Abstract
Background
Migrant populations such as mine workers contributed to the spread of HIV in sub-Saharan Africa. We used a mathematical model to estimate the community-wide impact of targeting treatment and prevention to male migrants.
Methods
We augmented an individual-based network model, EMOD-HIV v0.8, to include an age-dependent propensity for males to migrate. Migrants were exposed to HIV outside their home community, but continued to participate in HIV transmission in the community during periodic visits.
Results
Migrant-targeted interventions would have been transformative in the 1980s to 1990s, but post-2015 impacts were more modest. When targetable migrants comprised 2% of adult males, workplace HIV prevention averted 3.5% of community-wide infections over 20 years. Targeted treatment averted 8.5% of all-cause deaths among migrants. When migrants comprised 10% of males, workplace prevention averted 16.2% of infections in the community, one-quarter of which were among migrants. Workplace prevention and treatment acted synergistically, averting 17.1% of community infections and 11.6% of deaths among migrants. These estimates do not include prevention of secondary spread of HIV or tuberculosis at the workplace.
Conclusions
Though cost-effective, targeting migrants cannot collapse generalized epidemics in their home communities. Such a strategy would only have been possible prior to the early 1990s. However, migrant-targeted interventions synergize with general-population expansion of HIV services.
Keywords: Epidemiology, HIV/AIDS, HIV prevention, Migration, Modeling, Southern Africa
Introduction
A majority of the world's HIV infections occur in generalized epidemic countries in sub-Saharan Africa, where HIV transmission is thought to be sustained by sexual behavior in the general population and would persist despite effective programs for high-risk sub-populations.1 In contrast, concentrated epidemics are those in which high-risk sub-populations are essential to sustaining transmission. In India, programs targeting sex workers have successfully reduced the spread of HIV in the general population.2 Buoyed by these signs of success in India, HIV prevention researchers have begun to ask whether such approaches could be translated to generalized epidemic settings, where efficient strategies for HIV prevention are desperately needed.
Recent studies have underscored the continuing role of concentrated epidemics embedded within generalized epidemics3 based on behavioral,4,5 biological6,7 and spatial8 heterogeneities in HIV transmission seen in sub-Saharan Africa well after aggregate HIV incidence rates have declined from their peak. These findings raise hope that focusing HIV interventions to those most at risk of propagating infections could produce a disproportionately large reduction in HIV incidence in the general population.9,10 A sub-population of interest for such strategies is migrant laborers.11 The patterns of migration with periodic visits to the home community, dubbed circular or oscillating migration, contributed to the early spread of HIV.12,13
In this analysis, we chose to focus on migrant mine workers as a potential target group for intensified HIV services. Mine workers have been identified as an important group to target with HIV prevention and treatment for multiple reasons. Migration itself is associated with elevated HIV prevalence among migrant individuals14,15 and their non-migrant partners16–18 even after HIV had spread in their home communities. In addition, migrant mine workers are at especially high risk of AIDS because of TB associated with dense housing and work conditions in mines. Further, mine workers are predominantly male, and in the era of widespread availability of antiretroviral therapy (ART), males have lagged behind females in access to HIV testing and ART.19 Migration of these males adds even greater barriers to continuity of care.
Increased private-sector involvement in HIV care in the mining sector20 speaks to the feasibility of intensifying HIV services for migrant mine workers beyond the scope of available public health care. A recent analysis of coal mining in South Africa revealed that providing HIV testing and treatment services for employees was cost-saving for coal companies due to reduced absenteeism and employee turnover.21 Because of the precedent for workplace HIV programs for mine workers22 and the ongoing research on prevention of HIV and TB for mine workers,23 we designed our model scenarios around the relevant target population of male migrant mine workers.
We used an individual-based network model of HIV transmission in rural South Africa to estimate the impact of targeting HIV prevention and treatment to male migrant workers on HIV incidence and mortality in the migrant population and the rural community from which they migrate. Though the term ‘migration’ is used for brevity, this study focuses on mine migration by males who comprise less than 1% of the South African resident population.24 In Lesotho, mine workers comprised approximately 10% of the adult population in the mid-1990s, most of whom were international migrants working in South Africa's gold mines,25 but the number of mine workers has since declined to less than 5% of the adult population,26 paralleling contraction of the gold mining industry.
