Abstract
The concept of a matricellular protein was first proposed by Paul Bornstein in the mid-1990s to account for the non-lethal phenotypes of mice with inactivated genes encoding thrombospondin-1, tenascin-C, or SPARC. It was also recognized that these extracellular matrix proteins were primarily counter or de-adhesive. This review reappraises the matricellular concept after nearly two decades of continuous investigation. The expanded matricellular family as well as the diverse and often unexpected functions, cellular location, and interacting partners/receptors of matricellular proteins are considered. Development of therapeutic strategies that target matricellular proteins are discussed in the context of pathology and regenerative medicine.
Keywords: Matricellular, Thrombospondins, SPARC, Hevin, Tenascins, CCN, Osteopontin, Fibulin, Periostin, R-spondin, Extracellular matrix, Cell adhesion, Regenerative medicine
1. Introduction
The concept of a matricellular protein arose nearly twenty years ago from Paul Bornstein's group at the University of Washington with the advent of data from several laboratories showing that some secreted and/or extracellular matrix (ECM) proteins were “de-adhesive” (Murphy-Ullrich and Hook, 1989; Lane and Sage, 1990; Murphy-Ullrich et al., 1991; Sage and Bornstein, 1991; Murphy-Ullrich et al., 1995). Substrata composed of these proteins failed to support cell adhesion characterized by the formation of focal adhesions and stress fibers in contrast to adhesive extracellular matrix proteins such as fibronectin, vitronectin, and collagen. These matricellular proteins also antagonized cell adhesion when presented to cells as soluble molecules and induced reorganization of focal adhesions and actin stress fibers, a state termed intermediate adhesion (reviewed in (Sage and Bornstein, 1991; Lane and Sage, 1994; Sage, 1997b, 2001; Murphy-Ullrich, 2001)). It was also disturbing that mice with targeted inactivation of these genes were born alive and, on first glance, had no apparent or only subtle phenotypes (Erickson, 1993). There was even speculation that these extracellular proteins did not have any important role in cell biology. Fortunately, work over the past twenty years has quelled this speculation. Indeed, the importance of matricellular proteins to development, health, and disease has never been more apparent. In July 2013, the 6th conference dedicated to matricellular proteins was held in Saxtons River, Vermont. The articles in this themed issue reflect the profound and broad importance of matricellular proteins in diverse cellular processes and diseases as well as the incredible complexity of their regulation and functions.
In this review, we will re-visit the initial matricellular concept as defined by Paul Bornstein in 1995 in the context of recent findings from the matricellular field, with an emphasis on data presented at the FASEB Scientific Research Conference on Matricellular Proteins in Development, Health, and Disease (Bornstein, 1995).
1.1. Early history of the matricellular idea
The concept of “matricellular” is credited to Paul Bornstein, M.D. (1934–2013) and members of his laboratory who worked on two prototypes that defined this novel family of proteins — SPARC and thrombospondin (TSP)-1. This idea, nearly 20 years in development, started in 1975 when Paul spent sabbatical time in Jon Singer's laboratory at Cal Tech, where immunofluorescence techniques revealing cell-surface and ECM components were being used for the first time in biochemistry and cell biology. Paul was fascinated by what he called “cell coats” and set several of his postdocs to defining the fibroblast and endothelial cell integuments. The work led to his concept of “dynamic reciprocity” to explain the apparent influence exerted by the ECM on the very cells that initially produced it (Bornstein et al., 1982). In a recent review on wound healing and tissue regeneration, dynamic reciprocity is defined as “an ongoing, bidirectional interaction among cells and their surrounding microenvironment. Such cell-extracellular matrix interactions not only guide and regulate cellular morphology, but also cellular differentiation, migration, proliferation, and survival during tissue development, including, e.g., embryogenesis, angiogenesis, as well as during pathologic processes including cancer, diabetes, hypertension, and chronic wound healing” (Schultz et al., 2011). It was initially envisioned that certain fibrillar ECM proteins (e.g., collagen types I and III and fibronectin) interact in some manner with the cytoskeleton (admittedly indirectly) to effect changes in cell shape, ion fluxes, secretory patterns, and mitosis (Bornstein et al., 1982). Subsequently, two important discoveries lent credence to this early model: integrin receptors spanning the plasma membrane provided a link between extracellular macromolecules and the cytoskeleton/signaling cascades, and matricellular proteins as extracellular but non-structural components provided specific functions and a vastly expanded dimension to what a somewhat inert ECM was formerly thought to comprise. In 1995, Bornstein published the seminal article defining matricellular proteins: “Matricellular is used in this analysis to refer to a group of modular, extracellular proteins whose functions are achieved by binding to matrix proteins as well as to cell surface receptors, or to other molecules such as cytokines and proteases that interact, in turn, with the cell surface”. Furthermore, although matricellular proteins “can be associated with structural elements such as collagen fibrils and basement membranes, it is assumed that they do not contribute to the structural integrity of these elements” (Bornstein, 1995). An in-depth review of this topic can be found in Bornstein and Sage (2002). The dynamic nature of “established” ECM has also evolved accordingly (Hynes, 2009).
The original matricellular triumvirate consisted of SPARC (secreted protein, acidic and rich in cysteine, also known as osteonectin and BM-40), thrombospondin (now TSP-1), and tenascin (now tenascin-C) (TN-C) (Sage and Bornstein, 1991). Although unrelated in primary structure, their unifying characteristics were that they were 1) secreted by diverse types of cells, 2) associated with, but not necessarily a part of, the insoluble/fibrillar ECM, 3) counter-adhesive for cells under various conditions, 4) prevalent in areas of tissue remodeling associated with normal and pathologic processes, and 5) featured prominently in mammalian and avian embryogenesis. As the literature expanded with additions to the matricellular group as well as to its individual protein families (e.g., hevin/SC-1 of the SPARC family and TSP-2 of the thrombospondin family), screening became more difficult with the discovery of new functions appropriate for matricellular membership and classification. There could be no greater compliment to Paul Bornstein's scientific career than the growth and success of this family, the FASEB Symposium in 2013 devoted to these interesting and important proteins, and their recognition in this themed issue of Matrix Biology.
1.2. SPARC as a matricellular prototype
Although not the first of the matricellular group to be described, SPARC became known as its prototype due in part to the relative simplicity of its structure (a monomer of Mr ~32,000 excluding the signal peptide and carbohydrate) and the apparent myriad of functions that it displayed (reviewed in (Sage, 2009)). Dominant features included its counter-adhesive activity, later described as a condition of “intermediate adhesion” by Murphy-Ullrich and colleagues in 1995 and an impetus for Bornstein's subsequent matricellular concept (reviewed in (Murphy-Ullrich, 2001)). Later studies identified the interaction of SPARC with integrin beta 1 and signaling through integrin-linked kinase and/or GSK-3 beta as effectors of cell shape, adhesion, and differentiation (Barker et al., 2005a; Weaver et al., 2008; Nie and Sage, 2009b).
Another compelling feature of SPARC was its abundant levels of secretion by cultured cells and, in vivo, at sites of tissue injury (e.g., wound healing), cancer, and remodeling (bone, gut epithelia, hair follicles, and steroid-producing organs). These data were suggestive of processes involving changes in cell shape, cell cycle, protein secretion, and motility, all of which were subsequently verified experimentally (Lane and Sage, 1994; Sage, 1997b; Bradshaw and Sage, 2001; Emerson et al., 2006). Related lines of evidence also pointed to the interactions of SPARC with ECM components such as collagens (principally types I and IV) and with growth factors (e.g., VEGF-A and plateletderived growth factor) that inhibited their binding to cognate receptors. The consequences of these activities became apparent in later developmental studies with mice harboring an inactivated SPARC gene (Delany et al., 2000; Yan et al., 2002; Bradshaw et al., 2003a, 2003b; Gruber et al., 2005). Indeed, with their thin skins and brittle bones (impaired collagen I production and fibrillogenesis), progressive cataracts (poorly assembled collagen IV in the lens capsule), accumulation of excessive adipose tissue (compromised osteoblast formation and survival), and intervertebral disc degeneration, the SPARC-null mice appeared to be aging well before their time. These characteristics reflecting alterations in tissues during development became more evident, or were exacerbated, in disease models. For example, Brekken et al., reported enhanced growth of pancreatic tumors in SPARC-null mice, due to a compromised ECM, poor encapsulation of the tumor, reduced infiltration of macrophages, and attenuated levels of tumor cell apoptosis (Brekken et al., 2003). The reduced foreign body response in mice lacking SPARC was similarly characterized by a reduction of ECM deposition (Puolakkainen et al., 2003).
As most (if not all) of the matricellular proteins have a modular primary structure, we had proposed that, if specific proteinases could be identified, cleavage of SPARC into bioactive peptides might not only reveal new functions for the protein but also could present potential therapeutic targets in the treatment of certain pathologies (Sage, 1997a). To this end, Lane et al., identified copper-binding peptides of SPARC that regulated angiogenesis, and subsequent studies showed that matrix metalloproteinase 3 (stromelysin) released polypeptides from SPARC with similar activity (Lane et al., 1994; Sage et al., 2003). Moreover, the copper-binding domain of SPARC was identified as a mediator of cell survival in vitro via its interaction with integrin beta 1 and signaling through integrin-linked kinase (Weaver et al., 2008); similar peptides have been implicated in the inhibition of angiogenesis in neuroblastoma (Chlenski et al., 2004). Clearly, there is a future for SPARC (and its homolog hevin, see below) in the diagnosis and treatment of cancers, as indicated by gene array analyses (Clark and Sage, 2008; Sage, 2009).
Since the first descriptions of SPARC/osteonectin/BM-40, several new family members, based on the signature ECM calcium-binding (EC) domain, have been added to the fold: hevin (also known as SPARC-like 1, SC-1, Mast 9, Ecm 1 SMOC 1 and 2, and several of the testicans. Hevin particularly has received attention as a potential tumor suppressor expressed abundantly in certain tumor cells, their stroma, and their neovasculature (reviewed in (Sullivan and Sage, 2004)). Both counter-adhesive and implicated in neuronal migration, it was surprising that hevin-null mice initially appeared “normal.” However, Sullivan et al. later found that mice lacking hevin had an unusually stiff dermis with a high tensile modulus and aberrant collagen fibrils, due to the impaired regulation of the collagen-binding accessory proteoglycan decorin (Sullivan et al., 2006). Other similarities between hevin-null and SPARC-null mice were the appearance of cataracts (albeit at different ages), enhanced growth of solid tumors, and alterations in dermal wound repair (Sullivan et al., 2008; Sage, 2009). Using hevin/SPARC single- and double-null mice in a model of the foreign body response, Barker et al. showed that hevin alone suppressed inflammation, whereas both proteins diminished angiogenesis (Barker et al., 2005b; Sullivan et al., 2008). Because the copper-binding and EC domains of SPARC and hevin are similar, one might predict that a synergy occurs, especially at the peptide level that would result in an enhanced angiogenic response.
From the description of a SPARC homolog in Caenorhabditis elegans that influenced the morphology and mobility of this invertebrate (Schwarzbauer and Spencer, 1993), hevin appeared to be an excellent candidate to test the hypothesis that hevin and SPARC might compensate for each other developmentally through the release of peptides or domains by endogenous proteolysis at evolutionarily conserved sites. Although more examples of this interesting phenomenon are required, Weaver et al. showed that a SPARC-like fragment of hevin (termed SLF) was indeed produced by matrix metalloproteinase 3 and was found in the neovasculature of murine glioma (Weaver et al., 2011).
This section is intended to provide an historical perspective for the continued study of the matricellular proteins, and has used one of them, SPARC, as an example of the varied functions attributed to this structurally diverse group of extracellular (and in some cases, intracellular) macro-molecules. Many of the functions (e.g., counter-/intermediate adhesion, regulation of ECM deposition, inhibition of growth factor activity, signaling via integrins) appear to be overlapping, yet it is clear that specificmolecular targets and mechanisms exist for each matricellular entity. The articles presented in this themed issue of Matrix Biology attest to the broad spectrum of biological activities facilitated by the matricellular proteins. In retrospect, a need for this functional class was correctly perceived, as evidenced by the recent studies, discussed in succeeding sections that correlate the early and basic observations with the complex mechanisms that operate in health and disease.
2. Members of the matricellular family
The original members of the matricellular family included SPARC, TSP-1, and TN-C (Bornstein, 1995), previously grouped as secreted proteins that modulated cell-ECM interactions (Sage and Bornstein, 1991). It had become clear that there were additional molecules in the ECM that interacted with cells, but did not play a primarily physical role in the structure of the ECM. The family now includes additional members of the SPARC (hevin/SC-1), TSP (TSPs1–4, TSP-5 or cartilage oligomeric protein) (COMP), and tenascin families (tenascins-C, R, W, X, and Y) (Sage and Bornstein, 1991; Brekken and Sage, 2001; Sullivan et al., 2008; Sage, 2009; Acharya et al., 2014-in this issue; Chiquet-Ehrismann et al., 2014-in this issue; Posey et al., 2014-in this issue; Resovi et al., 2014-in this issue). Furthermore, many other components of the ECM microenvironment have been proposed to be matricellular: most are readily identified as components of the ECM, but some are not typically categorized as ECM components. The expanded matricellular family includes osteopontin, members of the CCN family (Cyr61, CCN2, CCN3), periostin, R-spondins, the short fibulins including hemicentin, galectins, small leucine rich proteoglycans (SLRPs), autotaxin, pigment epithelium derived factor (PEDF), and plasminogen activator inhibitor-1 (PAI-1) (Giachelli and Steitz, 2000; Moiseeva et al., 2000; Sodek et al., 2002; Czekay et al., 2003; Leask and Abraham, 2006; Yeger and Perbal, 2007; Hamilton, 2008; Perbal, 2008; Yuelling and Fuss, 2008; Merline et al., 2009; Yanagisawa et al., 2009; Hankenson et al., 2010; Kular et al., 2011; Fitchev et al., 2013; Bedore et al., 2014-in this issue; Knight and Hankenson, 2014-in this issue; Liu et al., 2014-in this issue; Papke and Yanagisawa, 2014-in this issue; Shevde and Samant, 2014-in this issue). The SLRPs are the subject of an extensive recent review and as the SLRPs are usually considered in the context of proteoglycans, they will not be addressed further in this review (Iozzo et al., 2011). Although these proteins are structurally diverse, most, but not all, contain repeats of common ECM structural motifs such as thrombospondin type 1 repeats (TSRs), fibronectin type III repeats, EGF-like repeats, among others, and many are also calcium binding proteins (Adams and Engel, 2007; Mosher and Adams, 2012). As reviewed previously (Martinek et al., 2007; Mosher and Adams, 2012), matricellular proteins with thrombospondin and SPARC-like structures appeared in early metazoans. Despite their diversity, common to all matricellular proteins is the capacity to interact with cellular receptors, ECM macromolecules, growth factors, and proteases and thereby to directly or indirectly regulate cellular function, either at the plasma membrane, intracellularly, in body fluids, or from the ECM.
