Abstract
Electrophysiological studies have shown the enhanced response of anterior cingulate cortex (ACC) to colorectal distension in viscerally hypersensitive (VH) rats, which can be observed up to 7 weeks following colonic anaphylaxis, independent of colon inflammation, suggesting a mechanism for learning and triggering of pain memories in the ACC neuronal circuitry. Activity-dependent plasticity in synaptic strength may serve as a key mechanism that reflects cortical plasticity. However, only a few reports have indicated the synaptic plasticity of ACC in vivo. In the present study, electrophysiological recording showed long-lasting potentiation of local field potential in the medial thalamus (MT)-ACC synapses in VH rats. Theta burst stimulation in the MT reliably induced long-term potentiation in the MT-ACC pathway in normal rats, but was occluded in the VH state. Further, repeated tetanization of MT increased ACC neuronal activity and visceral pain responses of normal rats, mimicking VH rats. In conclusion, we demonstrated for the first time that visceral hypersensitivity is associated with alterations of synaptic plasticity in the ACC. The ACC synaptic strengthening in chronic visceral pain may engage signal transduction pathways that are in common with those activated by electrical stimulation, and serves as an attractive cellular model of functional visceral pain.
Keywords: anterior cingulate cortex, basal synaptic transmission, long-term potentiation, medial thalamus, visceral hypersensitivity
Introduction
Visceral hypersensitivity is a key factor in the pathophysiology of gastrointestinal functional disorders. Human brain imaging studies (Mayer et al. 2006) and growing experimental evidence have revealed new roles of cortical neuronal networks in chronic visceral pain (Zhuo 2008). Our series of published observations have characterized the neural electrophysiological activity of anterior cingulate cortex (ACC) during processing of visceral nociceptive stimulation. Using the viscerally hypersensitive (VH) rat model, we identified perigenual anterior cingulate cortex (pACC) sensitization. Peripheral afferent signals are not required for maintaining the increased ACC spontaneous firing and the enhanced responses to glutamate (Gao et al. 2006; Wu et al. 2008). Allodynia and hyperalgesia in VH rats appear to be mediated by enhanced glutamate N-methyl-d-aspartate (NMDA)-receptor 2B activities in the pACC (Cao et al. 2008; Fan et al. 2009; Li et al. 2012). Moreover, hypersensitivity to colorectal distension (CRD) can be observed up to 7 weeks after the initiation of colonic anaphylaxis and is independent of mucosal inflammation, suggesting mediation by a mechanism for the learning and triggering of pain memories in the ACC neuronal circuitry (Fan et al. 2009). Recent studies have further demonstrated that pACC activation is critical for the memory processing involved in long-term negative affective states (Yan et al. 2012). However, the mechanisms underlying how visceral nociceptive input is encoded within the ACC have not yet been explored. The synaptic substrates in the ACC neuronal circuitry responsible for storing nociceptive information for a prolonged period of time (e.g., by use-dependent change in synaptic strength) have not been identified.
The responses of neocortical neurons can be persistently modified by alterations in sensory experience. Such modifications reflect changes in synaptic transmission that shape cortical circuits and store information. Activity-dependent plasticity in synaptic strength, such as long-term potentiation (LTP) and long-term depression (LTD), may serve as key synaptic mechanisms reflecting cortical plasticity. While there have been studies involving synaptic plasticity in the ACC in vitro (Sah and Nicoll 1991; Hedberg and Stanton 1995), only a few reports have indicated the plastic properties of the ACC in vivo (Wei and Zhuo 2001; Zhuo 2007). We hypothesize that functional visceral pain is regulated by changes in the strength of the synapses in ACC neural circuits. The ACC LTP-like changes may serve as one of the basic neuronal synapse functions that underlie the mechanisms responsible for learning and memory storage for long-lasting functional visceral pain.
In the current study, ACC local field potentials (LFP) elicited by the electrical stimulation of the medial thalamus (MT) were used as a quantitative measure of synaptic strength. We first identified long-lasting potentiation of LFP in the MT-ACC synapses in the sensitized rats, suggesting an enhancement of basal synaptic transmission. Recording of ACC-LFP was combined with reverse microdialysis of NMDA and non-NMDA receptor antagonists to identify which receptor subtype mediates ACC synaptic plasticity. We then showed that theta burst stimulation (TBS) reliably induces LTP-like plasticity in the MT-ACC pathway in normal rats. However, in the VH state, the expression of LTP-like plasticity in MT-ACC synapses was smaller or occluded. Further, chronic repeated tetanization applied on MT markedly increased the ACC neuronal responses to visceral distension and the visceral pain responses of normal rats, which were mediated by NMDA receptor 2B subunit and CaMKII activities. Consistent with growing evidence that the sensory cortex remains plastic under appropriate circumstances (Zhou 2007, 2008; Shyu and Vogt 2009), our studies demonstrated for the first time that visceral hypersensitivity is associated with alterations of the properties of synaptic plasticity in the ACC. The upregulation of long-lasting synaptic transmission contributes to the enhanced ACC response to noxious visceral stimulation and pain responses. Further, together with data obtained from experiments by repeated tetanization of MT, our observations demonstrated that ACC nociceptive sensitization, chronic functional visceral pain, and classical LTP in the MT-ACC synapses share a similar mechanism.
Materials and Methods
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (TinHang Ltd, HK). Experiments were performed on adult male Sprague-Dawley rats (250–350 g). The animals were kept in their home cages and maintained on a 12:12 h light-dark cycle and provided with food and water ad libitum for at least 5 days prior to use. All the experimental procedures were approved by the Committee on the Use and Care of Animals at City University of Hong Kong and the licensing authority for conducting experiments of the Department of Health of Hong Kong (No. 10-4 in DH/HA&P/8/2/5).
Viscerally Hypersensitive Rat Model
Visceral hypersensitivity in rats was induced by colonic anaphylaxis. This animal model has been reliably used in previous work, and the detailed procedures were described in our previous publications (Gao et al. 2006; Cao et al. 2008; Fan et al. 2009). Briefly, the rats were injected (i.p.) with 1 mL saline, containing egg albumin (10 μg) as the antigen and aluminum hydroxide (10 mg) as the adjuvant. Beginning on day 3, colorectal anaphylaxis was induced. Antigen solution (egg albumin, 10 µg/mL) was perfused through a silastic enema tube. Then, CRD (30 mmHg for 30 s with a 3-min interval, and repeated 5 times) was performed. This complete procedure was performed once a day for 3 consecutive days. Rats injected with 1 mL saline (i.p.) served as controls. The electrophysiological recording studies were performed 5–7 days after the entire procedure.
Electrophysiology
The rats were anesthetized with urethane (i.p. 1.5 g/kg) and maintained at a constant level with intravenous supplements of anesthetic (one-fourth of the initial dosage) given every 3.5 h. Adequate depth of anesthesia was detected in a separate series of experiments without immobilization, by frequent observation of the absence of heart rate changes, and withdrawal reflexes after pinching the skin or subcutaneous electrical stimulation. After anesthesia, the rats were paralyzed with pancuronium (0.3 mg/h, i.v.) and artificially ventilated (3–3.5 mL, 55–65 strokes/min). Body temperature was maintained at 36.5 ± 0.5 °C using a heating blanket. The heart rate and electrocardiogram were monitored continuously. All the animals were put in a stereotaxic frame.
