Abstract
Heightened areas of spatial relative risk for ASD, or ASD hotspots, in Utah were identified using adaptive kernel density functions. Children ages four, six and eight with ASD from multiple birth cohorts were identified by the Utah Registry of Autism and Developmental Disabilities (URADD). Each ASD case was gender-matched to 20 birth cohort controls. Demographic and socioeconomic characteristics of children born inside versus outside ASD hotspots were compared. ASD hotspots were found in the surveillance area for all but one birth cohort and age group sample; maximum relative risk in these hotspots ranged from 1.8 to 3.0. Associations were found between higher socioeconomic status (SES) and birth residence in an ASD hotspot in five out of six birth cohort and age group samples.
Keywords: ascertainment age, Autism Spectrum Disorders, diagnostic age, maternal residential birth address, socioeconomic status, spatial analysis, race/ethnicity
Autism Spectrum Disorders (ASD) are neurodevelopmental disorders with a complex etiology characterized by deficits in social, communicative, and behavioral functioning. The measured prevalence of ASD has risen sharply over the past three decades in the U.S. with early studies reporting estimates of 0.7 (Treffert 1970) to 3.3 (Burd et al.1987) cases per 10,000 and more recent studies reporting 147 cases per 10,000 (CDC 2014). Similar increases have been observed in Utah where initial surveillance studies based on DSM III diagnostic criteria identified four autism cases per 10,000 in the mid-1980’s (Ritvo et al. 1989) and recent studies based on DSM-IV-TR criteria identified 186 ASD cases per 10,000 (CDC 2014).
Genetics play a central role in ASD’s etiology (Sutcliffe 2008; Abrahams and Geschwind 2008); however, genetic risk factors alone fail to fully explain ASD’s occurrence (London and Etzel 2000; Hallmayer et al. 2011). Numerous extrinsic risk factors have been implicated in the development of ASD suggesting environment-by-genetic causal mechanisms (Persico and Bourgeron 2006; Altevogt et al. 2008). Environmental risk factors currently associated with ASD include prenatal and perinatal factors such as advanced parental age, breech presentation, maternal pregnancy weight gain, and maternal fever during pregnancy (e.g. Croen et al. 2007; Bilder et al. 2009; Grether et al. 2009; Bilder et al. 2013; Zerbo et al. 2013), chemical and pollutant exposures including hazardous air pollutants, heavy metals and pesticides (e.g. Windham et al. 2006; Roberts et al. 2007; Kalkbrenner et al. 2010; Volk et al. 2013), and prescription medications such as valproic acid, thalidomide and selective serotonin reuptake inhibitors (e.g. Bromley et al. 2008; Strömland et al. 1994; Croen et al. 2011). Collectively, however, studies examining environmental ASD risk factors have found modest or inconclusive effects. Thus, the search for substantial environmental risk factors remains an active area of intense inquiry.
The discovery of plausible environmental exposure risk factors for ASD may be hastened through the application of spatial analysis and disease mapping tools to identify localized regions of heightened risk. These exploratory geographical tools which include cluster detection tests (e.g. Kuldorff 1997; Besag and Newell 1991), kernel smoothing methods (Kelsall and Diggle 1995a; 1995b), and generalized additive models (Tibshirani 1990; Webster et al. 2006) are used to monitor for non-random groupings of disease occurrences in count, cohort or case-control data. Findings from an exploratory geographical analysis are commonly used to generate hypotheses concerning disease etiology or to test for the influence of specific causal mechanisms in producing disease clusters.
Spatial ASD clusters may be induced by non-environmental exposure factors related to ASD which also follow a non-random geographical distribution such as familial risk or socioeconomic status. Residential segregation which is the sorting of individuals into neighborhoods according to cultural, racial, ethnic or economic drivers has been shown to produce non-random risk patterns for health conditions in the U.S. including low birth weight (Grady 2006; Walton 2009), preterm birth (Osypuk and Acevedo-Garcia 2008; Mason et al. 2009), psychological well-being (Lee 2009), and developmental disabilities (Fiscella and Williams 2004). A heterogeneous relationship has been reported between ASD and indicators of elevated socioeconomic class across studies conducted outside of the U.S. (Rai et al. 2012; Larsson 2005; Fombonne et al. 1997); however, ASD has been more consistently associated with higher socioeconomic status (SES) in recent U.S. based studies (Bhasin and Schendel 2007; Croen et al. 2002; Durkin et al. 2010; Windham et al. 2011). In Utah, mixed findings have been found between ASD and socioeconomic indicators with one study reporting no association between ASD risk and higher maternal education, a common SES proxy, (p = 0.06; Pinborough-Zimmerman et al. 2011) and a second study confirming an increased ASD risk associated with higher maternal education (p = 0.03; Bilder et al. 2009).
Previous geographical analyses of ASD have been limited to studies conducted statewide in California (Van Meter et al. 2010; Mazumdar et al. 2010; Mazumdar et al. 2012) and in an eight county area of North Carolina (Hoffman et al. 2012; Hoffman et al. 2013). The California-based studies applied spatial cluster detection tests to look for higher incidence of autism at birth (Mazumdar et al. 2010; Mazumdar et al. 2012; Van Meter et al. 2010) and at time of diagnosis (Mazumdar et al. 2012) using data from the California Department of Developmental Services. All three studies found clusters of increased autism risk. Methods employed in these studies to examine the association of known SES-related ASD risk factors with ASD clusters included covariate adjustment (Mazumdar et al. 2010; Mazumdar et al. 2012) and mixed Poisson regression modeling (Van Meter et al. 2010). In North Carolina-based studies, generalized additive models were formulated to predict ASD prevalence of children aged eight using data from the North Carolina Autism and Developmental Disability Monitoring (ADDM) site; the relationship between ASD prevalence and known ASD predictors was examined by adjusting the models with individual-level ASD risk factors (Hoffman et al. 2012; Hoffman et al. 2013). Although the majority of prior studies found evidence of an association between individual and neighborhood-level SES risk factors and ASD spatial risk patterns (Van Meter et al. 2010; Mazumdar et al. 2010; Hoffman et al. 2012), areas of excess risk persisted after adjusting for known SES-related ASD risk factors (Mazumdar et al. 2010; Mazumdar et al. 2012; Hoffman, Vieira and Daniels 2013). This suggests that contextual, social and/or environmental drivers beyond SES also contribute to spatial ASD risk patterns.
Utah offers a unique location to examine the spatial distribution of ASD risk because of its consistently high measured ASD prevalence (Pinborough-Zimmerman et al. 2012; CDC 2012; CDC 2014), and an exploratory spatial analysis may clarify the mediating role of SES and demographic factors on ASD risk in Utah. Limitations of previous studies exploring ASD birth clusters include aggregation of data to areal units (Mazumdar et al. 2010; Mazumdar et al. 2012), the collapsing of multiple birth cohorts into one sample (Van Meter et al. 2010), and the absence of diagnostic age effects in statistical modeling (Mazumdar et al. 2010; Van Meter et al. 2010). These limitations justify further spatial analyses of ASD risk that address these confounding issues.
The current study uses a case-control design and point-level geocoded data to identify heightened areas of spatial ASD risk in successive birth cohorts ascertained at ages four, six, and eight in a three county surveillance region of Utah. Our objectives were: (1) to identify significantly heightened areas of ASD spatial relative risk at birth in five birth cohorts, and (2) to evaluate the degree to which spatial relative risk patterns are related to SES and demographic variables by analyzing individual-level socioeconomic and demographic correlates of ASD cases and controls born within versus outside heightened areas of relative risk.