2.1. Matricellular proteins: functions within and without
Matricellular proteins were first identified as transient, rather than constitutive, components of the ECM. They are incorporated into the ECM of remodeling tissues during development, wound healing, and in response to injury and stress, especially in fibrotic ECM and tumorassociated stroma. In addition, many matricellular proteins are present in body fluids and can bind cells as soluble ligands. Matricellular proteins can also bind soluble growth factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and (latent) transforming growth factor-β (TGF-β) and can modulate the actions of these factors through presentation to their receptors, by blocking receptor binding, or by induction of conformational alterations to modulate activity. Interestingly, two different matricellular proteins, TSP1 and tenascin-X can convert latent TGF-β to its biologically active form, indirectly regulation ECM expression (Schultz-Cherry and Murphy-Ullrich, 1993; Alcaraz et al., 2014). Matricellular proteins can also directly or indirectly alter growth factor receptor signaling (Schultz-Cherry and Murphy-Ullrich, 1993; Brekken and Sage, 2001; Schiemann et al., 2003; Nozaki et al., 2006; Iyer et al., 2007; Liu et al., 2009; Garg et al., 2011; Kazerounian et al., 2011).
Recent evidence suggests that some matricellular proteins can regulate cell function from intracellular compartments. Intracellular SPARC co-localized with intracellular collagen IV in the endoplasmic reticulum (ER) of adipocytes from Drosophila larvae (Martinek et al., 2007, 2008): SPARC is thought to be important for basement membrane formation during development (Yan et al., 2002). OPN is also located intracellularly on the inner surface of the plasma membrane as part of the CD44-ezrin/radixin/moesin complex where it regulates cellular functions related to cytoskeletal organization (Zohar et al., 2000). Thrombospondins also play a role in the ER. TSP4 binds to ATF6α in the ER and regulates its ER-nuclear shuttling and ER adaptive responses in the myocardium (Lynch et al., 2012). TSP-1 also binds to STIM1 (stromal interaction molecule 1), an ER and plasma membrane protein that detects calcium depletion, at the platelet plasma membrane and potentially regulates STIM1 calcium sensing that is important for platelet aggregation (Ambily et al., 2014). Multiple TSPs (TSP-1, TSP-4, and COMP) were shown to bind to the N-terminal domain of STIM1, which is exposed to the ER lumen and on the external side of the plasma membrane. TSPs bind to STIM1 through the TSP C-terminal signature domains, independent of the presence of calcium (Duquette et al., 2014-in this issue). Overexpression of COMP promoted arachidonate-regulated calcium channel activity which is regulated by plasma membrane STIM1 (Duquette et al., 2014-in this issue). These provocative findings indicate that the capacity of TSPs to bind to multiple receptors and molecules affects the functions of macromolecular assemblies not only in the ECM, but also at the cell membrane and in intracellular compartments. Mutations in COMP associated with pseudoachondroplasia result in accumulation of misfolded COMP in the ER which induces ER stress and chondrocyte apoptosis and also leads to abnormal intra-ER assembly of ECM proteins (Posey et al., 2009; Coustry et al., 2012). Given the capacity of matricellular proteins to bind other ECM components, their putative roles as chaperones for these molecules and perhaps growth factors are reasonable (Chlenski et al., 2004; Emerson et al., 2006). Some matricellular proteins have also been identified in nuclear compartments (SPARC, CCN5, amino-domain truncated CCN3) (Gooden et al., 1999; Planque et al., 2006; Wiesman et al., 2010), although the nuclear roles of matricellular proteins remain unclear. Nonetheless, the possibility that matricellular proteins act as intracellular targeting or transport molecules warrants further investigation.
2.2. Regulation of matricellular protein expression
Since the initial report that TSP-1 and SPARC secretion are inversely proportional to cell density (Mumby et al., 1984), the precise regulation of matricellular protein expression has been appreciated. Not unexpectedly, regulation of matricellular proteins is complex. Many labs have characterized the regulation of expression of specific matricellular proteins through characterization of promoter regions and their interactions with transcription factors: the regulation of TSPs and tenascins is discussed extensively in reviews in this issue (Chiquet-Ehrismann et al., 2014-in this issue; Stenina-Adognravi, 2014-in this issue). These proteins can also be regulated post-transcriptionally, at the level of translation, and via secretory pathways. These points are extensively discussed for the TSPs in a review in this issue by Stenina-Adognravi (2014-in this issue). Matricellular proteins are also regulated by microRNA and other epigenetic mechanisms (DNA methylation, histone acetylation/deacetylation) (Whitcomb et al., 2003; Suzuki et al., 2005; Rodriguez-Jimenez et al., 2007; Hanna et al., 2009; Dews et al., 2010; Greco et al., 2010; Galvez et al., 2011; Lindner et al., 2013; Qin et al., 2013; Dogar et al., 2014; Stein et al., 2014; Wang et al., 2014). Matricellular proteins can also be auto-regulated through their receptors: It has recently been shown that TSP-1 expression is attenuated by CD47, and that genetic disruption of CD47 results in increased TSP-1 expression and downstream increases in TGF-β activity (Soto-Pantoja et al., 2014-in this issue). One interesting area that has not been fully explored is the regulation of matricellular proteins by circadian clock genes. Clock genes have been implicated in many cellular processes including metabolic regulation, cancer, and immune responses, cellular events in which matricellular proteins play key roles (Li et al., 2012; P. Chen et al., 2013; Kelleher et al.,2014).The aryl hydrocarbon receptor, a member of the clock gene family, has been shown to regulate glucose dependent stimulation of TSP-1 (Dabir et al., 2008); otherwise, there is little information concerning the regulation of matricellular proteins by clock genes. In recent years, extracellular matrix stiffness and cellular contractility have been shown to regulate cellular differentiation and gene expression, especially of genes involved in fibrosis (Wells and Discher, 2008; Brown et al., 2013; Godbout et al., 2013; Karsdal et al., 2013). There is strong evidence that mechanical forces regulate TN-C expression (Fluck et al., 2008; Maier et al., 2008; Jiang et al., 2009; Asparuhova et al., 2011). There are reports of cell contractility, shear forces, or mechanical stretch increasing TSP-1 expression under fibrotic conditions (Warburton and Kaartinen, 2007; Chen et al., 2011; Holliday et al., 2011), suggesting possible amplification of TSP-1 in fibrotic tissues. Mechanical strain also enhanced SPARC expression by podocytes, with implications for glomerulosclerosis (Durvasula and Shankland, 2005). In contrast to increased forces, osteopontin was decreased by microgravity (Kumei et al., 2006). Mechanical regulation of matricellular proteins is likely to regulate stem cell differentiation in tissue specific niches and also has implications for tissue engineering (Chiquet-Ehrismann et al., 2014-in this issue).
2.3. Alternate splicing and post-translational modifications of matricellular proteins
Further contextual specificity of matricellular protein interactions and functions can be derived from mRNA splicing or post-translational modifications such as specialized forms of glycosylation, gammacarboxylation, and phosphorylation. It has long been known that the transcript for TN-C can undergo splicing to generate “large” and “small” forms with different tissue-specific associations for the TN-C isoforms containing or lacking the alternatively-spliced domain (Borsi et al., 1992; Ghert et al., 2002; Keller et al., 2007). A recent paper reports that overexpression in skin of a splicing factor SRSF6, which promotes exon inclusion, increases expression of “large” TN-C associated with a hyperplasic wound healing response, consistent with observations that the large form is a marker of tumor invasion (Jensen et al., 2014). Splice variants of osteopontin and its receptor CD44 are also associated with malignant tumor progression (Gimba and Tilli, 2013; Tang et al., 2013; Shevde and Samant, 2014-in this issue). Matricellular proteins are also subject to post-translational modifications. In addition to the more common O-glycosylation, the type I (TSR) repeats of TSP-1 are subject to C-mannosylation on tryptophan residues and O-fucosylation (Hofsteenge et al., 2001; Leonhard-Melief and Haltiwanger, 2010). The EGF-like module 1 of TSP-1 is also modified by O-linked N-acetylglucosamine (Hoffmann et al., 2012). Although, the functional significance of TSP glycosylation is not clear, there is evidence that C-mannosylated peptides of the WSPW motif enhance lipopolysaccharide-induced signaling (Muroi et al., 2007) and that C-mannosylation is increased in diabetic tissues (Ihara et al., 2005). O-fucosylation of TSRs might regulate epithelial differentiation during gastrulation (Du et al., 2010). The role of glycosylation of the type 1 repeats in binding of ligands such as heparin and TGF-β to TSP-1 has not been addressed. Periostin is γ-carboxylated on glutamic acids, a post-translational modification common to vitamin K dependent clotting factors (Coutu et al., 2008). In addition, phosphorylation, glycosylation, tyrosine sulfation, and sialylation of osteopontin are known to regulate its interactions with cells (Kazanecki et al., 2007; Wang and Denhardt, 2008; Shevde and Samant, 2014-in this issue).
2.4. Receptors for matricellular proteins
Matricellular proteins bind to a diverse array of receptors (Table 1) (see reviews in this issue (Acharya et al., 2014-in this issue; Bedore et al., 2014-in this issue; Chiquet-Ehrismann et al., 2014-in this issue; Resovi et al., 2014-in this issue)). Most matricellular proteins bind to and activate signaling through multiple integrins. In at least one case, fibulin-5 binds to α5β1 and α4β1 integrins, but does not activate signaling via these receptors that mediate focal adhesion formation or stress fiber formation (Lomas et al., 2007; Papke and Yanagisawa, 2014-in this issue). In addition to integrins, multiple matricellular proteins bind to cell membrane heparan sulfate proteoglycans, particularly syndecan-4: these include TSP-1, TSP-2, TN-C, CCN1, CCN2, and R-spondins. Matricellular proteins also bind to scavenger receptors such as LDL-receptor related protein 1 (LRP-1) and CD36. Matricellular protein binding to scavenger receptors is consistent with a mechanism to spatially and temporally limit the action of matricellular proteins and/or their binding partners, such as matrix metalloproteinases. TSPs-1 and 2 and CCN2 bind to LRP-1, which mediates endocytosis of TSPs and CCN2 (Godyna et al., 1995; Segarini et al., 2001; Mason, 2013). Alternately, LRP-1 can also act as a co-receptor for a cell surface form of the TSP binding protein calreticulin to mediate activation of G-protein-dependent ERK and PI3K signaling (Orr et al., 2003). CCN2 binding to LRP-1 can also stimulate phosphorylation of the cytoplasmic tail of LRP-1 (Yang et al., 2004). Stabilin-1, a scavenger receptor expressed on alternatively-activated macrophages and on endothelial cells during inflammation, mediates the endocytic clearance of SPARC (Kzhyshkowska et al., 2006; Workman and Sage, 2011). R-spondins bind to Wnt-receptors LRP6, LGR4 and LGR 5 (leucine rich repeat G protein coupled receptors) and through membrane E3 ubiquitin ligases ZNRF3 (Jin and Yoon, 2012; Xie et al., 2013; Knight and Hankenson, 2014-in this issue). PEDF binds to ECM components and to a cell membrane protein with phospholipase activity, a laminin receptor, F1 ATP synthase, and LRP6 (Becerra and Notario, 2013). Autotaxin, a matricellular protein with phosphodiesterase activity binds to P2Y12 on oligodendrocytes (Dennis et al., 2012). Matricellular proteins can bind multiple receptors on a cell and there is evidence that binding of one domain of a matricellular protein to a particular receptor and its net downstream signaling can be regulated through binding to receptors or binding ligands in other domains of the matricellular protein. For example, fibronectin binding to the stalk region of TSP-1 affects the ability of the TSP-1 N-terminus to bind integrin α3β1 (Rodrigues et al., 2001). The complement of receptors on a particular cell type can also govern cellular responses to a matricellular ligand. Furthermore, the availability of a particular matricellular receptor binding domain can be modulated by the binding of soluble matricellular ligands which sterically block matricellular protein–receptor interactions, such as the ability of heparin to prevent binding of the N-terminus of TSP-1 to calreticulin (Goicoechea et al., 2000). More recently described functions of matricellular proteins have been reported to occur through signaling from receptors not typically associated with ECM proteins. These include the calcium channel gabapentin receptor (α2δ-1) for TSP-1 and TSP-4 and toll-like receptor 4 (TLR4) for TN-C (Eroglu et al., 2009; Midwood et al., 2009; Risher and Eroglu, 2012; Chiquet-Ehrismann et al., 2014-in this issue). Like TLR4, CD36, a receptor for TSPs, can also act as a pattern recognition receptor, suggesting a broader role for matricellular proteins in innate immunity (Areschoug and Gordon, 2009; Sheedy et al., 2013). The role of osteopontin in inflammation and autoimmune diseases has long been appreciated (Uede, 2011). The binding of many of the matricellular proteins to these diverse receptors has been shown to regulate most known signaling pathways, including those activated by VEGF receptors and nitric oxide (Nozaki et al., 2006; Lawler and Lawler, 2012; Roberts et al., 2012; Rogers et al., 2014-in this issue). Matricellular proteins can either directly (TN-C) or indirectly (TSP-1) stimulate signaling through the epidermal growth factor receptor (Swindle et al., 2001; Liu et al., 2009; Garg et al., 2011).
