Abstract
The choroid plexus produces cerebrospinal fluid and plays an important role in brain homeostasis both pre and postnatally. In vitro studies have suggested that cells from adult choroid plexus have stem/progenitor cell-like properties. Our initial aim was to investigate whether such a cell population is present in vivo during development of the choroid plexus, focusing mainly on the chick choroid plexus. Cells expressing neural markers were indeed present in the choroid plexus of chick and also those of rodent and human embryos, both within their epithelium and mesenchyme. ß3-tubulin-positive cells with neuronal morphology could be detected as early as at E8 in chick choroid plexus and their morphological complexity increased with development. Whole mount immunochemistry demonstrated the presence of neurons throughout choroid plexus development and they appeared to be mainly catecholaminergic, as indicated by tyrosine-hydroxylase reactivity. The presence of cells co-labeling for BrdU and the neuroblast marker, doublecortin, in organotypic choroid plexus cultures supported the hypothesis that neurogenesis can occur from neural precursors within the developing choroid plexus. Furthermore, we found that extrinsic innervation is present in the developing choroid plexus, unlike previously suggested. Altogether, our data are consistent with the presence of neural progenitors within the choroid plexus, suggest that at least some of the choroid plexus neurons are born locally, and show for the first time that choroid plexus innervation occurs prenatally. Hence, we propose the existence of a complex neural regulatory network within the developing choroid plexus that may play a crucial role in modulating its function during development as well as throughout life.
Keywords: chick, choroid plexus, development, innervation, neurogenesis, neural stem cell
Introduction
The choroid plexus (CP) is important for the secretion of cerebrospinal fluid (CSF) and plays an important role in brain development, homeostasis and disease. The CP, found in lateral, third and fourth ventricles of the brain, consists of a highly vascularized stroma with loose connective tissue surrounded by a single layer of simple cuboidal epithelium. The CP epithelium, considered to be a specialized ependymal epithelium, is continuous with the ependyma lining the brain of lateral, III and IV ventricles. It originates from the neuroepithelium, whereas the inner CP stromal core is believed to originate from the mesenchyme (Lehtinen et al., 2013; Liddelow et al., 2013).
The CP develops early during embryogenesis and which CP is first clearly visible depends on the species. In mouse and humans, the IV ventricle CP anlage is the first one observed at approximately E10.5 and 41 days of gestation, respectively (Dziegielewska et al., 2001; Hunter and Dymecki, 2007). In the chick embryo, it has been indicated that a CP anlage is first detectable in the lateral ventricles between E6 and E8. The chick lateral ventricle CP rapidly grows, lengthening and branching out many times with a dip in its growth reported at around E15–E16 followed by its thinning and flattening (Smith, 1966; Stastny and Rychter, 1976).
The CP constitutes the blood-CSF barrier that is established by the tight junctions of the CP epithelial cells. The CP is a very active organ that continuously secretes CSF from early development throughout life (Johansson et al., 2008; Liddelow et al., 2012; Lehtinen et al., 2013). It has been proposed that its secretory function is controlled by its autonomic innervation (Edvinsson and Lindvall, 1978; Lindvall and Owman, 1981). CSF secretion could be inhibited by stimulation of sympathetic nerves in the neck (Lindvall et al., 1978c) and via activation of β2 receptors (Nathanson, 1980). Sympathetic denervation in rabbits resulted in hydrocephalus and was fatal (Lindvall and Owman, 1981). It has been assumed that nerves enter the CP only after birth (Tsuker, 1947; Edvinsson et al., 1973; Lindvall et al., 1977a, 1978a,b,c; Edvinsson and Lindvall, 1978), but there is only one study where this issue was specifically addressed and that suggests that the CP becomes innervated postnatally (Lindvall and Owman, 1978). This seems peculiar, as innervation tends to occur at early stages of organ development, before a high degree of complexity is achieved. We therefore postulated that this would be the case also for the CP and set to test this hypothesis.
A variety of stem/progenitor cells have been hypothesized to reside within the CP. Nataf and co-workers have reported that myeloid progenitors in the CP are capable of differentiation toward macrophage or dendritic cell phenotypes (Nataf et al., 2006). In addition, the CP has been reported to contain neural stem-like cells able to give rise to neural cells in vitro and upon transplantation into a spinal cord injury model (Kitada et al., 2001). The transcriptome of the CP from adult mouse has revealed the presence of genes important for neural development (Itokazu et al., 2006; Marques et al., 2011). The neural stem cell potential observed in the CP has been suggested to reside in the CP epithelium on the basis of in vitro studies (Itokazu et al., 2006). The localization of those putative neural stem cell population(s) was not extensively studied in vivo. In another study in rat pups, the presence of nestin-expressing cells just beneath the CP epithelium, that decreased in number with age, was taken to indicate the presence of neural stem cells in the CP stroma (Huang et al., 2011). We hypothesized that the developing CP harbors a neurogenic niche that may account for the proposed “neurogenicity” of the CP.
We assessed the presence of neural progenitors and time of innervation in developing CPs, focusing on the chick CP in vivo and in organotypic cultures. We show for the first time that the CP mesenchymal compartment contains neural progenitor-like cells that may be the precursors of the neurons identified in this compartment, and that innervation of the CP is an early developmental event. Hence, we suggest a model where a neural regulatory network is present within the CP and may play a crucial role in modulating its function during development as well as throughout life.
Materials and methods
Unless otherwise specified all general reagents were from Sigma and tissue culture reagents from Gibco.
Chicken embryos and isolation of choroid plexus (CP)
Fertilized Brown Leghorn chicken eggs were purchased from Henry Stewart & Co. Ltd (Lincolnshire, UK). On arrival, eggs were stored at 15°C in the egg fridge (JENCONS Ltd., USA) and used within 1 week. They were maintained on cardboard egg racks in a humidified forced flow incubator at 38°C (MARSH automatic incubator, LYON electric company, USA) until the required developmental stages. Chick embryos were decapitated and brains removed. The meninges were carefully peeled off because its connective tissue normally attaches to the pineal gland which also connects to the 3rd ventricle CP. The isolated CP was used for whole mount immunohistochemistry, organotypic culture, or RNA extraction. Unless otherwise indicated, lateral ventricle CPs were used.
Human and mouse embryos
Mouse embryonic brains were isolated and fixed in 4% paraformaldehyde (PFA) overnight at 4°C, cryo-protected by incubation in 30% sucrose containing 0.02% sodium azide in PBS (phosphate buffered saline) at 4°C for approximately 24 h, OCT embedded, and immunohistochemistry performed. Paraffin sections of human brains at Carnegie stage 23 (Cs23; 56 days of gestation) were obtained from the Human Developmental Biology Resource (HDBR).
Immunohistochemistry and whole mount labeling
Embryonic chick or mouse brains were fixed in 4% PFA overnight at 4°C, cryo-protected by incubation in 30% sucrose containing 0.02% sodium azide in PBS (phosphate buffered saline) at 4°C for approximately 24 h, OCT embedded and cryosectioned (14–16 μm thick). Isolated CPs were fixed in 4% PFA overnight at 4°C and washed several times with PBS for whole mount labeling. For wax removal and epitope unmasking, human embryonic brain sections were immersed in 1:20 Declere® solution (Sigma) in PBS, heated in a microwave oven at 720 watt for 5 min and then at 270 watt for 20 min, rinsed in PBS and then processed as cryosections. Brain sections or whole mount CPs were permeabilized (0.5% Triton X-100 in PBS) and blocked in 20% BSA in PBS for 1 h at room temperature. Sections were incubated with primary antibodies (Table 1) diluted in blocking solution for 3–4 h at room temperature or overnight at 4°C. After several washes with PBS, sections were incubated with appropriate secondary antibodies for 1–2 h. Citiflour® solution (Citifluor Limited) was used as a mounting medium in most of immunohistochemistry for cryosections and paraffin sections. In this case, cell nuclei were visualized with Hoechst 33258 (2.5 μg/ml final concentration). Stained section and whole mount CPs were visualized and digitally scanned using an Axiophot 2 (Zeiss) with Hamamatsu ORCA-ER digital camera or by confocal laser scanning microscopy using an LSM 710 (Zeiss). Image analysis was performed using ImageJ software (Rasband, 1997-2009).
Table 1.