Methods
Case ascertainment
ASD cases were identified by the Utah Registry of Autism and Developmental Disabilities (URADD). In study year (SY) 2002, ASD ascertainment was conducted in children aged eight living in Davis, Salt Lake and Utah counties (see Figure 1 for map of Utah and the URADD surveillance region). In study years 2006 and 2008, the surveillance age range was expanded to include children aged four, six and eight residing in Davis, Salt Lake, and Utah counties (see Table 1).
Figure 1.
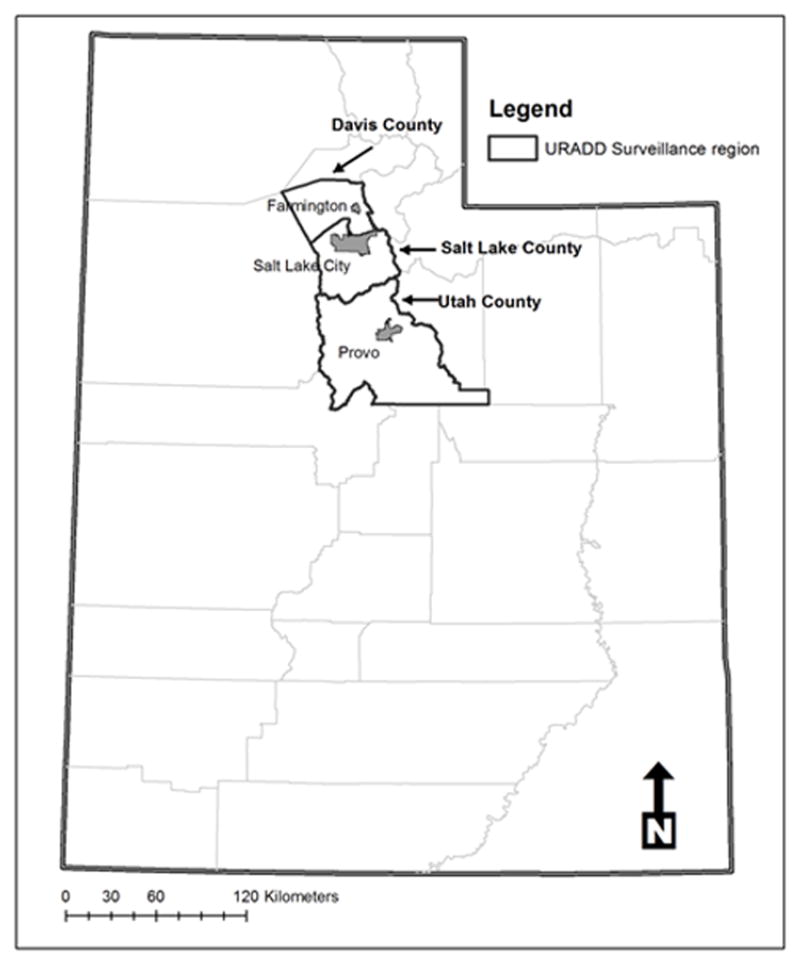
Utah and the URADD surveillance area in SY2002, 2006 and 2008. County seats are indicated in dark grey: Farmington in Davis County, Salt Lake City in Salt Lake County, and Provo in Utah County.
Table 1.
Description of ASD study population and relative risk (RR) hotspots
| Sample | Birth Cohort | Study Year | Agea | Surveillance Populationb (n) | Cases (n) | Controls (n) | Hotspots (n) | Maximum RR | Cases in hotspots (n) | Total area of hotspot(km2)c |
|---|---|---|---|---|---|---|---|---|---|---|
| 1994–8 | 1994 | 2002 | 8 | 26,213 | 132 | 2640 | 1 | 2.3 | 42 | 69.0 |
| 1998–8 | 1998 | 2006 | 8 | 29,494 | 182 | 3560 | 0 | 2.7 | 0d | 0d |
| 2000–6 | 2000 | 2006 | 6 | 32,801 | 223 | 4460 | 2 | 1.8 | 48 | 154.52 |
| 2000–8 | 2000 | 2008 | 8 | 33,757 | 295 | 5900 | 2 | 2.6 | 18 | 41.22 |
| 2002–4 | 2002 | 2006 | 4 | 33,955 | 166 | 3320 | 2 | 2.5 | 33 | 261.92 |
| 2002–6 | 2002 | 2008 | 6 | 34,368 | 289 | 5780 | 3 | 3.0 | 66 | 359.29 |
| 2004–4 | 2004 | 2008 | 4 | 35,803 | 186 | 3720 | 1 | 2.0 | 43 | 142.78 |
Age at surveillance
ASD surveillance conducted in Davis, Salt Lake and Utah counties
Total area of surveillance region=9,274.6 km2
No evidence of heightened areas of relative risk in this birth cohort*ascertainment age sample
Children with ASD were identified by querying administrative records of all major health and education sources in the ascertainment area. Specifically, medical sources such as the Utah Department of Health (UDOH), private and public clinics and hospitals, and behavioral health centers reported children who received ASD diagnostic codes including ICD-9 299.00, 299.01, 299.80, and 299.90 as mandated under Utah Health Code Chapter 26 Title 7 Section 4. Similarly, the Utah State Office of Education (USOE) provided counts of children receiving special education services under an autism special educational classification. A child was classified as an ASD case by meeting at least one of two criteria: (1) received an ASD medical diagnosis from a qualified provider such as a developmental pediatrician, child psychiatrist, or clinical psychologist, and/or (2) received special education services under an autism educational classification (see Pinborough-Zimmerman et al. 2012 for further detail).
Data linkage and selection of control population
Birth certificate vital records were obtained from the UDOH Office of Vital Records and Statistics for birth years 1994, 1998, 2000, 2002 and 2004. ASD cases were linked to their birth certificate using a deterministic linkage approach in SAS 9.2 (SAS Institute, Cary NC, USA) with successful linkage rates ranging from 61%–69%. There were no differences in the sex or race/ethnicity between children with ASD who were linked to their birth certificates versus children who were not linked to their birth certificates. Linkage success rates varied across years with no indication of improved matching over time. Overall, our linkage rates were low compared to ASD studies conducted in other US states (e.g. Mazumdar et al. 2012) but consistent with other Utah-based studies (e.g. Bilder et al. 2009). The majority of children not linked to their birth certificates were born outside of the surveillance area.
Birth certificate variables used in the analysis included sex, mother’s age at birth (maternal age), father’s age at birth (paternal age), mother’s level of educational attainment at birth (maternal education), father’s level of educational attainment at birth (paternal education), mother’s race/ethnicity, father’s race/ethnicity, and geocoded maternal residential birth address (see Table 2). The pre-, peri- and post-natal periods are largely thought to represent the critical windows of development for ASD (Hertz-Picciotto et al. 2006); the maternal birth address is commonly used to approximate a child’s location of exposure during this period when finer scale data is unavailable (e.g. residential and maternal work history questionnaire data). Maternal residential birth addresses were geocoded by the UDOH Environmental Public Health Tracking Program as point locations in the Universal Transverse Mercator geographic coordinate system. Twenty controls were randomly selected per case from birth certificates using a weighted scheme that matched based on gender, birth cohort, and age. The probability of selection was weighted based on the distribution of births by US postal zip code to ensure that the control population’s spatial distribution reflected the geographical distribution of the background population. Separate control populations were derived for each birth cohort*age at ascertainment sample (see the spatial analysis section for further description of the samples). ASD cases were removed from the pool of potential controls and were not eligible for selection as part of the control population. Institutional Review Board approval to conduct this research was obtained from the University of Utah and UDOH.