Methods
We augmented an individual-based network model, EMOD-HIV v0.8, to include a migrant population that imports the initial source of HIV infections that initiate simulated epidemic. The patterns of heterosexual partnerships used to construct the contact network were modeled after a rural hyperendemic setting in KwaZulu-Natal, South Africa.27 The model was calibrated to the South African national HIV epidemics, including age- and gender-disaggregated HIV prevalence, population size, distribution of CD4 count at treatment initiation, and proportion of the population tested for HIV. The baseline scenario (without targeting of services to migrants) includes ART expansion as well as HIV prevention services such as male circumcision and provision of condoms. Model assumptions, parameter values, data sources, and methods of model fitting are available as open-access publications28–31 and model assumptions, parameters, and sources most relevant to this study are listed in Supplementary Table 1. The baseline model trajectories and projections for general-population treatment scale-up have been compared to those of 11 other mathematical models of HIV in South Africa as part of a series of projects by the HIV Modelling Consortium.32,33
EMOD-HIV v0.8 was modified in several ways to enable the analysis described here. First, a configurable percentage of males in the model were labeled as eligible to become migrants, as illustrated in Figure 1. These males began to exhibit migratory behavior at a specified age, which was configured to match the age distribution of migrants observed in a rural community in South Africa.34 The age to begin migration was chosen randomly for each migrant-eligible individual from the distribution shown in Figure 2A. Because not all males began migration at age 15, the proportion of adult males migrating at any time was approximately half the percentage of males who would eventually begin migration (Figure 2B). For example, if the model was configured so that 20% of males would eventually migrate sometime after the age of 15, approximately 10% of males age ≥15 would be migratory at a given time, because some wait until older ages to begin migration. The proportion migrating fluctuated over the course of the epidemic due to elevated AIDS mortality among migrants, followed by reduced mortality due to increasing ART availability.
Figure 1.
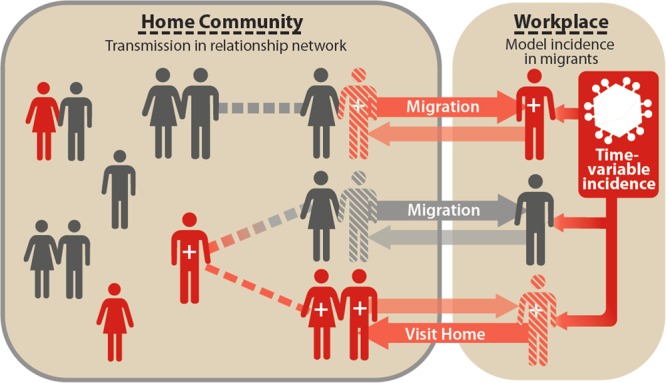
Schematic of migration in the EMOD-HIV v0.8 individual-based model. The network of sexual contacts (dashed lines) is explicitly modeled for the home community (left box encircled by gray line), whereas HIV acquisition at the workplace (right box) is represented as an external incidence rate (dark box with virus symbol). Whether infected at home or in the workplace, the model tracks the HIV status of individual migrants (dark: infected, light: uninfected) as well as their current location (horizontal arrows). Migrants can potentially infect home partners with HIV, and vice versa, during visits home (cross-hatched figure at workplace location). Visits were assumed to occur monthly for 3 days per visit, but in sensitivity analysis we made visits perpetual (without reducing externally-acquired infections) to test the maximum possible role of migrants. When at the workplace location (cross-hatched figure at the home location), migrants do not expose or acquire HIV from home partners. This figure is available in black and white in print and in color at International Health online.
Figure 2.