List of antibodies used for immunohistochemistry and whole mount staining.
| Dilution | Host | Types | Suppliers | |
|---|---|---|---|---|
| ANTIGEN (PRIMARY ANTIBODIES)* | ||||
| Aquaporin 1 (Aq-1) | 1: 200 | Mouse | IgG | Bioscience |
| βTub3 | 1: 1000 | Mouse | mAb IgG | Promega, |
| BrdU | 1: 100 | Mouse | IgG | DSHB |
| Clusterin | 1: 100 | Rabbit | pAb IgG | Santa cruz (SC) |
| Doublecortin (Dcx) | 1: 100 | Goat | IgG | Cell signaling |
| Flk-1 | 1: 200 | Rabbit | pAb, IgG | Fisher scientific |
| Glial fibrillary acidic protein (GFAP) | 1: 1000 | Rabbit | pAb | Chemicon |
| Laminin | 1: 200 | Rabbit | pAb | Milipore |
| Myelin basic protein | 1: 100 | Mouse | IgG | Chemicon |
| Nestin (human) | 1: 1000 | Rabbit | pAb IgG | Milipore |
| Nestin (rat, mouse) | 1: 200 | Mouse | IgG | Developmental studies hybridoma bank (DSHB) |
| NF200 | 1: 100 | Rabbit | IgG | Sigma |
| Otx2 | 1: 200 | Rabbit | pAb IgG | Abcam |
| P75NTR | 1: 500 | Rabbit | IgG | Sigma |
| Pax6 | 1: 100 | Mouse | mAb IgG1 | DSHB |
| Phosphohistone3 (pH3) | 1: 100 | Rabbit | IgG | Upstate |
| Sox2 | 1: 1000 | Rabbit | pAb | Milipore |
| Synaptic vesicles 2 (SV2) | 1: 100 | Mouse | IgG1 | DSHB |
| Transitin (chick) | 1: 100 | Mouse | mAb IgG1 | DSHB |
| Tyrosine hydroxylase | 1: 400 | Sheep | pAb | Millipore |
| VEGF | 1: 100 | Mouse | mAb IgG1 | SC |
| Vimentin | 1: 100 | Mouse | IgG | DAKO |
| ZO-1 | 1: 100 | Rabbit | pAb IgG | Molecular Probe |
| SECONDARY ANTIBODIES | ||||
| Anti-mouse IgG1a Alexa Fluor® 568 | 1: 400 | Goat | – | Molecular probe |
| Goat anti-mouse IgM Alexa Fluor® 594 | 1: 400 | Goat | – | Molecular probe |
| Anti-rabbit Alexa Fluor® 594 | 1: 400 | Goat | – | Molecular probe |
| Anti-mouse IgG Alexa Fluor® 488 | 1: 400 | Goat | – | Molecular probe |
| Anti-sheep Alexa Fluor® 488 | 1: 1000 | Rabbit | – | Molecular probe |
mAb, monoclonal antibody; pAb, polyclonal antibody.
The same antibodies were used in the different species unless the species is indicated in brackets.
Organotypic culture of chick CPs
E12 chick embryo CPs were used to set up organotypic cultures. CPs from lateral and third ventricles were dissected and grown onto a 0.4-μM-Millipore®-CM culture plate insert in MEM (minimal essential medium) containing Glutamax, 5% Earle's balanced salt solution (EBSS), 36 mM D-glucose, 1% penicillin/streptomycin and 25% horse serum.
MTT assay and PI staining of organotypic chick CP cultures
Propidium iodide (PI) was used to identify cell death in organotypic CP cultures. Briefly, the cultures were incubated with culture medium containing 5 μg/ml PI for 1 h at 37°C in an atmosphere of 95% O2 and 5% CO2. Organotypic cultures were incubated with 0.5 mg/ml MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution for 1 h at 37°C to assess cell viability. The appearance of blue/purple formazan crystals is indicative of cell viability and structural integrity.
BrdU incorporation in organotypic chick CP cultures
Organotypic CP preparations were treated with 10 μM BrdU for 3 days and then processed for BrdU detection and immunohistochemistry. CPs were incubated in 1N HCl for 10 min on ice, followed by incubation in 2N HCl for 10 min at room temperature, and then for 20 min at 37°C. The CPs were then washed three times in 0.1M borate buffer at room temperature, once in PBS, and immunostained as described above.
Polymerase chain reaction (RT-qPCR)
RNA was extracted from chick lateral ventricle CP and midbrain at different developmental stages (see Results) using Tri-Reagent (Life Technologies) according to the manufacturer's instructions. RNA was retro-transcribed with Moloney murine leukemia virus reverse transciptase (Promega, Madison, WI). The annealing temperature for RT-PCR was 54°C for Sox2 and Otx2 (30 cycles) and 66°C for GAPDH (25 cycles). Real time quantitative polymerase chain reaction (RT-qPCR) was preformed with ABI Prism 7500 sequence detection system (Applied Biosystems) using the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Gene expression data were normalized using GAPDH housekeeping gene as a reference using the 2-ΔΔCt method. The primers used for RT-PCR and RT-qPCR are listed in Table 2.
Table 2.
Primers used for analysis of gene expression in chick choroid plexus and midbrain by RT-PCR and RT-qPCR.
| Gene | Primers (5′-3′) |
|---|---|
| GAPDH | For CCAGGTTGTCTCCTGTGACT Rev CACAACACGGTTGCTGTAT |
| Transitin | For CTGGAGCAGGAGAAGCAGAG Rev CTGTTGGCCAGCTTGAACTC |
| GFAP | For CCAGGTTGTCTCCTGTGACT Rev CACAACACGGTTGCTGTAT |
| Doublecortin (Dcx) | For CCCATTCGTTTGAGCAAG TT Rev CCTGTGCATAGCGGAATTTT |
| ß3-tubulin | For TCTCACAAGTACGTGCCTCG Rev CCCCGCTCTGACCGAAAATG |
| Sox2 | For AGGCTATGGGATGATGCAAG Rev GTAGGTAGGCGATCCGTTCA |
| Otx2 | For CCACCTCAACCAGTCTCCAG Rev TTCCATGAGGATGTCTGGTC |
Statistical analysis
Data are presented as mean ±SEM. The statistical analysis was performed using GraphPad Prism version 5.00 for Windows. Statistical significance was evaluated by One-Way ANOVA. A p-value equal to or less than 0.05 was considered as statistically significant.