Table 2.
Characteristics of URADD ASD cases and controls
| Characteristic | n (%) | Chi-square p-valuea | |
|---|---|---|---|
| Control (n=27,541) | ASD case (n=1,090) | ||
| Gender | 0.42 | ||
| Male | 22,134 (80.4) | 888 (81.5) | |
| Female | 5,407 (19.6) | 202 (18.5) | |
| Race/ethnicity | 0.001 | ||
| White non-Hispanic | 21,231 (77.1) | 902 (82.8) | |
| Hispanic | 4,503 (16.4) | 144 (13.2) | |
| Other | 1,184 (4.3) | 29 (2.7) | |
| Missing | 623 (2.3) | 15 (1.4) | |
| Maternal age | 0.005 | ||
| <21 years | 3,339 (12.1) | 114 (10.5) | |
| 21–33 years | 20,817 (75.6) | 807 (74.0) | |
| 34+ years | 3,384 (12.3) | 169 (15.5) | |
| Missing | 1 (<0.01) | 0 (0.0) | |
| Paternal age | 0.42 | ||
| <21 years | 1,108 (4.0) | 35 (3.2) | |
| 21–33 years | 18,910 (68.7) | 744 (68.3) | |
| 34+ years | 5,666 (20.6) | 228 (20.9) | |
| Missing | 1,857 (6.7) | 83 (7.6) | |
| Maternal education | 0.001 | ||
| <12 years | 3,853 (14.0) | 108 (9.9) | |
| 12 or 13 years | 10,898 (39.6) | 464 (42.6) | |
| 14+ years | 12,451 (45.2) | 504 (46.2) | |
| Missing | 339 (1.2) | 14 (1.3) | |
| Paternal education | 0.001 | ||
| <12 years | 2,688 (9.8) | 73 (6.7) | |
| 12 or 13 years | 8,326 (30.2) | 368 (33.8) | |
| 14+ years | 13,818 (50.2) | 523 (48.0) | |
| Missing | 2,709 (9.8) | 126 (11.6) | |
Adjusted using the Benjamini-Hochberg procedure
Characterization of study population
We examined differences in children’s race/ethnicity, maternal age, paternal age, maternal education and paternal education between ASD cases and controls. Maternal age, paternal age, maternal education, and paternal education were converted into categorical variables with three levels (see Table 2) which were chosen based on previously published Utah studies (e.g. Pinborough-Zimmerman et al. 2011). Differences between ASD cases and controls among these variables were tested using χ2 goodness-of-fit tests. P-values from these tests were adjusted for multiple comparisons with the Benjamini-Hochberg procedure (Bejamini and Hochberg 1995) using the multtest procedure in SAS software, version 9.2 (SAS Institute, Cary NC, USA). An alpha level of 0.05 was assumed for all statistical tests. The non-spatial analyses were conducted using SAS software, version 9.2 (SAS Institute, Cary NC, USA).
Spatial analysis
Adaptive kernel density functions (Davies and Hazelton 2010) were used to measure variation in spatial relative risk for ASD in the three county surveillance region (Davis, Salt Lake, and Utah counties). This approach tested the null hypothesis that the risk for ASD did not vary spatially across the surveillance region and that ASD cases were located independently of one another. First, the adaptive kernel densities of ASD cases and controls were separately estimated. Kernel densities represent the relative intensity of a point pattern process across a two-dimensional grid surface. Here, the point pattern process is the distribution of either ASD cases or their set of matched controls. At each grid point, the kernel density estimate assigns a probability of encountering a case or a control by finding a weighted average of case or control intensities across neighboring case or control locations (see Hazelton and Davies 2009 and Fernando and Hazelton 2014 for more information concerning kernel density estimation of relative risk).
Estimation of adaptive kernel densities requires the selection of one or more smoothing bandwidths which vary across the spatial extent of the surveillance region as a function of the density of case and control locations (Silverman 1986). In public health applications, adaptive kernel density functions are often preferred over fixed kernel density functions as they support varying levels of smoothing in response to a heterogeneously distributed population. The bandwidth parameter, hi, was estimated using least squares cross validation which has been shown to produce unbiased estimates of the mean integrated square error (Scott and Terrell 1986). Separate bandwidth parameters were estimated for each sample. Kernel density functions were corrected for edge-effects to avoid negative bias around the surveillance area boundary.
Next, the spatial relative risk function was computed as the ratio of the case to control adaptive kernel densities (Bithell 1990). We examined both the raw and the log of the spatial relative risk but used the log-risk function to test for significantly heightened areas of relative risk (Kelsall and Diggle 1995a, 1995b). Tolerance contours were constructed based on p-value surfaces using a z-test statistic approach (Hazelton and Davies 2009) to identify heightened areas of relative risk or relative risk “hotspots” (Davies and Hazelton 2010). Upper tailed tests for heightened areas of relative risk were conducted corresponding to a p-value of 0.05. Although not a component of this study, two-tailed hypothesis tests can also be conducted using the kernel density approach to investigate areas of reduced risk for ASD which may be valuable for identifying factors that decrease a child’s likelihood of receiving an ASD diagnosis.
The analysis was conducted within polygons constructed to minimize the inclusion of unpopulated areas to reduce the possibility of identifying artefactual relative risk hotspots. Separate spatial analyses were conducted for each ascertainment age group in each surveillance year for a total of seven birth cohort* ascertainment age samples. For example, the 1994 birth cohort that was ascertained for ASD in 2002 at the age of 8 was labelled and referred to as the 1994–8 sample (see Table 1 for description of all samples and associated labels). The problem of multiple comparison testing was minimized by conducting single hypothesis tests across the entire surveillance area, selecting sensible smoothing bandwidths, and minimizing the inclusion of areas where data was absent. The spatial relative risk analysis was conducted in R (R Development Core Team 2012) using the sparr package (Davies, Hazelton, and Marshall 2011).
Association with individual-level demographic and SES variables
We used single and multiple logistic regression models to examine the association between being born in an ASD hotspot (independent of case versus control status) with individual-level demographic and SES factors known to be associated with ASD risk. The boundaries of the heightened relative risk contours and the ASD case and control birth addresses were projected onto surveillance area maps in ESRI ArcGIS 10. The ASD cases and controls that fell within hotspot boundaries were identified by birth cohort, age at surveillance and surveillance year. Individual-level demographic and SES variables included in the models were child’s sex, child’s race/ethnicity, maternal age, and maternal education. Paternal age and paternal education were not included in the models due to their strong correlation with maternal age and maternal education as indicated by Pearson correlation coefficients (Pearson’s r > 0.50). First, associations were examined between each individual-level demographic and SES variable with birth in an ASD hotspot (single variable analysis). Next, all individual-level ASD variables were included in multiple logistic regression models (multiple variables analysis). Separate models were formulated for each birth cohort*ascertainment age sample.
To test for residual risk of ASD not explained by demographic or SES variables, we used multiple logistic regression models to examine the association between ASD case and control status (probability of being an ASD case or control) and birth in an ASD hotspot while adjusting the models for individual-level variables including child’s race/ethnicity, maternal age, and maternal education. In this analysis, the finding of a significant relationship between ASD case versus control status and birth in an ASD hotspot after controlling for individual-level demographic and socioeconomic factors associated with ASD would suggest that additional, untested factors exist that are related to the ASD hotspots.
