Abstract
Background:
Quince seed mucilage (QSM) has been used in Iranian folk medicine in the treatment of wounds and burns. Experimental and clinical studies showed its wound healing activity. However, the mechanism by which this agent affects cells involved in the wound healing process is unknown.
Objectives:
In this study, we investigated the effects of QSM at concentrations of 50, 100, 200, and 400 µg/mL on human skin fibroblast proliferation as an aspect of promotion of wound healing.
Materials and Methods:
Human skin fibroblast cell line (HNFF-P18) was used in the experiment. Cell proliferation assay was measured by a MTT assay.
Results:
Cells treated with QSM at concentrations less than 400 µg/mL increased their proliferative activity. The concentration of 50 µg/mL was the most effective dose after 72 hours treatment.
Conclusions:
QSM has the ability to stimulate proliferation of human skin fibroblast. This effect suggests that this compound can act as a wound healing agent.
Keywords: Fibroblast Proliferation Inhibitor, Wound Healing, Plant Mucilage
1. Background
Wound healing is a complex biological process, which results in the restoration of tissue integrity. Physiologically, it can be broken down into four distinct phases of haemostasis, inflammation, proliferation and tissue remodeling (1). Fibroblasts are critical in supporting normal wound healing, involved in key processes such as breaking down the fibrin clot, creating new extra cellular matrix (ECM) and collagen structures to support other cells associated with effective wound healing, as well as contracting the wound (2). It has been demonstrated that increasing the number of fibroblasts in an artificial dermal substitute leads to improved healing in experimental wounds (3).
Several herbal and natural products have been investigated for promotion of wound healing. Quince seed mucilage (QSM) has been used for healing skin wounds in Iranian folk medicine. Experimental and clinical studies have shown the wound healing activity of QSM, which support the traditional use of this natural product (4-6).
Quince (Cydonia oblonga Miller) from Rosaceae family is a deciduous tree cultivated as a medicinal and nutritional plant in the Middle East, South Africa and central Europe (7). Different parts of the plant have been used in folk medicine for the treatment of variety of diseases. The leaves have been used after decoction for their sedative, antipyretic and antidiarrheic properties. The fruits were used for dysentery (8). The seeds soaked or boiled in water, release the mucilage from the seed coat and make a jelly-like consistency used for sore throats and bronchitis and as a bulk laxative. Mucilage is also applied externally to minor burns, etc. (9). During the past years, several experimental or clinical studies were performed to evaluate biological effects of quince and its derivatives such as antiradical (10), antiproliferative (11), antihemolytic (12) and antiallergic properties (13); moreover, lipid lowering (14), antidiabetic (15) and healing effects of quince (5, 6) were investigated.
2. Objectives
The objective of this study was to investigate the effects of QSM on human skin fibroblast proliferation rate as an aspect of promotion of wound healing. The results from this study would explain possible mechanism of QSM on wound healing. To the best of our knowledge, this is the first study on the effects of QSM on fibroblast proliferation.
3. Materials and Methods
3.1. Plant Material
Quince fruits (Cydonia oblonga Miller) were collected during late summer from a garden in the city of Isfahan, central Iran. The plant was taxonomically identified in the Department of Botany, School of Sciences, Ahvaz Shahid Chamran University, Ahvaz, Iran.
3.2. Mucilage Preparation
Seeds were separated from the fresh pulps. The prepared seeds were dried in shade at a temperature of 25 - 30°C. Then, 200 grams of quince seeds was added to 2500 mL distilled water then heated at 50 - 60°C and mixed for 30 minutes. After 30 minutes, the beaker containing the quince mucilage was left to reach near 40°C. Afterwards it was filtered through a clean linen cloth and mucilage was separated. The mucilage was then heated in an oven at 40°C to form a dry powder. For use in experiments, the mucilage was dissolved in distilled water to give final concentrations of 50, 100, 200 and 400 µg/mL.