Results
Expression of neural markers in the developing choroid plexus (CP)
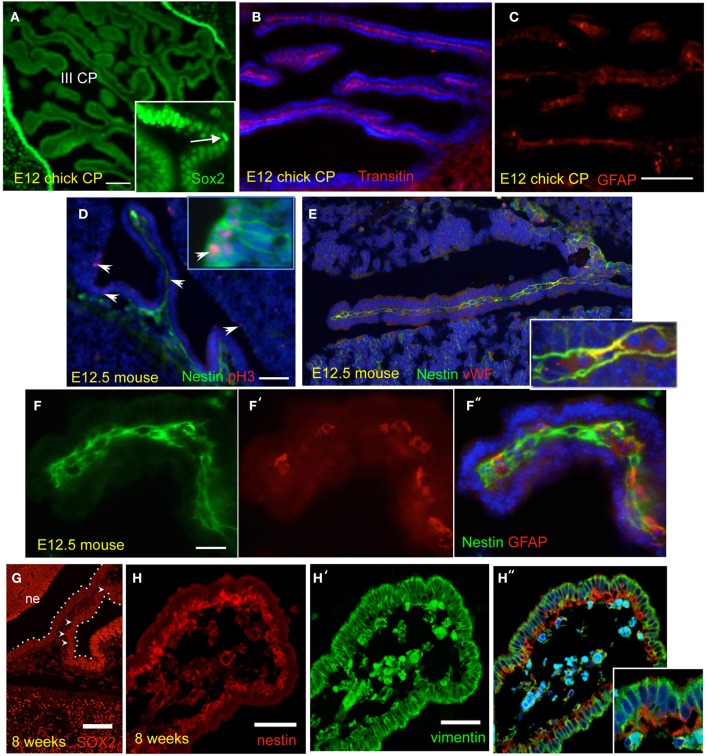
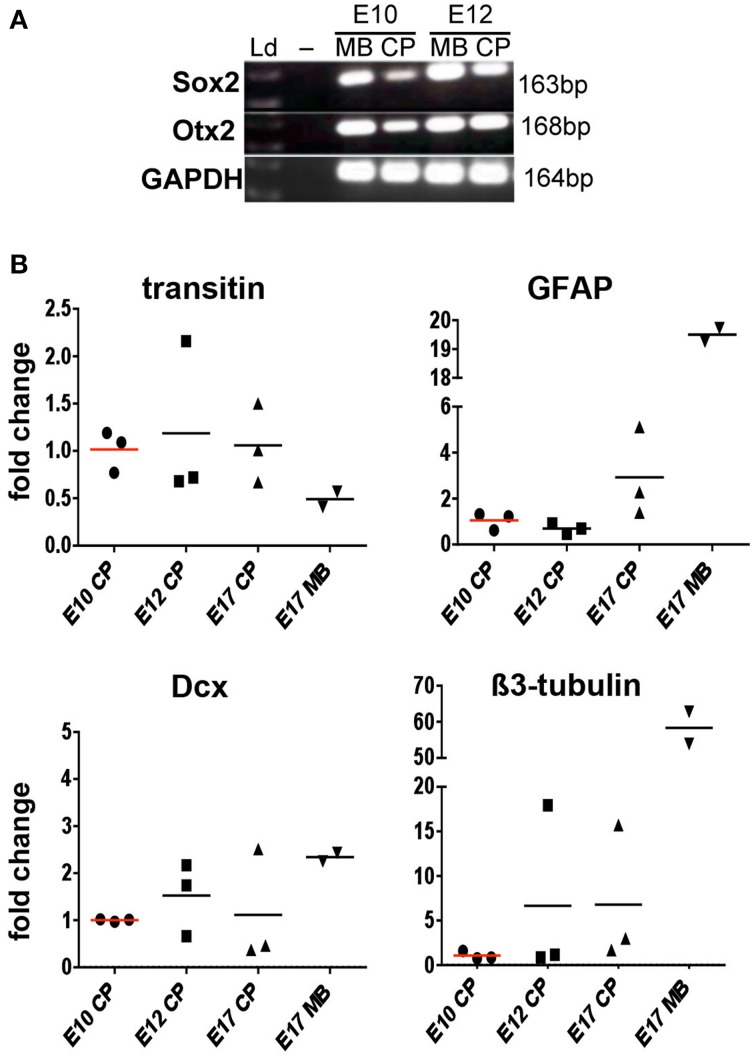
Expression of markers of neural stem/progenitor cells, such as Sox2 and nestin, in the CP was first studied by fluorescent immunohistochemistry in sections from E12 chick brains (Figure 1 and Table 3). In the E12 CP, cells positive for the neural progenitor marker, Sox2, were detected in the CP epithelium of both lateral and 3rd ventricles (Figure 1A). The staining intensity in the CP was lower than in the neuroepithelium and a gradient of Sox2 expression, from high, in the neuroepithelium, to low, in the CP epithelium, was apparent (Figure 1A, Table 3). Interestingly, Sox2 expression in cells located at presumed CP branching points was stronger than in adjacent cells (see insert Figure 1A). The early CP epithelium was also positive for Otx2 and Pax6, and for several other markers that have been reported to be expressed in neural stem cells (Table 3). In contrast to the CP epithelium, the CP stroma was Sox2-negative. However, the CP mesenchyme contained cell expressing other neural markers, transitin, the chick nestin-like protein, and GFAP, a marker of astrocytes, radial glia and neural stem cells (Figures 1B,C). Expression of neural progenitor-associated proteins in the chick CP was consistent with expression of their transcripts detected by RT-PCR (Figure 2).
Figure 1.
Neural progenitor markers are expressed in the developing choroid plexus (CP), in different species. Unless otherwise indicated, micrographs show the CP of lateral ventricles. (A–C) E12 Chick CP stained for Sox2, transitin, and GFAP. III CP: third ventricle CP. (D–F) E12.5 mouse CP stained for pH3 (phosphorylated-histone 3), nestin, von Willebrand factor, and GFAP, either alone or in combination. Arrowheads in (D) point at proliferating cells in the CP and in the neuropepithelium (insert); only the CP is shown in (F). (G,H) Human CP at 8 weeks of gestation (CS23) stained for Sox2, nestin, and vimentin. The CP is outlined and some of the brighter SOX2-positve cells in the CP are indicated by arrowheads; note the gradient of SOX2 staining in the CP; ne, neuroepithelium. Nuclei are counterstained with Hoechst dye (blue). Scale bars are: (A) = 50 μm; (B,C) (same magnification) = 100 μm; (D,E) (same magnification) = 100 μm; (F) = 100 μm; (G) = 100 μm; (H) = 50 μm.
Table 3.
Summary of protein expression in the developing chick choroid plexus.
| Stroma | Epithelium | |||
|---|---|---|---|---|
| E12 | E19/20 | E12 | E19/20 | |
| NEURAL STEM/PROGENITOR CELL | ||||
| Sox2 | – | nd | ✓ | nd |
| Otx2 | – | – | ✓ | ✓ |
| Vimentin* | ✓ (low) | ✓ (low) | ✓ (high) | ✓ (high) |
| Transitin | ✓ | √ | – | – |
| GFAP | ✓ | ✓ | – | – |
| Cytokeratin | - | nd | ✓ | nd |
| Doublecortin | ✓ | ✓ | – | – |
| NEURONAL | ||||
| ß3-tubulin | ✓ | ✓ | – | – |
| Neurofilament | ✓ | ✓ | – | – |
| Synaptic vesicles (SV2) | ✓ | ✓ | – | – |
| Tyrosine hydroxylase | ✓ | nd | – | nd |
| TIGHT JUNCTION PROTEINS | ||||
| ZO-1 | – | – | ✓ | ✓ |
| ASPP2 | ✓ | ✓ | ✓ | ✓ |
| Connexin-34 | – | – | ✓ | ✓ |
| OTHERS | ||||
| Laminin | ✓ | nd | – | nd |
| Aquaporin-1 | – | nd | ✓ | nd |
| Clusterin* | ✓ (low) | ✓ (low) | ✓ (high) | ✓ (high) |
–, negative; nd, not done;
particularly low levels of expression in the CP stroma and high levels of expression in the CP epithelium are indicated.
Figure 2.
Expression of neural transcripts in the chick lateral ventricle choroid plexus (CP) at E10, E12, and E17 detected by RT-PCR and RT-qPCR. (A) Detection of Sox2 and Otx2 by RT-PCR in the CP and midbrain (MB, positive control). (B) Detection of transitin, GFAP, Dcx, and β3-tubulin by RT-qPCR. Fold changes in individual CPs or MB normalized against GAPDH are shown. Note that transitin, GFAP, Dcx, and β3-tubulin transcripts are detected in the CP at all developmental stages examined; no statistically significant differences in expression levels are observed.
In order to establish whether neural progenitor-like cells were present only in the chick or also in mammals, we studied their expression in mouse CP at embryonic day 12.5 (E12.5) and in the Cs23 (56 days of gestation) human CP. Nestin and GFAP protein expression was found in the stromal compartment of the mouse CP paralleling that in the chick (Figures 1D,E). Proliferating nestin-positive cells were observed in the CP as well as in the neuroepithelium (Figure 1D). Expression of nestin and GFAP appeared to be mutually exclusive (Figure 1F), but some overlap of nestin immunoreactivity with an endothelial marker, the von Willebrand factor, was observed suggesting expression of nestin in some endothelial cells (Figure 1E). The developing human CP at Cs23 also displayed a gradient of SOX2 staining, as observed in the chick, and was also positive for vimentin and nestin (Figures 1G,H). Nestin staining in the human CP appeared to be sub-epithelial and vimentin expression was very high in the CP epithelium but not restricted to it (Figure 1H). This paralleled the vimentin pattern of expression observed in the chick CP (Table 3). In mouse CP at comparable stages of development, vimentin was detected in the CP stroma (not shown).
Analysis of neurogenesis in the developing chick choroid plexus (CP)
Given the expression of several neural markers observed in the CP, we investigated whether neurons were generated within the CP focusing on the chick CP.