Results
Study population characteristics
Pooling all study samples, the case and control populations were significantly different in their racial/ethnic composition (p=.0001), and in their frequencies of maternal age (p=.005), maternal education (p=.001), and paternal education (p=.001) (Table 2). However, when analyzed separately, we did not find uniform case-control differences in SES characteristics within each individual sample. The strongest evidence of socioeconomic differences between cases and controls were observed in the 1998 and 2000 birth cohorts (the 1998–8, 2000–6 and 2000–8 samples) (see Online Resource 1).
Spatial analysis
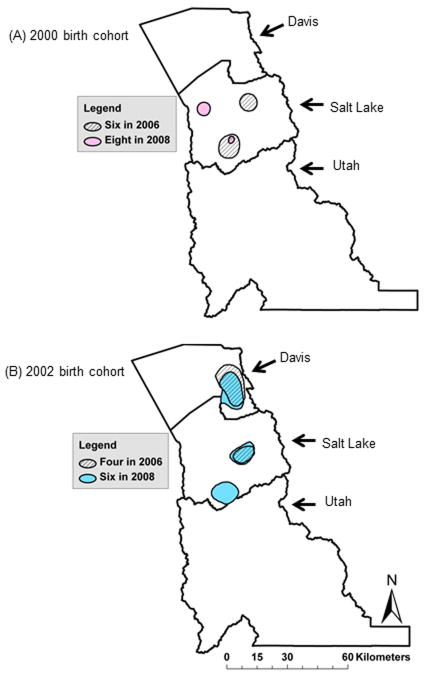
ASD hotspots were identified in the surveillance region for each birth cohort*age sample with the exception of the 1998–8 sample (Table 1). As shown in Figure 2 and Table 1, the number of unique ASD hotspots ranged between one (1994–8 and 2004–4 samples) to three (2002–6 sample). Maximum relative risk in the hotspots ranged between 1.8 (2000–6 sample) to 3.0 (2002–6 sample). The areas encompassed by the ASD hotspots ranged from 41.22 km2 (2000–8 sample) to 359.29 km2 (2002–6 sample). The geographical stability of hotspots decreased with increasing ascertainment age across (Figure 2) and within (Figure 3) birth cohorts. All but one ASD hotspot were located in Salt Lake County. Utah County did not contain any ASD hotspots.
Figure 2.
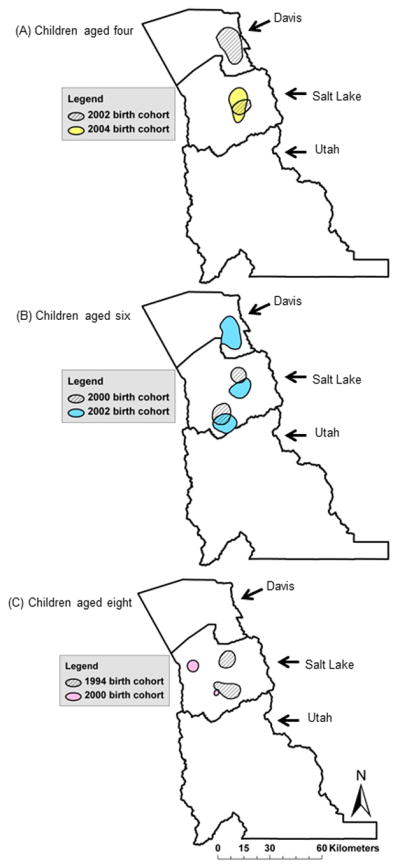
Heightened areas of ASD relative risk in Utah by age group and surveillance year: A) children aged four at the time of surveillance in SY2006 (2002–4 sample; diagonal hatch) and SY2008 (2004–4 sample; yellow), B) children aged six at the time of surveillance in SY2006 (2000–6 sample; diagonal hatch) and SY2008 (2002–6 sample; blue), and C) children aged eight at the time of surveillance in SY2002 (1994–8 sample; diagonal hatch) and SY2008 (2000–8 sample; pink).
Figure 3.
Heightened areas of relative risk in Utah by birth cohort and surveillance year: A) children born in 2000 and ascertained in SY2006 at six years of age (2000–6 sample; diagonal hatch) and SY2008 at eight years of age (pink; 2000–8 sample), and B) children born in 2002 and ascertained in SY2006 at four years of age (2002–4 sample; diagonal hatch) and SY2008 at six years of age (2002–6 sample; blue).
Individual-level demographic and SES models
Higher SES was associated with birth in an ASD hotspot, regardless of ASD case status in four out of six samples as indicated by the association between maternal education (a common proxy for SES) and birth in an ASD hotspot (Table 3). [Insert Table 3 here] Mothers of ASD cases and controls born within ASD hotspots were more likely to have acquired 14 or more years of education compared to mothers of ASD cases and controls born outside of ASD hotspots in the 2000–6 (AOR = 1.56, 95 % CI 1.25–1.96), 2002–4 (AOR = 1.38, 95 % CI 1.06–1.80), 2002–6 (AOR = 1.25, 95 % CI 1.05–1.49), and the 2002–4 (AOR = 1.29, 95 % CI 1.04–1.61) samples. Maternal education greater than 14 years was not associated with increased odds of birth in an ASD hotspot in the 1998–8 sample (AOR = 0.93, 95 % CI 0.64–1.35) and was protective in the 2000–8 sample (AOR = 0.35, 95 % CI 0.22–0.56). Differences were measured in five out of six samples in the demographic composition of births in versus outside of ASD hotspots. ASD cases and controls born within an ASD hotspot were less likely to be Hispanic than White non-Hispanic in the 1994–8 (AOR = 0.35, 95 % CI 0.14–0.89), 2002–4 (AOR = 0.48, 95 % CI 0.28–0.80) and 2002–6 (AOR = 0.55, 95 % CI 0.41–0.74) samples. Mothers of children born in an ASD hotspot were more likely to be at least 34 years of age in the 2000–8 (AOR = 1.71, 95 % CI 1.00–2.90), 2002–4 (maternal age AOR = 1.82, 95 % CI 1.32–2.51), 2002–6 (AOR = 1.3, 95% CI 1.04–1.62) and 2004–4 samples (AOR = 1.44, 95 % CI 1.09–1.89). Child’s gender was not found to be associated with birth in an ASD hotspot in any of the samples.
Table 3.