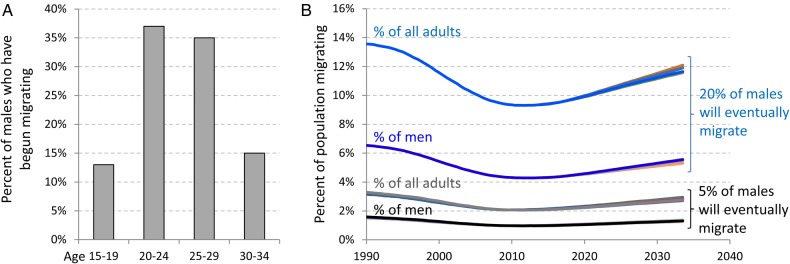
Age at the start of migration and proportion of adults who are targetable migrants in the EMOD-HIV v0.8 model. (A) Histogram showing the age distribution at which males began mining, according to the age distribution of migrants in Agincourt, South Africa.34 A fixed proportion of males (5 or 20%) were labeled as destined to migrate, and the age at which their migratory behavior began was sampled from this histogram. (B) Because males had to reach the specified age to begin migrating, the proportion of males actively migrating was approximately half of the proportion of males who would eventually migrate (dark curves). The proportion fluctuates over the course of the epidemic due to elevated AIDS mortality among migrants, followed by reduced mortality due to increasing ART availability. Including adult women in the denominator halved the proportion the proportion, so that the proportion of all adults who were migrating was one-quarter the proportion of males who would migrate, and fluctuated with the same trend (light curves). The intervention scenario had little impact on the proportion migrating (underlaid lines). This figure is available in black and white in print and in color at International Health online.
Migrants spent time at the home community and at their workplace location. The network of sexual partnerships in the community was only explicitly modeled for the home location; to represent the workplace location, the migrants were exposed to a configurable and time-variable incidence rate. This external incidence rate was allowed to vary over time to modulate the timing of introduction of HIV into the population, and was held at 1.2% per person-year after 2006 unless altered by the workplace prevention intervention.
We simulated two hypothetical interventions targeted to male migrant workers: primary prevention directed at sexual contacts at the workplace location, and secondary prevention in the form of antiretroviral treatment for infected migrants (which also provides a direct health benefit to the recipients). Primary prevention was operationalized in the model by removing the source of externally-acquired HIV incidence. Because the relationships and risk behaviors outside the home community were not modeled explicitly, this approach does not specify which modality of primary prevention would be used, e.g., behavior change, condoms, STI prevention, circumcision, and/or HIV prophylaxis.
The external source of HIV incidence in migrants constituted the sole source of initial HIV infections in the model, initiating the early HIV epidemic. The true distribution of the sources of initial HIV infections introduced into rural communities is not known, but ours was an extreme assumption made to maximize the hypothetical impact of migrant-targeted interventions on the home community. As we will see, the impact on the home community is modest, so pushing this assumption to its extreme provides confidence that our results are robust to unknowns such as the proportion of infections brought into the community by the specific sub-population targeted with intensified HIV services.
The rates of relationship formation and dissolution of migrants not present in the community were assumed to be the same as for non-migrants. However, migrants were assumed to put their home partners at risk only while visiting the home community. Visits were assumed to occur monthly and last three days. This strategy for simulating migrants is illustrated in Figure 1. Active relationships in the home community were consummated at least once and an average of two times per home visit. In sensitivity analysis, we experimented with lengthening the duration of home visits without attenuating the rate of incidence coming from the workplace.
The baseline EMOD-HIV v0.8 model includes the possibility for relationship concurrency.29,30 Briefly, individuals are assigned ‘flags’ that prevent uptake of additional partnerships when engaged in an existing partnership of a given type. Individuals could uptake additional concurrent partners as long as this was not prohibited by their flags and as long as they did not exceed a maximum of one marital, two informal, and three transitory partnerships.30
To simulate increases in risk behavior among partners of migrants, we modified this concurrency model such that migrants would not count against the limitations on partnership concurrency. For example, a home partner whose flags prohibit additional partnerships could nonetheless acquire one additional non-migrant partner after her existing partner begins a pattern of circular migration.
Results
We estimated the impact of workplace HIV prevention, which protects migrants from externally acquired infections but not infections from partners in the home community, as well as a test-and-treat strategy targeting migrants. Both interventions were assumed to start in 2015, during a mature epidemic that had already spread in the general population. Other intervention start years are also explored, including years in the past when HIV had not yet spread through the general population.
Table 1 summarizes three outcome measures accumulated over a 5-year and 20 time period (2015–2020 and 2015–2035, respectively): the number of infections averted in the entire community (both migrants and non-migrants), all-cause deaths averted in the entire community, and all-cause deaths averted among migrants.
Table 1 .