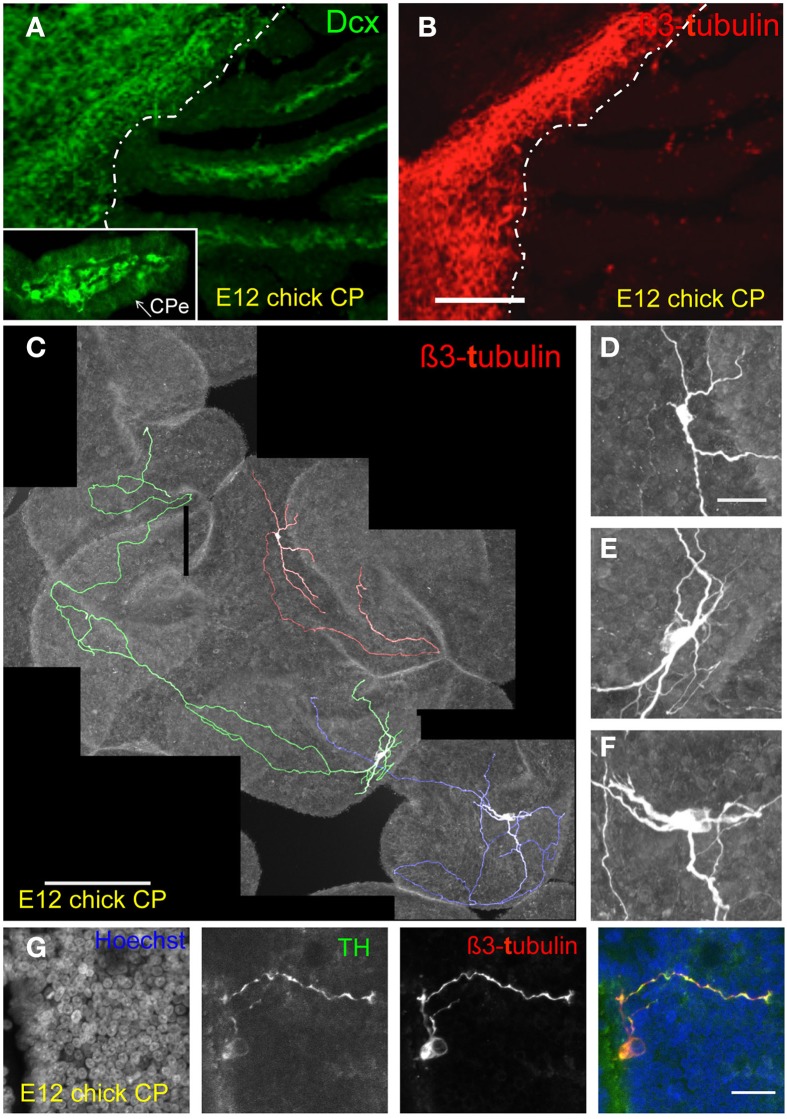
E12 chick lateral ventricle CPs were immunostained for the neuroblast marker, doublecourtin (Dcx), and the neuronal marker, β3-tubulin. Extensive Dcx reactivity was detected even at a low magnification within the CP mesenchyme. Some scattered β3-tubulin reactivity was observed in the E12 CP sections (Figures 3A,B). Both Dcx and β3-tubulin transcripts were detected in the CP (Figure 2). The presence of cells double-labeled for Dcx and β3-tubulin was also consistent with birth of neurons within the CP (Supplementary Figure 1). As it was difficult to visualize what the anti-β3-tubulin antibody staining in CP sections, particularly at later stages of development when the CPs are highly branched, most of the subsequent analysis was carried out in whole mount CP preparations. Analysis of whole mount E12 CPs stained for β3-tubulin clearly showed the presence of β3-tubulin-positive cells with extensive branching and a variety of neuronal morphologies (Figures 3 C–F, Movie 1).
Figure 3.
Identification of neuroblasts and neurons in the developing chick CP. (A,B) E12 lateral CP section stained for doublecortin (Dcx) and ß3-tubulin in E12 chick CP. Note Dcx reactivity in the CP stroma (insert in A) as well as in the brain; only some punctuated staining is observed in the CP with the anti-ß3-tubulin antibody whereas the brain tissue is strongly labeled. The approximate boundary between brain and CP is indicated by the dotted line. CPe, CP epithelium. (C–F) Examples of extensive branching (individual neurons in (C) are shown in different colors for ease of visualization) and neuronal morphologies detected by ß3-tubulin staining. (G) Staining of the E12 CP for tyrosine-hydroxylase (TH), ß3-tubulin and Hoechst dye (nuclei) and merged image; note the presence of a positive TH neuron in proximity of the CP epithelium. Scale bars: (A,B) (same magnification) = 200 μm; (C) = 200 μm; (D–F) (same magnification) = 30 μm; (G) = 20 μm.
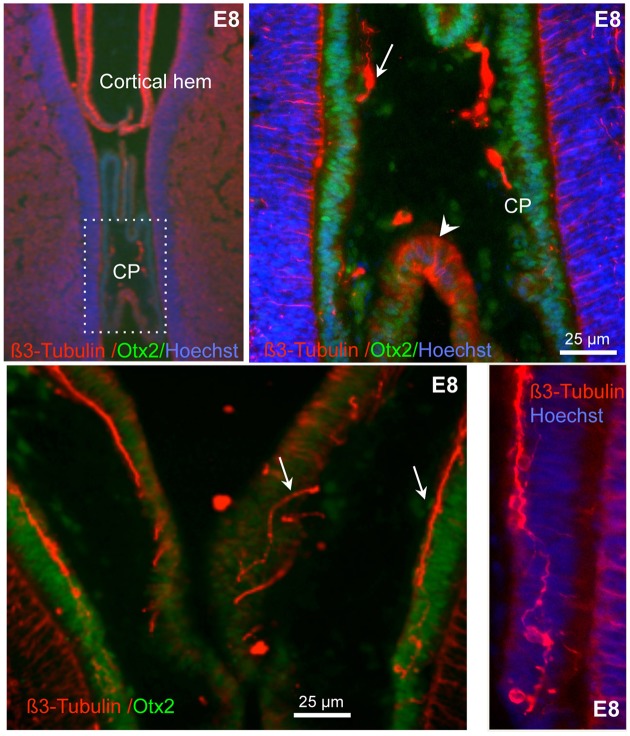
We then wished to establish when these neurons first appeared in the CP and whether they were a transient or a stable feature of the CP. Hence we stained chick CPs for β3-tubulin at E6, E8, E10, E18, E19, and E20 (Figures 4–6, Supplementary Figure 1). We were unable to identify the CP at E6, but at E8 a small CP was clearly visible, and it was found to contain neurons as indicated by β3-tubulin staining and morphological appearance (Figure 4). At this stage β3-tubulin staining was observed also in some cells spanning the CP epithelium and contacting the CSF; some thin β3-tubulin fibers that appeared to project into the CSF were also observed at E8 as well as at later stages when double-labeling for ß3-tubulin and the tight junction marker, ZO-1, was carried out (Figure 4, Movie 2). Furthermore, nerve fibers that appeared to be entering the CP mesenchyme were observed at E8 as well as at E12 (Figures 4, 5).
Figure 4.
Expression of β3-tubulin in E8 chick CP. Sections double-labeled for ß3-tubulin and Otx2, a marker of the CP epithelium. High magnification images are shown in the right panels. Note the presence of nerves and neurons (some indicated by arrows) in the CP as well as of cells spanning across the CP epithelium (arrowhead). Nuclei are counterstained with Hoechst (blue). The bottom right panel is at the same magnification as the one at its left.
Figure 5.
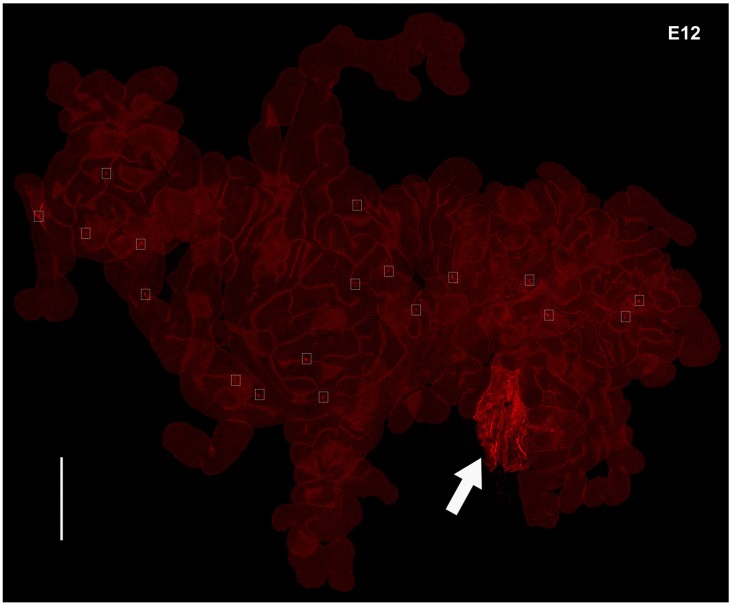
Expression of β3-tubulin in whole mount E12 chick CP. The nerve bundle in the CP stalk is indicated by the arrow and some neurons within the CP are boxed. Scale bars = 500 μm.