Crude and adjusted odds of being born in an ASD hotspot associated with demographic and socioeconomic variablesa
| Covariate | 1994–8 sampleb
|
2000–6 samplec
|
2000–8 sampled
|
2002–4 samplee
|
2002–6 samplef
|
2004–4 sampleg
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) |
p | Adjusted OR (95% CI) |
p | Crude OR (95% CI) |
p | Adjusted OR (95% CI) |
p | Crude OR (95% CI) |
p | Adjusted OR (95% CI) |
p | Crude OR (95% CI) |
p | Adjusted OR (95% CI) |
p | Crude OR (95% CI) |
p | Adjusted OR (95% CI) |
p | Crude OR (95% CI) |
p | Adjusted OR (95% CI) |
p | |
| Gender | ||||||||||||||||||||||||
| Female | 0.83 (0.490–1.42) | 0.5 | 0.90 (0.52–1.53) | 0.69 | 1.06 (0.85–1.32) | 0.62 | 1.04 (0.82–1.32) | 0.75 | 0.90 (0.56–1.45) | 0.67 | 0.97 (0.59–1.59) | 0.91 | 0.89 (0.65–1.22) | 0.47 | 0.88 (0.63–1.22) | 0.43 | 0.95 (0.78–1.16) | 0.62 | 0.94 (0.77–1.16) | 0.58 | 0.93 (0.74–1.17) | 0.56 | 0.91 (0.71–1.17) | 0.46 |
| Male (Reference) | ||||||||||||||||||||||||
| Race/ethnicity | ||||||||||||||||||||||||
| Hispanic | 0.40 (0.18–0.92) | 0.03 | 0.35 (0.14–0.89) | 0.03 | 0.61 (0.46–0.81) | <.01 | 0.72 (0.51–1.01) | 0.06 | 1.03 (0.63–1.67) | 0.92 | 1.04 (0.60–1.79) | 0.9 | 0.32 (0.20–0.50) | <.01 | 0.48 (0.28–0.80) | <.01 | 0.41 (0.32–0.53) | <.01 | 0.55 (0.41–0.74) | <.01 | 0.86 (0.67–1.10) | 0.24 | 0.99 (0.73–1.33) | 0.94 |
| White non-Hispanic (Reference) | ||||||||||||||||||||||||
| Other | 0.17 (0.02–1.20) | 0.08 | 0.20 (0.03–1.43) | 0.11 | 1.17 (0.73–1.87) | 0.52 | 1.24 (0.74–2.07) | 0.41 | 0.98 (0.36–2.69) | 0.97 | 0.86 (0.27–2.75) | 0.8 | 1.10 (0.67–1.83) | 0.71 | 0.97 (0.56–1.69) | 0.91 | 0.91 (0.66–1.26) | 0.57 | 0.95 (0.68–1.34) | 0.78 | 1.25 (0.85–1.84) | 0.27 | 1.39 (0.93–2.08) | 0.11 |
| Maternal age | ||||||||||||||||||||||||
| <21 years | 0.85 (0.50–1.44) | 0.55 | 0.63 (0.31–1.28) | 0.2 | 0.68 (0.50–0.94) | 0.02 | 0.72 (0.47–1.12) | 0.15 | 1.38 (0.82–2.31) | 0.23 | 1.14 (0.62–2.09) | 0.67 | 0.83 (0.55–1.27) | 0.4 | 1.27 (0.74–2.18) | 0.38 | 0.65 (0.49–0.86) | <.01 | 0.95 (0.67–1.35) | 0.78 | 0.90 (0.64–1.26) | 0.54 | 1.07 (0.70–1.64) | 0.76 |
| 21–33 years (Reference) | ||||||||||||||||||||||||
| 34+ years | 1.38 (0.88–2.17) | 0.16 | 1.47 (0.93–2.32) | 0.1 | 1.31 (1.01–1.71) | 0.04 | 1.26 (0.95–1.66) | 0.11 | 1.58 (0.96–2.59) | 0.07 | 1.71 (1.01–2.90) | 0.05 | 1.83 (1.34–2.51) | <.01 | 1.82 (1.3–2.51) | <.01 | 1.32(1.07–1.63) | 0.01 | 1.3 (1.04–1.62) | 0.02 | 1.38 (1.06–1.80) | 0.02 | 1.44 (1.09–1.89) | <.01 |
| Maternal education | ||||||||||||||||||||||||
| <12 years | 0.69 (0.37–1.30) | 0.25 | 1.2 (0.58–2.49) | 0.63 | 1.01 (0.74–1.37) | 0.98 | 1.18 (0.78–1.79) | 0.44 | 0.70 (0.41–1.19) | 0.19 | 0.64 (0.33–1.27) | 0.2 | 0.39 (0.22–0.67) | <.01 | 0.60 (0.31–1.17) | 0.13 | 0.49 (0.36–0.66) | <.01 | 0.62 (0.42–0.90) | 0.01 | 1.05 (0.77–1.42) | 0.77 | 1.04 (0.70–1.53) | 0.87 |
| 12–13 years (Reference) | ||||||||||||||||||||||||
| 14+ years | 1.01 (0.71–1.44) | 0.94 | 0.93 (0.64–1.35) | 0.7 | 1.59 (1.30–1.95) | <.01 | 1.56 (1.25–1.96) | <.01 | 0.39 (0.26–0.61) | <.01 | 0.35 (0.22–0.56) | <.01 | 1.58 (1.22–2.04) | <.01 | 1.55 (1.17–2.05) | <.01 | 1.38 (1.18–1.63) | <.01 | 1.25 (1.05–1.49) | 0.01 | 1.35 (1.10–1.65) | <.01 | 1.29 (1.04–1.61) | 0.02 |
The 1998–8 sample is not represented in the table because ASD hotspots were not identified for this sample.
N in cluster = 135; N out of cluster = 2330
N in cluster = 442; N out of cluster = 3625
N in cluster = 105; N out of cluster =5310
N in cluster = 271; N out of cluster =2841
N in cluster = 708; N out of cluster = 4696
N in cluster = 468; N out of cluster = 2981
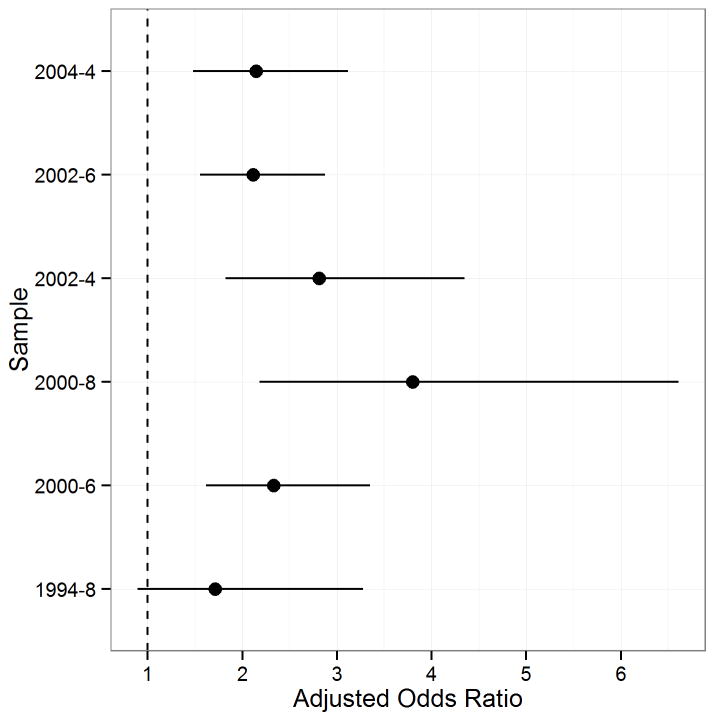
Figure 4 displays the odds of ASD associated with birth in an ASD hotspot after adjusting for individual-level demographic and socioeconomic factors related to ASD. An increased odds of ASD was associated with birth in an ASD hotspot following adjustment for demographic and socioeconomic variables for all samples with the exception of the 1994–8 sample (AOR: 1.71, 95 % CI 0.89–3.28).
Figure 4.
Adjusted odds ratios of Autism Spectrum Disorder (ASD) associated with birth in an ASD hotspot. Models are adjusted for sex, race/ethnicity, mother’s age at child’s birth, and mother’s education at child’s birth.