Synaptic Transmission and Synaptic Plasticity in MT-ACC Pathway
Previous studies suggest that the MT is the location that relays the nociceptive inputs to ACC (Kung and Shyu 2002; Vogt et al. 2003; Yang et al. 2006). A bipolar tungsten electrode was placed in the stereotaxic coordinates of the MT nuclei (2.5–4 mm posterior to bregma; 0.5–3.0 mm lateral to the midline; and 5–7 mm ventral to brain surface), ipsilateral to the recording sites in the ACC (Kung and Shyu 2002). The unit responses to CRD (50 mmHg) were determined. A neuron was defined as CRD-excited if its spike-firing rate increased by at least 10% from its predistension baseline activity. After the CRD-excited MT neurons were located, the electrode position was fixed, and the bipolar tungsten electrode connection was switched to the stimulator. A silver wire was placed in the connective tissues of the scalp as a reference electrode.
Field Potential Recording and Input–Output Studies
A tungsten-recording electrode was placed at stereotaxic coordinates (2.7 mm anterior to bregma; and 0.8 mm lateral to the midline) (Kung and Shyu 2002) and penetrated the surface of the cortex, avoiding the numerous blood vessels near the midline, and advanced into the ACC (0.4–0.8 mm ventral to brain surface) until the maximal response to MT stimulus (400 µA, square wave pulse, duration: 0.2 ms, 30 s interval) was observed. After stabilization of the ACC-LFP amplitude to MT stimulation, input–output (I/O) curves were generated by varying the stimulus current (from 50 to 1000 µA, increasing in 50-µA intervals) to evaluate synaptic potency. Stimulus pulses were delivered to the MT every 30 s, and 10 stimuli were given at each current level. The evoked responses were digitized (10 kHz) and amplified (×100) by a high-input impedance amplifier, and sent to a PC-based data acquisition system for online A/D conversion (Axon, Digidata 1440A) and digital analysis (Axonscope and Clampfit). The amplitude of the LFP was measured for the downward peak. The amplitudes of 10 responses recorded at each current level were averaged to construct the I/O curves.
LTP Induction
Electrophysiological recordings from animals and humans have revealed that ACC neurons are likely to fire action potentials at 4–7 Hz (θ) during various behavioral tests (Hutchison et al. 1999). LTP was induced by applying TBS (3 sets of 10 trains at 10 s intervals between each train; each train consisted of 10 bursts at 5 Hz, with each burst containing 5 pulses at 100 Hz) to the MT. The test stimulation intensity used in the present study was able to evoke about 50% of the maximum amplitude of field potentials (FPs) and was delivered to MT at 30 s intervals. FPs were recorded from the ACC every 5 min until 40 min after the TBS to the MT. The LTP-like plasticity was measured as an increase in LFP amplitude. Potentiation was measured as a percentage change from baseline and was analyzed using ANOVA. As the stimulation intensity used to elicit a baseline response in the normal control and VH groups of rats was different, in separate experiments on VH rats, a lower intensity of stimulation that was able to evoke 15% of the maximum LFP amplitude in VH rats was delivered to MT. This lower intensity evoked ACC-LFP amplitude in the VH rats, similar to 50% of maximum LFP amplitude in control rats.
ACC Neuronal Activity in Response to CRD
The details of the recording were described previously (Gao et al. 2006; Wu et al. 2008; Fan et al. 2009). Tungsten electrodes were lowered into the rostral ACC using a micromanipulator (2.0–3.8 mm anterior to bregma; 0.5–1.0 mm lateral to midline; and 1.5–3.5 mm ventral to brain surface). ACC neuronal spontaneous discharge was monitored for 2 min to confirm the stability of the basal firing frequency. A neuron was deemed responsive to CRD if its spike-firing rate increased or decreased by at least 10% from its predistension baseline activity, and only the CRD-excited neurons were tested further. The graded CRD pressures (20, 40, and 60 mmHg) were produced by rapidly injecting saline into the balloon over 1 s and maintaining the distension for 30 s with 5-min intervals. Neuronal discharge rates were measured for 30 s before, 30 s during, and 120 s after CRD, and evaluated on a time histogram (5 s bin width).
Pharmacological Blockade
For local pharmacological manipulations of MT-ACC synaptic transmission in vivo, in separate groups of control and VH rats, we performed LFP recording in the ACC at the same time as reverse microdialysis of NMDA receptor antagonist Aminophosphonopentanoic acid (AP5), AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), NMDA receptor 2B subunit antagonist (Ro25-6981), and CaMK II inhibitor Antennapedia-CaMK II Ntide (Ant-CaMK II Ntide). In a separate group of rats, GABAA receptor antagonist bicuculline methobromide (BIC, 100 µM) was applied to the ACC through reverse microdialysis before LTP induction in the MT-ACC synapse. The drug concentrations were chosen based on our previous studies (Wu et al. 2008; Fan et al. 2009) and reports by others (Galiñanes et al. 2011). This technique allows the administration of drugs in vivo with no change in tissue pressure or volume, and no current or voltage stimuli. Detailed procedures were described in our previous study (Wu et al. 2008). Microdialysis probes (Bioanalytical Systems, West Lafayette, IN, USA) with 3–4 mm of exposed membrane (320 μm diameter, ∼6000 Da permeability) were implanted into the ACC (1.5–3.8 mm anterior to bregma; and 0.3–1.0 mm lateral to midline at 30°) with a micromanipulator at 5 μm/s. AP5 and CNQX were dissolved in artificial cerebrospinal fluid (ACSF). The dosage of the drug was chosen in accordance with previous study, which showed that a similar dosage of glutamate receptor antagonist reduced CRD-induced ACC neuronal firing in VH rats (Wu et al. 2008), inhibiting ACC laminar transmembrane currents during noxious electrical stimulation of the MT (Yang et al. 2006). Recording microelectrodes were lowered into the rostral ACC about 1 mm lateral or rostral to the probe and angled at 10° toward the probe. The distance between the microdialysis probe and the recording electrode was 0.1–0.5 mm. Electrophysiological recordings were initiated about 2 h after probe implantation. Basal synaptic transmission and I/O curve studies were performed as described above.
Chronic Theta-Patterned Tetanization at the MT
Rats were anesthetized with pentobarbital (i.p. 50 mg/kg) and placed in a stereotaxic frame. Using bregma as the origin, a twisted bipolar Teflon-coated stainless steel (125 µm diameter in exposed tips) was inserted into the MT (3.5–4.0 mm posterior to bregma; and 1.0–1.5 mm lateral to the midline, at a depth of 5.0–7.0 mm below the cortical surface) as a stimulating electrode. The implanted electrodes were sealed with dental cement on the skull to ensure the correct location for further experiments. Five days after surgery, chronic electrical stimulation (6 sets of TBS at an intensity of 400 µA with 15-min intervals repeated for 3 days) was delivered to the MT. Then, at the end of the chronic stimulation, the ACC neuronal response to CRD and visceral pain behavior were examined. In control rats, the same surgery was performed, but with no theta-patterned tetanization.