The morphology of the CP neurons became more complex with development. At E8 neurons were mainly bipolar, at E10 some were bipolar, some tripolar and other had several branches, and from E12 the neuronal processes appeared to be longer and more convoluted (Figures 3C–F, 4, Movie 1). Staining for tyrosine-hydroxylase at E12 indicated that at least some of the neurons present in the developing CPs are catecholaminergic (Figure 3G, Supplementary Figure 2A). The morphology of some of these neurons resembles the drawing by Clark (1928) shown in Supplementary Figure 2B, though this author interpreted the bulbous structures as nerve terminals.
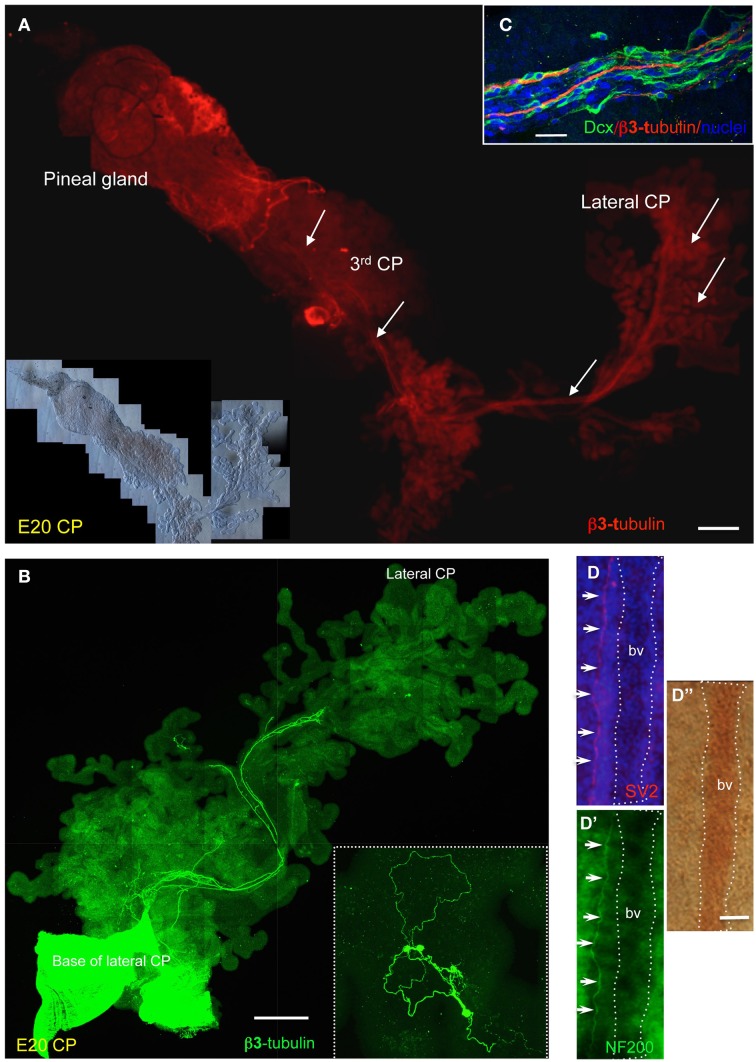
Nerves coursing the length of the CPs were detected at all stages studied and in all CPs, with dense clusters of neurons or fibers located at the base of the lateral ventricle CP and at branching points, where groups of neurons with highly convoluted morphology could be observed (Figures 4, 5, 6A,B). The CP neural fibers did not appear to be myelinated, as staining for myelin basic protein was only detected in the nerve bundle at the base of the CP stalk (Supplementary Figure 3). Dcx-positive cells were still present at late developmental stages, consistent with detection of Dcx mRNA by RT-qPCR (Figure 6C). A high density of nerve terminals/fibers was observed in close proximity to blood vessels, as indicated by staining with the SV2 (synaptic vesicle protein 2) antibody that detects a glycoprotein important for synaptic vesicle function, and NF200; close correspondence between SV2 and NF200 staining could be observed, notwithstanding some background with the NF200 antibody in whole mount CPs (Figure 6D) (Xu and Bajjalieh, 2001).
Figure 6.
Expression of neuronal proteins in chick CPs. (A,B) Examples of β3-tubulin staining in whole mount E20 CPs; note the extensive network connecting lateral ventricle CP, 3rd ventricle CP and pineal gland. The arrows point at some of the neurons observed at high magnification. (C) E19 CP stained for doublecortin (Dcx) and ß3-tubulin; nuclei are counterstained with Hoechst dye. (D–D”) Whole mount E19 chick CP double-labeled for the synaptic vesicle 2 marker, SV2, and NF200 and corresponding bright field image; nuclei are counterstained with Hoechst dye. The dotted line indicates a blood vessel (bv). Scale bars: (A) = 200 μm; (B) = 500 μm; (C,D) = 25 μm.
Whole mount analysis of E20 pineal gland, 3rd and lateral ventricle CPs isolated together revealed an interesting neural network between these structures (Figure 6B). Interconnecting nerve fibers were clearly observed between the pineal gland and the 3rdV CP and between the 3rd and lateral ventricle CPs.
Analysis of choroid plexus (CP) organotypic cultures
Although expression of neural progenitor markers within the CP suggested that the Dcx-positive cells are born within the CP, it could not be ruled out that post-mitotic Dcx-positive neuroblasts had migrated into the CP from other brain regions. In vivo experiments cannot address this issue as BrdU would label both neural precursor cells born and matured in the CP and neuroblasts originating from adjacent brain regions that had migrated into the CP. Hence to address this issue, we set up CP organotypic cultures from E12 chick embryo that allow one to specifically study events occurring within the CP. As indicated by MTT metabolic assay, PI (Figures 7A–C) and Otx2 (not shown), the E12 organotypic CP cultures showed very good viability and maintenance of the CP phenotype over 7 days in culture. After 3 days labeling with BrdU, the CPs were stained either for BrdU and Dcx or BrdU and NF200 (Figures 7D–E). BrdU-positive cells were observed in the CP stroma, and some were found to be Dcx-positive; also a few BrdU and NF200 positive cells were observed. This suggests that the neuroblasts had been born within the CP.
Figure 7.
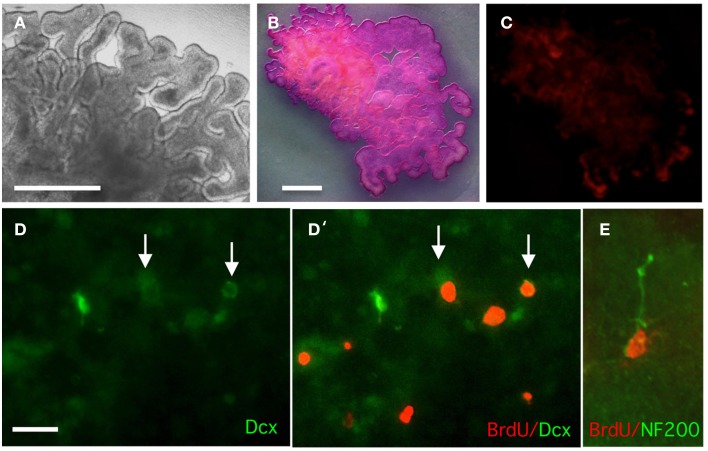
Analysis of survival, proliferation, and doublecortin (Dcx) expression in organotypic cultures of E12 lateral CP. (A) Phase image of CP after 7 days in culture. (B) MTT staining of live CP after 7 days in culture; extensive staining is indicative cell viability. (C) Propidium iodide staining of live CP after 7 days in culture to detect dead cells; only very limited staining is observed. (D,D') CP organotypic culture treated with BrdU for 3 days and double-stained for BrdU and doublecortin (Dcx). Some double-labeled cells are observed. (E) Detection of NF200 and BrdU in an organotypic culture. Scale bars: (A–C) = 200 μm (B and C are at the same magnification); (D,E) = 50 μm.