Table 1.
Receptors for matricellular proteins.
3. Roles in diseases
It is not surprising that matricellular proteins have been shown to play important roles in disease. Because the early studies established that the original matricellular proteins (TSP-1, TN-C, and SPARC) are primarily de-adhesive and pro-migratory, their roles in wound healing and cancer were initial targets for investigation. From a myriad of studies, it became apparent that gene inactivation, overexpression, or antagonism of a matricellular protein receptor changed the course of wound healing, tumor growth and/or metastasis, and the production of connective tissue proteins such as collagen. Because results of these studies were highly contextual, it has been difficult to draw broad conclusions regarding the role of a specific matricellular protein in a given pathology. Instead, as additional functions of matricellular proteins in biological processes were elucidated, such as the regulation of growth factor and soluble mediator activity, cell death, cell senescence, cell–cell adhesion and vascular permeability, inflammation, innate and acquired immune function, fibrosis, modulation of ECM assembly, and angiogenesis, the context-specific roles of a given matricellular protein in a certain disease environment on a specific cell or tumor type have become better appreciated and more clearly defined. It is now well established that various matricellular proteins play critical roles in disease processes of all organ systems, including the stem cell niche and in stem cell fate (Gattu et al., 2013; Qin et al., 2013; Chiquet-Ehrismann et al., 2014; Lee et al., 2014b): many of the reviews and articles in this issue will deal with particular roles of specific matricellular proteins in development and disease in detail. Typically matricellular proteins are involved in disease because of abnormal regulation leading to either over or underexpression of the protein, but there are examples of disease due to inherited mutations or gene deficiency. Mutations leading to protein misfolding and ER retention of mutant COMP result in skeletal defects such as pseudoachondroplasia and multiple epiphyseal dysplasia (Posey et al., 2009, 2014-in this issue). Tenascin-X deficiency is associated with the Ehlers-Danlos syndrome (Burch et al., 1997; Schalkwijk et al., 2001). Single nucleotide polymorphisms of TSP-4 are associated with increased cardiovascular risk (Pluskota et al., 2005; Corsetti et al., 2011), a decrease in TSP-2 polymorphisms in plaque erosion (Burke et al., 2010), and a polymorphism of TSP-1 with increased corneal allograft rejection (Winton et al., 2014). Mutations in the THBS1 type 1 repeats are associated with familial pulmonary arterial hypertension (Maloney et al., 2012). Because some matricellular proteins are components of the ECM, we have traditionally thought of them in the context of solid tissues, particularly in connective tissue compartments. However, matricellular proteins are also secreted proteins that can influence cell behavior or disease progression as soluble moieties interacting with cell surface receptors or ECM components implicating matricellular proteins in both paracrine and autocrine cellular regulation. Recent findings that matricellular proteins, particularly TN-C, function as damage-associated molecular pattern (DAMP) molecules in tissue damage-induced inflammation expand our concept of the matricellular protein in tissue remodeling (Udalova et al., 2011; Ruhmann et al., 2012; Chiquet-Ehrismann et al., 2014). Matricellular proteins can also regulate multiple components of the immune system as seen for TSP-1 in Sj gren's syndrome in the eye and in multiple myeloma (Kukreja et al., 2009; Turpie et al., 2009). Matricellular proteins also are involved in metabolic diseases such as diabetes and obesity. The roles of matricellular proteins, such as CCN2, osteopontin, TSP-1, and SPARC, in fibrotic diabetic complications is well-established (Taneda et al., 2003; Belmadani et al., 2007; Daniel et al., 2007; Mason, 2009; Lu et al., 2011; Yoshida et al., 2011; Sorenson et al., 2013). Moreover, the involvement of matricellular proteins such as TSP-1 in insulin sensitivity and obesity-induced inflammation and fibrosis and of SPARC in insulin resistance and adipose tissue accumulation is emerging (Nie and Sage, 2009a; Kos and Wilding, 2010; Li et al.,2011; Olerud et al., 2011; Drott et al., 2012; Finlin et al., 2013; M. Inoue et al., 2013; Kong et al., 2013; Gattu et al., 2014). Over the last several years, unexpected roles of thrombospondins, tenascin-R, and SPARC family members in synapse formation and maturation and of NOV/CCN3, TSP-4, and SPARC in chronic and neuropathic pain have emerged (Kucukdereli et al., 2011; Kim et al., 2012; Kular et al., 2012; Millecamps et al., 2012; Risher and Eroglu, 2012; Li et al., 2014; Xu et al., 2014). With the recent findings for intracellular roles of matricellular proteins (Martinek et al., 2007; Chlenski et al., 2011; Lynch et al., 2012; Duquette et al., 2014-in this issue; Posey et al., 2014-in this issue), there is the distinct possibility that matricellular proteins regulate additional cellular functions relevant to disease.
4. Matricellular proteins as biomarkers
Matricellular proteins play key roles in tissue remodeling, inflammation, and in immune responses and therefore, it is not surprising that their levels will be altered in various disease states. Many matricellular proteins are present in body fluids as soluble proteins and are used clinically and experimentally to monitor disease progression. Tenascins, particularly TN-C, have been shown to be markers of tumor progression, cardiovascular disease, tumor angiogenesis, inflammation in arthritis, and in infection in the oral cavity (Orend and Chiquet-Ehrismann, 2006; Midwood et al., 2009; Ozcakir-Tomruk et al., 2012; K. Inoue et al., 2013; Karatas et al., 2013; Sakamoto et al., 2014; Chiquet-Ehrismann et al., 2014). Serum periostin is an indicator of eosinophilic airway inflammation and response to certain therapeutics (Jia et al., 2012; Parulekar et al., 2014). Periostin has also been described as a biomarker of systemic sclerosis and of cancer progression (Lv et al., 2013; Yamaguchi et al., 2013). Levels of SPARC were increased in the sera of patients after bariatric surgery and were also associated with hemoglobin A1c levels, suggesting a possible role for SPARC as an indicator of metabolic state (Kotani et al., 2011; Lee et al., 2014a). Osteopontin levels are measured as indicators of bone density, cardiovascular disease, and tumor progression (Cho et al., 2013; Kadoglou et al., 2013; Mardani et al., 2013). Fibulin-3 peptides are markers of osteoarthritis (Henrotin et al., 2012). Serum COMP levels correlate with early joint damage in both osteoarthritis and rheumatoid arthritis (Andersson et al., 2013; Verma and Dalal, 2013). As many matricellular proteins regulate different aspects of angiogenesis, they have been deemed biomarkers of pre-eclampsia (TSP-1, TSP-2, CCN1, CCN3) (Gellhaus et al., 2007; Stenczer et al., 2011; Stenczer et al., 2012). Nearly all of the matricellular proteins have been identified as tissue markers of specific tumors, grades of tumor, or prognosis and there is an abundant of literature on this topic. Exciting are the studies showing that expression of a particular matricellular protein is predictive of response to therapy. For example, Farina et al. showed that RNA levels of COMP and TSP-1 were part of a four gene biomarker panel that correlates with the modified Rodnan Skin Score, which is used as a clinical indicator of skin disease severity in systemic sclerosis (Farina et al., 2010), suggesting that this four gene panel might have predictive value as a surrogate marker in clinical trials.
5. Translational potential and challenges
Matricellular proteins represent an underappreciated target for therapeutic interventions in a wide spectrum of diseases. The importance of matricellular proteins in disease is strongly supported by a wealth of literature from animal models and data from patients. It is less clear, however, how to target a specific function of a particular matricellular protein in a disease setting. As multifunctional proteins with multiple receptors, genetic ablation or the use of a blocking antibody to a particular matricellular protein raises the potential for adverse effects due to concomitant attenuation of beneficial functions in addition to ablation of the disease-inducing function. However, the use of antibody-based therapeutics directed against a matricellular protein has achieved some clinical success. For example, a monoclonal antibody against CCN2, also known as connective tissue growth factor (CTGF) (FG-3019) has been successful in phase I clinical trials in patients with diabetes/albuminuria and in pancreatic cancer and is entering phase 2 trials for idiopathic pulmonary fibrosis and liver fibrosis (www.ClinicalTrials.gov). Preclinical studies with FG-309 have shown utility in models of glaucoma and acute lymphoblastic leukemia (Lu et al., 2013; Wallace et al., 2013, 2014-in this issue). A number of pharmaceutical firms are testing anti-sense approaches to reduce CCN2 expression for treatment of skin fibrosis. The use of synthetic microRNAs has been proposed to regulate TSP1 expression (Dogar et al., 2014). In some cases, there is functional antagonism between members of a matricellular protein family. For example, TSP-2 overexpression has been attempted as a means to block TSP-1-dependent TGF-β activation, because TSP-2 lacks the TGF-β activating sequence (Schultz-Cherry et al., 1995). Whereas TSP-2 overexpression does attenuate TGF-β activity in a model of diabetic nephropathy and in acute glomerulosclerosis, long-term overexpression of TSP-2 resulted in vascular rarefaction due to the potent anti-angiogenic effect of TSP-2 (Daniel et al., 2009, 2013). A more successful application of this idea is the overexpression of CCN3 to counteract the profibrotic effects of CCN2 in diabetic nephropathy (Riser et al., 2010). That many receptors for matricellular proteins, such as integrins, are promiscuous and bind multiple ligands, also raise the possibility of non-specific and unwanted side effects. More specific approaches, such as delivery of agonists and antagonists of a functional sequence of a matricellular protein, represent a more specific therapeutic approach. Second-generation octapeptides based on ABT-510 (peptide mimetics of the TSP-1 binding site for CD36) have been used in human and canine clinical trials to block angiogenesis for treatment of soft tissue sarcomas (Sahora et al., 2012). A synthetic fragment of the follistatin domain of SPARC has been used to inhibit tumor angiogenesis and another SPARC peptide enhances chemo sensitivity of tumors (Chlenski et al., 2004; Rahman et al., 2011). Another approach is the inhibition of matricellular protein interactions with receptors for pathways implicated in disease. For example, humanized anti-CD47 antibodies are in development for vascular and ischemic diseases (Rogers et al., 2014-in this issue). PEDF peptidomimetics are being tested for oncologic applications (Becerra and Notario, 2013; Craword et al., 2013). Disease-specific expression of matricellular protein splice variants, in particular osteopontin and TN-C, also presents new targets for antibody-based therapies (Weidle et al., 2011). Finally, one could potentially modify post-translational modifications through the blocking of key processing enzymes, for example blocking periostin γ-carboxylation through use of Coumadin, although this type of approach would affect multiple targets.
6. Matricellular proteins in tissue engineering and responses to biomaterials
Adhesive and structural extracellular matrix proteins such as collagens and fibronectin have been used to develop biomimetic scaffolds for tissue engineering. The incorporation of matricellular proteins into biomaterials has received less attention, however, possibly because of the relative unfamiliarity of these specialized ECM components to the tissue engineering community. Given their role in the regulation of processes important for development, stem cell differentiation, and tissue repair, matricellular proteins represent a promising resource for engineered “smart” biomaterials. The ability to incorporate specific functional domains of matricellular macromolecules into scaffolds provides another level of control over cellular responses to selected biomaterials. Matricellular-based engineered biomaterials could conceivably regulate angiogenesis, growth factor and protease activity, cell survival, migration, and differentiation. In addition, matricellular proteins such as TSPs-1 and 2, fibulins, OPN, and SPARC can regulate assembly and organization of other extracellular matrix components and thus modify cellular responses to biomaterials (Bale and Mosher, 1986; Giachelli et al., 1995; Kyriakides et al., 1999; Bradshaw et al., 2003b; Papke and Yanagisawa, 2014-in this issue). As matricellular proteins can regulate stem cell differentiation in different niches, these proteins or fragments thereof are candidates for biomaterial applications (Bailey Dubose et al., 2012; Kaur et al., 2013; Liu and Leask, 2013; Wang et al., 2013; Chiquet-Ehrismann et al., 2014; Jose et al., 2014; Lee et al., 2014b). Decellularized tissue-specific extracellular matrices have been widely used in regenerative medicine (Faulk et al., 2014). Artificial matrices derived from tissues with cells either lacking or overexpressing a particular matricellular molecule could direct host cellular responses via alteration of tissue repair processes such as angiogenesis, matrix assembly, and myofibroblast induction (Barker et al., 2005b; Elliott et al., 2012; Calabro et al., 2014; Morris and Kyriakides, 2014-in this issue). For example, coating either smooth or etched titanium implants with periostin increased cell adhesion as compared to metal surfaces without periostin, although periostin only improved osteoblastic differentiation of cells adherent to smooth titanium surface, suggesting that perhaps the surface bound conformation of periostin can differentially regulate cell behavior (Galli et al., 2013). One can also take advantage of sequence-specific matricellular interactions with growth factors or receptors to target molecules to biologic scaffolds: one of the TGF-β binding motifs of TSP-1 (GGWSHW) was coupled to a scaffold to localize TGF-β to hydrogels for local growth factor delivery (McCall et al., 2011).