3.3. Cell Culture
Human skin fibroblast cell line (HNFF-P18) was purchased from the National Cell Bank of Iran (NCBI, Pasteur Institute, Tehran, Iran). This cell line was obtained from the foreskin of a male newborn in NCBI. Cells were grown to confluence in culture flasks containing the Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) containing 10% heated inactivated fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin (Sigma, USA) in a 5% CO2 incubator at 37°C. Confluent fibroblast monolayers were propagated by trypsinization (0.1% trypsin and 0.02% EDTA, Sigma, USA) and subcultured in a ratio of 1:2. The cells for the experiments were used in passages 4 - 8.
3.4. Proliferation Assay
Cell proliferation assay was measured using the MTT-microculture tetrazolium assay as earlier described (16). Briefly, cells at the exponential growth phase were harvested, centrifuged and incubated in a 5% humidified CO2 incubator at 37°C. The cell number was determined by hemocytometer. The mucilage was dissolved in distilled water and administered (10 µL) to give final concentrations of 50, 100, 200 and 400 µg/mL. In the control group, 10 µL of distilled water was added. Cells were collected at 12, 24, 48, and 72 hours. MTT [stock solution 5 mg/mL, phosphate-buffered saline (PBS, Sigma, USA)] was added and the plates were again incubated for four hours. Then, the plates were read immediately in a microplate reader (Power WaveX, Bio-Tek Instruments, USA) at 540 nm. Wells with complete medium, test agent and MTT but without cells were used as blanks.