Discussion
This study shows that neurons are present from early stages of CP development and that they appear to originate from progenitor cells within the CP. Furthermore, it challenges the view that the CPs become innervated only postnatally, by clearly demonstrating innervation of the CP at early developmental stages.
Proteins normally associated with neural progenitors are expressed in the immature developing CP
We have shown that the developing chick CPs express markers that are found in the neuroepithelium and/or radial glial cells of the developing central nervous system. Markers expressed in the neuroepithelium at early stages of development, including Sox2, vimentin, and ASPP2 (Bennett and Dilullo, 1985; Uwanogho et al., 1995; Sottocornola et al., 2010), have been found to be expressed in the epithelium of chick CPs. Expression of Sox2 in the prenatal mouse CP epithelium as well as in a subset of CP epithelial cells in the adult mouse has been previously reported (Ferri et al., 2004). This, together with our observations in chick and human CPs, indicates that expression of Sox2 in the CP is a feature conserved across species. Interestingly, whereas Sox2 staining intensity in both chick and human embryonic CPs is overall lower than in the neuroepithelium, some strongly Sox2-positive cells, mainly localized at CP branching points, are present. Differences in Sox2 levels have also been observed in the developing mouse telencephalon, with “high Sox2” radial glial cells displaying higher neurosphere forming ability, growth rate, and self-renewal capability than “low Sox2” intermediate progenitor cells (Hutton and Pevny, 2011). To establish whether the “high-Sox2” cells within the CPs are landmarks important for CP branching morphogenesis, and/or represent a neural stem cells population from which nestin and/or GFAP-positive cells in the stroma originate is an important and challenging question that will require extensive investigation.
In contrast to Sox2 and vimentin, the chick nestin-like protein, transitin, expressed in early radial glial cells that have neuronal differentiation potential (Kriegstein and Alvarez-Buylla, 2009), was not detected in the chick CP epithelium, but only in the mesenchyme. This was the case also for nestin in mouse and human CPs, where the nestin positive cells were located just beneath the CP epithelium as reported in neonatal rats (Huang et al., 2011). Cells expressing GFAP, another protein expressed in neural stem cells as well as in astrocytes, were found in the mesenchymal compartment of the chick CPs. Because of antibody species of origin, we could not double stain the chick CP for transitin and GFAP. However, double-staining of mouse E12.5 CPs for nestin and GFAP indicated that at least in this species the developing CP contains two distinct populations of “neural” cells in the stroma, identified by nestin and GFAP reactivity. Consistent with population(s) of neural progenitors within the CP was the presence of cells expressing the neuroblast marker, Dcx. The finding that BrdU incorporation in Dcx-positive cells was observed in isolated CPs, supports the view that the neurons present in the CP are born within the CP itself and originate from neural progenitors, rather than having migrated from the neighboring neuroepithelium. This is consistent with previous studies suggesting that cells isolated from the CP have neural potential, as they could form neurosphere-like structures and differentiated into neuronal-like cells (Itokazu et al., 2006).
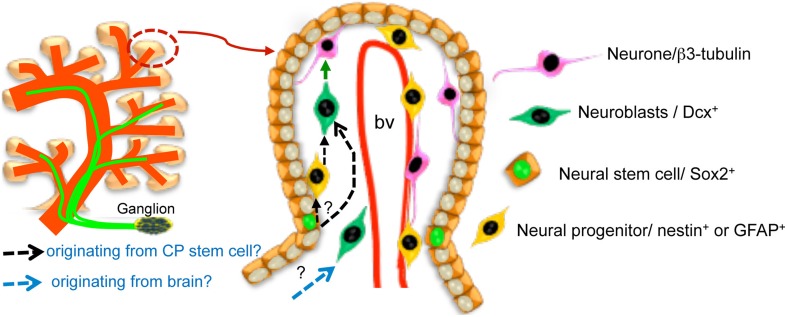
The presence of subsets of highly Sox2-positive cells in the CP epithelium, that is also vimentin-positive, and of nestin-positive cells beneath it, raises the possibility, summarized in Figure 8, that the CP may be akin to the neurovascular niche in the subventricular region, where the neural stem cells reside close to the ependyma, in contact with blood vessels and extend their primary cilia into the CSF of the brain ventricles (Doetsch et al., 1997).
Figure 8.
Schematic representation of the CP neural components (innervation from ganglia outside the CP and neurons within the CP) and of the model proposing the existence of a neurovascular niche within the developing chick CP. It is not currently known whether nestin-like-positive cells reflect a transition from high-Sox2/vimentin-positive cells to neuroblasts (black dotted arrows) nor whether some of the CP neurons are born from neuroblasts migrating into the CP from the brain rather than having been generated within it (blue dotted arrow). Please note that only the subset of highly Sox2-positive putative progenitors in the CP epithelium is indicated in the cartoon. The two sources are not mutually exclusive. bv, blood vessel.
Neurogenesis occurs within the developing CP
We have demonstrated for the first time that single neurons reside within the chick CP stroma from early stages of development. We have also shown that at least some of the neurons present in the developing CPs are catecholaminergic as indicated by co-expression of tyrosine-hydroxylase and β3-tubulin. This is consistent with the presence of dopamine receptors in the epithelium and smooth muscle cells of the blood vessels of the adult rat CP (Mignini et al., 2000).
Because of the difficulties in carrying out accurate quantification on neurons in whole mounts and variability among animals, we cannot be sure when neurons stop being born in the CP. Analysis of neurons in several preparations, and the fact that Dcx staining was more widespread around E12, would suggest that most neurons are born around this stage of development. However, some neurogenesis appeared to occur also at later stages, as some Dcx-BrdU labeled cells were observed after in vivo BrdU labeling at E15 (not shown).
The morphology of the CP neurons appears to change with development and the complexity of their branching appears to increase with embryonic age. CSF secretion is crucial for brain development and its production is at least in part controlled by the nervous system (Edvinsson and Lindvall, 1978; Lindvall and Owman, 1981). Projection into the CSF of fibers from individual neurons could be detected in some of the chick whole mount preparations, and fibers in very close proximity to the CP epithelium have been detected by light and transmission electron microscopy in adult CPs (Clark, 1928; Edvinsson et al., 1975; Nakamura and Milhorat, 1978). It is conceivable that these neurons act as sensory or autonomic neurons that integrate signals between the CP/CSF and the nerves entering the CP. The presence of numerous CSF-contacting axons has been reported in the ependyma and periventricular organs (Tramu et al., 1983; Michaloudi and Papadopoulos, 1996) and a mechanism for non-synaptic signal transmission in the brain has been proposed (Vigh et al., 2004).
Innervation of the CP occurs pre-natally
The presence of nerves in adult CPs has been described in various species including mice, rats, hedgehogs, guinea-pigs, rabbits, cats, cows, and monkeys; differences in density of innervation were reported in different CPs, with the 3rd ventricle CP being the most densely innervated, and across species, with the lowest density of innervation observed in the mouse CP (Edvinsson et al., 1973; Lindvall et al., 1977a,b, 1978c; Lindvall, 1979). Notwithstanding its complexity, innervation of the CP was believed to occur postnatally (Tsuker, 1947; Lindvall and Owman, 1978). Our data clearly demonstrate that in the chick the nerves enter the CP before birth, apparently as early as at E8. The previously reported lack of evidence for prenatal innervation of the CP is likely due to the fact that it is very difficult to detect nerves, as well as neurons, in CP sections; apart from very early stages of development, clear visualization of CP innervation can be achieved only by using whole mount CP preparations and confocal microscopy; furthermore, nerves are easily pulled out of the CP during dissection, hence this step requires very careful handling. Expression of synaptic vesicle proteins involved in the release of neurotransmitters is indicative of neural activity within the CP well before birth, consistent with a role for innervation in the developing CP. Hence, innervation of the CP during development is in line with that of other secretory structures, such as the salivary glands that become innervated by parasympathetic nerves during organogenesis (Knox et al., 2010). As intestinal epithelial stem/progenitor cells in the crypts are controlled by mucosal afferent nerves (Lundgren et al., 2011), it is conceivable that the nerves might play a role in proliferation and branching of the CP. However, whether during development the nerves' main role is in controlling CSF secretion or also in CP growth remains to be established.