Histological Identification of Stimulating and Recording Sites
At the end of experiments, a small electronic lesion (50 µA for 30 s, anodal DC current) was made by the tip of the stimulating and recording electrodes in the MT and ACC. Rats were fixed by perfusion with saline followed by 4% paraformaldehyde. Brains were sliced at 50 µm using a freezing microtome and stained with Cresyl violet. Drawings were made of sections showing electrode tracks related to the structure of the ACC and MT. A standard rat atlas (Paxinos and Watson 1998) was used as a reference for reconstruction of the stimulating and recording sites (Kung and Shyu 2002; Gao et al. 2006).
Chronic ACC Cannulation
Bilateral pACC cannulation was performed using the following coordinates: 4.2–3.0 mm anterior to bregma; 0.7 mm lateral to the midline; and 2.5 mm ventral to the brain surface as described previously (Cao et al. 2008). The visceromotor response (VMR) experiments were performed 6 days postoperatively. The location of the point of the termination of the cannula track was determined by histological studies. Serial coronal sections (50 µm) were cut with a cryostat along the path of the cannula, mounted on gelatin-coated slides, and stained with thionine.
Visceromotor Response to CRD
Details of this protocol have been described previously (Cao et al. 2008; Fan et al. 2009; Li et al. 2012). Briefly, 32-gauge stainless steel wires were implanted in the external oblique pelvic muscles 4–6 days before the beginning of the experimental procedures. Graded-pressure CRD (20, 40, and 60 mm Hg) was produced by rapidly injecting saline into a colonic balloon over 1 s and maintaining the distention for 30 s. The results of electromyography were quantified by calculating the area under the curve (AUC). Statistical comparisons of the VMR in various groups were made using 2-way ANOVA, followed by multiple comparisons adjusted by the Bonferroni's test.
Data Analysis and Statistics
Results of the LFP studies, induction of LTP, and various antagonist tests between the control group and the VH group were compared by 2-way ANOVA, followed by multiple comparisons adjusted by the Bonferroni's test. For ACC neuronal firing, single neuronal responses were examined using Datapac 2000 (RUN Technologies, Mission Viejo, CA, USA). The ACC neuronal responses to graded CRD pressures between the 2 groups were compared by 1-way repeated-measures ANOVA, followed by multiple comparisons of the Bonferroni's test. Results are expressed as mean ± SE. P < 0.05 was considered statistically significant.
Results
Facilitation of Basal Synaptic Transmission at the MT-ACC Synapses in VH Rats
A total of 8 control rats and 10 VH rats were tested. By unit recording of CRD-excited MT neurons, we identified that most of the MT neurons were located in the central lateral and medial dorsal region of the MT, according to the histological analysis.
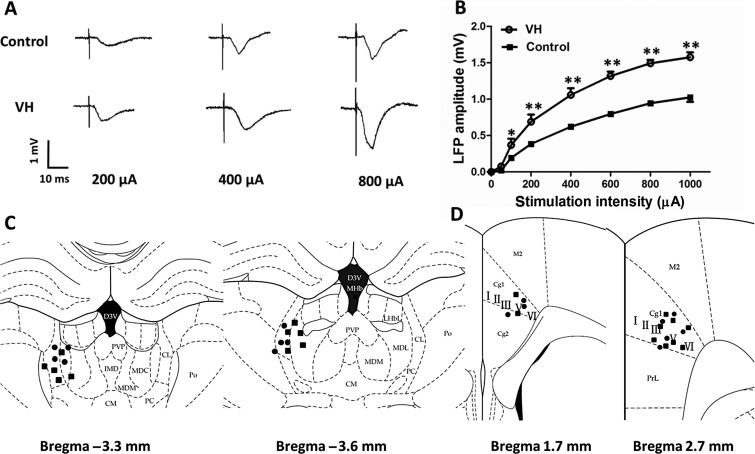
In both control (n = 8) and VH rats (n = 10), various stimuli (50–1000 µA) in the MT elicited gradually increasing ACC FP amplitudes. Representative original tracings of the ACC-LFP amplitudes in the control and VH rats are shown in Figure 1A. The VH rats showed markedly enhanced LFP response evoked by MT stimulations compared with that of control rats (Fig. 1B). A clear left shift of the I/O curve was observed in VH rats, suggesting long-lasting potentiation of MT-ACC synaptic transmission after induction of visceral hypersensitivity. Histological studies confirmed the localization of stimulating sites in the MT (Fig. 1C) and the recording sites in the ACC (layer III–VI of Cg1 region) (Fig. 1D). This result was consistent with the increased ACC neuronal spontaneous firings and the increased response to CRD observed in VH rats in our previous studies (Gao et al. 2006; Wu et al. 2008).
Figure 1.
LFP recording and input–output (I/O) curve in the ACC of control and VH rats. (A) Representative curves of LFP responses in the ACC to different stimuli intensities (200, 400, and 800 µA) to the MT in control and VH rats. (B) Left shift of I/O curve in the ACC of VH rats. (C) Stimulation sites in the MT in control (solid circles) and VH (black squares) rats. CL, central lateral; MDL, mediodorsal lat; and PC, paracentral. (D) Field potential recording sites in control (solid circles) and VH (black squares) rats. Cg1, cingulate cortex, area 1; Cg2, cingulate cortex, area 2; M2, secondary motor cortex; and PrL, prelimbic cortex. Results are presented as mean ± SEM, n = 8 for control rats, and n = 10 for VH rats. Statistical significance was determined by 2-way ANOVA, followed by multiple comparisons adjusted by the Bonferroni's test, *P < 0.05, **P < 0.01.
Effects of Glutamate Receptor Antagonists on MT-ACC Basal Synapse Transmission
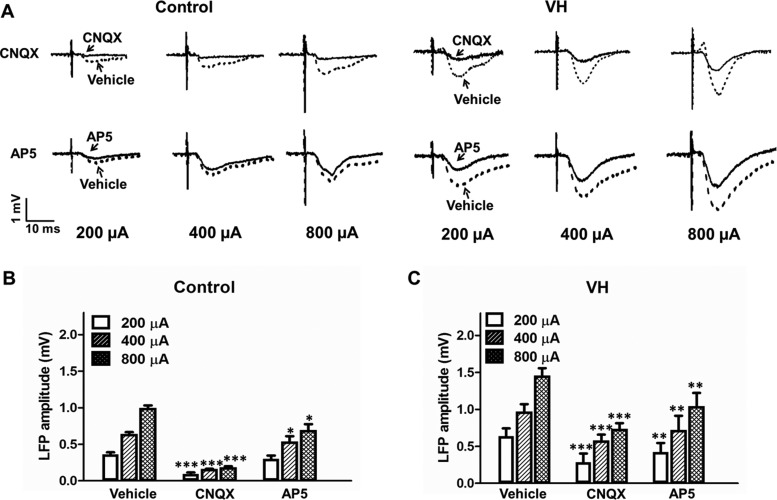
Representative recording curves of LFP amplitudes after application of CNQX or AP5 in control and VH rats are shown in Figure 2A. In control rats (n = 14), application of AMPA receptor antagonist CNQX (1.0 mM, n = 7) into the ACC significantly decreased the basal LFP amplitudes in response to 200, 400, and 800 µA stimulations in the MT (Fig. 2B). In contrast, microdialysis of NMDA receptor antagonist AP5 (2.0 mM, n = 7) mildly reduced the LFP amplitudes in response to 200, 400, and 800 µA stimulations in the MT (Fig. 2B). These results indicate that AMPA/kainate receptor plays a major role in modulating MT-ACC basal synaptic transmission under normal conditions.