The assay included:
Blank wells containing medium only.
Control wells containing untreated cells
Test wells containing treated cells
From the absorbance reading from each well:
Proliferation rate (%) = (A (sample) –A (b))/(A (c) –A (b)) × 100
A (b) = Absorbance of blank
A (c) = Absorbance of negative control
3.5. Statistical Analysis
The statistical analysis was performed by two-way analysis of variances (ANOVA). A Dunnett’s multiple comparison test was used for post-hoc analysis. P < 0.05 was considered statistically significant.
4. Results
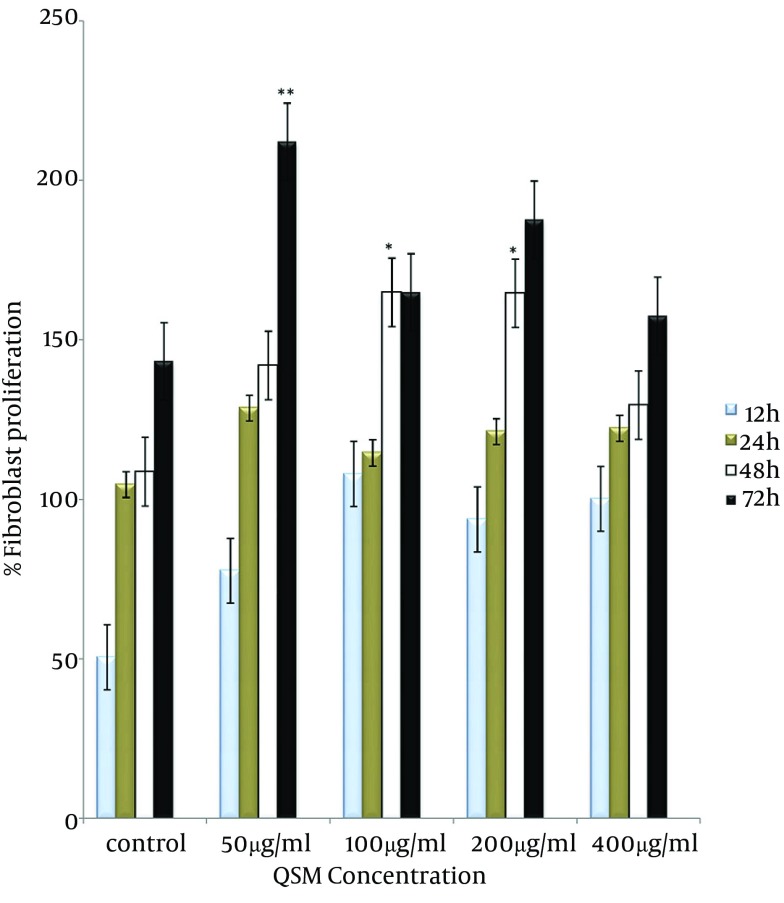
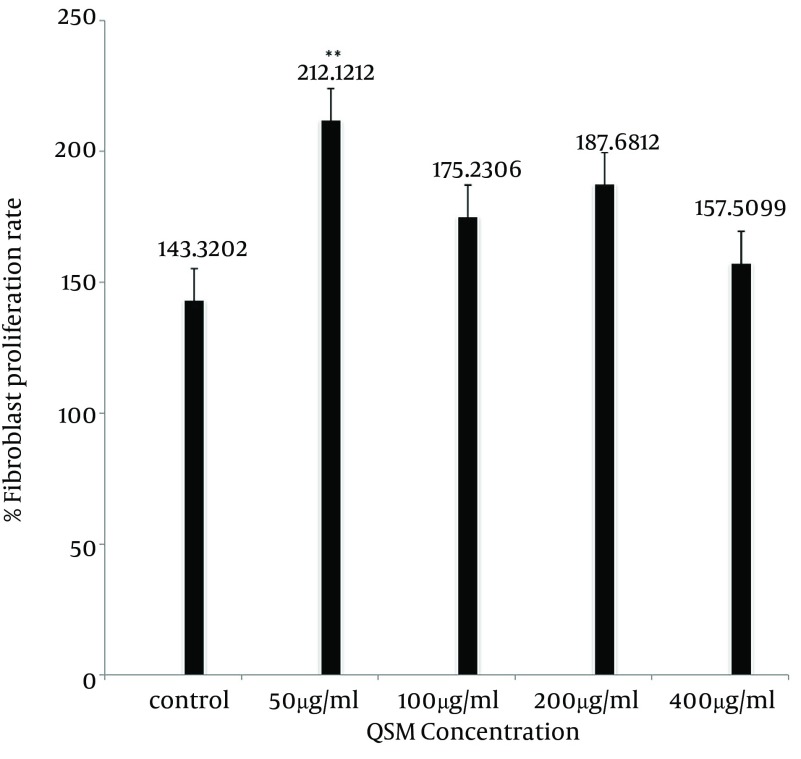
The QSM cream enhanced human fibroblasts proliferation (Figure 1). This effect of QSM in concentration of 100 and 200 µg/mL was visible by MTT assay after 48 hours of treatment (P < 0.05) (Figure 2). QSM at concentration of 50 µg/mL significantly enhanced the proliferation of fibroblast compared to the untreated group after 72 hours (P < 0.01) (Figure 3). The concentration of 400 µg/mL was ineffective.
Figure 1. Comparison of Microscopic Aspects of Human Skin Fibroblasts Submitted to Different Concentrations of QSM After 72 Hours of Incubation.
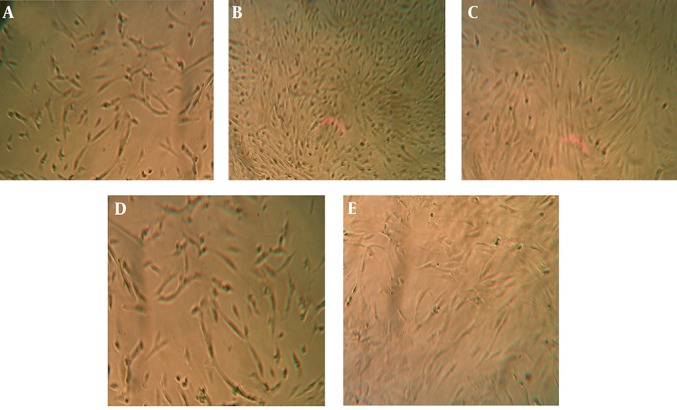
A) Control group. B) QSM 50 µg/mL treated cells. C) QSM 100 µg/mL treated cells. D) QSM 200 µg/mL treated cells. E) QSM 400 µg/mL treated cells.