The nerves in the E20 CP did not appear to be myelinated, as indicated by lack of staining for myelin basic protein, and this is consistent with a transmission electron microscopy study of adult rat CP (Edvinsson et al., 1975; Nakamura and Milhorat, 1978).
The nature of CP innervation has been examined in a variety of species including adult rat, guinea-pig, hedgehog, rabbit, cat, and man; noradrenergic and cholinergic innervation, as well as substance P-positive fibers, have been described in adult CPs from different species, whereas the presence of serotoninergic fibers has so far been reported only in the rat and hedgehog CPs (Napoleone et al., 1982; Edvinsson et al., 1983; Nilsson et al., 1990; Michaloudi and Papadopoulos, 1996). However, most of these studies have not investigated comprehensively the presence of different types of fibers and their localization within the same CP. Hence, it will be important to carry out systematic comparative studies on the type of fibers present in developing and adult CP and innervation using whole mount preparations.
Another important finding that has emerged from staining whole mount preparations for β3-tubulin concerns the identification of a neural network between pineal gland, 3rd ventricle CP and lateral ventricle CP. This neural network may be important for development and maintenance of the CP throughout life as suggested in other organs, such as the developing teeth (Luukko et al., 1997), kidney (Tiniakos et al., 2004), pancreas (Burris and Hebrok, 2007), and heart (Hildreth et al., 2009). It is also tempting to speculate that in the developing chick, the neuron cluster between the 3rd and lateral ventricle CPs may serve as a relay station for integrating signals from the CSF and co-ordinating secretory activity throughout the ventricular system.
Together, as schematically summarized in Figure 8, the presence of innervation and of neurons adjacent to blood vessels, or projecting into the ventricles, is consistent with the hypothesis that the ventricular system contains a neural network that resembles to some extent the enteric nervous system and is important for CP development and homeostasis.
Author contributions
WP designed and performed experiments, analyzed data and wrote the manuscript; BV and ZA performed experiments and critical reading of the manuscript; NJ performed experiments; PF planned research, analyzed data, and wrote the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Higher Educational Strategic Scholarship for Frontier Research Network from the Commission on Higher Education, Royal Thai Government and the Great Ormond Street Hospital Children's Charity. The human embryonic material was provided by the Human Developmental Biology Resource (http://hdbr.org) jointly funded by the Medical Research Council (grant G070089) and The Wellcome Trust (grant GR082557).
Glossary
Abbreviations
- Aq-1
aquaporin-1
- ASPP2
apoptosis-stimulating of p53 protein 2
- BrdU
5-bromo-2′-deoxyuridine
- bv
blood vessel
- CP
choroid plexus
- CSF
cerebrospinal fluid
- Dcx
doublecortin
- EBSS
Earle's balanced salt solution
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- HDBR
human developmental biology resource
- ne
neuroepithelium
- NF200
neurofilament 200 kDa
- MB
midbrain
- MBP
myelin basic protein
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Otx2
orthodenticle homeobox 2
- PFA
paraformaldehyde
- pH3
phosphorylated-histone 3
- PI
propidium iodide
- RT-PCR
reverse transcription polymerase chain reaction
- RT-qPCR
quantitative reverse transcription polymerase chain reaction
- Sox2
SRY (sex determining region Y)-box 2
- SV2
synaptic vesicles 2
- TH
tyrosine-hydroxylase
- vWF
von Willebrand factor
- ZO-1
zonula occludens-1.
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fnins.2015.00103/abstract
Neuron detected by ß3tubulin staining (red) in an E12 whole mount chick CP. Nuclei are counterstained with Hoechst dye (blue). Scale bar = 100 μm.
Neuron detected by ß3tubulin staining (red) coursing across the CP epithelium stained by the tight junction protein, ZO-1 (green), in an E12 whole mount chick CP. Nuclei are counterstained with Hoechst dye (blue). Scale bar = 20 μm.
References
- Bennett G. S., Dilullo C. (1985). Transient expression of a neurofilament protein by replicating neuroepithelial cells of the embryonic chick brain. Dev. Biol. 107, 107–127. 10.1016/0012-1606(85)90380-X [DOI] [PubMed] [Google Scholar]
- Burris R. E., Hebrok M. (2007). Pancreatic innervation in mouse development and beta-cell regeneration. Neuroscience 150, 592–602. 10.1016/j.neuroscience.2007.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. L. (1928). Nerve endings inthe choroid plexus of the fourth ventricle. J. Comp. Neurol. 47, 1–21 10.1002/cne.900470102 [DOI] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M., Varez-Buylla A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Ek J., Habgood M. D., Saunders N. R. (2001). Development of the choroid plexus. Microsc. Res. Tech. 52, 5–20. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Hakanson R., Lindvall M., Owman C., Svensson K. G. (1975). Ultrastructural and biochemical evidence for a sympathetic neural influence on the choroid plexus. Exp. Neurol. 48, 241–251. 10.1016/0014-4886(75)90154-5 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Lindvall M. (1978). Autonomic vascular innervation and vasomotor reactivity in the choroid plexus. Exp. Neurol. 62, 394–404. 10.1016/0014-4886(78)90063-8 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Nielsen K. C., Owman C. (1973). Cholinergic innervation of choroid plexus in rabbits and cats. Brain Res. 63, 500–503. 10.1016/0006-8993(73)90132-7 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Rosendal-Helgesen S., Uddman R. (1983). Substance P: localization, concentration and release in cerebral arteries, choroid plexus and dura mater. Cell Tissue Res. 234, 1–7. 10.1007/BF00217397 [DOI] [PubMed] [Google Scholar]
- Ferri A. L., Cavallaro M., Braida D., Di Cristofano A., Canta A., Vezzani A., et al. (2004). Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131, 3805–3819. 10.1242/dev.01204 [DOI] [PubMed] [Google Scholar]
- Hildreth V., Anderson R. H., Henderson D. J. (2009). Autonomic innervation of the developing heart: origins and function. Clin. Anat. 22, 36–46. 10.1002/ca.20695 [DOI] [PubMed] [Google Scholar]
- Huang S. L., Shi W., Jiao Q., He X. J. (2011). Change of neural stem cells in the choroid plexuses of developing rat. Int. J. Neurosci. 121, 310–315. 10.3109/00207454.2011.556282 [DOI] [PubMed] [Google Scholar]
- Hunter N. L., Dymecki S. M. (2007). Molecularly and temporally separable lineages comprise the hindbrain roof plate and contribute differentially to the choroid plexus. Development 134, 3449–3460. 10.1242/dev.003095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton S. R., Pevny L. H. (2011). SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal telencephalon. Dev. Biol. 352, 40–47. 10.1016/j.ydbio.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Itokazu Y., Kitada M., Dezawa M., Mizoguchi A., Matsumoto N., Shimizu A., et al. (2006). Choroid plexus ependymal cells host neural progenitor cells in the rat. Glia 53, 32–42. 10.1002/glia.20255 [DOI] [PubMed] [Google Scholar]
- Johansson P. A., Dziegielewska K. M., Liddelow S. A., Saunders N. R. (2008). The blood-CSF barrier explained: when development is not immaturity. Bioessays 30, 237–248. 10.1002/bies.20718 [DOI] [PubMed] [Google Scholar]
- Kitada M., Chakrabortty S., Matsumoto N., Taketomi M., Ide C. (2001). Differentiation of choroid plexus ependymal cells into astrocytes after grafting into the pre-lesioned spinal cord in mice. Glia 36, 364–374. 10.1002/glia.1123 [DOI] [PubMed] [Google Scholar]