Figure 2.
Effects of glutamate receptor antagonists on ACC-LFP in control and VH rats. (A) Representative curves of LFP responses in the ACC to different MT stimuli (200, 400, and 800 µA) after reverse microdialysis of vehicle (ACSF, dotted line), AMPA receptor antagonist CNQX (solid line), and NMDA receptor antagonist AP5 (solid line) in control and VH rats. (B) In control rats, mean LFP amplitudes to different MT stimuli (200, 400, and 800 µA) were significantly decreased after CNQX (n = 7) or AP5 (n = 7) administration compared with the vehicle. (C) In VH rats, mean LFP amplitudes to different MT stimuli (200, 400, and 800 µA) were significantly decreased after CNQX (n = 7) or AP5 (n = 7) administration compared with the vehicle. Results are presented as mean ± SEM. Statistical significance was determined by 2-way ANOVA, followed by multiple comparisons adjusted by the Bonferroni's test, *P < 0.05, **P < 0.01, ***P < 0.001.
In VH rats (n = 14), administration of CNQX (1.0 mM, n = 7) reduced the ACC-LFP amplitudes in response to 200, 400, and 800 µA stimulations in the MT (Fig. 2C). Meanwhile, microdialysis of NMDA receptor antagonist AP5 at a concentration of 2.0 mM (n = 7) reduced the LFP amplitudes in response to 200, 400, and 800 µA stimulations in the MT, respectively (Fig. 2C). These results showed that both AMPA receptor and NMDA receptor antagonist could partially inhibit the enhanced MT-ACC basal synaptic transmission in VH rats.
Effects of CaMK II Inhibitor and NR2B Receptor Antagonist on MT-ACC Basal Synaptic Transmission
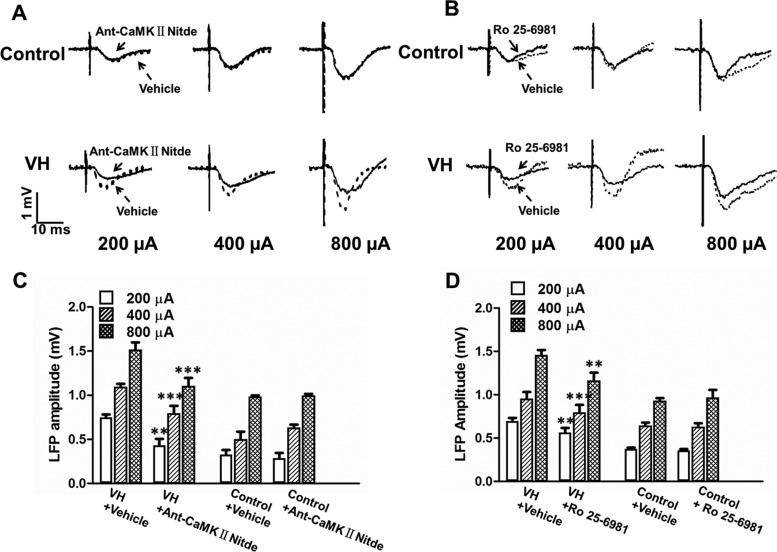
Previous studies have suggested that calcium/calmodulin-dependent protein kinase (CaMKII) binding to the NR2B receptor plays an important role in synaptic plasticity and memory formation (Lisman et al. 2002; Li et al. 2012). Here we showed that, in the control group, application of CaMKII inhibitor Ant-CaMK II Ntide (50 µM, n = 6, Fig. 3C) or NR2B receptor antagonist Ro25-6981(500 µM, n = 6, Fig. 3D) did not change the LFP amplitude in the ACC compared with vehicle in response to different intensities of MT stimuli. In contrast, in VH rats, microdialysis of Ant-CaMK II Ntide (50 µM, n = 6, Fig 3C) or NR2B receptor antagonist Ro25-6981(500 µM, n = 6, Fig 3D) both significantly reduced the LFP amplitude in the ACC compared with vehicle. Representative recording curves in response to different intensities of MT stimuli in control and VH rats with Ant-CaMK II Ntide or Ro25-6981 administration are shown in Figure 3A,B. This result demonstrated that both CaMKII and NR2B receptor activity participate in modulating the MT-ACC basal synaptic transmission in VH rats.
Figure 3.
Effect of CaMKII inhibitor and NR2B antagonist on ACC-LFP in control and VH rats. (A) Typical recordings of LFP in the ACC to different intensities of MT stimuli (200, 400, and 800 µA) in control and VH rats after application of vehicle (dotted line) and CaMKII inhibitor Antennapedia-CaMKIINtide (solid line). (B) Typical recordings of LFP in the ACC to different intensities of MT stimuli (200, 400, and 800 µA) after application of vehicle and NR2B receptor antagonist Ro25-6981 (solid line). (C) Application of Antennapedia-CaMKIINtide had no effect on ACC-LFP in control rats, whereas it significantly decreased ACC-LFP in VH rats compared with vehicle. (D) Application of Ro25-6981 significantly decreased ACC-LFP in VH rats compared with vehicle. No effects were observed in control rats. Results are presented as mean ± SEM. Statistical significance was determined by 2-way ANOVA, followed by multiple comparisons adjusted by the Bonferroni's test, *P < 0.05, **P < 0.01, ***P < 0.001.
Induction of LTP-Like Plasticity at the MT-ACC Synapses in Control Rats
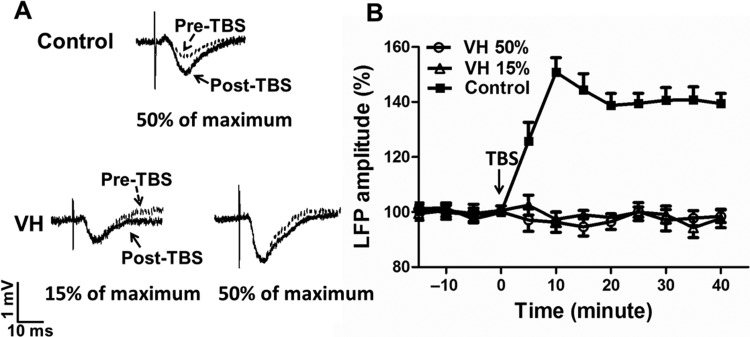
In control rats (n = 8), TBS to the MT induced a robust and long-lasting LTP-like increase in LFP amplitude in the ACC in response to the MT stimuli (400 µA, evoked about 50% of the maximum LFP amplitude) compared with the baseline level, reflecting the potentiation of the MT-ACC pathway. The increased LFP amplitude reached a peak level (146.9 ± 6.7% of pre-TBS) at 10 min and remained at this level for at least 40 min (Fig. 4). The average LFP amplitude after TBS was 140.1 ± 5.3% over pre-TBS. These observations suggest that LTP could be reliably induced in the MT-ACC synapses in control rats.