Impact of targeting HIV prevention or treatment to migrants
| Proportion of males who migrate | 5% |
20% |
5% |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Monthly 3-day visits to home community | Yes |
Yes |
No–perpetual visits | ||||||
| HIV prevention at workplace location | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes |
| HIV treatment uptake by 80% of migrants | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Over 5 years | |||||||||
| New HIV infections averted | 2.5% | 0.1% | 2.6% | 12.6% | 1.3% | 13.4% | 3.1% | 1.3% | 4.0% |
| Total deaths averted (all-cause) | 0.1% | 0.1% | 0.2% | 0.6% | 0.4% | 0.8% | 0.1% | 0.0% | 0.2% |
| Migrant deaths averted (all-cause) | 1.3% | 4.6% | 4.5% | 1.2% | 4.3% | 4.9% | 0.0% | 4.1% | 5.6% |
| Over 20 years | No | ||||||||
| New HIV infections averted | 3.5% | 0.1% | 3.9% | 16.2% | 1.1% | 17.1% | 4.6% | 2.1% | 5.6% |
| Total deaths averted (all-cause) | 0.3% | 0.1% | 0.4% | 1.5% | 0.7% | 1.8% | 0.3% | 0.2% | 0.5% |
| Migrant deaths averted (all-cause) | 6.8% | 8.9% | 12.1% | 6.9% | 8.5% | 11.6% | 4.9% | 10.4% | 13.1% |
Impact of workplace HIV prevention
We first examined a scenario in which 5% of males in the community would become eligible to migrate between ages 15 and 35, such that approximately 2–3% of males aged 15 and older were targetable migrants at any point in time (Figure 2B). We examined the impact of workplace HIV prevention programs for migrants, which had the effect of preventing workplace-acquired HIV infections, but not community-acquired HIV infections. In the model, this amounted to shutting off the externally-acquired incidence of infections, but still allowing migrants to potentially become infected during visits to the home community. This was compared to a baseline scenario in which migrants' externally-acquired HIV incidence rate was maintained at 1.2% per person year.
Complete prevention of new HIV infections at the workplace averted 80.3% of infections among migrants over 5 years and 77.9% of infections among migrants over 20 years. Not all infections by migrants were averted by workplace prevention because migrants could still become infected by partners in the home community, and were put at increased risk of HIV acquisition from home partners due to their increased propensity to have a concurrent partner.
HIV prevention at the workplace averted 1.3% of all-cause deaths among migrants over 5 years, and 6.8% of all-cause deaths among migrants over 20 years (Table 1, left section). Because the mean survival time with untreated HIV infection is approximately a decade—far longer than the 5-year time horizon—the proportion of deaths averted by prevention was much greater when examined over a 20-year time horizon. The health benefit to the migrant population of HIV prevention at the workplace underscores the potential benefits of such programs to employees and their employers, consistent with a recent analysis that HIV services provided by private coal mining companies in South Africa are cost-saving to the employers due to reduced absenteeism and employee turnover caused by illness and death.21
In the whole home community (including non-migrants and migrants), prevention services for migrants at the workplace averted 2.5% of infections and 0.2% of deaths (from any cause) over five years, and 3.5% of all HIV infections in the 0.3% of deaths over 20 years (Table 1, left section).
Impact of a migrant-targeted test-and-treat strategy
We next examined the impact of providing treatment as prevention for migrants beginning in 2015, assuming that 80% of migrants are tested for HIV at an average rate of once per year, and that all those testing positive through this program initiate treatment that reduces infectiousness by 92%.35 This intervention reduced all-cause deaths among migrants by 8.5% over 20 years, but had no significant impact on infections or deaths in the community. A combination of workplace prevention and treatment for migrants had more impact than prevention or treatment alone, averting 12.1% of deaths among migrants, 0.4% of deaths in the community, and 3.9% of infections in the community over 20 years. However, the effect at the community level was still small compared to the effect on the target group.
To test whether the impact of targeting migrants could be greater under assumptions that exaggerate the role of this population, we increased the proportion of males who migrate to 20% (Table 1, middle section) or increased the duration of visits home to its maximum (perpetual visits) without reducing workplace-acquired HIV incidence (Table 1, right section).