Matricellular proteins also play important roles in tissue responses to biomaterials, for example TSP-2 and SPARC in host responses to foreign bodies (Kyriakides and Bornstein, 2003; Puolakkainen et al., 2003; Barker et al., 2005a; Kyriakides and Maclauchlan, 2009; Tian and Kyriakides, 2009; Morris and Kyriakides, 2014-in this issue). Pallero et al. showed that metal ions such as those released from metal-based implants stimulate increased TSP-1 expression and TGF-β activation, suggestive of a potential role in implant failure and encapsulation (Pallero et al., 2010). Because these matricellular proteins generally promote collagen deposition and fibrotic responses to foreign materials, attenuation of these activities could enhance successful tissue integration. This idea contrasts with the potentially beneficial roles of matricellular-based biomaterials in the promotion of “wound healing” and cellular differentiation, and highlights the complexity of matricellular function and especially, the mode of matricellular presentation to cells.
7. Being matricellular in 2014 and questions for the future
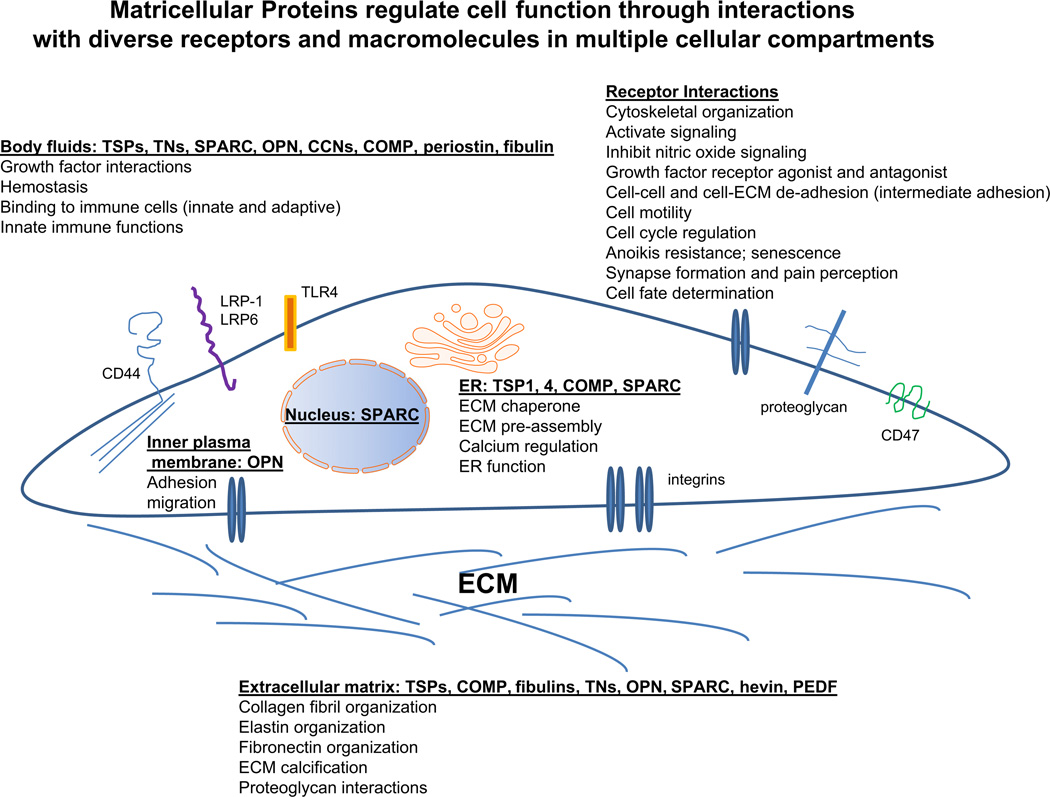
Matricellular proteins are important to more cellular functions and disease processes than we could have proposed in 1995. The original definition of matricellular still largely holds; today we have a greater appreciation of the significance of these modular proteins in the regulation of cell functions through an array of complex interactions with cellular receptors, soluble molecules, and ECM components. The context-dependent nature and the complexity of their roles and interactions are indeed greater than we initially thought (Fig. 1). If any part of Bornstein's original definition can be challenged, it is the finding that matricellular proteins can also have intracellular roles. However, with the exception of intracellular OPN which binds to the cytoplasmic domain of CD44, these roles to date have been primarily limited to locations in the nucleus and in the lumens or at the membranes of intracellular vesicular structures such as the endoplasmic reticulum. In this context, such vesicular lumens can be thought of as “inland lakes” mimicking the larger extracellular ocean where the matricellular protein can function as a soluble molecule or “at the shore” in the context of receptor binding at the plasma membrane. The chaperone function of matricellular proteins in the endoplasmic reticulum can similarly be considered as “intracellular matrix assembly;” e.g., Drosophila SPARC has been shown to be a chaperone facilitating the assembly of basement membrane (type IV) collagen (Martinek et al., 2008). The function(s) of nuclear SPARC remain elusive, despite reports of its cell-cycle dependent association with the nuclear matrix and of its uptake and translocation to the nuclei of lens epithelial cells (Gooden et al., 1999; Yan et al., 2005).
Fig. 1.
Matricellular proteins in multiple extracellular, cell membrane, and intracellular locations regulate cell function and ECM organization. This cartoon depicts the complexity of matricellular protein interactions with cell receptors and other interacting macromolecules in multiple extracellular and intracellular compartments and the resulting cellular functions regulated by these interactions. This diagram is not meant to be comprehensive, but it is intended to highlight examples of actions of matricellular proteins.
However, the translational potential of matricellular proteins has not been fully realized, partly due to their complexity and multifunctionality. What new knowledge do we need and how can we better frame our questions to utilize the potential therapeutic power of matricellular proteins? Is our current working definition of “matricellular” accurate, especially considering the intracellular roles of some of these proteins? Are there additional proteins or fragments of structural ECM proteins that could be considered “matricellular?” What can we learn from the study of one matricellular protein that applies or is relevant to other matricellular proteins? Clearly it is important to appreciate that the actions of one matricellular protein might oppose that of another. These questions and others are necessary to guide our thinking over the second twenty years of matricellular protein research and to advance knowledge and human health through matricellular-targeted therapeutics.
Acknowledgments
The authors acknowledge support from NIH (NIAMS and NIDDK) which supported the 2013 meeting (R13DK100244). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors express their appreciation to Dr. Mariya Sweetwyne, University of Pennsylvania, for her assistance with conceptualization of the figure. This issue is a celebration of the scientific legacy of Dr. Paul Bornstein and his contributions to the study of extracellular matrix and matricellular proteins.
Abbreviations
- CCN
cyr61-CTGF-NOV
- COMP
cartilage oligomeric protein
- CTGF
connective tissue growth factor
- DAMP
damage-associated molecular pattern
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- LRP
Low density lipoprotein receptor related protein
- PAI-1
plasminogen activator inhibitor 1
- PEDF
pigment epithelium derived factor
- SLRPs
small leucine rich proteoglycans
- SPARC
secreted protein acid and rich in cysteine
- STIM1
stromal interaction molecule 1
- TGF-β
transforming growth factor-β
- TN
tenascin
- TSP
thrombospondin
- TLR4
toll-like receptor 4
- VEGF
vascular endothelial growth factor
References
- Acharya C, Yik JH, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–111. doi: 10.1016/j.matbio.2014.06.001. in this issue. [DOI] [PubMed] [Google Scholar]
- Adams JC, Engel J. Bioinformaric analysis of adhesion proteins. Methods Mol. Biol. 2007;370:147–172. doi: 10.1007/978-1-59745-353-0_12. [DOI] [PubMed] [Google Scholar]
- Adams JC, Kureishy N, Taylor AL. A role for syndecan-1 in coupling fascin spike formation by thrombospondin-1. J. Cell Biol. 2001;152:1169–1182. doi: 10.1083/jcb.152.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E, Aymerich MS, Becerra SP. Binding of pigment epithelium-derived factor (PEDF) to retinoblastoma cells and cerebellar granule neurons. Evidence for a PEDF receptor. J. Biol. Chem. 1999;274:31605–31612. doi: 10.1074/jbc.274.44.31605. [DOI] [PubMed] [Google Scholar]
- Alcaraz LB, Exposito JY, Chuvin N, Pommier RM, Cluzel C, Martel S, Sentis S, Bartholin L, Lethias C, Valcourt U. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J. Cell Biol. 2014;205:409–428. doi: 10.1083/jcb.201308031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambily A, Kaiser WJ, Pierro C, Chamberlain EV, Li Z, Jones CL, Kassouf N, Gibbins JM, Authi KS. The role of plasma membrane STIM1 and Ca(2+)entry in platelet aggregation. STIM1 binds to novel proteins in human platelets. Cell. Signal. 2014;26:502–511. doi: 10.1016/j.cellsig.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson ML, Svensson B, Petersson IF, Hafstrom I, Albertsson K, Forslind K, Heinegard D, Saxne T. Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2013;14:229. doi: 10.1186/1471-2474-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol. 2009;11:1160–1169. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Asch AS, Silbiger S, Heimer E, Nachman RL. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem. Biophys. Res. Commun. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Asparuhova MB, Ferralli J, Chiquet M, Chiquet-Ehrismann R. The transcriptional regulator megakaryoblastic leukemia-1 mediates serum response factor-independent activation of tenascin-C transcription by mechanical stress. Faseb J. 2011;25:3477–3488. doi: 10.1096/fj.11-187310. [DOI] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF. Fispl2/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol. Cell. Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey Dubose K, Zayzafoon M, Murphy-Ullrich JE. Thrombospondin-1 inhibits osteogenic differentiation of human mesenchymal stem cells through latent TGF-beta activation. Biochem. Biophys. Res. Commun. 2012;422:488–493. doi: 10.1016/j.bbrc.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale MD, Mosher DF. Effects of thrombospondin on fibrin polymerization and structure. J. Biol. Chem. 1986;261:862–868. [PubMed] [Google Scholar]
- Barker TH, Baneyx G, Cardo-Vila M, Workman GA, Weaver M, Menon PM, Dedhar S, Rempel SA, Arap W, Pasqualini R, Vogel V, Sage EH. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005a;280:36483–36493. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- Barker TH, Framson P, Puolakkainen PA, Reed M, Funk SE, Sage EH. Matricellular homologs in the foreign body response: hevin suppresses inflammation, but hevin and SPARC together diminish angiogenesis. Am J. Pathol. 2005b;166:923–933. doi: 10.1016/S0002-9440(10)62312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea G, Grumet M, Milev P, Silvennoinen O, Levy JB, Sap J, Schlessinger J. Receptor tyrosine phosphatase beta is expressed in the form of proteoglycan and binds to the extracellular matrix protein tenascin. J. Biol. Chem. 1994;269:14349–14352. [PubMed] [Google Scholar]
- Barry ST, Ludbrook SB, Murrison E, Horgan CM. Analysis of the alpha4beta1 integrin-osteopontin interaction. Exp. Cell Res. 2000;258:342–351. doi: 10.1006/excr.2000.4941. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Meininger GA, Scholtz JM, Davis GE. Osteopontin is a ligand for the alpha4betal integrin. J. Cell Sci. 1998;111(Pt 9):1165–1174. doi: 10.1242/jcs.111.9.1165. [DOI] [PubMed] [Google Scholar]
- Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev. Cancer. 2013;13:258–271. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedore J, Leask A, Seguin CA. Targeting the extracellular matrix: matricellular proteins regulate cell-extracellular matrix communication within distinct niches of the intervertebral disc. Matrix Biol. 2014;37:124–130. doi: 10.1016/j.matbio.2014.05.005. in this issue. [DOI] [PubMed] [Google Scholar]
- Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am. J. Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Gao-Li J, Franco CA, Bouceba T, Huet A, Li Z. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J. Biol. Chem. 2009;284:10480–10490. doi: 10.1074/jbc.M809259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J. Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P, McPherson J, Sage H. Synthesis and secretion of structural macromolecules by endothelial cells in culture. In: Nossel HL, Vogel HJ, editors. Pathobiology of the Endothelial Cell. New York: Academic Press; 1982. pp. 215–228. [Google Scholar]
- Borsi L, Carnemolla B, Nicolo G, Spina B, Tanara G, Zardi L. Expression of different tenascin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int. J. Cancer. 1992;52:688–692. doi: 10.1002/ijc.2910520504. [DOI] [PubMed] [Google Scholar]
- Bourdon MA, Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J. Cell Biol. 1989;108:1149–1155. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc. Natl. Acad. Sci. U. S. A. 2003a;100:6045–6050. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J. Investig. Dermatol. 2003b;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J. Clin. Invest. 2003;111:487–495. doi: 10.1172/JCI16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Fiore VF, Sulchek TA, Barker TH. Physical and chemical microenvi-ronmental cues orthogonally control the degree and duration of fibrosis-associated epifhelial-to-mesenchymal transitions. J. Pathol. 2013;229:25–35. doi: 10.1002/path.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch GR, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet. 1997;17:104–108. doi: 10.1038/ng0997-104. [DOI] [PubMed] [Google Scholar]
- Burke A, Creighton W, Tavora F, Li L, Fowler D. Decreased frequency of the 3’UTR T>G single nucleotide polymorphism of thrombospondin-2 gene in sudden death due to plaque erosion. Cardiovasc. Pathol. 2010;19:e45–e49. doi: 10.1016/j.carpath.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Calabro NE, Kristofik NJ, Kyriakides TR. Thrombospondin-2 and extracellular matrix assembly. Biochim. Biophys. Acta. 2014;1840:2396–2402. doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, Roberts DD. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J. Biol. Chem. 2003;278:40679–40687. doi: 10.1074/jbc.M302014200. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, Mosher DF, Roberts DD. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J. Biol. Chem. 2004;279:41734–41743. doi: 10.1074/jbc.M406267200. [DOI] [PubMed] [Google Scholar]
- Chen H, Strickland DK, Mosher DF. Metabolism of thrombospondin 2. Binding and degradation by 3t3 cells and glycosaminoglycan-variant Chinese hamster ovary cells. J. Biol. Chem. 1996;271:15993–15999. doi: 10.1074/jbc.271.27.15993. [DOI] [PubMed] [Google Scholar]
- Chen Y, Abraham DJ, Shi-Wen X, Pearson JD, Black CM, Lyons KM, Leask A. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol. Biol. Cell. 2004;15:5635–5646. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FH, Thomas AO, Hecht JT, Goldring MB, Lawler J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J. Biol. Chem. 2005;280:32655–32661. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Leask A, Abraham DJ, Kennedy L, Shi-Wen X, Denton CR, Black CM, Verjee LS, Eastwood M. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 2011;4:9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Kakan X, Wang S, Dong W, Jia A, Cai C, Zhang J. Deletion of clock gene Per2 exacerbates cholestatic liver injury and fibrosis in mice. Exp. Toxicol. Pathol. 2013;65:427–432. doi: 10.1016/j.etp.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Chen PH, Chen X, Lin Z, Fang D, He X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013;27:1345–1350. doi: 10.1101/gad.219915.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. Tenascins in stem cell niches. Matrix Biol. 2014;37:112–123. doi: 10.1016/j.matbio.2014.01.007. in this issue. [DOI] [PubMed] [Google Scholar]