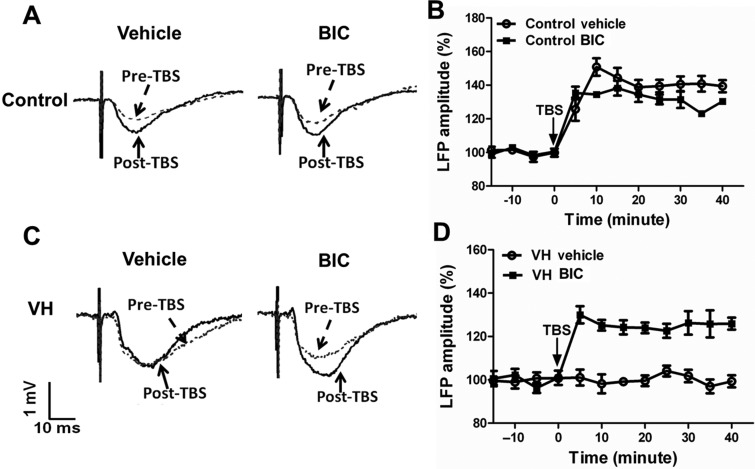
Figure 4.
LTP-like plasticity in the MT-ACC synapse in control and VH rats. (A) Representative curves of LFP responses in the ACC to MT stimuli before TBS and 40 min after TBS in control and VH rats. (B) LTP-like plasticity was reliably induced in the ACC by MT-TBS in control rats (n = 8). In VH rats, the induction of LTP-like plasticity in the ACC was occluded when tested with either stimulation intensity that evoked about 50% of the maximum LFP amplitude (n = 10) or a lower intensity (evoked about 15% of the maximum LFP amplitude, n = 7) after MT-TBS. Results are expressed as mean ± SEM.
LTP-Like Plasticity at MT-ACC Synapses Was Blocked in VH Rats
In contrast to normal rats, TBS to the MT failed to facilitate the induction of LTP-like plasticity in VH rats (n = 10). Representative recording curves are shown in Figure 4A. There was no obvious change of LFP amplitude tested in pre-TBS and post-TBS conditions at every time point (Fig. 4B). The LFP amplitude was 97.2 ± 2.9% of pre-TBS values. As the baseline LFP values were higher in VH rats compared with the control rats, we performed an additional study by using low-intensity stimulus (100 µA, evoked 15% of maximum amplitude of the LFP), which evoked response in VH rats that is comparable to the response induced by 400 µA (induced 50% of maximum amplitude of the LFP) in control rats. We found that low-intensity stimulus in VH rats also failed to elicit increases in LFP amplitude following TBS conditioning (98.5 ± 6.6% of pre-TBS values, Fig. 4B). These observations suggest that the induction of LTP-like plasticity in the MT-ACC synapses was blocked in the VH states. It appears that, in the VH state, transduction signals in the MT-ACC synapses are not available for subsequent electrical recruitment, suggesting that the synaptic strengthening occurring in the VH state engages signal transduction pathways that are in common with those activated by electrical stimulation.
Effects of GABAA Receptor Antagonist on the Induction of LTP-Like Synaptic Plasticity
In separate groups of control (n = 8) and VH rats (n = 10), GABAA receptor antagonist BIC (100 µM) was applied to the ACC through reverse microdialysis before induction of LTP-like plasticity by TBS to the MT. Representative recording curves of LFP in response to MT stimulation (400 µA, evoked 15% of maximum amplitude of the LFP) pre- and post-TBS in control and VH rats treated with vehicle (ACSF) or BIC are shown in Figure 5A,C. In our experimental conditions, there were no significant changes in the LTP-like synaptic plasticity in control rats after BIC application (n = 4) compared with those treated with vehicle (n = 4) (Fig 5B). However, we found that, in VH rats, LTP-like synaptic plasticity was significantly facilitated only if GABAAergic tone was reduced by local application of GABAA receptor antagonist (n = 5), but not vehicle (n = 5). The LFP amplitude following TBS conditioning reached 125.9 ± 2.7% of pre-TBS values and lasted for at least 40 min in VH rats (Fig. 5D).
Figure 5.
Effects of GABAA receptor antagonist on LTP-like synaptic plasticity in control and VH rats. (A) Representative curves of LFP responses in the ACC to MT stimuli pre- and post-TBS after vehicle or BIC application in control rats. (B) There was no obvious change in LTP-like plasticity in the MT-ACC synapse in control rats. (C) Representative curves of LFP responses in the ACC to MT stimuli pre- and post-TBS after vehicle or BIC application in VH rats. (D) LTP-like plasticity in the MT-ACC synapse was facilitated in VH rats after BIC application. Results are expressed as mean ± SEM.
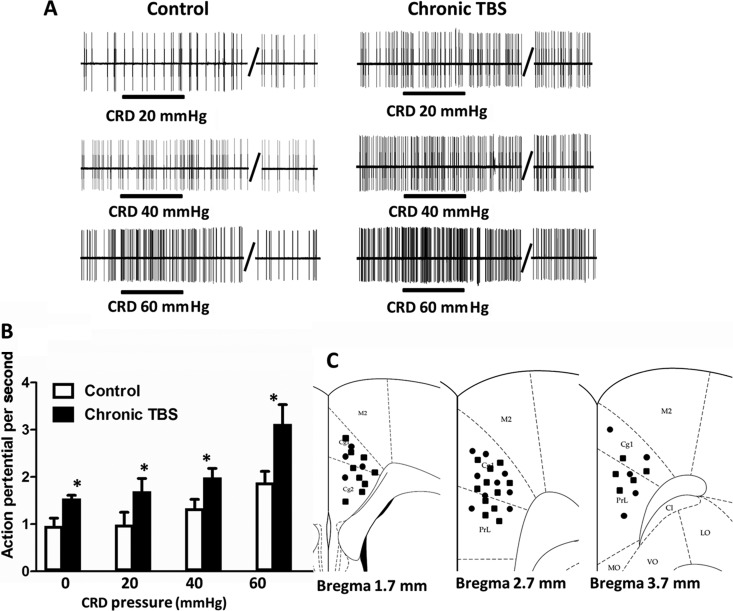
Chronic Theta-Patterned Tetanization at MT Facilitates ACC Neuronal Response to CRD
In control rats (n = 7) with sham stimulation, a total of 74 neurons were tested: 53 neurons showed no response, 3 neurons showed inhibited response, and 18 neurons (24.3%) showed excited response to CRD. Only the CRD-excited neurons were further examined. Representative single unit recordings of ACC neuronal response to graded CRD pressures are shown in Figure 6A. Basal ACC neuronal activity was assessed over 30 s, and the spontaneous activity in control rats was characterized as 0.96 ± 0.17 spikes/s. The firing rates in response to 20, 40, and 60 mmHg CRD pressures were gradually increased (Fig. 6B). In rats (n = 8) treated with chronic theta-patterned tetanization at the MT, a total of 79 neurons were tested: 50 neurons showed no response, 4 neurons showed inhibited response, and 25 neurons (31.6%) were identified as CRD-excited neurons. The average spontaneous firing rate of these 25 CRD-excited neurons was 1.54 ± 0.07 spikes/s, markedly enhanced compared with control rats (0.96 ± 0.17 spikes/s). The ACC neuronal firing rates were also significantly increased in response to gradually increased CRD pressures compared with those of control rats (Fig. 6B). Representative recordings are shown in Figure 6A. This result indicated that repeated artificial induction of LTP in the MT-ACC synapse by chronic repeated TBS to MT significantly enhanced the ACC neuronal activities at baseline and also in response to graded CRD stimuli. This phenomenon mimics the characteristic of ACC sensitization in VH rats, which has been reported in our previous publications (Gao et al. 2006; Wu et al. 2008). The location of the CRD-excited neurons recorded in the ACC of control and chronic TBS rats are shown in Figure 6C.