The assumption that 20% of males begin migratory behavior between ages 15 and 35 meant that, at any time, approximately 10% of males age 15+ (or approximately 5% of all adults age 15+) were migrants who could be targeted by the intervention. The impact of treatment and prevention on the migrant sub-population remained similar, but the impact on the community became more exaggerated. Over 20 years, workplace prevention averted 16.2% of infections in the community (migrant and non-migrants), and a combination of workplace prevention and treatment averted 17.1% of infections in the community. Only 24.8% and 23.6% of these infections, respectively, were among the migrants themselves.
Treatment as prevention targeted to migrants averted 1.1% of community infections and 0.7% of community deaths over 20 years when 20% of males were eligible to migrate, compared to only 0.1% or infections and deaths when only 5% of males were eligible to migrate. The effect of the intervention was magnified by more than four fold because the increased risk behaviors of the home partners of migrants caused them to play a disproportionately large role in transmission at the home community.
Extending the duration of migrants' visits home to its theoretical maximum (perpetual visits) increased the rate of transfer of new infections between migrants and their partners in the home community. In this extreme example, migrants effectively did not migrate, but nonetheless retained the externally-acquired incidence and the increased risk behavior of home partners that would normally be associated with migration. The purpose of turning the duration of visits to their maximum value was to explore the extent to which the decoupling of migrants from the home community, due to their reduced presence in the community, could have modulated their impact on the ongoing epidemic and thus attenuated the impacts observed in the previous analysis.
This increased coupling between the migrant and home communities amplified the impact of treatment as prevention for migrants, so that 2.1% of community infections were averted by treatment when migrants had perpetual visits, compared to only 0.1% of infections when migrants had monthly 3-day visits to the home community.
The increased exposure to HIV from home partners meant that prevention of workplace-derived infections averted more infections at the community level, but it also made workplace prevention less beneficial to the migrants themselves. Deaths among migrants did not drop significantly in the first 5 years of prevention, and dropped by only 4.9% over 20 years, compared to 6.8% when migrants had monthly 3-day visits to the home community.
The reduced benefit to migrants with frequent visits reflects that HIV prevention at the workplace did not reduce the migrants' HIV exposure from home partners. A limitation of the scenario is that it did not capture potential behavior change associated with increasing the proportion of time migrants spend in the home community. If home partners reduced their risk behavior in the perpetual presence of the migrant partner, we would have expected greater benefits to migrants.
Although our model assumptions were optimistic about the uptake and efficacy of a hypothetical migrant-targeted prevention or treatment intervention, the impact of this intervention was modest on a community level. Even after stretching the assumptions of the model to further exaggerate the role of migrants in the community, the optimistic interventions were a far cry from reversing the generalized epidemic at the community level.
When in history could migrant targeting have collapsed generalized epidemics?
Workplace prevention and test-and-treat for migrants starting in 2015 were unable to reverse the generalized epidemic in the home community within 20 years. To understand why targeting migrants had so little impact at the community level, we experimented with the timing of the migrant-targeted interventions compared to the growth and stabilization of the generalized epidemic. Rather than starting workplace HIV prevention in 2015, we experimented with start dates in earlier years, moving back the start date in one-year increments as far back as the year 1984, when it was assumed that the first migrants introduced HIV into the community (Figure 3).
Figure 3.
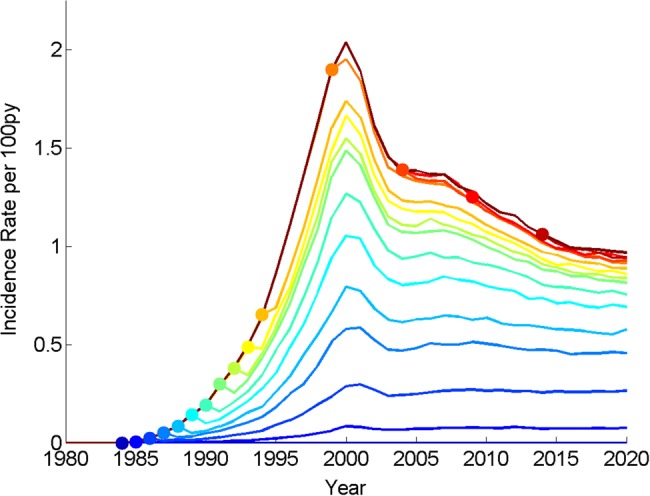
HIV incidence rates among adults age 15+ in the home community after prevention of workplace-acquired HIV infection begins in different years throughout the history of the HIV epidemic. This method of representing of hypothetical past interventions is analogous to Johnson and White's 2011 analysis of hypothetical reductions in concurrency over the history of the HIV epidemic.37 Dots represent the year that workplace prevention begins, and lines of the same shading emanating from these dots represent the impact of sustained workplace prevention on future HIV incidence. Targeting migrants in present or future years would fail to collapse generalized epidemics in their home communities. Such a strategy would only have been successful prior to the early 1990s. This figure is available in black and white in print and in color at International Health Online.