- Knox S. M., Lombaert I. M., Reed X., Vitale-Cross L., Gutkind J. S., Hoffman M. P. (2010). Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329, 1645–1647. 10.1126/science.1192046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A., Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen M. K., Bjornsson C. S., Dymecki S. M., Gilbertson R. J., Holtzman D. M., Monuki E. S. (2013). The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J. Neurosci. 33, 17553–17559. 10.1523/JNEUROSCI.3258-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Dziegielewska K. M., Ek C. J., Habgood M. D., Bauer H., Bauer H. C., et al. (2013). Mechanisms that determine the internal environment of the developing brain: a transcriptomic, functional and ultrastructural approach. PLoS ONE 8:e65629. 10.1371/journal.pone.0065629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Temple S., Mollgard K., Gehwolf R., Wagner A., Bauer H., et al. (2012). Molecular characterisation of transport mechanisms at the developing mouse blood-CSF interface: a transcriptome approach. PLoS ONE 7:e33554. 10.1371/journal.pone.0033554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall M. (1979). Fluorescence histochemical study on regional differences in the sympathetic nerve supply of the choroid plexus from various laboratory animals. Cell Tissue Res. 198, 261–267. 10.1007/BF00232009 [DOI] [PubMed] [Google Scholar]
- Lindvall M., Alumets J., Edvinsson L., Fahrenkrug J., Hakanson R., Hanko J., et al. (1978a). Peptidergic (VIP) nerves in the mammalian choroid plexus. Neurosci. Lett. 9, 77–82. 10.1016/0304-3940(78)90051-4 [DOI] [PubMed] [Google Scholar]
- Lindvall M., Edvinsson L., Owman C. (1977a). Histochemical study on regional differences in the cholinergic nerve supply of the choroid plexus from various laboratory animals. Exp. Neurol. 55, 152–159. 10.1016/0014-4886(77)90166-2 [DOI] [PubMed] [Google Scholar]
- Lindvall M., Edvinsson L., Owman C. (1977b). Histochemical, ultrastructural and functional evidence for a neurogenic control of cerebrospinal fluid production from the choroid plexus. Acta Physiol. Scand. Suppl. 452, 77–86. [PubMed] [Google Scholar]
- Lindvall M., Edvinsson L., Owman C. (1978b). Histochemical, ultrastructural, and functional evidence for a neurogenic control of CSF production from the choroid plexus. Adv. Neurol. 20, 111–120. [PubMed] [Google Scholar]
- Lindvall M., Edvinsson L., Owman C. (1978c). Sympathetic nervous control of cerebrospinal fluid production from the choroid plexus. Science 201, 176–178. 10.1126/science.663649 [DOI] [PubMed] [Google Scholar]
- Lindvall M., Owman C. (1978). Early development of noradrenaline-containing sympathetic nerves in the choroid plexus system of the rabbit. Cell Tissue Res. 192, 195–203. 10.1007/BF00220738 [DOI] [PubMed] [Google Scholar]
- Lindvall M., Owman C. (1981). Autonomic nerves in the mammalian choroid plexus and their influence on the formation of cerebrospinal fluid. J. Cereb. Blood Flow Metab. 1, 245–266. 10.1038/jcbfm.1981.30 [DOI] [PubMed] [Google Scholar]
- Lundgren O., Jodal M., Jansson M., Ryberg A. T., Svensson L. (2011). Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS ONE 6:e16295. 10.1371/journal.pone.0016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukko K., Arumae U., Karavanov A., Moshnyakov M., Sainio K., Sariola H., et al. (1997). Neurotrophin mRNA expression in the developing tooth suggests multiple roles in innervation and organogenesis. Dev. Dyn. 210, 117–129. [DOI] [PubMed] [Google Scholar]
- Marques F., Sousa J. C., Coppola G., Gao F., Puga R., Brentani H., et al. (2011). Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS 8:10. 10.1186/2045-8118-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloudi H. C., Papadopoulos G. C. (1996). Catecholaminergic and serotoninergic fibres innervate the ventricular system of the hedgehog CNS. J. Anat. 189(Pt 2), 273–283. [PMC free article] [PubMed] [Google Scholar]
- Mignini F., Bronzetti E., Felici L., Ricci A., Sabbatini M., Tayebati S. K., et al. (2000). Dopamine receptor immunohistochemistry in the rat choroid plexus. J. Auton. Pharmacol. 20, 325–332. 10.1046/j.1365-2680.2000.00198.x [DOI] [PubMed] [Google Scholar]
- Nakamura S., Milhorat T. H. (1978). Nerve endings in the choroid plexus of the fourth ventricle of the rat: electron microscopic study. Brain Res. 153, 285–293. 10.1016/0006-8993(78)90407-9 [DOI] [PubMed] [Google Scholar]
- Napoleone P., Sancesario G., Amenta F. (1982). Indoleaminergic innervation of rat choroid plexus: a fluorescence histochemical study. Neurosci. Lett. 34, 143–147. 10.1016/0304-3940(82)90166-5 [DOI] [PubMed] [Google Scholar]
- Nataf S., Strazielle N., Hatterer E., Mouchiroud G., Belin M. F., Ghersi-Egea J. F. (2006). Rat choroid plexuses contain myeloid progenitors capable of differentiation toward macrophage or dendritic cell phenotypes. Glia 54, 160–171 10.1002/glia.20373 [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. (1980). beta-Adrenergic-sensitive adenylate cyclase in choroid plexus: properties and cellular localization. Mol. Pharmacol. 18, 199–209. [PubMed] [Google Scholar]
- Nilsson C., Ekman R., Lindvall-Axelsson M., Owman C. (1990). Distribution of peptidergic nerves in the choroid plexus, focusing on coexistence of neuropeptide Y, vasoactive intestinal polypeptide and peptide histidine isoleucine. Regul. Pept. 27, 11–26. 10.1016/0167-0115(90)90201-7 [DOI] [PubMed] [Google Scholar]
- Rasband W. (1997-2009). ImageJ. Bethesda, MD: U. S. National Institutes of Health; Available online at: http://rsb.info.nih.gov/ij [Google Scholar]
- Smith D. E. (1966). Morphological changes occurring in the developing chick choroid plexus. J. Comp. Neurol. 127, 381–388. 10.1002/cne.901270306 [DOI] [PubMed] [Google Scholar]
- Sottocornola R., Royer C., Vives V., Tordella L., Zhong S., Wang Y., et al. (2010). ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev. Cell 19, 126–137. 10.1016/j.devcel.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Stastny F., Rychter Z. (1976). Quantitative development of choroid plexuses in chick embryo cerebral ventricles. Acta Neurol. Scand. 53, 251–259. 10.1111/j.1600-0404.1976.tb04346.x [DOI] [PubMed] [Google Scholar]
- Tiniakos D., Anagnostou V., Stavrakis S., Karandrea D., Agapitos E., Kittas C. (2004). Ontogeny of intrinsic innervation in the human kidney. Anat. Embryol. (Berl) 209, 41–47. 10.1007/s00429-004-0420-3 [DOI] [PubMed] [Google Scholar]
- Tramu G., Pillez A., Leonardelli J. (1983). Serotonin axons of the ependyma and circumventricular organs in the forebrain of the guinea pig. An immunohistochemical study. Cell Tissue Res. 228, 297–311. 10.1007/BF00204880 [DOI] [PubMed] [Google Scholar]
- Tsuker M. (1947). Innervation of the choroid plexus. Arch. Neurol. Psychiatry 58, 474–483. 10.1001/archneurpsyc.1947.02300330086007 [DOI] [PubMed] [Google Scholar]
- Uwanogho D., Rex M., Cartwright E. J., Pearl G., Healy C., Scotting P. J., et al. (1995). Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech. Dev. 49, 23–36. 10.1016/0925-4773(94)00299-3 [DOI] [PubMed] [Google Scholar]
- Vigh B., Manzano e Silva M. J., Frank C. L., Vincze C., Czirok S. J., Szabo A., et al. (2004). The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histol. Histopathol. 19, 607–628. Available online at: http://www.hh.um.es/pdf/Vol_19/19_2/Vigh-19-607-628-2004.pdf [DOI] [PubMed] [Google Scholar]
- Xu T., Bajjalieh S. M. (2001). SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat. Cell Biol. 3, 691–698. 10.1038/35087000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuron detected by ß3tubulin staining (red) in an E12 whole mount chick CP. Nuclei are counterstained with Hoechst dye (blue). Scale bar = 100 μm.
Neuron detected by ß3tubulin staining (red) coursing across the CP epithelium stained by the tight junction protein, ZO-1 (green), in an E12 whole mount chick CP. Nuclei are counterstained with Hoechst dye (blue). Scale bar = 20 μm.