Figure 2. Proliferation Rate in Fibroblasts Treated With QSM for 12, 24, 48, and 72 hours.
Data are presented as Mean ± SE. Values significantly different from the control group are indicated as * P < 0.05, ** P < 0.01.
Figure 3. The Percentages of Fibroblast Proliferation in Studied Groups After 72 Hours of Incubation.
Data are presented as Mean ± SE. Values significantly different from the control group are indicated as ** P < 0.01.
5. Discussion
Wound healing is a dynamic and interactive process involving soluble mediators, blood cells, extracellular matrix and parenchymal cells (17). Extracellular matrix (ECM) components play a key role during the angiogenic process for a correct development of blood vessels. Fibroblasts are the main cell type involved in the regulation of ECM protein production (18). These cells proliferate to expand and migrate into the wound area and synthesize ECM, also express thick actin bundles as myofibroblasts, which represent key players in physiological reconstruction of connective tissue after injury (19, 20).
In this study, we tested the effects of QSM on human dermal fibroblast at doses of 50, 100, 200 and 400 µg/mL using MTT assay. The selection of the agent was based on its traditional medicinal use and its wound healing activity in animal model and human as reported in experimental and clinical studies.
Our results revealed that QSM significantly enhanced the proliferation rate of fibroblasts at concentrations less than 400 µg/mL. This finding is consistent with previous studies that QSM has the ability to accelerate wound healing (5, 6). QSM at 400 µg/mL, the maximum concentration used in this study was not toxic to human fibroblasts. The concentrations of 100 µg/mL and 200 µg/mL of QSM significantly increased the proliferation of fibroblasts after 48 hours of treatment compared to the control group. Nevertheless, at concentration of 50 µg/mL, the effect was observed only after 72 hours, which was significantly greater compared to other groups. It means that QSM is effective in the proliferation phase of wound healing at low concentrations.
This finding could explain in part the observed wound healing effects of QSM. It presumably stimulates fibroblasts of the tissue around the wound to proliferate, expresses appropriate integrin receptors and migrates into the wound space (21). In fact, the appearance of fibronectin and appropriate integrin receptors that bind fibronectin, fibrin, or both on fibroblasts appears to be the rate-limiting step in the formation of granulation tissue (22).
Enhancement of fibroblasts proliferation indicates that QSM may contain growth promoting factor(s). These components are likely to candidates for the effects of QSM on proliferation of fibroblasts in the present study. Quince seed presents a phenolic profile composed of 3-O-caffeoylquinic, 4-O-caffeoylquinic, 5-O-caffeoylquinic and 3, 5-dicaffeoylquinic acids, lucenin-2, vicenin-2, stellarin-2, isoschaftoside, schaftoside, 6-C-pentosyl-8-C-glucosyl chrysoeriol and 6-C-glucosyl-8-C-pentosyl chrysoeriol (22). The caffeoylquinic acid is a potent antioxidant. Radical scavenging and antioxidant activity of quince seed may explain the accelerative effect of QSM on fibroblast proliferation.
We concluded that enhancement of fibroblast proliferation by QSM supports scientific basis of using this natural product in Iranian folk medicine in the treatment of wounds and burns.
Acknowledgments
We would like to express our special thanks to the Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences. This study was issued from the PhD thesis of Dr. Pari Tamri (Grant No. PRC 123).