Beginning workplace prevention in 1984 or earlier would, by definition, prevent the epidemic in our model, because it was assumed that the workplace-derived HIV infections were introduced into the community by migrants beginning in this year. However, delaying the intervention by even a few years after HIV introduction into the community would have failed to avoid a generalized epidemic in the model. To halve the incidence rate in 2020, prevention would have had to begin in 1989. Intervening in 2015 with workplace prevention provided too little, too late to reverse the generalized epidemic in the home community.
An important caveat to the analysis in Figure 3 is that it does not capture the changing role of migrant labor over the history of South Africa. The frequency of return visits, percentage of community involved in migrant economy, and the proportion of women having migrant and non-migrant partners likely underwent dramatic changes during the dismantling of apartheid. Thus, model estimates pertaining to the early HIV epidemic, including year of intervention required to halve incidence (Figure 3), should be interpreted with caution. If these changes had been accounted for, the year of intervention required to halve incidence would likely have been later than suggested by our simplified model.
Discussion
We have modeled the impact of a targeted intervention on a generalized HIV epidemic that had already spread and stabilized in a population. The model scenarios were inspired by research in southern Africa analyzing the circular migration patterns of mine workers between rural home communities and dormitories or, increasingly, dense settlements proximal to places of employment.
We found that migrant-targeted interventions would have been transformative in the 1980s to 1990s, but post-2015 impacts were modest from the point of view of the hyper-endemic home community. Even under extreme assumptions that targetable migrants constituted 10% of the communities' males (with 20% of males destined to become migrant workers); that these males constituted the sole external source of HIV into the community; and that primary prevention could eliminate all workplace-acquired infections, a combination of primary prevention and test-and-treat for migrants averted less than 20% of infections in the community.
These extreme assumptions were designed to exaggerate the possible role of male migrant mine workers, to explore whether an extreme assumption could be found in which targeting migrants could collapse the home community's HIV epidemic. For example, we assumed that male migrant workers constituted the sole source of HIV infections brought into the home community, and that primary HIV prevention could completely eliminate externally-acquired HIV infections. Attenuating these assumptions would have reduced the impact of the interventions, which we already found to be modest at the population level in the home community. Thus, the extreme assumptions lend confidence to the robustness of our main finding.
Another way in which we exaggerated the role of migrants was by increasing the proportion of males destined to migrate four-fold: from 5 to 20%. In the 20% scenario, approximately 5% of adults would be employed in mining at any point in time–an estimate that may have been reasonable for Lesotho at the height of South African gold mining operations, but that is not representative across southern Africa.25 However, demographic surveillance studies in rural South Africa have found that the proportion of individuals migrating away from the home can be much higher (20%) especially among older males.34,36 The potential impact of a broad migrant-targeting intervention that is not industry-specific is therefore beyond the scope of this analysis.
A shortcoming of this analysis is its focus on the home community to which migrants belong, and not on the network of transmission outside the home community. Workplace-acquired HIV was modeled as an external source of HIV incidence in migrants, and we assumed that workplace HIV prevention programs could modulate this incidence rate. We did not estimate the role of migrants in the spread of HIV at the workplace site. Intensified HIV services for migrant workers and their partners away from home could potentially have a great impact on HIV transmission in settlements proximal to places of employment, but much remains to be learned about the networks of transmission that sustain such epidemics. Another shortcoming of the historical analysis is that it did not account for political or historical changes influencing the population or behavior of migrants and their partners. Thus, the model can only coarsely approximate the historical timing of a hypothetical intervention that could have interrupted HIV transmission.
Conclusions
Targeting HIV acquisition by migrants in their place of work provides too little, too late to reverse the HIV epidemic in the home community. However, our model did not capture the network of transmission at the workplace, and therefore does not estimate potential benefits to those put at risk outside the home community.