- Chlenski A, Liu S, Baker LJ, Yang Q, Tian Y, Salwen RR, Cohn SL. Neuroblastoma angiogenesis is inhibited with a folded synthetic molecule corresponding to the epidermal growth factor-like module of the follistatin domain of SPARC. Cancer Res. 2004;64:7420–7425. doi: 10.1158/0008-5472.CAN-04-2141. [DOI] [PubMed] [Google Scholar]
- Chlenski A, Guerrero LJ, Salwen HR, Yang Q, Tian Y, Morales La Madrid A, Mirzoeva S, Bouyer PG, Xu D, Walker M, Cohn SL. Secreted protein acidic and rich in cysteine is a matrix scavenger chaperone. PLoS One. 2011;6:e23880. doi: 10.1371/journal.pone.0023880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EH, Cho KH, Lee HA, Kim SW. High serum osteopontin levels are associated with low bone mineral density in postmenopausal women. J. Korean Med. Sci. 2013;28:1496–1499. doi: 10.3346/jkms.2013.28.10.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J. Cell Biol. 1994;126:539–548. doi: 10.1083/jcb.126.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol. Biol. Cell. 1996;7:883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment—where there's SPARC, there's fire. J. Cell. Biochem. 2008;104:721–732. doi: 10.1002/jcb.21688. [DOI] [PubMed] [Google Scholar]
- Corsetti JP, Ryan D, Moss AJ, McCarthy J, Goldenberg I, Zareba W, Sparks CE. Thrombospondin-4 polymorphism (A387P) predicts cardiovascular risk in postinfarction patients with high HDL cholesterol and C-reactive protein levels. Thromb. Haemost. 2011;106:1170–1178. doi: 10.1160/TH11-03-0206. [DOI] [PubMed] [Google Scholar]
- Coustry F, Posey KL, Liu P, Alcorn JL, Hecht JT. D469del-COMP retention in chondrocytes stimulates caspase-independent necroptosis. Am. J. Pathol. 2012;180:738–748. doi: 10.1016/j.ajpath.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J. Biol. Chem. 2008;283:17991–18001. doi: 10.1074/jbc.M708029200. [DOI] [PubMed] [Google Scholar]
- Craword SE, Fitchev P, Veliceasa D, Volpert OV. The many facets of PEDF in drug discovery and disease: a diamond in the rough or split personality disorder? Expert Opin. Drug Discov. 2013;8:769–792. doi: 10.1517/17460441.2013.794781. [DOI] [PubMed] [Google Scholar]
- Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J. Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ. Res. 2008;102:1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- Daniel C, Wagner A, Hohenstein B, Hugo C. Thrombospondin-2 therapy ameliorates experimental glomerulonephritis via inhibition of cell proliferation, inflammation, and TGF-beta activation. Am. J. Physiol. Ren. Physiol. 2009;297:F1299–F1309. doi: 10.1152/ajprenal.00254.2009. [DOI] [PubMed] [Google Scholar]
- Daniel C, Vogelbacher R, Stief A, Grigo C, Hugo C. Long-term gene therapy with thrombospondin 2 inhibits TGF-beta activation, inflammation and angiogenesis in chronic allograft nephropathy. PLoS One. 2013;8:e83846. doi: 10.1371/journal.pone.0083846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen M, Brellier F, Kain R, Ruiz C, Terracciano L, Orend G, Chiquet-Ehrismann R. Tenascin-W is a novel marker for activated tumor stroma in low-grade human breast cancer and influences cell behavior. Cancer Res. 2007;67:9169–9179. doi: 10.1158/0008-5472.CAN-07-0666. [DOI] [PubMed] [Google Scholar]
- Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Invest. 2000;105:1325. doi: 10.1172/JCI7039C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda S, Reichardt LF, Muller U. Identification of osteopontin as a novel ligand for the integrin alpha8 betal and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol. Biol. Cell. 1998;9:1425–1435. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J, Morgan MK, Graf MR, Fuss B. P2Y12 receptor expression is a critical determinant of functional responsiveness to ATX's MORFO domain. Purinergic Signal. 2012;8:181–190. doi: 10.1007/s11302-011-9283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande M, Notari L, Subramanian P, Notario V, Becerra SP. Inhibition of tumor cell surface ATP synthesis by pigment epithelium-derived factor: implications for antitumor activity. Int J. Oncol. 2012;41:219–227. doi: 10.3892/ijo.2012.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews M, Fox JL, Hultine S, Sundaram P, Wang W, Liu YY, Furth E, Enders GH, El-Deiry W, Schelter JM, Cleary MA, Thomas-Tikhonenko A. The myc-miR-17-92 axis blunts TGF{beta} signaling and production of multiple TGF{beta}-dependent antiangiogenic factors. Cancer Res. 2010;70:8233–8246. doi: 10.1158/0008-5472.CAN-10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogar AM, Semplicio G, Guennewig B, Hall J. Multiple microRNAs derived from chemically synthesized precursors regulate thrombospondin 1 expression. Nucleic Acids Ther. 2014;24(2):149–159. doi: 10.1089/nat.2013.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drott CJ, Olerud J, Emanuelsson H, Christoffersson G, Carlsson PO. Sustained beta-cell dysfunction but normalized islet mass in aged thrombospondin-1 deficient mice. PLoS One. 2012;7:e47451. doi: 10.1371/journal.pone.0047451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Takeuchi H, Leonhard-Melief C, Shroyer KR, Dlugosz M, Haltiwanger RS, Holdener BC. O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation. Dev. Biol. 2010;346:25–38. doi: 10.1016/j.ydbio.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette M, Nadler M, Okuhara D, Thompson J, Shuttleworth T, Lawler J. Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity. Matrix Biol. 2014;37:15–24. doi: 10.1016/j.matbio.2014.05.004. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula RV, Shankland SJ. Mechanical strain increases SPARC levels in podocytes: implications for glomerulosclerosis. Am. J. Physiol. Ren. Physiol. 2005;289:F577–F584. doi: 10.1152/ajprenal.00393.2004. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Exposito JY, Garrone R, Lethias C. Cell adhesion to tenascin-X mapping of cell adhesion sites and identification of integrin receptors. Eur. J. Biochem. 1999;263:840–848. doi: 10.1046/j.1432-1327.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, Leask A, Conway SJ, Hamilton DW. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J. Cell Sci. 2012;125:121–132. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RO, Sage EH, Ghosh JG, Clark JL. Chaperone-like activity revealed in the matricellular protein SPARC. J. Cell. Biochem. 2006;98:701–705. doi: 10.1002/jcb.20867. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Gene knockouts of c-src, transforming growth factor beta 1, and tenascin suggest superfluous, nonfunctional expression of proteins. J. Cell Biol. 1993;120:1079–1081. doi: 10.1083/jcb.120.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62:580–588. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. J. Cell. Physiol. 2014;229(8):984–989. doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- Ferrari do Outeiro-Bernstein MA, Nunes SS, Andrade AC, Alves TR, Legrand C, Morandi V. A recombinant NH(2)-terminal heparin-binding domain of the adhesive glycoprotein, thrombospondin-1, promotes endothelial tube formation and cell survival: a possible role for syndecan-4 proteoglycan. Matrix Biol. 2002;21:311–324. doi: 10.1016/s0945-053x(02)00010-0. [DOI] [PubMed] [Google Scholar]
- Finlin BS, Zhu B, Starnes CP, McGehee RE, Jr, Peterson CA, Kern PA. Regulation of thrombospondin-1 expression in alternatively activated macrophages and adipocytes: role of cellular cross talk and omega-3 fatty acids. J. Nutr. Biochem. 2013;24:1571–1579. doi: 10.1016/j.jnutbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchev P, Chung C, Plunkett BA, Brendler CB, Crawford SE. Pedf & stem cells: niche vs. nurture. Curr. Drug Deliv. 2013;24(2):149–159. doi: 10.2174/156720181105140922122754. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fluck M, Mund SI, Schittny JC, Klossner S, Durieux AC, Giraud MN. Mechano-regulated tenascin-C orchestrates muscle repair. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13662–13667. doi: 10.1073/pnas.0805365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C, Piergianni M, Piemontese M, Lumetti S, Ravanetti E, Cacchioli A, Macaluso GM, Passed G. Periostin improves cell adhesion to implantable biomaterials and osteoblastic differentiation on implant titanium surfaces in a topography-dependent fashion. J. Biomed. Mater. Res. A. 2013 doi: 10.1002/jbm.a.35056. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Galvez AF, Huang L, Magbanua MM, Dawson K, Rodriguez RL. Differential expression of thrombospondin (THBS1) in tumorigenic and nontumorigenic prostate epithelial cells in response to a chromatin-binding soy peptide. Nutr. Cancer. 2011;63:623–636. doi: 10.1080/01635581.2011.539312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Garg P, Yang S, Liu A, Pallero MA, Buchsbaum DJ, Mosher DF, Murphy-Ullrich JE, Goldblum SE. Thrombospondin-1 opens the paracellular pathway in pulmonary microvascular endothelia through EGFR/ErbB2 activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;301:L79–L90. doi: 10.1152/ajplung.00287.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood J, Rigato F, Heck N, Faissner A. Tenascin glycoproteins and the complementary ligand DSD-1-PG/phosphacan—structuring the neural extracellular matrix during development and repair. Restor. Neurol. Neurosci. 2001;19:51–64. [PubMed] [Google Scholar]
- Gattu AK, Swenson ES, Iwakiri Y, Samuel VT, Troiano N, Berry R, Church CD, Rodeheffer MS, Carpenter TO, Chung C. Determination of mesenchymal stem cell fate by pigment epithelium-derived factor (PEDF) results in increased adiposity and reduced bone mineral content. Faseb J. 2013;27:4384–4394. doi: 10.1096/fj.13-232900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattu AK, Birkenfeld AL, Iwakiri Y, Jay S, Saltzman M, Doll J, Protiva P, Samuel VT, Crawford SE, Chung C. Pigment epithelium-derived factor (PEDF) suppresses IL-lbeta-mediated c-Jun N-terminal kinase (JNK) activation to improve hepatocyte insulin signaling. Endocrinology. 2014 doi: 10.1210/en.2013-1785. en20131785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellhaus A, Schmidt M, Dunk C, Lye SJ, Winterhager E. The circulating proangiogenic factors CYR61 (CCN1) and NOV (CCN3) are significantly decreased in placentae and sera of preeclamptic patients. Reprod. Sci. 2007;14:46–52. doi: 10.1177/1933719107309816. [DOI] [PubMed] [Google Scholar]
- Ghert MA, Qi WN, Erickson HP, Block JA, Scully SP. Tenascin-C expression and distribution in cultured human chondrocytes and chondrosarcoma cells. J. Orthop. Res. 2002;20:834–841. doi: 10.1016/S0736-0266(01)00172-3. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Schwartz SM, Liaw L. Molecular and cellular biology of osteopontin: potential role in cardiovascular disease. Trends Cardiovasc. Med. 1995;5:88–95. doi: 10.1016/1050-1738(95)00005-T. [DOI] [PubMed] [Google Scholar]
- Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V) beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- Gimba ER, Tilli TM. Human osteopontin splicing isoforms: known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013;331:11–17. doi: 10.1016/j.canlet.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Godbout C, Follonier Castella L, Smith EA, Talele N, Chow ML, Garonna A, Hinz B. The mechanical environment modulates intracellular calcium oscillation activities of myofibroblasts. PLoS One. 2013;8:e64560. doi: 10.1371/journal.pone.0064560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J. Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea S, Orr AW, Pallero MA, Eggleton P, Murphy-Ullrich JE. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J. Biol. Chem. 2000;275:36358–36368. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- Gooden MD, Vernon RB, Bassuk JA, Sage EH. Cell cycle-dependent nuclear location of the matricellular protein SPARC: association with the nuclear matrix. J. Cell. Biochem. 1999;74:152–167. [PubMed] [Google Scholar]
- Greco SA, Chia J, Jnglis KJ, Cozzi SJ, Ramsnes I, Buttenshaw RL, Spring KJ, Boyle GM, Worthley DL, Leggett BA, Whitehall VL. Thrombospondin-4 is a putative tumour-suppressor gene in colorectal cancer that exhibits age-related methylation. BMC Cancer. 2010;10:494. doi: 10.1186/1471-2407-10-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PM, Ludbrook SB, Miller DD, Horgan CM, Barry ST. Structural elements of the osteopontin SWYGLR motif important for the interaction with alpha(4) integrins. FEBS Lett. 2001;503:75–79. doi: 10.1016/s0014-5793(01)02690-4. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Sage EH, Norton HJ, Funk S, Ingram J, Hanley EN., Jr Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J. Histochem. Cytochem. 2005;53:1131–1138. doi: 10.1369/jhc.5A6687.2005. [DOI] [PubMed] [Google Scholar]
- Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J. Cell Commun. Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankenson KD, Sweetwyne MT, Shitaye H, Posey KL. Thrombospondins and novel TSR-containing proteins, R-spondins, regulate bone formation and remodeling. Curr. Osteoporos. Rep. 2010;8:68–76. doi: 10.1007/s11914-010-0017-0. [DOI] [PubMed] [Google Scholar]
- Hanna M, Liu H, Amir J, Sun Y, Morris SW, Siddiqui MA, Lau LF, Chaqour B. Mechanical regulation of the proangiogenic factor CCN1/CYR61 gene requires the combined activities of MRTF-A and CREB-binding protein histone acetyltransferase. J. Biol. Chem. 2009;284:23125–23136. doi: 10.1074/jbc.M109.019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin Y, Gharbi M, Mazzucchelli G, Dubuc JE, De Pauw E, Deberg M. Fibulin 3 peptides Fib3-1 and Fib3-2 are potential biomarkers of osteoarthritis. Athritis Rheum. 2012;64:2260–2267. doi: 10.1002/art.34392. [DOI] [PubMed] [Google Scholar]
