Abstract
Visual deprivation is reported to prevent or delay the development of mature receptive field (RF) properties in primary visual cortex (V1) in several species. In contrast, visual deprivation neither prevents nor delays refinement of RF size in the superior colliculus (SC) of Syrian hamsters, although vision is required for RF maintenance in the SC. Here, we report that, contrary to expectation, visual cortical RF refinement occurs normally in dark-reared animals. As in the SC, a brief period of visual experience is required to maintain V1 RF refinement in adulthood. Whereas in the SC, 3 days of visual experience within a sensitive period (P37–40) was sufficient to protect RFs from deprivation-induced enlargement in adulthood, 7 days (P33–40) were required for RF size maintenance in V1. Thus, spontaneous activity is sufficient for RF refinement at these 2 levels of the visual pathway, and visual input is necessary only to prevent deprivation-induced RF enlargement in adulthood. These studies show that sensory experience during a late juvenile sensitive period protects the visual pathway against sensory deprivation in adulthood, and suggest that more importance may have been placed on the role of early visual experience in visual RF development than is warranted.
Keywords: adult plasticity, critical period, dark rearing, rodent, visual deprivation
Introduction
A major question in developmental neuroscience is how early sensory experience contributes to the development of adult brain function. Experience can be in the form of light-evoked or spontaneously generated neural activity. There has been considerable debate about what the relative roles of these 2 types of activity are in visual system development (Ruthazer and Aizenman 2010). Early developmental events rely on spontaneous activity before eye opening (Galli and Maffei 1988; Wong et al. 1993; Feller et al. 1996; Weliky and Katz 1999; Wong 1999; Chiu and Weliky 2001; Chandrasekaran et al. 2005; Mrsic-Flogel et al. 2005; Xu et al. 2011; Ackman et al. 2012) and in some cases, light through unopened eyelids (Akerman et al. 2002; Colonnese et al. 2010). Light-evoked visual experience after eye opening plays a role in the development of some visual cortical receptive field (RF) properties but not others, for reasons that are unclear. An initial development of orientation selectivity and ocular dominance occurs without visual experience, but sharpening and maintenance of these properties require visual experience (for review see Huberman et al. 2008; Espinosa and Stryker 2012). In contrast, direction selectivity requires visual experience for even rudimentary development in ferrets (Li et al. 2006), but not in mice (Rochefort et al. 2011), illustrating that species differences also exist.
RF refinement is an important aspect of the progressive improvement in visual perceptual acuity and thus, may be especially critical for survival. There is a lack of consensus regarding the time course of RF refinement and its dependence on visual experience. One part of this lack of clarity may arise from species differences and differences in the dependence of different brain regions on visual experience. In rat primary visual cortex (V1), the majority of RF refinement occurs by P30 (Fagiolini et al. 1994), whereas there is little refinement by that age in mouse V1 (Ko et al. 2013) or hamster superior colliculus (SC; Carrasco et al. 2005) even when differences in the rate of development are taken into account (Clancy et al. 2001). Synaptogenesis in V1 proceeds normally in enucleated monkeys or preterm monkeys exposed to light, but visual experience is required for fine tuning the location of dendritic spines (Bourgeois et al. 1989; Bourgeois and Rakic 1996), and thus, is likely to be necessary for RF refinement.
The role of visual experience in the development and plasticity of RFs has received little study. RF refinement is an activity-dependent process (Meyer 1983; Thompson and Holt 1989; Cook et al. 1999; Chandrasekaran et al. 2005; Guido 2008; for review see Espinosa and Stryker 2012). Previous studies have reported that RFs in V1 of dark-reared (DR) animals are enlarged (Fagiolini et al. 1994; Gianfranceschi et al. 2003) and behavioral measures show reduced visual acuity (Timney et al. 1978), but it is unclear whether the RFs never refined or whether they refined but were not maintained in a refined state.