Our findings are consistent with the notion that targeting high-risk groups late in the course of a generalized epidemic would yield health benefits to both the risk groups and their home community, but would fail to reverse the generalized epidemic in the home community. Such a targeted intervention would have been impactful during the early spread of HIV, but now provides too little, too late.
Supplementary data
Acknowledgments
Authors' contributions: DJK, PAE, and AB conceived the study and designed the model scenarios; DJK and AB carried out the analysis and interpretation of results; AB drafted the manuscript; DJK, PAE, and AB critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. DJK and AB are guarantors of the paper.
Acknowledgements: The authors thank Bill & Melinda Gates for their active support of this work and their sponsorship through the Global Good Fund.
Funding: This work was supported by the Global Good Fund.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Wilson D, Halperin DT. ‘Know your epidemic, know your response’: a useful approach, if we get it right. Lancet. 2008;372:423–6. doi: 10.1016/S0140-6736(08)60883-1. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Gakidou E, Levin-Rector A, et al. Assessment of population-level effect of Avahan, an HIV-prevention initiative in India. Lancet. 2011;378:1643–52. doi: 10.1016/S0140-6736(11)61390-1. [DOI] [PubMed] [Google Scholar]
- 3.Tanser F, de Oliveira T, Maheu-Giroux M, Bärnighausen T. Concentrated HIV subepidemics in generalized epidemic settings: Curr Opin HIV. AIDS. 2014;9:115–25. doi: 10.1097/COH.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scorgie F, Chersich MF, Ntaganira I, et al. Socio-demographic characteristics and behavioral risk factors of female sex workers in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16:920–33. doi: 10.1007/s10461-011-9985-z. [DOI] [PubMed] [Google Scholar]
- 5.Bellan SE, Fiorella KJ, Melesse DY, et al. Extra-couple HIV transmission in sub-Saharan Africa: a mathematical modelling study of survey data. Lancet. 2013;381:1561–9. doi: 10.1016/S0140-6736(12)61960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1–serodiscordant couples. J Infect Dis. 2012;205:358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray RH, Wawer MJ. Probability of heterosexual HIV-1 transmission per coital act in sub-Saharan Africa. J Infect Dis. 2012;205:351–2. doi: 10.1093/infdis/jir751. [DOI] [PubMed] [Google Scholar]
- 8.Mee P, Collinson MA, Madhavan S, et al. Evidence for localised HIV related micro-epidemics associated with the decentralised provision of antiretroviral treatment in rural South Africa: a spatio-temporal analysis of changing mortality patterns (2007–2010) J Glob Health. 2014;4:010403. doi: 10.7189/jogh.04.010403. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4073250/ [accessed 9 August 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra S, Steen R, Gerbase A, et al. Impact of high-risk sex and focused interventions in heterosexual HIV epidemics: a systematic review of mathematical models. PLoS ONE. 2012;7:e50691. doi: 10.1371/journal.pone.0050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aral SO, Cates W. Coverage, context and targeted prevention: optimising our impact. Sex Transm Infect. 2013;89:336–40. doi: 10.1136/sextrans-2012-050707. [DOI] [PubMed] [Google Scholar]
- 11.Anarfi JK. Reversing the spread of HIV/AIDS: What role has migration? Int Migr Millenn Dev Goals. 2005;99 [Google Scholar]
- 12.Lurie MN, Williams BG, Zuma K, et al. The impact of migration on HIV-1 transmission in South Africa: a study of migrant and nonmigrant men and their partners. Sex Transm Dis. 2003;30:149–56. doi: 10.1097/00007435-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Banati P. Risk amplification: HIV in migrant communities. Dev South Afr. 2007;24:205–23. [Google Scholar]
- 14.Barnighausen T, Hosegood V, Timaeus IM, Newell M-L. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS. 2007;21(Suppl 7):S29–38. doi: 10.1097/01.aids.0000300533.59483.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffee M, Lurie MN, Garnett GP. Modelling the impact of migration on the HIV epidemic in South Africa. Aids. 2007;21:343–50. doi: 10.1097/QAD.0b013e328011dac9. [DOI] [PubMed] [Google Scholar]