Figure 6.
Facilitated ACC neuronal response to graded CRDs after chronic TBS to MT. (A) Representative recordings of ACC CRD-excited neurons in response to graded CRDs (20, 40, and 60 mmHg) in normal rats with and without chronic TBS at MT. (B) The ACC neuronal spontaneous firings (0 mmHg CRD) and firing rates in response to 20, 40, and 60 mmHg CRD were markedly increased in rats with chronic TBS at MT. (C) Locations of identified CRD-excited neurons in the ACC in control (solid circles) and chronic TBS rats (black squares). Cg1, cingulate cortex, area 1; Cg2, cingulate cortex, area 2; M2, secondary motor cortex; and PrL, prelimbic cortex. Results are presented as mean ± SEM. Statistical significance was determined by 2-way ANOVA, followed by multiple comparisons adjusted by the Bonferroni's test, *P < 0.05.
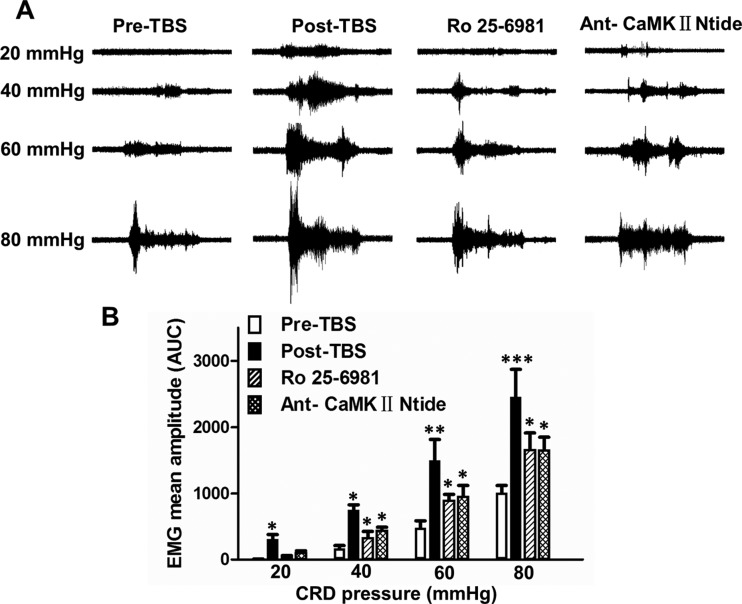
Visceromotor Responses to CRD after Chronic Theta-Patterned Tetanization at MT
To determine if chronic TBS tetanization at the MT modulates visceral pain, we tested the VMR to graded CRD pressures in normal rats before and after chronic TBS to the MT. Original recordings of electromyography are shown in Figure 7A. All of the rats showed CRD pressure-dependent increases of VMR measured before and after chronic TBS. A significant VMR to the lowest distension pressure tested (20 mmHg) after chronic TBS and an absence of response to the same pressure in normal state (prechronic TBS) suggest a reduced pressure threshold in rats after chronic TBS. Compared with normal condition (prechronic TBS), chronic TBS to the MT led to greatly enhanced VMR postchronic TBS in response to CRD pressures of 20, 40, 60, and 80 mmHg, respectively (Fig. 7B). These results provide evidence of enhanced visceral pain responses in rats after chronic artificial induction of LTP in MT-ACC synapse.
Figure 7.
VMR to CRD after chronic TBS to MT and effects of NR2B receptor antagonist and CaMKII inhibitor. (A) Representative EMG recordings to graded CRDs (20, 40, 60, and 80 mmHg) in normal rats pre- and postchronic TBS at MT, and after administration of NR2B receptor antagonist Ro 25-6981 or CaMKII inhibitor Ant-CaMKII Ntide. (B) EMG mean amplitudes (AUC) in response to graded CRDs (20, 40, 60, and 80 mmHg) were significantly increased after chronic TBS at MT, which could be reduced by application of either Ro 25-6981 or Ant- CaMKII Ntide. Results are presented as mean ± SEM. Statistical significance was determined by 2-way ANOVA, followed by multiple comparisons adjusted by the Bonferroni's test, *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of NR2B Receptor Antagonist and CaMK II Inhibitor on VMR to CRD
The original recording and mean amplitudes expressed as AUC in rats before and after chronic TBS to the MT are shown in Figure 7. After Ro25-6981 administration, the EMG amplitude was decreased in rats postchronic TBS. Application of Ant-CaMK II Ntide also significantly reduced the VMR in response to CRD pressures of 20, 40, 60, and 80 mmHg in rats postchronic TBS (Fig. 7B). These results indicate that NR2B and CaMK II mediate the enhanced visceral pain response induced by chronic TBS to the MT, and also suggest that chronic repeated artificial LTP in the MT-ACC synapses inducing visceral pain shares a similar molecular mechanism with colonic anaphylaxis elicited visceral hypersensitivity in VH rats.
Discussion
Studies of both human and animals consistently suggest that the ACC and its related areas are important for processing pain perception (Vogt et al. 2003). Patients with irritable bowel syndrome (IBS) show enhanced activation of the dorsal ACC, and a reduction of this pattern is associated with a reduction in IBS symptoms (Mertz et al. 2000). These findings suggest a possible dysfunction of the emotional and sensory components of the brain-pain experience system in IBS. Previously, we have provided direct electrophysiological evidence of the sensitization of ACC neurons in VH rats (Gao et al. 2006; Wu et al. 2008). Additionally, the NMDA receptor activation of sensitized ACC neurons plays a causative role in the long-lasting visceral pain responses, which are independent of inflammation in the colon in the functional VH rats (Cao et al. 2008; Fan et al. 2009). Recently, using a rodent visceral pain assay that combines the CRD-induced VMR with conditioning-place avoidance, we showed that pACC activation is critical for the memory processing involved in long-term negative affective states and the prediction of aversive stimuli by contextual cues (Yan et al. 2012). Taken together, these observations suggest that the facilitation of visceral pain responses following a brief noxious stimulus (colonic anaphylaxis in our model) has clear parallels with memory, where information needs to be stored and retrieved. ACC neuronal circuitry may store information for prolonged periods of time (e.g., by use-dependent changes in synaptic strength).