Discussion
ASD hotspots were identified in four out of five birth cohorts and two out of three Utah counties studied. The relative risk for ASD in the hotspots ranged from 1.8–3.0 indicating that children born inside of the hotspots were up to three times the risk for ASD than children living elsewhere in the surveillance region. This relative risk range is comparable to estimates from California-based studies which found that children born in certain areas of California were at 1.7–4 times the risk for ASD (Van Meter et al. 2010; Mazumdar et al. 2010; Mazumdar et al. 2012).
The current study identified indicators of higher SES associated with children born within ASD hotspots compared to those born outside ASD hotspots, regardless of ASD case status. This association was evident for nearly all birth cohort*ascertainment age samples. In this study, a significant link was identified between SES, birth residence, and ASD risk, suggesting that the presence of ASD hotspots may be, at least in part, attributable to higher SES. Previous spatial analyses of ASD have also implicated SES’s associated effect with heightened ASD spatial risk (Van Meter et al. 2010; Mazumdar et al. 2010; Mazumdar et al. 2012; Hoffman et al. 2012). However, some of these previous studies (Van Meter et al. 2010; Mazumdar et al. 2010) did not explicitly account for what effects the inclusion in their samples of children diagnosed at different ages might have on birth ASD risk. In our analysis, diagnostic age impacted the identification of heightened areas of spatial relative risk for ASD at birth as reflected by the differences in spatial ASD risk patterns found among children from the same birth cohort identified at various ages. This suggests a contribution to spatial ASD birth risk patterns of factors associated with both ASD birth risk and the age at which a child with ASD is recognized.
The connection between ASD spatial relative risk and higher SES may reflect ascertainment bias favoring identification of ASD in higher SES classes (Fombonne 2003; Newschaffer et al. 2007). If so, this association may be expected to weaken as children age into public education settings where diagnostic services may be more accessible for those with lower SES. Previously published data on the current sample has shown that although the majority of ASD cases were ascertained from medical sources, a small proportion were ascertained exclusively through school sources (Pinborough-Zimmerman et al. 2012). Not surprisingly, the proportion of exclusively school-ascertained cases in this study was found to rise with increasing age. The 2000 and 2002 birth cohorts were ascertained at two different ages (six and eight years in the 2000 birth cohort and four and six years in the 2002 birth cohort), providing an opportunity to assess the relationship between SES and ASD hotspots across ascertainment ages. The association between birth in an ASD hotspot and higher SES was present in the four and six year old 2000 and 2002 birth cohort samples but not in the eight year old 2000 birth cohort sample. In addition, another eight year old sample (1998–8) did not contain an ASD hotspot and the hotspots identified in the 1994–8 sample were not associated with higher SES. These findings support the hypothesis that SES and spatial ASD risk at younger ages may be linked, in part, through earlier access to diagnostic services.
Membership in an ASD hotspot was associated with being White, non-Hispanic in the 1994, 1998 and 2002 birth cohorts which may be related to residential segregation in our surveillance area by race/ethnicity. The Latino/White, non-Hispanic racial residential segregation pattern is pronounced in Salt Lake County where it follows a west-east gradient (Downey and Timberlake 2006). Recent U.S. based studies show that measured ASD prevalence is consistently lower in Hispanic versus White, non-Hispanic populations (CDC 2012; Liptak et al. 2008; Mandell et al. 2009; Pedersen et al. 2012). Although wide in Utah, this prevalence gap may be starting to shrink as indicated by comparisons of measured prevalence over time (CDC 2012). If racial residential segregation has indeed played a primary role in producing ASD hotspots, than we can expect the strength of the relationship between being White, non-Hispanic and membership in an ASD hotspot to diminish in future birth cohorts as ascertainment of ASD improves in Utah’s Hispanic population.
Conversely, multiple findings from our study suggest caution in inferring a causal SES or residential segregation mechanism for ASD hotspots including 1) the size and location of ASD hotspots varied by birth cohort, 2) the amount of hotspot overlap decreased as the gap in years between birth cohorts increased, 3) the strength of the relationship between high SES indicators and birth in an ASD hotspot was inconsistent across samples, 4) the association between ASD risk and birth in an ASD hotspot persisted after adjusting for demographic and SES factors related to ASD. These findings suggest that factors in addition to SES, such as social-influence effects (e.g. information diffusion through social networks) (Liu, King and Bearman 2010), variables related to diagnosis (Mazumdar et al. 2012), and/or local area environmental exposures may also drive spatial patterns of spatial ASD relative risk. In the URADD surveillance region, potential sources of environmental exposures during the prenatal and early postnatal periods include ambient air pollution, altitude, and, agricultural pesticides (e.g. Roberts et al. 2007; Kalkbrenner et al. 2010; Volk et al. 2013). Utah’s unique geographical and meteorological conditions merit a further examination of the association between these environmental exposures and ASD risk.
Maternal residential birth address was used to represent the geographic location of potential exposure to extrinsic risk factors for ASD during fetal development and immediately following birth. Although commonly used in geographical analyses of developmental disorders and birth defects (Rushton and Lolonis 1996; Gardner, Strickland and Correa 2007) weaknesses are associated with using maternal addresses to indicate exposure location. The exposure window for ASD is hypothesized to range from the prenatal to early post-natal period; however, the maternal residential birth address may not be an appropriate proxy for exposure during this entire range due to maternal mobility during pregnancy. Maternal mobility studies have found that 12–33% of mothers change residencies at some point between conception and birth (Canfield et al. 2006). In addition, maternal birth residence is a poor proxy for exposures occurring at locations outside the home such as the workplace. Another limitation was the use of birth certificate data for control group selection. Although residence within the surveillance area was confirmed for ASD cases at the time of surveillance, such confirmation could not be conducted for controls. There may have been ASD cases within the control group who moved out of the surveillance area prior to case ascertainment or were not identified by our surveillance system despite being residents in our surveillance area. The surveillance area’s largely urban setting presented an additional study limitation because the study’s findings may not be generalizable to rural areas. In SY2010, the URADD surveillance area expanded to include a rural county in Utah; future spatial analyses will address this limitation.
Despite these limitations, our study has numerous strengths, including the use of point-level data, the application of the kernel density estimator approach, and the inclusion of ascertainment age. The use of point-level data in spatial analyses is considered superior to using aggregated case and control data because it avoids the modifiable areal unit problem (Waller and Gotway 2004) and accommodates areas with small numbers of cases and controls (Gatrell 2002). Yet, hotspot detection studies that are conducted at the individual scale remain a rarity in health geography. The spatial kernel density approach provides the flexibility to identify heightened areas of spatial relative risk while accommodating significant contours and/or irregular shapes.
Differences identified in the size and location of ASD hotspots for individual birth cohorts illustrate the impact of a child’s age at ASD diagnosis on spatial analysis results and challenge the assumption that birth hotspots reflect factors exclusively present at birth. The spatial variation found among different ascertainment ages within single birth cohorts may also suggest diagnostic or ascertainment bias within the surveillance region, especially among younger children. Diagnostic bias could reflect variation in the distribution of ASD severity in the Utah surveillance area because of the well-established inverse relationship between age at diagnosis and ASD severity (Mandell et al. 2005; Shattuck et al. 2009). Unfortunately, we do not have a measure of severity associated with ASD case status, and cannot examine how ASD severity impacts spatial relative risk patterns at birth. However, one option would be to use the presence of co-morbid intellectual disability (ID) as a proxy for ASD severity and conduct a spatial analysis for ASD cases with and without co-occurring ID, similar to the North Carolina study (Hoffman et al. 2012). We speculate that some discrepancies in spatial risk patterns related to children’s age may also be attributed to improved identification of higher functioning children with ASD by age eight and the contribution of data from education sources, as described earlier.