- 16.Lurie MN, Williams BG, Zuma K, et al. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. AIDS. 2003;17:2245–52. doi: 10.1097/00002030-200310170-00013. [DOI] [PubMed] [Google Scholar]
- 17.Lurie MN. The epidemiology of migration and HIV/AIDS in South Africa. J Ethn Migr Stud. 2006;32:649–66. [Google Scholar]
- 18.Vearey J. Learning from HIV: Exploring migration and health in South Africa. Glob Public Health. 2012;7:58–70. doi: 10.1080/17441692.2010.549494. [DOI] [PubMed] [Google Scholar]
- 19.Shand T, Thomson-de Boor H, van den Berg W, et al. The HIV blind spot: men and HIV testing, treatment and care in sub-Saharan Africa. IDS Bull. 2014;45:53–60. [Google Scholar]
- 20.Croucher R, Miles L. Corporate governance and employees in South Africa. J Corp Law Stud. 2010;10:367–89. [Google Scholar]
- 21.Meyer-Rath G. Company-level provision of universal HIV testing and treatment in a mining workforce in South Africa is cost-saving. 2013 [Google Scholar]
- 22.Wachman R. The business of fighting AIDS. The Guardian (London). 3 November 2011. http://www.theguardian.com/business/2011/nov/03/anglo-american-medical-officer-brian-brink-interview?INTCMP=SRCH. [accessed 23 October 2014] [Google Scholar]
- 23.Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–10. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 24.Statistics South Africa. Quarterly Labour Force Survey: Quarter 2 (April to June), 2014 Press Statement. Pretoria: Statistics South Africa. 2014 http://beta2.statssa.gov.za/?p=2951. [accessed 24 October 2014]. [Google Scholar]
- 25.Corno L, de Walque D. Mines, Migration and HIV/AIDS in Southern Africa. J Afr Econ. 2012;21:465–98. [Google Scholar]
- 26.Rees D, Murray J, Nelson G, Sonnenberg P. Oscillating migration and the epidemics of silicosis, tuberculosis, and HIV infection in South African gold miners. Am J Ind Med. 2010;53:398–404. doi: 10.1002/ajim.20716. [DOI] [PubMed] [Google Scholar]
- 27.Ott MQ, Bärnighausen T, Tanser F, et al. Age-gaps in sexual partnerships: seeing beyond ‘sugar daddies. AIDS. 2011;25:861–3. doi: 10.1097/QAD.0b013e32834344c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bershteyn A, Klein DJ, Wenger E, Eckhoff PA. Ithaca: Cornell University Library; 2012. Description of the EMOD-HIV Model v0.7. http://arxiv.org/abs/1206.3720. [accessed] [Google Scholar]
- 29.Klein DJ. Relationship formation and flow control algorithms for generating age-structured networks in HIV modeling; pp. 1041–6. IEEE 51st Annual Conference on Decision and Control (CDC), 10–13 December 2012, Maui, Hawaii. [Google Scholar]
- 30.Bershteyn A, Klein DJ, Eckhoff PA. Age-dependent partnering and the HIV transmission chain: a microsimulation analysis. J R Soc Interface. 2013;10:20130613. doi: 10.1098/rsif.2013.0613. doi:10.1098/rsif.2013.0613http://rsif.royalsocietypublishing.org/content/10/88/20130613. [accessed 6 September 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein DJ, Bershteyn A, Eckhoff PA. Dropout and re-enrollment: implications for epidemiological projections of treatment programs. AIDS. 2014;28:S47–59. doi: 10.1097/QAD.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 32.Eaton JW, Johnson LF, Salomon JA, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaton JW, Menzies NA, Stover J, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2014;2:e23–34. doi: 10.1016/S2214-109X(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 34.Collinson MA, Wolff B, Tollman SM, Kahn K. Trends in internal labour migration from rural Limpopo Province, male risk behaviour, and implications for the spread of HIV/AIDS in rural South Africa. J Ethn Migr Stud. 2006;32:633–48. doi: 10.1080/13691830600610023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camlin CS, Hosegood V, Newell M-L, et al. Gender, migration and HIV in rural KwaZulu-Natal, South Africa. PLoS ONE. 2010;5:e11539. doi: 10.1371/journal.pone.0011539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson LF, White PJ. A review of mathematical models of HIV/AIDS interventions and their implications for policy. Sex Transm Infect. 2011;87:629–34. doi: 10.1136/sti.2010.045500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