Activity-induced persistent synaptic modifications are generally thought to be the cellular mechanism underlying developmental refinement of neuronal connections (Zhang et al. 2001), as well as learning and memory in mature animals (Bliss and Collingridge 1993; Chapman et al. 1999; Martin et al. 2000). Studies of synaptic plasticity have shown that repetitive electrical activity can rapidly induce persistent changes in the strength of synaptic transmissions, known as LTP and LTD (Chapman et al. 1998).
The MT serves as a major relay in the medial pain system and in the conveyance of nociceptive information to the ACC (Shyu and Vogt 2009; Vogt et al. 2003). Studies using electrical stimulation of the MT have revealed the temporal events and the pharmacological sensitivities of MT-ACC activation (Castro-Alamancos 1997; Shyu and Vogt 2009). LFP is a low-frequency (40–130 Hz) component of the electrophysiological signal. It reflects the superposition of synchronized dendritic currents, averaged over a large variety of interneurons and intracortical activity. FP recording is one way to study artificially induced synaptic plasticity (Martin et al. 2000; Shyu and Vogt 2009). In the current study, the ACC FPs elicited by electrical stimulation of the MT were used as a quantitative measure of synaptic strength. I/O curves generated by a gradual increase of the stimulus intensity (50–1000 µA) showed significant increases in LFP in the sensitized rat, suggesting enhancement of basal synaptic transmission in the thalamo-ACC synapses after induction of visceral hypersensitivity; this is mediated by both NMDA and AMPA receptor activity.
LTP has received a tremendous amount of attention in the roughly 40 years since it was first described by Bliss and Lomo (1973). LTP is an activity-dependent process that involves a persistent increase in synaptic efficacy. LTP has been demonstrated in both the hippocampus (Bliss and Collingridge 1993) and neocortex (Zhang and Poo 2001) following the administration of brief, high-frequency bursts of stimulation to excitatory afferents. This phenomenon has also been shown in a behavioral model related to fear emotion (Garcia et al. 1998). While there have been studies involving synaptic plasticity in the posterior cingulate cortex (Hedberg and Stanton 1995) and in the ACC in vitro (Sah and Nicoll 1991), only a few reports have examined the plastic properties of the ACC in vivo (Wei and Zhuo 2001; Zhuo 2007). The literature is devoid of a definitive study that demonstrates the activities and synaptic plasticity of ACC neuronal circuitry for processing visceral nociceptor stimulation.
Electrophysiological recordings from animals and humans have revealed that ACC neurons are likely to fire action potentials at 4–7 Hz (theta) during various behavioral tests. In this study, we showed that, in the normal rats, delivering TBS to the MT induced a robust and long-lasting increase of the amplitude of the evoked FP in the ACC, reflecting the potentiation of the MT-ACC pathway. Therefore, LTP-like plasticity is reliably induced in rat MT-ACC synapses in intact rats by TBS condition stimulation.
A key feature of synaptic plasticity is that it can be modulated by prior synaptic activity. This property is termed metaplasticity (i.e., high-order synaptic plasticity) (Bliss and Collingridge 1993; Abraham and Bear 1996; Chapman et al. 1999), which describes all phenomena in which previous synaptic activity modifies the degree or direction of long-term changes in synaptic strength caused by subsequent artificial stimuli (Abraham and Bear 1996). In the present study, the data demonstrate for the first time that induction of visceral hypersensitivity effectively blocks the expression of LTP at thalamo-ACC synapses in vivo. It appears that induction of visceral hypersensitivity produces a change in the ability to induce subsequent synaptic plasticity at the MT-ACC pathway, supporting the notion that visceral hypersensitivity and electrically induced LTP share common mechanisms, that is, the synaptic strengthening that occurs in the ACC in the chronic visceral pain state may engage signal transduction pathways that are in common with those activated by electrical stimulation. This occlusion demonstrates that the VH condition activates transduction signals that are not available for subsequent electrical recruitment. This occlusion approach has been commonly used to investigate whether the plasticity obtained in vitro by electrical stimulation is a reliable model for studying the mechanisms of learning and memory (Martin et al. 2000). The physiological role of occlusion of LTP in thalamo-ACC synapses may represent a neuroprotective mechanism that prevents an overstimulation of already-potentiated synapses. In line with the present results, a similar LTP occlusion has been found after a fear paradigm in amygdala (Tsvetkov et al. 2002) and hippocampus (Sacchetti et al. 2002). Furthermore, we found that application of GABAA receptor antagonist significantly facilitated induction of LTP-like plasticity in VH rats, but had no obvious effects in control rats. It seems conceivable that homeostatic metaplasticity is present in the VH state and, perhaps, in inhibitory intracortical circuits. Inhibitory mechanisms may play a significant role in controlling the magnitude of subsequent plasticity of the excitatory MT-ACC pathways in chronic pain state.
Microstimulation of the thalamus can evoke visceral pain experiences, such as angina or labor pain, sometimes years after the original episode (Lenz et al. 1994; Davis et al. 1995). These observations highlight the integrative role of the thalamus in processing memories of pain and the existence of long-lived neural mechanisms that are capable of storing the results of a previous painful experience for many years. The pain memories evoked in these studies are of visceral pain, presumably because they are common in the general population and tend to be fairly intense experiences. To further characterize whether LTP-like synaptic plasticity in the MT-ACC synapses contributes to the learning and memory of visceral pain in a VH state, we showed that repeated applying theta-patterned tetanization in the MT in the awake normal rats (Werk and Chapman 2003) resulted in enhancement of ACC responses to CRD and facilitation of behavioral visceral pain, which mimic ACC sensitization and visceral allodynia and hyperalgesia in the VH model as we demonstrated previously (Gao et al. 2006; Cao et al. 2008; Fan et al. 2009). The facilitated visceral pain responses were attenuated by administration of NR2B receptor antagonist and CaMKII inhibitor. These observations lend support to the theory that the responses of neocortical neurons can be persistently modified by alterations in visceral sensory experience. The enhanced long-lasting transmission at the MT-ACC synapses causally contributes to visceral pain. It appears that visceral hypersensitivity, pACC sensitization, and long-lasting enhanced synaptic transmission in the VH state are expressed by the same core mechanisms as TBS-induced canonical LTP in rats.