Conclusions
Increased ASD risk is associated with higher SES in the majority of ASD hotspots identified in this study. Differences among ASD hotspots within single birth cohorts occurred as a function of ascertainment age, underscoring the importance of considering diagnostic age in future studies of ASD risk at birth. Further spatial analysis studies are merited to investigate additional risk factors and replicate Utah’s findings across larger, more diversified regions of the US.
Supplementary Material
Acknowledgments
Autism surveillance data was obtained through Centers for Disease Control and Prevention Cooperative Agreement UR3DD000685-03. Research analysis was supported by the Utah Registry of Autism and Developmental Disabilities, the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH094400, and University of Utah Department of Psychiatry funds. Thank you to Drs. Harper Randall, Paul Carbone, Marc Babitz, Eric Fombonne, Barry Nangle and Sam LeFevre for feedback on earlier versions of this manuscript. Brian Robison provided editorial assistance. We are extremely grateful to our health and education data sources for their data contributions. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health. The final publication is available at Springer via http://dx.doi.org/10.1007/s10803-014-2253-0.
Footnotes
Changes in the author’s affiliation subsequent to the time of the study:
None
Integrity of research and reporting
Approval to conduct this research was granted by the University of Utah and Utah Department of Health’s Institutional Review Boards. This manuscript does not contain clinical studies or patient data.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Contributor Information
Amanda V. Bakian, Department of Psychiatry, University of Utah, Salt Lake City, Utah, USA.
Deborah Bilder, Department of Psychiatry, University of Utah, Salt Lake City, Utah, USA.
Hilary Coon, Department of Psychiatry, University of Utah, Salt Lake City, Utah, USA.
William McMahon, Department of Psychiatry, University of Utah, Salt Lake City, Utah, USA.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of new neurobiology. Nature Reviews Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Hanson SL, Leshner AI. Autism and the environment: challenges and opportunities for research. Pediatrics. 2008;121(6):1225–1229. doi: 10.1542/peds.2007-3000. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57(1):289–300. [Google Scholar]
- Besag J, Newell J. The detection of clusters in rare diseases. Journal of the Royal Statistical Society. Series A. 1991;154(Part 1):143–155. [Google Scholar]
- Bhasin TK, Schendel D. Sociodemographic risk factors for autism in a US metropolitan area. Journal of Autism and Developmental Disorders. 2007;37(4):667–677. doi: 10.1007/s10803-006-0194-y. [DOI] [PubMed] [Google Scholar]
- Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorder. Pediatrics. 2009;123(5):1293–1300. doi: 10.1542/peds.2008-0927. [DOI] [PubMed] [Google Scholar]
- Bilder DA, Bakian AV, Viskochil J, Clark EAS, Botts EB, Smith KR, et al. Maternal prenatal weight gain and autism spectrum disorders. Pediatrics. 2013 doi: 10.1542/peds.2013-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bithell JF. An application of density estimation to geographical epidemiology. Statistics in Medicine. 1990;9(6):691–701. doi: 10.1002/sim.4780090616. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71(23):1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- Burd L, Fisher W, Kerbeshian J. A prevalence study of pervasive developmental disorders in North Dakota. Journal of the American Academy of Child & Adolescent Psychiatry. 1987;26(5):700–703. doi: 10.1097/00004583-198709000-00014. [DOI] [PubMed] [Google Scholar]
- Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. Journal of Exposure Science and Environmental Epidemiology. 2006;16(6):538–543. doi: 10.1038/sj.jes.7500501. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Autism and Developmental Disabilities Monitoring Network Year 2008 Principal Investigators (CDC) Prevalence of autism spectrum disorders-Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveillance Summary. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Autism and Developmental Disabilities Monitoring Network Year 2010 Principal Investigators (CDC) Prevalence of autism spectrum disorder among children aged 8 years-Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveillance Summary. 2014;63(2):1–21. [PubMed] [Google Scholar]
- Croen LA, Grether JK, Selvin S. Descriptive epidemiology of autism in a California population: who is at risk? Journal of Autism and Developmental Disorders. 2002;32(3):217–224. doi: 10.1023/a:1015405914950. [DOI] [PubMed] [Google Scholar]
- Croen LA, Najjar DV, Bireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Archives of Pediatrics and Adolescent Medicine. 2007;161(4):334–340. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood Autism Spectrum Disorders. Archives of General Psychiatry. 2011;68(11):1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- Davies TM, Hazelton ML. Adaptive kernel estimation of spatial relative risk. Statistics in Medicine. 2010;29(23):2423–2437. doi: 10.1002/sim.3995. [DOI] [PubMed] [Google Scholar]
- Downey DJ, Timberlake MF. Diversity in Deseret: Race/Ethnic Segregation and Inequality in Utah. In: Zick CD, Smith KS, editors. Utah in the New Millennium: A Demographic Perspective. Salt Lake City, UT: University of Utah Press; 2006. pp. 203–215. [Google Scholar]
- Durkin MS, Maenner MJ, Meaney FJ, Levy SE, DiGuiseppi C, Nicholas JS, et al. Socioeconomic inequality in the prevalence of Autism Spectrum Disorder: evidence from a U.S. cross-sectional study. PLoS One. 2010;5(7):1–8. doi: 10.1371/journal.pone.0011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando WTPS, Hazelton ML. Generalizing the spatial relative risk function. Spatial and Spatio-temporal Epidemiology. 2014;8:1–10. doi: 10.1016/j.sste.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Williams DR. Health disparities based on socioeconomic inequalities: implications for urban health care. Academic Medicine. 2004;79(12):1139–1147. doi: 10.1097/00001888-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Du Mazaubrun C, Cans C, Grandjean H. Autism and associated medical disorders in a French epidemiological survey. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(11):1561–1569. doi: 10.1016/S0890-8567(09)66566-7. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of Autism and Developmental Disorders. 2003;33(4):365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Gardner BR, Strickland MJ, Correa A. Application of the automated spatial surveillance program to birth defects surveillance data. Birth Defects Research Part A. 2007;79(7):559–564. doi: 10.1002/bdra.20363. [DOI] [PubMed] [Google Scholar]
- Gatrell AC. Geographies of Health: An Introduction. Oxford, United Kingdom: Wiley-Blackwell; 2002. [Google Scholar]
- Grady SC. Racial disparities in low birthweight and the contribution of residential segregation: A multilevel analysis. Social Science & Medicine. 2006;63(12):3013–3029. doi: 10.1016/j.socscimed.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Grether JK, Anderson MC, Croen LA, Smith D, Windham GC. Risk of autism and increasing maternal and paternal age in a large North American population. American Journal of Epidemiology. 2009;170(9):1118–1126. doi: 10.1093/aje/kwp247. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Generalized Additive Models. New York: Chapman and Hall; 1990. [DOI] [PubMed] [Google Scholar]
- Hazelton ML, Davies TM. Inference based on kernel estimates of the relative risk function in geographical epidemiology. Biometrical Journal. 2009;51(1):98–109. doi: 10.1002/bimj.200810495. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Delwiche L. The rise in autism and role of age at diagnosis. Epidemiology. 2009;20(1):84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Kalbrenner AE, Vieira VM, Daniels JL. The spatial distribution of known predictors of autism spectrum disorders impacts geographic variability in prevalence in central North Carolina. Environmental Health. 2012;11:80. doi: 10.1186/1476-069X-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Vieira VM, Daniels JL. Brief report: diminishing geographic variability in autism spectrum disorders over time? Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Chen J, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21(5):631–641. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsall JE, Diggle PJ. Kernel estimation of relative risk. Bernoulli. 1995a;1(1–2):3–16. [Google Scholar]