- Hoffmann BR, Liu Y, Mosher DF. Modification of EGF-like module 1 of thrombospondin-1, an animal extracellular protein, by O-linked N-acetylglucosamine. PLoS One. 2012;7:e32762. doi: 10.1371/journal.pone.0032762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, Peter-Katalinic J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J. Biol. Chem. 2001;276:6485–6498. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- Holliday CJ, Ankeny RF, Jo R, Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J. Physiol. Heart Circ. Physiol. 2011;301:H856–H867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61:8586–8594. [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Manabe S, Kanda M, Kawano H, Nakayama T, Sekine I, Kondo T, Ito Y. Increased expression of protein C-mannosylation in the aortic vessels of diabetic Zucker rats. Glycobiology. 2005;15:383–392. doi: 10.1093/glycob/cwi012. [DOI] [PubMed] [Google Scholar]
- Inoue K, Jinnin M, Hara Y, Makino K, Kajihara I, Makino T, Sakai K, Fukushima S, Inoue Y, Ihn H. Serum levels of tenascin-C in collagen diseases. J. Dermatol. 2013;40:715–719. doi: 10.1111/1346-8138.12218. [DOI] [PubMed] [Google Scholar]
- Inoue M, Jiang Y, Barnes II RH, Tokunaga M, Martinez-Santibanez G, Geletka L, Lumeng CN, Buchner DA, Chun TH. Thrombospondin 1 mediates high-fat dietinduced muscle fibrosis and insulin resistance in male mice. Endocrinology. 2013;154:4548–4559. doi: 10.1210/en.2013-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Goldoni S, Berendsen AD, Young MF. Small leucine-rich proteoglycans. In: Mecham RP, editor. The Extracellular Matrix: An Overview, Biology of Extracellular Matrix. Berlin Heidelberg: Springer-Verlag; 2011. pp. 197–229. [Google Scholar]
- Iyer AK, Tran KT, Borysenko CW, Cascio M, Camacho CJ, Blair RC, Bahar I, Wells A. Tenascin cytotactin epidermal growth factor-like repeat binds epidermal growth factor receptor with low affinity. J. Cell. Physiol. 2007;211:748–758. doi: 10.1002/jcp.20986. [DOI] [PubMed] [Google Scholar]
- Jensen MA, Wilkinson JE, Krainer AR. Splicing factor SRSF6 promotes hyperplasia of sensitized skin. Nat Struct Mol. Biol. 2014;21(2):189–197. doi: 10.1038/nsmb.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, Bradding P, Fahy JV, Woodruff PG, Harris JM, Arron JR. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J. Allergy Clin. Immunol. 2012;130(647–654):e610. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Wei XF, Yi DH, Xu P, Liu H, Chang Q, Yang SM, Li ZE, Gao HB, Hao GJ. Synergistic effects of cyclic strain and Th1-like cytokines on tenascin-C production by rheumatic aortic valve interstitial cells. Clin. Exp. Immunol. 2009;155:216–223. doi: 10.1111/j.1365-2249.2008.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YR, Yoon JK. The R-spondin family of proteins: emerging regulators of WNT signaling. Int. J. Biochem. Cell Biol. 2012;44:2278–2287. doi: 10.1016/j.biocel.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MW, Annis DS, Mosher DF. Alpha(M)beta(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am J. Respir. Cell Mol. Biol. 2013;48:503–510. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J. Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose S, Hughbanks ML, Binder BY, Ingavle GC, Kent Leach J. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014;10(5):1955–1964. doi: 10.1016/j.actbio.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoglou NP, Kottas G, Lampropoulos S, Vitta L, Liapis CD. Serum levels of fetuin-A osteoprotegerin and osteopontin in patients with coronary artery disease: effects of statin (HMGCoA-reductase inhibitor) therapy. Clin. Drug Investig. 2013;34(3):165–171. doi: 10.1007/s40261-013-0157-y. [DOI] [PubMed] [Google Scholar]
- Karatas Z, Baysal T, Alp H, Toker A. Serum tenascin-C: a novel biomarker for diagnosis and predicting prognosis of rheumatic carditis? J. Trap. Pediatr. 2013;59:476–482. doi: 10.1093/tropej/fmt058. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Nielsen MJ, Sand JM, Henriksen K, Genovese F, Bay-Jensen AC, Smith V, Adamkewicz JL, Christiansen C, Leeming DJ. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev. Technol. 2013;11:70–92. doi: 10.1089/adt.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri YU, Sleeman J, Fujii H, Herrlich R, Hotta R, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers AF, Uede T. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- Katoh D, Nagaharu K, Shimojo N, Hanamura N, Yamashita M, Kozuka Y, Imanaka-Yoshida K, Yoshida T. Binding of alphavbetal and alphavbeta6 integrins to tenascin-C induces epithelial-mesenchymal transition-like change of breast cancer cells. Oncogenesis. 2013;2:e65. doi: 10.1038/oncsis.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Jsenberg JS, Roberts DD. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci. Rep. 2013;3:1673. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J. Cell. Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- Kazerounian S, Duquette M, Reyes MA, Lawler JT, Song K, Perruzzi C, Primo L, Khosravi-Far R, Bussolino F, Rabinovitz I, Lawler J. Priming of the vascular endothelial growth factor signaling pathway by thrombospondin-1, CD36, and spleen tyrosine kinase. Blood. 2011;117:4658–4666. doi: 10.1182/blood-2010-09-305284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher FC, Rao A, Maguire A. Orcadian molecular clocks and cancer. Cancer Lett. 2014;342:9–18. doi: 10.1016/j.canlet.2013.09.040. [DOI] [PubMed] [Google Scholar]
- Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol. Vis. Sci. 2007;48:1164–1172. doi: 10.1167/iovs.06-0875. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE, Weissleder R. SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration. J. Leukoc. Biol. 2007;81:748–756. doi: 10.1189/jlb.1105664. [DOI] [PubMed] [Google Scholar]
- Kim DS, Li KW, Boroujerdi A, Peter Yu Y, Zhou CY, Deng P, Park J, Zhang X, Lee J, Corpe M, Sharp K, Steward O, Eroglu C, Barres B, Zaucke F, Xu ZC, Luo ZD. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J. Neurosci. 2012;32:8977–8987. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MN, Hankenson KD. R-spondins: novel matricellular regulators of the skeleton. Matrix Biol. 2014;37:156–160. doi: 10.1016/j.matbio.2014.06.003. in this issue. [DOI] [PubMed] [Google Scholar]
- Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am. J. Physiol. Endocrinol. Metab. 2013;305:E439–E450. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos K, Wilding JP. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat. Rev. Endocrinol. 2010;6:225–235. doi: 10.1038/nrendo.2010.18. [DOI] [PubMed] [Google Scholar]
- Kotani K, Yamada T, Taniguchi N. The association between circulating secreted protein acidic and rich in cysteine (SPARC) and glycosylated haemoglobin (HbA(1c)) during lifestyle-modified weight reduction intervention in obese male subjects. J. Int. Med. Res. 2011;39:528–532. doi: 10.1177/147323001103900221. [DOI] [PubMed] [Google Scholar]
- Krutzsch HC, Choe BJ, Sipes JM, Guo N, Roberts DD. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J. Biol. Chem. 1999;274:24080–24086. doi: 10.1074/jbc.274.34.24080. [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins hevin and SPARC. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: implications for bone disease. Blood. 2009;114:3413–3421. doi: 10.1182/blood-2009-03-211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: a new class of inflammation modulators? Biochimie. 2011;93:377–388. doi: 10.1016/j.biochi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Kular L, Rivat C, Lelongt B, Calmel C, Laurent M, Pohl M, Kitabgi P, Melik-Parsadaniantz S, Martinerie C. NOV/CCN3 attenuates inflammatory pain through regulation of matrix metalloproteinases-2 and -9. J. Neuroinflammation. 2012;9:36. doi: 10.1186/1742-2094-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumei Y, Morita S, Katano H, Akiyama H, Hirano M, Oyha K, Shimokawa H. Microgravity signal ensnarls cell adhesion, cytoskeleton, and matrix proteins of rat osteoblasts: osteopontin, CD44, osteonectin, and alpha-tubulin. Ann. N. Y. Acad. Sci. 2006;1090:311–317. doi: 10.1196/annals.1378.034. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 2003;90:986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J. Cell Commun. Signal. 2009;3:215–225. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4449–4454. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kzhyshkowska J, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Gratchev A, Krusell L, Goerdt S, Sage EH. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J. Immunol. 2006;176:5825–5832. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- Lane TF, Sage EH. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J. Cell Biol. 1990;111:3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. Faseb J. 1994;8:163–173. [PubMed] [Google Scholar]
- Lane TF, Iruela-Arispe ML, Johnson RS, Sage EH. SPARC is a source of copperbinding peptides that stimulate angiogenesis. J. Cell Biol. 1994;125:929–943. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell. Mol. Life Sci. 2011;68:3149–3163. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Hynes RO. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989;74:2022–2027. [PubMed] [Google Scholar]
- Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and −2. Cold Spring Harb. Perspect. Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J. Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Heo YS, Park HS, Lee SH, Lee SK, Jang YJ. Serum SPARC and matrix metalloproteinase-2 and metalloproteinase-9 concentrations after bariatric surgery in obese adults. Obes. Surg. 2014a;24:604–610. doi: 10.1007/s11695-013-1111-z. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014b;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard-Melief C, Haltiwanger RS. O-fucosylation of thrombospondin type 1 repeats. Methods Enzymol. 2010;480:401–416. doi: 10.1016/S0076-6879(10)80018-7. [DOI] [PubMed] [Google Scholar]
- Lethias C, Elefteriou F, Parsiegla G, Exposito JY, Garrone R. Identification and characterization of a conformational heparin-binding site involving two fibronectin type III modules of bovine tenascin-X. J. Biol. Chem. 2001;276:16432–16438. doi: 10.1074/jbc.M010210200. [DOI] [PubMed] [Google Scholar]
- Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6:e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Li CM, Wang Z. The role of circadian clocks in metabolic disease. Yale J. Biol. Med. 2012;85:387–401. [PMC free article] [PubMed] [Google Scholar]
- Li KW, Kim DS, Zaucke F, Luo ZD. Trigeminal nerve injury-induced thrombospondin-4 up-regulation contributes to orofacial neuropathic pain states in a rat model. Eur. J. Pain. 2014;18(4):489–495. doi: 10.1002/j.1532-2149.2013.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw L, Lindner V, Schwartz SM, Chambers AF, Giachelli CM. Osteopontin and beta 3 integrin are coordinately expressed in regenerating endothelium in vivo and stimulate Arg-Gly-Asp-dependent endothelial migration in vitro. Circ. Res. 1995a;77:665–672. doi: 10.1161/01.res.77.4.665. [DOI] [PubMed] [Google Scholar]
- Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM, Giachelli CM. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J. Clin. Invest. 1995b;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J. Biol. Chem. 2005;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- Lindner DJ, Wu Y, Haney R, Jacobs BS, Fruehauf JP, Tuthill R, Borden EC. Thrombospondin-1 expression in melanoma is blocked by methylation and targeted reversal by 5-Aza-deoxycytidine suppresses angiogenesis. Matrix Biol. 2013;32:123–132. doi: 10.1016/j.matbio.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Leask A. CCN2 modulates hair follicle cycling in mice. Mol. Biol. Cell. 2013;24:3939–3944. doi: 10.1091/mbc.E13-08-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Garg P, Yang S, Gong P, Pallero MA, Annis DS, Liu Y, Passaniti A, Mann D, Mosher DF, Murphy-Ullrich JE, Goldblum SE. Epidermal growth factor-like repeats of thrombospondins activate phospholipase Cgamma and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J. Biol. Chem. 2009;284:6389–6402. doi: 10.1074/jbc.M809198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014;37:150–155. doi: 10.1016/j.matbio.2014.04.007. in this issue. [DOI] [PubMed] [Google Scholar]
- Lomas AC, Mellody KT, Freeman LJ, Bax DV, Shuttleworth CA, Kielty CM. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. Biochem. J. 2007;405:417–428. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am. J. Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Kojima K, Battula VL, Korchin B, Shi Y, Chen Y, Spong S, Thomas DA, Kantarjian H, Lock RB, Andreeff M, Konopleva M. Targeting connective tissue growth factor (CTGF) in acute lymphoblastic leukemia preclinical models: anti-CTGF monoclonal antibody attenuates leukemia growth. Ann. Hematol. 2014;93:485–492. doi: 10.1007/s00277-013-1939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Wang W, Jia WD, Sun QK, Huang M, Zhou HC, Xia HH, Liu WB, Chen H, Sun SN, Xu GL. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur. J. Surg. Oncol. 2013;39:1129–1135. doi: 10.1016/j.ejso.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H, Prywes R, Lorenz JN, Mori K, Lawler J, Robbins J, Molkentin JD. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149:1257–1268. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Lutz R, Gelman L, Sarasa-Renedo A, Schenk S, Grashoff C, Chiquet M. Tenascin-C induction by cyclic strain requires integrin-linked kinase. Biochim. Biophys. Acta. 2008;1783:1150–1162. doi: 10.1016/j.bbamcr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Maloney JP, Stearman RS, Bull TM, Calabrese DW, Tripp-Addison ML, Wick MJ, Broeckel U, Robbins IM, Wheeler LA, Cogan JD, Loyd JE. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L541–L554. doi: 10.1152/ajplung.00282.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardani M, Andisheh-Tadbir A, Khademi B, Fattahi MJ, Shafiee S, Asad-Zadeh M. Serum levels of osteopontin as a prognostic factor in patients with oral squamous cell carcinoma. Tumour Biol. 2014;35:3827–3829. doi: 10.1007/s13277-013-1506-4. [DOI] [PubMed] [Google Scholar]