Most clinical specialists continue to treat visceral pain as only a symptom and not as a distinct neurological entity. The findings of studies with positron emission tomography lend support to the hypothesis that these syndromes may be the result of visceral hypersensitivity that causes patients to become more aware of gastrointestinal activity (Naliboff et al. 1997; Silverman et al. 1997). This heightened awareness may be the result of alterations of central processing that lead to increased activation of visceral nociceptive pathways (Cervero and Laird 1999). Our novel observations suggest that functional visceral pain is regulated by changes in the strength of the synapses in ACC neural circuits. ACC LTP-like changes may serve as one of the basic neuronal synapse functions that underlie the mechanisms responsible for learning and memory storage for long-lasting functional visceral pain. These results represent a significant step on the path to direct comparison of the course of the processes of neuron specialization during induction of visceral hypersensitivity with changes in synaptic efficiency in the same cells during the induction of canonical LTP. This suggests the possibility that the same synaptic mechanisms are involved in the processes of modifying ACC cells, reducing the pain threshold, amplifying affective responses to pain, and processing learning and memory in patients with IBS.
In summary, long-lasting ACC potentiation (basal synaptic transmission) occurs in the VH state. TBS of the MT reliably induces canonical LTP-like plasticity in the MT-ACC synapses in normal rats. However, in the VH state, the expression of TBS-induced LTP-like increases in LFP was smaller or occluded. Theta-patterned tetanization in thalamo-ACC synapses induced LTP-like plasticity, causally contributing to ACC sensitization and visceral pain, which mimics the VH rat model. We conclude that thalamo-ACC LTP (canonical LTP) has striking similarities to phenomena of ACC sensitization in the VH state, and serves as an attractive cellular model of functional visceral pain.
Funding
This work was supported by the Research Grants Council of Hong Kong (160810, 160811, and 160812 to Y.L.), the National Science Foundation of China (81170353 to Y.L.), City University of Hong Kong Neuroscience Research Infrastructure Grant (9610211 to Y.L.) and Centre for Biosystems, Neuroscience, and Nanotechnology (9360148 to S.P. and Y.L.). Funding to pay the Open Access publication charges for this article was provided by Centre for Biosystems, Neuroscience, and Nanotechnology (9360148 to S.P. and Y.L.).
Notes
Conflict of Interest: None declared.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZJ, Wu XY, Chen SL, Owyang C, Li Y. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology. 2008;134:535–543. doi: 10.1053/j.gastro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Short-term plasticity in thalamocortical pathways: cellular mechanisms and functional roles. Rev Neurosci. 1997;8:95–116. doi: 10.1515/revneuro.1997.8.2.95. [DOI] [PubMed] [Google Scholar]
- Cervero F, Larid JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Trepel C, Ivanco TL, Froc DJ, Wilson K, Racine RJ. Changes in field potentials and membrane currents in rat sensorimotor cortex following repeated tetanization of the corpus callosum in vivo. Cereb Cortex. 1998;8(8):730–742. doi: 10.1093/cercor/8.8.730. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Davis KD, Tasker RR, Kiss ZHT, Hutchison WD, Dostrovsky JO. Visceral pain evoked by thalamic microstimulation in humans. Neuroreport. 1995;6:369–374. doi: 10.1097/00001756-199501000-00035. [DOI] [PubMed] [Google Scholar]
- Fan J, Wu XY, Cao ZJ, Chen SL, Owyang C, Li Y. Upregulation of anterior cingulate cortex NR2B receptors contributes to visceral pain as measured by visceromotor responses in rats. Gastroenterology. 2009;136:1732–1740. doi: 10.1053/j.gastro.2009.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Galiñanes GL, Braz BY, Murer MG. Origin and properties of striatal local field potential responses to cortical stimulation: temporal regulation by fast inhibitory connections. PLoS One. 2011;6(12):e28473. doi: 10.1371/journal.pone.0028473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wu XY, Owyang C, Li Y. Enhanced responses of the anterior cingulate cortex neurons to colonic distension in viscerally hypersensitive rats. J Physiol. (London) 2006;570:169–184. doi: 10.1113/jphysiol.2005.096073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Garcia R, Tocco G, Baudry M, Thompson RF. Exposure to a conditioned aversive environment interferes with long-term potentiation induction in the fimbria–CA3 pathway. Neurosci. 1998;82:139–145. doi: 10.1016/s0306-4522(97)00285-6. [DOI] [PubMed] [Google Scholar]
- Hedberg TG, Stanton PK. Long-term potentiation and depression of synaptic transmission in rat posterior cingulate cortex. Brain Res. 1995;670:181–196. doi: 10.1016/0006-8993(94)01254-f. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2(5):403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- Kung JC, Shyu BC. Potentiation of local field potentials in the anterior cingulate cortex evoked by the stimulation of the medial thalamic nuclei in rats. Brain Res. 2002;953(1–2):37–44. doi: 10.1016/s0006-8993(02)03265-1. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Gracely RH, Hope EJ, Baker FH, Rowland LH, Dougherty PM, Richardson RT. The sensation of angina can be evoked by stimulation of the human thalamus. Pain. 1994;59:119–125. doi: 10.1016/0304-3959(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang X, Liu HY, Cao ZJ, Chen SL, Cao B, Liu J. Phosphorylated CaMK II postsynaptic binding to NR2B subunits in the anterior cingulate cortex mediates visceral pain in visceral hypersensitive rats. J Neurochem. 2012;121(4):662–671. doi: 10.1111/j.1471-4159.2012.07717.x. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMK II function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3(3):175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distension. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain. 4th ed. San Diego, CA: Academic; 1998. [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. Time-dependent inhibition of hippocampal LTP in vitro following contextual fear conditioning in the rat. Eur J Neurosci. 2002;15:143–150. doi: 10.1046/j.0953-816x.2001.01844.x. [DOI] [PubMed] [Google Scholar]
- Sah P, Nicoll RA. Mechanisms underlying potentiation of synaptic transmission in rat anterior cingulate cortex in vitro. J Physiol. 1991;433:615–630. doi: 10.1113/jphysiol.1991.sp018446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu BC, Vogt BA. Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway. Mol Pain. 2009;5:51. doi: 10.1186/1744-8069-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DHS, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Farber NB. Cingulate cortex and disease models. In: Paxinos G, editor. The rat nervous system. 3rd ed. San Diego: Elsevier; 2003. pp. 705–727. [Google Scholar]
- Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetized rat. Physiol. 2001;532:823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werk CM, Chapman CA. Long-term potentiation of polysynaptic responses in layer V of the sensorimotor cortex induced by theta-patterned tetanization in the awake Rat. Cereb Cortex. 2003;13:500–507. doi: 10.1093/cercor/13.5.500. [DOI] [PubMed] [Google Scholar]
- Wu XY, Gao J, Yan J, Fan J, Owyang C, Li Y. Role for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive rats. Am J Physiol. 2008;294:918–927. doi: 10.1152/ajpgi.00452.2007. [DOI] [PubMed] [Google Scholar]
- Yan N, Cao B, Xu JH, Hao C, Zhang X, Li Y. Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiol Learn Mem. 2012;97(1):156–164. doi: 10.1016/j.nlm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Yang JW, Shih HC, Shyu BC. Intracortical circuits in rat anterior cingulate cortex are activated by nociceptive inputs mediated by medial thalamus. J Neurophysiol. 2006;96(6):3409–3422. doi: 10.1152/jn.00623.2006. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zhuo M. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells. 2007;23:259–271. [PubMed] [Google Scholar]