. Tumour characteristics in colorectal cancer and their relationship to treatment and prognosis. Dis Colon Rectum. 1987;30:934–938. doi: 10.1007/BF02554279. http://dx.doi.org/10.1007/BF02554279. [DOI] [PubMed] [Google Scholar]
- 21.Tomoda H, Taketomi A, Baba H, Kohnoe S, Seo Y, Saito T. The clinicopathological characteristics and outcome of patients with right colon cancer. Oncol Rep. 1998;5:481–483. [PubMed] [Google Scholar]
- 22.Takeuchi K, Kuwano H, Tsuzuki Y, Ando T, Sekihara M, Hara T, et al. Clinicopathological characteristics of poorly differentiated adenocarcinoma of the colon and rectum. Hepatogastroenterology. 2004;51:1698–1702. [PubMed] [Google Scholar]
- 23.Matsuda T, Saito Y, Fujii T, Uraoka T, Nakajima T, Kobayashi N. Size does not determine the grade of malignancy of early invasive colorectal cancer. World J Gastroenterol. 2009;15:2708–2713. doi: 10.3748/wjg.15.2708. http://dx.doi.org/10.3748/wjg.15.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi Y, Yasuda K, Kakisako K, Sato K, Shiraishi N, Kitano S. Histopathologic criteria for local excision of colorectal cancer: multivariate analysis. Ann Surg Oncol. 1999;6:385–388. doi: 10.1007/s10434-999-0385-9. http://dx.doi.org/10.1007/s10434-999-0385-9. [DOI] [PubMed] [Google Scholar]
- 25.Tominaga K, Nakanishi Y, Nimura S, Yoshimura K, Sakai Y, Shimoda T. Predictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinoma. Dis Colon Rectum. 2005;48:92–100. doi: 10.1007/s10350-004-0751-4. http://dx.doi.org/10.1007/s10350-004-0751-4. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Kishida T, Kobayashi M. Serum iron and ferritin levels in patients with colorectal cancer in relation to the size, site, and disease stage of cancer. J Gastroenterol. 1999;3:195–199. doi: 10.1007/s005350050243. http://dx.doi.org/10.1007/s005350050243. [DOI] [PubMed] [Google Scholar]
- 27.Ju JH, Chang SC, Wang HS, Yang SH, Jiang JK, Chen WCK. Changes in disease pattern and treatment outcome of colorectal cancer: a review of 5,474 cases in 20 years. Int J Colorectal Dis. 2007;22:855–862. doi: 10.1007/s00384-007-0293-z. http://dx.doi.org/10.1007/s00384-007-0293-z. [DOI] [PubMed] [Google Scholar]
- 28.Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Dis. 2008;23:683–688. doi: 10.1007/s00384-008-0463-7. http://dx.doi.org/10.1007/s00384-008-0463-7. [DOI] [PubMed] [Google Scholar]