- Martinek N, Shahab J, Sodek J, Ringuette M. Is SPARC an evolutionarily conserved collagen chaperone? J. Dent. Res. 2007;86:296–305. doi: 10.1177/154405910708600402. [DOI] [PubMed] [Google Scholar]
- Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J. Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- Mason RM. Connective tissue growth factor(CCN2), a pathogenic factor in diabetic nephropathy. What does it do? How does it do it? J. Cell Commun. Signal. 2009;3:95–104. doi: 10.1007/s12079-009-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RM. Fell-Muir lecture: connective tissue growth factor (CCN2) — a pernicious and pleiotropic player in the development of kidney fibrosis. Int. J. Exp. Pathol. 2013;94:1–16. doi: 10.1111/j.1365-2613.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel P, Rauch U, Flad M, Margolis RK, Margolis RU. Phosphacan, a chondroitin sulfate proteoglycan of brain that interacts with neurons and neural cell-adhesion molecules, is an extracellular variant of a receptor-type protein tyrosine phosphatase. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2512–2516. doi: 10.1073/pnas.91.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JD, Lin CC, Anseth KS. Affinity peptides protect transforming growth factor beta during encapsulation in poly(ethylene glycol) hydrogels. Biomacromolecules. 2011;12:1051–1057. doi: 10.1021/bm101379v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucinerich proteoglycans (SLRPs) J. Cell Commun. Signal. 2009;3:323–335. doi: 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- Mikhailenko I, Kounnas MZ, Strickland DK. Low density lipoprotein receptorrelated protein/alpha 2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J. Biol. Chem. 1995;270:9543–9549. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Tajerian M, Naso L, Sage EH, Stone LS. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC-null mice. Pain. 2012;153:1167–1179. doi: 10.1016/j.pain.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Moiseeva EP, Javed Q, Spring EL, de Bono DP. Galectin 1 is involved in vascular smooth muscle cell proliferation. Cardiovasc. Res. 2000;45:493–502. doi: 10.1016/s0008-6363(99)00276-x. [DOI] [PubMed] [Google Scholar]
- Morris AH, Kyriakides TR. Matricellular proteins and biomaterials. Matrix Biol. 2014;37:181–189. doi: 10.1016/j.matbio.2014.03.002. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF, Adams JC. Adhesion-modulating/matricellular ECM protein families: a structural, functional and evolutionary appraisal. Matrix Biol. 2012;31:155–161. doi: 10.1016/j.matbio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Abbott-Brown D, Raugi GJ, Bornstein P. Regulation of thrombospondin secretion by cells in culture. J. Cell. Physiol. 1984;120:280–288. doi: 10.1002/jcp.1041200304. [DOI] [PubMed] [Google Scholar]
- Muroi E, Manabe S, Ikezaki M, Urata Y, Sato S, Kondo T, Ito Y, Ihara Y. C-mannosylated peptides derived from the thrombospondin type 1 repeat enhance lipopolysaccharide-induced signaling in macrophage-like RAW264.7 cells. Glycobiology. 2007;17:1015–1028. doi: 10.1093/glycob/cwm071. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J. Clin. Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Hook M. Thrombospondin modulates focal adhesions in endothelial cells. J. Cell Biol. 1989;109:1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Lightner VA, Aukhil I, Yan YZ, Erickson HP, Hook M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J. Cell Biol. 1991;115:1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J. Cell. Biochem. 1995;57:341–350. doi: 10.1002/jcb.240570218. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Nie J, Sage EH. SPARC functions as an inhibitor of adipogenesis. J. Cell Commun. Signal. 2009a;3:247–254. doi: 10.1007/s12079-009-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J. Biol. Chem. 2009b;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari L, Baladron V, Aroca-Aguilar JD, Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML, Sanchez-Sanchez F, Escribano J, Laborda J, Becerra SP. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J. Biol. Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Sakurai E, Raisler BJ, Baffi JZ, Witta J, Ogura Y, Brekken RA, Sage EH, Ambati BK, Ambati J. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J. Clin. Invest. 2006;116:422–429. doi: 10.1172/JCI26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes SS, Outeiro-Bernstein MA, Juliano L, Vardiero F, Nader HB, Woods A, Legrand C, Morandi V. Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1. J. Cell. Physiol. 2008;214:828–837. doi: 10.1002/jcp.21281. [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell. 2011;20:303–314. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, Carlsson PO. Thrombospondin-1: an islet endothelial cell signal of importance for beta-cell function. Diabetes. 2011;60:1946–1954. doi: 10.2337/db10-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006;244:143–163. doi: 10.1016/j.canlet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Orend G, Huang W, Olayioye MA, Hynes NE, Chiquet-Ehrismann R. Tenascin-C blocks cell-cycle progression of anchorage-dependent fibroblasts on fibronectin through inhibition of syndecan-4. Oncogene. 2003;22:3917–3926. doi: 10.1038/sj.onc.1206618. [DOI] [PubMed] [Google Scholar]
- Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcakir-Tomruk C, Chiquet M, Mericske-Stern R. Tenascin-C and matrix metalloproteinase-9 levels in crevicular fluid of teeth and implants. Clin. Implant. Dent. Relat. Res. 2012;14:672–681. doi: 10.1111/j.1708-8208.2010.00319.x. [DOI] [PubMed] [Google Scholar]
- Pallero MA, Talbert Roden M, Chen YF, Anderson PG, Lemons J, Brott BC, Murphy-Ullrich JE. Stainless steel ions stimulate increased thrombospondin-1-dependent TGF-beta activation by vascular smooth muscle cells: implications for in-stent restenosis. J. Vasc. Res. 2010;47:309–322. doi: 10.1159/000265565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke CL, Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: insights from mouse and human studies. Matrix Biol. 2014;37:142–149. doi: 10.1016/j.matbio.2014.02.004. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Lee K, Zhang B, Zhou T, He X, Gao G, Murray AR, Ma JX. Identification of a novel inhibitor of the canonical Wnt pathway. Mol. Cell. Biol. 2011;31:3038–3051. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parulekar AD, Atik MA, Hanania NA. Periostin, a novel biomarker of TH2-driven asthma. Curr. Opin. Pulm. Med. 2014;20:60–65. doi: 10.1097/MCP.0000000000000005. [DOI] [PubMed] [Google Scholar]
- Perbal B. Matricellular CCN proteins. J. Cell Commun. Signal. 2008;2:57. doi: 10.1007/s12079-008-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planque N, Long Li C, Saule S, Bleau AM, Perbal B. Nuclear addressing provides a clue for the transforming activity of amino-truncated CCN3 proteins. J. Cell. Biochem. 2006;99:105–116. doi: 10.1002/jcb.20887. [DOI] [PubMed] [Google Scholar]
- Pluskota E, Stenina OI, Krukovets I, Szpak D, Topol EJ, Plow EF. Mechanism and effect of thrombospondin-4 polymorphisms on neutrophil function. Blood. 2005;106:3970–3978. doi: 10.1182/blood-2005-03-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey KL, Veerisetty AC, Liu P, Wang HR, Poindexter BJ, Bick R, Alcorn JL, Hecht JT. An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am. J. Pathol. 2009;175:1555–1563. doi: 10.2353/ajpath.2009.090184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey KL, Alcorn JL, Hecht JT. Pseudoachondroplasia/COMP — translating from the bench to the bedside. Matrix Biol. 2014;37:166–171. doi: 10.1016/j.matbio.2014.05.006. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto AL, Edelman GM, Crossin KL. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc. Natl. Acad. Sci. U. S. A. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakkainen P, Bradshaw AD, Kyriakides TR, Reed M, Brekken R, Wight T, Bornstein P, Ratner B, Sage EH. Compromised production of extracellular matrix in mice lacking secreted protein, acidic and rich in cysteine (SPARC) leads to a reduced foreign body reaction to implanted biomaterials. Am. J. Pathol. 2003;162:627–635. doi: 10.1016/S0002-9440(10)63856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Qu C, Yamaza T, Yang R, Lin X, Duan XY, Akiyama K, Liu Y, Zhang Q, Chen C, Chen Y, Qi HH, Feng XH, Le AD, Shi S. Ossifying fibroma tumor stem cells are maintained by epigenetic regulation of a TSP1/TGF-beta/SMAD3 autocrine loop. Cell Stem Cell. 2013;13:577–589. doi: 10.1016/j.stem.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rahman M, Chan AP, Tai IT. A peptide of SPARC interferes with the interaction between caspase8 and Bcl2 to resensitize chemoresistant tumors and enhance their regression in vivo. PLoS One. 2011;6:e26390. doi: 10.1371/journal.pone.0026390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014;37:83–91. doi: 10.1016/j.matbio.2014.01.012. in this issue. [DOI] [PubMed] [Google Scholar]
- Riser BL, Najmabadi F, Perbal B, Rambow JA, Riser ML, Sukowski E, Yeger H, Riser SC, Peterson DR. CCN3/CCN2 regulation and the fibrosis of diabetic renal disease. J. Cell Commun. Signal. 2010;4:39–50. doi: 10.1007/s12079-010-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31:170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Haverstick DM, Dixit VM, Frazier WA, Santoro SA, Ginsburg V. The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J. Biol. Chem. 1985;260:9405–9411. [PubMed] [Google Scholar]
- Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock MJ, Holden P, Horton WA, Cohn DH. Cartilage oligomeric matrix protein promotes cell attachment via two independent mechanisms involving CD47 and alphaVbeta3 integrin. Mol. Cell. Biochem. 2010;338:215–224. doi: 10.1007/s11010-009-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues RG, Guo N, Zhou L, Sipes JM, Williams SB, Templeton NS, Gralnick HR, Roberts DD. Conformational regulation of the fibronectin binding and alpha 3beta 1 integrin-mediated adhesive activities of thrombospondin-1. J. Biol. Chem. 2001;276:27913–27922. doi: 10.1074/jbc.M009518200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Jimenez FJ, Caldes T, Iniesta P, Vidart JA, Garcia-Asenjo JL, Benito M. Overexpression of SPARC protein contrasts with its transcriptional silencing by aberrant hypermethylation of SPARC CpG-rich region in endometrial carcinoma. Oncol. Rep. 2007;17:1301–1307. [PubMed] [Google Scholar]
- Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101. doi: 10.1016/j.matbio.2014.01.002. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhmann M, Piccinini AM, Kong PL, Midwood KS. Endogenous activation of adaptive immunity: tenascin-C drives interleukin-17 synthesis in murine arthritic joint disease. Arthritis Rheum. 2012;64:2179–2190. doi: 10.1002/art.34401. [DOI] [PubMed] [Google Scholar]
- Sadvakassova G, Dobocan MC, Congote LF. Osteopontin and the C-terminal peptide of thrombospondin-4 compete for CD44 binding and have opposite effects on CD133+ cell colony formation. BMC Res. Notes. 2009;2:215. doi: 10.1186/1756-0500-2-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage EH. Pieces of eight: bioactive fragments of extracellular proteins as regulators of angiogenesis. Trends Cell Biol. 1997a;7:182–186. doi: 10.1016/S0962-8924(97)01037-4. [DOI] [PubMed] [Google Scholar]
- Sage EH. Terms of attachment: SPARC and tumorigenesis. Nat. Med. 1997b;3:144–146. doi: 10.1038/nm0297-144. [DOI] [PubMed] [Google Scholar]
- Sage EH. Regulation of interactions between cells and extracellular matrix: a command performance on several stages. J. Clin. Invest. 2001;107:781–783. doi: 10.1172/JCI12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage EH. Discovery of SPARC as a prototype for matricellular proteins. In: Pasqualini R, Arap W, editors. Protein Discovery Technologies. New York: CRC Press; 2009. pp. 223–237. [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J. Biol. Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Sage EH, Reed M, Funk SE, Truong T, Steadele M, Puolakkainen P, Maurice DH, Bassuk JA. Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J. Biol. Chem. 2003;278:37849–37857. doi: 10.1074/jbc.M302946200. [DOI] [PubMed] [Google Scholar]
- Sahora AI, Rusk AW, Henkin J, McKeegan EM, Shi Y, Khanna C. Prospective study of thrombospondin-1 mimetic peptides, ABT-510 and ABT-898, in dogs with soft tissue sarcoma. J. Vet. Intern. Med. 2012;26:1169–1176. doi: 10.1111/j.1939-1676.2012.00966.x. [DOI] [PubMed] [Google Scholar]
- Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, Ohwaki T, Nagao O, Kamiya S, Hayashi R, Kodama H, Handa H, Yoshida T, Fukai F. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J. Biol. Chem. 2007;282:34929–34937. doi: 10.1074/jbc.M705608200. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, T akagi M, Li CL, Perbal B, Katsube K. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J. Biol. Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Hoshino Y, Misaka T, Mizukami H, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Takeishi Y. Serum tenascin-C level is associated with coronary plaque rupture in patients with acute coronary syndrome. Heart Vessels. 2014;29(2):165–170. doi: 10.1007/s00380-013-0341-2. [DOI] [PubMed] [Google Scholar]