Footnotes
Funding/Support:This research was financially supported by Physiology Research Center, Ahvaz Jundishapur University of Medical Sciences, (Grant No: PRC123), Ahvaz, Iran
References
- 1.Clark R. The Molecular and Cellular Biology of Wound Repair. New York: Springer; 1996. [Google Scholar]
- 2.Stortelers C, Kerkhoven R, Moolenaar WH. Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics. 2008;9:387. doi: 10.1186/1471-2164-9-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaim SF, Henderson RA. Wound dressing materials and topical medication. Small animal wound management. Philadelphia: Lea and Febiger; 1990. [Google Scholar]
- 4.Adderson JE, Evans WC, Trease GE. The pharmacognosy of the root of Rauwolfia ligustrina Roem. and Schult. J Pharm Pharmacol. 1961;13:224–39. doi: 10.1111/j.2042-7158.1961.tb11816.x. [DOI] [PubMed] [Google Scholar]
- 5.Hemmati AA, Mohammadian F. An investigation into the effects of mucilage of quince seeds on wound healing in rabbit. J Herb Spices Med Plant. 2000;7(4):41–6. [Google Scholar]
- 6.Hemmati AA, Kalantari H, Jalali A, Rezai S, Zadeh HH. Healing effect of quince seed mucilage on T-2 toxin-induced dermal toxicity in rabbit. Exp Toxicol Pathol. 2012;64(3):181–6. doi: 10.1016/j.etp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Zargari A. Medicinal plants. Tehran: Tehran University; 1986. [Google Scholar]
- 8.Duke JA, Ayensu S. Medicinal plants of china. Medicinal plants of world. Michigan: Algonac; 1985. pp. 154–15. [Google Scholar]
- 9.Grieve M. A Modern Herbal: The Medicinal, Culinary, Cosmetic and Economic Properties, Cultivation and Folk-lore of Herbs, Grasses, Fungi, Shrubs, & Trees with All Their Modern Scientific Uses. New York: Dover Publications; 1971. [Google Scholar]
- 10.Alesiani D, Canini A, D’Abrosca B, DellaGreca M, Fiorentino A, Mastellone C, et al. Antioxidant and antiproliferative activities of phytochemicals from Quince (Cydonia vulgaris) peels. Food Chem. 2010;118(2):199–207. [Google Scholar]
- 11.Carvalho M, Silva BM, Silva R, Valentao P, Andrade PB, Bastos ML. First report on Cydonia oblonga Miller anticancer potential: differential antiproliferative effect against human kidney and colon cancer cells. J Agric Food Chem. 2010;58(6):3366–70. doi: 10.1021/jf903836k. [DOI] [PubMed] [Google Scholar]
- 12.Magalhaes AS, Silva BM, Pereira JA, Andrade PB, Valentao P, Carvalho M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem Toxicol. 2009;47(6):1372–7. doi: 10.1016/j.fct.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Shinomiya F, Hamauzu Y, Kawahara T. Anti-allergic effect of a hot-water extract of quince (Cydonia oblonga). Biosci Biotechnol Biochem. 2009;73(8):1773–8. doi: 10.1271/bbb.90130. [DOI] [PubMed] [Google Scholar]
- 14.Khademi F. [The efficacy of quince leave extract on atherosclerotic plaques induced by atherogenic diet in coronary and aorta, hyperlipidemia and liver in rabbit]. Tabriz University of Medical Sciences; 2009. [Google Scholar]
- 15.Aslan M, Orhan N, Orhan DD, Ergun F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J Ethnopharmacol. 2010;128(2):384–9. doi: 10.1016/j.jep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–42. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 18.Broughton G2, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 19.Falanga V. Wound healing and chronic wounds. J Cutan Med Surg. 1998;3:S1–1-5. [PubMed] [Google Scholar]
- 20.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Clark RA. Extracellular matrix alters PDGF regulation of fibroblast integrins. J Cell Biol. 1996;132(1-2):239–49. doi: 10.1083/jcb.132.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;110 ( Pt 7):861–70. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]