- Kelsall JE, Diggle PJ. Non-parametric estimation of spatial variation in relative risk. Statistics in Medicine. 1995b;14(21–22):2335–2342. doi: 10.1002/sim.4780142106. [DOI] [PubMed] [Google Scholar]
- Kuldorff M. Spatial scan statistic. Communications in Statistics-Theory and Methods. 1997;26(6):1481–1496. [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology. 2005;161(10):916–925. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- Lee M. Neighborhood residential segregation and mental health: A multilevel analysis on Hispanic Americans in Chicago. Social Science & Medicine. 2009;68(11):1975–1984. doi: 10.1016/j.socscimed.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Liptak GS, Benzoni LB, Mruzek DW, Nolan KW, Thingvoll MA, Wade CM, et al. Disparities in diagnosis and access to health services for children with autism: data from the National Survey of Children’s Health. Journal of Developmental & Behavioral Pediatrics. 2008;29(3):152–160. doi: 10.1097/DBP.0b013e318165c7a0. [DOI] [PubMed] [Google Scholar]
- Liu KY, King M, Bearman PS. Social influence and the autism epidemic. American Journal of Sociology. 2010;115(5):1387–1434. doi: 10.1086/651448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E, Etzel RA. The environment as an etiologic factor in autism: a new direction for research. Environmental Health Perspectives. 2000;108(Suppl 3):401–404. doi: 10.1289/ehp.00108s3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with Autism Spectrum Disorders. Pediatrics. 2005;116(6):1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Wiggins LD, Carpenter LA, Daniels J, DiGuiseppi C, Durkin MS, et al. Racial/ethnic disparities in the identification of children with autism spectrum disorders. American Journal of Public Health. 2009;99(3):493–498. doi: 10.2105/AJPH.2007.131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SM, Messer LC, Laraia BA, Mendola P. Segregation and preterm birth: The effects of neighborhood racial composition in North Carolina. Health & Place. 2009;15(1):1–9. doi: 10.1016/j.healthplace.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar S, King M, Zerubavel N, Bearman PS. The spatial structure of Autism in California, 1993–2001. Health & Place. 2010;16(3):539–546. doi: 10.1016/j.healthplace.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar S, Winter A, Liu K, Bearman P. Spatial clusters of autism births and diagnoses point to contextual drivers of increased prevalence. Social Science & Medicine. 2012 doi: 10.1016/j.socscimed.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annual Review of Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Osypuk TL, Acevedo-Garcia D. Are racial Disparities in preterm birth larger in hypersegregated areas? American Journal of Epidemiology. 2008;167(11):1295–1304. doi: 10.1093/aje/kwn043. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Pettygrove S, Meaney FJ, Mancilla K, Gotschall K, Kessler DB, et al. Prevalence of Autism Spectrum Disorders in Hispanic and Non-Hispanic White Children. Pediatrics. 2012;129(3):e629–e635. doi: 10.1542/peds.2011-1145. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends in Neurosciences. 2006;29(7):349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Pinborough-Zimmerman J, Bilder D, Bakian A, Satterfield R, Carbone PS, Nangle BE, et al. Sociodemographic risk factors associated with Autism Spectrum Disorders and Intellectual Disability. Autism Research. 2011;4(6):1–11. doi: 10.1002/aur.224. [DOI] [PubMed] [Google Scholar]
- Pinborough-Zimmerman J, Bakian AV, Fombonne E, Bilder D, Taylor J, McMahon WM. Changes in the administrative prevalence of autism spectrum disorders: contribution of special education and health from 2002–2008. Journal of Autism and Developmental Disabilities. 2012;42(4):521–530. doi: 10.1007/s10803-011-1265-2. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. downloaded from http://www.R-project.org. [Google Scholar]
- Rai D, Lewis G, Lundberg M, Araya R, Svensson A, Dalman C, et al. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(5):467–476. doi: 10.1016/j.jaac.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Breeman BJ, Pingree C, Mason-Brothers A, Jorde L, Jenson WR, et al. The UCLA-University of Utah Epidemiologic Survey of Autism: Prevalence. American Journal of Psychiatry. 1989;146(2):194–199. doi: 10.1176/ajp.146.2.194. [DOI] [PubMed] [Google Scholar]
- Roberts EM, Gross R, Weiser M, Bresnahan M, Silverman J, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environmental Health Perspectives. 2007;115(1):1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton G, Lolonis P. Exploratory spatial analysis of birth defect rates in an urban population. Statistics in Medicine. 1996;15(7–9):717–726. doi: 10.1002/(sici)1097-0258(19960415)15:7/9<717::aid-sim243>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS version 9.2. Cary, North Carolina, USA: SAS Institute; 2008. [Google Scholar]
- Scott DJ, Terrell GR. Technical Report # 23. Department of Statistics, Stanford University; CA: 1986. Biased and unbiased cross-validation in density estimation. [Google Scholar]
- Silverman BW. Density estimation for statistics and data analysis. London, UK: Chapman and Hall; 1986. [Google Scholar]
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, et al. Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(5):474–483. doi: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömland K, Nordin V, Miller M, Akerström B, Gillberg C. Autism in thalidomide embryopathy: A population study. Developmental Medicine & Child Neurology. 1994;36(4):351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS. Insights into the pathogenesis of autism. Science. 2008;321(5886):208–209. doi: 10.1126/science.1160555. [DOI] [PubMed] [Google Scholar]
- Treffert DA. Epidemiology of infantile autism. Archives of General Psychiatry. 1970;22(5):431–438. doi: 10.1001/archpsyc.1970.01740290047006. [DOI] [PubMed] [Google Scholar]
- Waller LA, Gotway CA. Applied Spatial Statistics for Public Health Data. New York: John Wiley; 2004. [Google Scholar]
- Walton E. Residential segregation and birth weight among racial and ethnic minorities in the United States. Journal of Health and Social Behavior. 2009;50(4):427–442. doi: 10.1177/002214650905000404. [DOI] [PubMed] [Google Scholar]
- Webster T, Vieira V, Weinberg J, Aschengrau A. Method for mapping population-based case-control studies: an application using generalized additive models. International Journal of Health Geography. 2006;5:26. doi: 10.1186/1476-072X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environmental Health Perspectives. 2006;114(9):1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Anderson MC, Croen LA, Smith KS, Collins J, Grether JK. Birth prevalence of autism spectrum disorders in the San Francisco Bay area by demographic and ascertainment source characteristics. Journal of Autism and Developmental Disabilities. 2011;41(10):1362–1372. doi: 10.1007/s10803-010-1160-2. [DOI] [PubMed] [Google Scholar]
- Van Meter KC, Christiansen LE, Delwiche LD, Azari R, Carpenter TE, Hertz-Picciotto I. Geographic distribution of Autism in California: A retrospective birth cohort analysis. Autism Research. 2010;3(1):19–29. doi: 10.1002/aur.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. Archives of General Psychiatry. 2013;70(1):71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Iosif A, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (Childhood Autism Risks from Genetics and Environment) Study. Journal of Autism and Developmental Disorders. 2013;43:25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.