- Salmivirta M, Elenius K, Vainio S, Hofer U, Chiquet-Ehrismann R, Thesleff I, Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J. Biol. Chem. 1991;266:7733–7739. [PubMed] [Google Scholar]
- Schalkwijk J, Zweers MC, Steijlen PM, Dean WB, Taylor G, van Vlijmen IM, van Haren B, Miller WL, Bristow J. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N. Engl. J. Med. 2001;345:1167–1175. doi: 10.1056/NEJMoa002939. [DOI] [PubMed] [Google Scholar]
- Scherberich A, Tucker RP, Degen M, Brown-Luedi M, Andres AC, Chiquet-Ehrismann R. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNF-alpha induced expression in vitro. Oncogene. 2005;24:1525–1532. doi: 10.1038/sj.onc.1208342. [DOI] [PubMed] [Google Scholar]
- Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol. Biol. Cell. 2003;14:3977–3988. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J. Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE, Spencer CS. The Caenorhabditis elegans homologue of the extracellular calcium binding protein SPARC/osteonectin affects nematode body morphology and mobility. Mol. Biol. Cell. 1993;4:941–952. doi: 10.1091/mbc.4.9.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR, III, Carmichael DF. The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor is a receptor for connective tissue growth factor. J. Biol. Chem. 2001;276:40659–40667. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevde LA, Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014;37:131–141. doi: 10.1016/j.matbio.2014.03.001. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek J, Zhu B, Huynh MH, Brown TJ, Ringuette M. Novel functions of the matricellular proteins osteopontin and osteonectin/SPARC. Connect. Tissue Res. 2002;43:308–319. doi: 10.1080/03008200290001050. [DOI] [PubMed] [Google Scholar]
- Sorenson CM, Wang S, Gendron R, Paradis H, Sheibani N. Thrombospondin-1 deficiency exacerbates the pathogenesis of diabetic retinopathy. J. Diabetes Metab. 2013;Suppl:12. doi: 10.4172/2155-6156.S12-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Shih HB, Maxhimer JB, Cook KL, Ghosh A, Isenberg JS, Roberts DD. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol. 2014;37:25–34. doi: 10.1016/j.matbio.2014.05.003. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by alpha 2 beta 1 and alpha v beta 3 integrins. J. Cell Sci. 1993;105(Pt 4):1001–1012. doi: 10.1242/jcs.105.4.1001. [DOI] [PubMed] [Google Scholar]
- Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, Roberts DD, Mosher DF, Tuszynski GP, Marcinkiewicz C. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ. Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- Stein JJ, Iwuchukwu C, Maier KG, Gahtan V. Thrombospondin-1-induced vascular smooth muscle cell migration and proliferation are functionally dependent on microRNA-21. Surgery. 2014;155:228–233. doi: 10.1016/j.surg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Stenczer B, Molvarec A, Veresh Z, Gullai N, Nagy GR, Walentin S, Szijarto J, Rigo J., Jr Circulating levels of the anti-angiogenic thrombospondin 2 are elevated in preeclampsia. Acta Obstet. Gynecol. Scand. 2011;90:1291–1295. doi: 10.1111/j.1600-0412.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- Stenczer B, Molvarec A, Szabo G, Szarka A, Fugedi G, Szijarto J, Rigo J., Jr Circulating levels of thrombospondin-1 are decreased in HELLP syndrome. Thromb. Res. 2012;129:470–473. doi: 10.1016/j.thromres.2011.09.032. [DOI] [PubMed] [Google Scholar]
- Stenina-Adognravi O. Invoking the power of thrombospondins: regulation of thrombospondin expression. Matrix Biol. 2014;37:69–82. doi: 10.1016/j.matbio.2014.02.001. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MM, Sage EH. Hevin/SC1, a matricellular glycoprotein and potential tumor-suppressor of the SPARC/BM-40/osteonectin family. Int. J. Biochem. Cell Biol. 2004;36:991–996. doi: 10.1016/j.biocel.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Sullivan MM, Barker TH, Funk SE, Karchin A, Seo NS, Hook M, Sanders J, Starcher B, Wight TN, Puolakkainen P, Sage EH. Matricellular hevin regulates decorin production and collagen assembly. J. Biol. Chem. 2006;281:27621–27632. doi: 10.1074/jbc.M510507200. [DOI] [PubMed] [Google Scholar]
- Sullivan MM, Puolakkainen PA, Barker TH, Funk SE, Sage EH. Altered tissue repair in hevin-null mice: inhibition of fibroblast migration by a matricellular SPARC homolog. Wound Repair Regen. 2008;16:310–319. doi: 10.1111/j.1524-475X.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Mosher DF, Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J. Biol. Chem. 1989;264:2885–2889. [PubMed] [Google Scholar]
- Suzuki M, Hao C, Takahashi T, Shigematsu H, Shivapurkar N, Sathyanarayana UG, Iizasa T, Fujisawa T, Hiroshima K, Gazdar AF. Aberrant methylation of SPARC in human lung cancers. Br. J. Cancer. 2005;92:942–948. doi: 10.1038/sj.bjc.6602376. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J. Cell Biol. 2001;154:459–468. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Seki Y, Saito Y, Kamiya S, Fujita M, Okutsu H, Iyoda T, Takai T, Owaki T, Yajima H, Taira J, Hayashi R, Kodama H, Matsunaga T, Fukai F. Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin alpha5beta1. J. Biol. Chem. 2014;289:17699–17708. doi: 10.1074/jbc.M113.546622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneda S, Pippin JW, Sage EH, Hudkins KL, Takeuchi Y, Couser WG, Alpers CE. Amelioration of diabetic nephropathy in SPARC-null mice. J. Am. Soc. Nephrol. 2003;14:968–980. doi: 10.1097/01.asn.0000054498.83125.90. [DOI] [PubMed] [Google Scholar]
- Tang X, Li J, Yu B, Su L, Yu Y, Yan M, Liu B, Zhu Z. Osteopontin splice variants differentially exert clinicopathological features and biological functions in gastric cancer. Int. J. Biol. Sci. 2013;9:55–66. doi: 10.7150/ijbs.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Kyriakides TR. Thrombospondin 2-null mice display an altered brain foreign body response to polyvinyl alcohol sponge implants. Biomed. Mater. 2009;4:015010. doi: 10.1088/1748-6041/4/1/015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic V, Chen CC, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J. Cell Biol. 2005;171:559–568. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjogren's syndromelike ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 2009;175:1136–1147. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udalova IA, Ruhmann M, Thomson SJ, Midwood KS. Expression and immune function of tenascin-C. Crit. Rev. Immunol. 2011;31:115–145. doi: 10.1615/critrevimmunol.v31.i2.30. [DOI] [PubMed] [Google Scholar]
- Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011;61:265–280. doi: 10.1111/j.1440-1827.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, Reichardt LF. The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J. Orthop. Res. 2013;31:999–1006. doi: 10.1002/jor.22324. [DOI] [PubMed] [Google Scholar]
- Wahab NA, Weston BS, Mason RM. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J. Am. Soc. Nephrol. 2005;16:340–351. doi: 10.1681/ASN.2003100905. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Clark AF, Lipson KE, Andrews D, Crean JK, O'Brien CJ. Anti-connective tissue growth factor antibody treatment reduces extracellular matrix production in trabecular meshwork and lamina cribrosa cells. Invest. Ophthalmol. Vis. Sci. 2013;54:7836–7848. doi: 10.1167/iovs.13-12494. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Murphy-Ullrich JE, Downs JC, O'Brien CJ. The role of matricellular proteins in glaucoma. Matrix Biol. 2014;37:172–180. doi: 10.1016/j.matbio.2014.03.007. (in this issue) [DOI] [PubMed] [Google Scholar]
- Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Wang L, Zheng J, Du Y, Huang Y, Li J, Liu B, Liu CJ, Zhu Y, Gao Y, Xu Q, Kong W, Wang X. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ. Res. 2010;106:514–525. doi: 10.1161/CIRCRESAHA.109.202762. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu J, Wang Z, Huang Y, Liu W, Zhu X, Cai Y, Fang X, Lin S, Yuan L, Ouyang G. Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLoS One. 2013;8:e72962. doi: 10.1371/journal.pone.0072962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Tao J, Chen DD, Cai JJ, Irani K, Wang Q, Yuan H, Chen AF. MicroRNA miR-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2014;34:99–109. doi: 10.1161/ATVBAHA.113.302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Kaartinen V. When the lung is stretched, could it be thrombospondin via TGFbeta1 peptide activation? J. Physiol. 2007;584:365. doi: 10.1113/jphysiol.2007.144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J. Biol. Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M, Workman G, Schultz CR, Lemke N, Rempel SA, Sage EH. Proteolysis of the matricellular protein hevin by matrix metalloproteinase-3 produces a SPARClike fragment (SLF) associated with neovasculature in a murine glioma model. J. Cell. Biochem. 2011;112:3093–3102. doi: 10.1002/jcb.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- Weidle UH, Maisel D, Klostermann S, Weiss EH, Schmitt M. Differential splicing generates new transmembrane receptor and extracellular matrix-related targets for antibody-based therapy of cancer. Cancer Genomics Proteomics. 2011;8:211–226. [PubMed] [Google Scholar]
- Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci. Signal. 2008;1:pe13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb BP, Mutch DG, Herzog TJ, Rader JS, Gibb RK, Goodfellow PJ. Frequent HOXA11 and THBS2 promoter methylation, and a methylator phenotype in endometrial adenocarcinoma. Clin. Cancer Res. 2003;9:2277–2287. [PubMed] [Google Scholar]
- Wiesman KC, Wei L, Baughman C, Russo J, Gray MR, Castellot JJ. CCN5, a secreted protein, localizes to the nucleus. J. Cell Commun. Signal. 2010;4:91–98. doi: 10.1007/s12079-010-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton HL, Bidwell JL, Armitage WJ. Thrombospondin-1 polymorphisms influence risk of corneal allograft rejection. Invest. Ophthalmol. Vis. Sci. 2014;55:2115–2120. doi: 10.1167/iovs.13-13681. [DOI] [PubMed] [Google Scholar]
- Workman G, Sage EH. Identification of a sequence in the matricellular protein SPARC that interacts with the scavenger receptor stabilin-1. J. Cell. Biochem. 2011;112:1003–1008. doi: 10.1002/jcb.23015. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zamponi R, Charlat O, Ramones M, Swalley S, Jiang X, Rivera D, Tschantz W, Lu B, Quinn L, Dimitri C, Parker J, Jeffery D, Wilcox SK, Watrobka M, LeMotte P, Granda B, Porter JA, Myer VE, Loew A, Cong F. Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 2013;14:1120–1126. doi: 10.1038/embor.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JC, Xiao MF, Jakovcevski I, Sivukhina E, Hargus G, Cui YF, Irintchev A, Schachner M, Bernreuther C. The extracellular matrix glycoprotein tenascin-R regulates neurogenesis during development and in the adult dentate gyrus of mice. J. Cell Sci. 2014;127:641–652. doi: 10.1242/jcs.137612. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, Aihara M, Takahashi K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br. J. Dermatol. 2013;168:717–725. doi: 10.1111/bjd.12117. [DOI] [PubMed] [Google Scholar]
- Yan Q, Clark JI, Wight TN, Sage EH. Alterations in the lens capsule contribute to cataractogenesis in SPARC-null mice. J. Cell Sci. 2002;115:2747–2756. doi: 10.1242/jcs.115.13.2747. [DOI] [PubMed] [Google Scholar]
- Yan Q, Weaver M, Perdue N, Sage EH. Matricellular protein SPARC is translocated to the nuclei of immortalized murine lens epithelial cells. J. Cell. Physiol. 2005;203:286–294. doi: 10.1002/jcp.20226. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Schluterman MK, Brekken RA. Fibulin-5, an integrin-binding matricellular protein: its function in development and disease. J. Cell Commun. Signal. 2009;3:337–347. doi: 10.1007/s12079-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xiao ZC, Becker B, Hillenbrand R, Rougon G, Schachner M. Role for myelin-associated glycoprotein as a functional tenascin-R receptor. J. Neurosci. Res. 1999;55:687–701. doi: 10.1002/(SICI)1097-4547(19990315)55:6<687::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Yang M, Huang H, Li J, Li D, Wang H. Tyrosine phosphorylation of the LDL receptor-related protein (LRP) and activation of the ERK pathway are required for connective tissue growth factor to potentiate myofibroblast differentiation. Faseb J. 2004;18:1920–1921. doi: 10.1096/fj.04-2357fje. [DOI] [PubMed] [Google Scholar]
- Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J. Cell Commun. Signal. 2007;1:159–164. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, Sheppard D. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J. Biol. Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005;24:418–427. doi: 10.1016/j.matbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Erickson HP, Ikeda Y, Takada Y. Identification of amino acid sequences in fibrinogen gamma-chain and tenascin C C-terminal domains critical for binding to integrin alpha vbeta 3. J. Biol. Chem. 2000;275:16891–16898. doi: 10.1074/jbc.M000610200. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ishikawa K, Asato R, Arima M, Sassa Y, Yoshida A, Yoshikawa H, Narukawa K, Obika S, Ono J, Ohta S, Izuhara K, Kono T, Ishibashi T. Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2011;52:5670–5678. doi: 10.1167/iovs.10-6625. [DOI] [PubMed] [Google Scholar]
- Yuelling LM, Fuss B. Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim. Biophys. Acta. 2008;1781:525–530. doi: 10.1016/j.bbalip.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisch AH, D'Alessandri L, Ranscht B, Falchetto R, Winterhalter KH, Vaughan L. Neuronal cell adhesion molecule contactin/F11 binds to tenascin via its immunoglobulin-like domains. J. Cell Biol. 1992;119:203–213. doi: 10.1083/jcb.119.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J. Cell. Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]