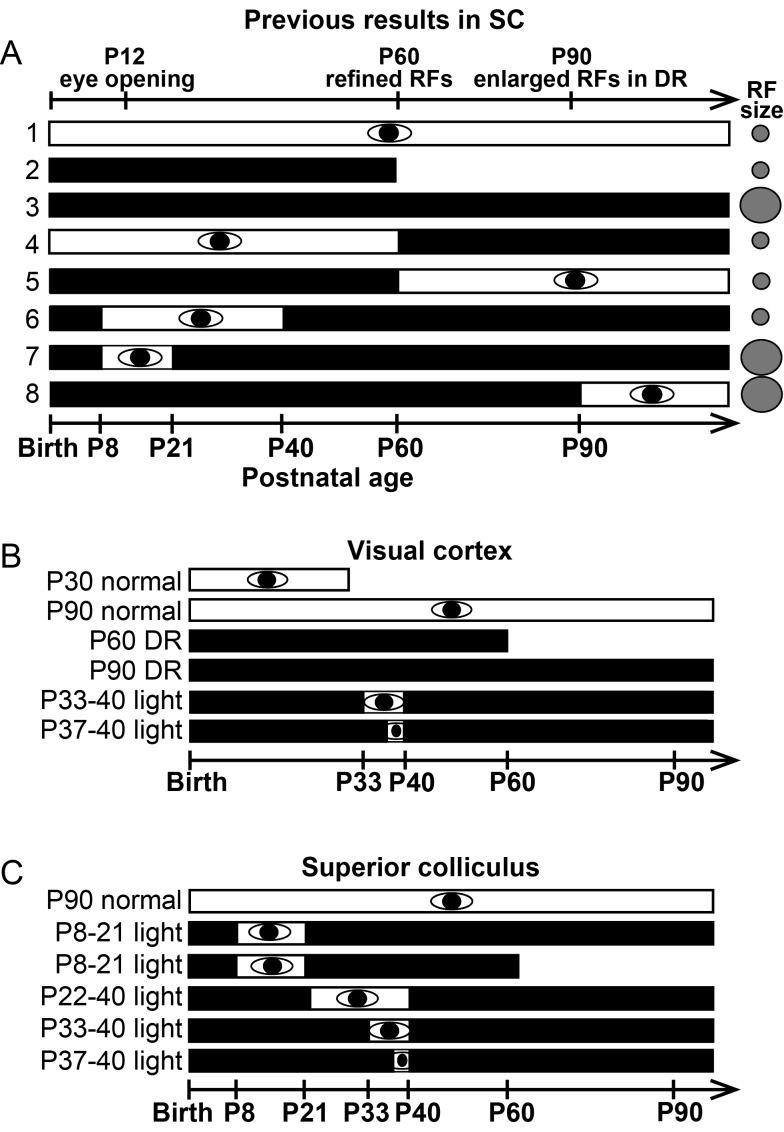
In contrast to the critical role of vision reported for several aspects of cortical development, early visual experience is not necessary for RF refinement in the developing visual midbrain (SC; Carrasco et al. 2005; Wang et al. 2010; Fig. 1A,1–2). In hamsters DR from birth, RFs in the SC refine normally by P60 (Fig. 1A,2) but become enlarged by P90 (Fig. 1A,3; Carrasco et al. 2005). A period of light-evoked activity after, but not before, P21 is sufficient to maintain the RFs in their refined state (Fig. 1A,4–7), but cannot rescue RFs that have already enlarged as a result of chronic light deprivation (Carrasco and Pallas 2006; Fig. 1A,8). These results from previous studies of the SC led us to challenge the notion that vision is necessary for RF refinement in V1 and that spontaneous retinal activity is not sufficient.
Figure 1.
(A) Summary of previous findings in the SC (Carrasco et al. 2005; Carrasco and Pallas 2006). The black bars represent DR, the white bars with eye symbol represent rearing under a normal light cycle, and the gray circles represent RF size. These experiments showed that RFs in the SC refine without visual experience, but that a period of early visual experience is necessary for maintenance of the refined RFs during DR that continues into adulthood. (B) Experimental design for V1 experiments. (C) Experimental design for SC experiments.
Here, we report that, contrary to previous thinking, spontaneous activity appears to be sufficient for the developmental refinement of RFs in both SC and V1, although late juvenile visual experience is essential to forestall the negative effects of chronic DR that occurs in adulthood. Our findings highlight the importance of spontaneous activity and the relative unimportance of visual experience in directing visual system development and suggest a heretofore unexamined role of visual experience in preventing future cortical plasticity.
Materials and Methods
Animals and Rearing Conditions
Syrian hamsters (Mesocricetus auratus) were used in this and the previous related studies due to their short gestation time, robust visual responses, and the abundance of data on their developmental plasticity (Razak et al. 2010). In total, 57 hamsters of both sexes were used in this study. All the procedures involving animals met or exceeded standards of humane care developed by the National Institutes of Health and the Society for Neuroscience and were approved by the Georgia State University Institutional Animal Care and Use Committee. Hamsters were obtained from Charles River Laboratories or Harlan and bred in-house. Normally reared hamsters were kept on a 14/10-h light/dark cycle. DR hamsters were maintained in a locked, light-tight dark room protected from the hallway lights by a locked, dark anteroom with blackout curtains at each door. Animals were exposed only briefly to a dim red light for husbandry purposes, at a wavelength not visible to Syrian hamsters (Huhman et al. 1999). Pregnant dams of DR pups were moved into a darkroom before or on the day of parturition (eye opening is ∼P12 in Syrian hamsters). DR hamsters that were exposed to light for 3–18 days were moved from the darkroom into a room with a 14/10-h light/dark cycle.
Surgery
Animals were prepared for terminal in vivo electrophysiological recordings as described previously (Pallas and Finlay 1989; Carrasco and Pallas 2006). Atropine (0.05 mg/kg) and dexamethasone (1 mg/kg) were administered preoperatively, and anesthesia was induced with urethane (2 g/kg, in 4 i.p. administrations). The depth of anesthesia was monitored during the experiments by checking withdrawal reflexes, pulse and breathing rates, and blood oxygenation, with supplemental doses of urethane given as needed. For recordings made in the SC, the overlying right cortex was removed by aspiration. Removal of the cortex has no observable effect on SC neuron RF properties in hamsters, except for a loss of direction tuning (Rhoades and Chalupa 1978a; Razak and Pallas 2005). The left eye was stabilized with a suture through the conjunctiva and protected with a plano contact lens.
Electrophysiology
Single units were recorded extracellularly with Teflon-coated tungsten electrodes (1–3 MΩ) in vivo. The SC recordings were made within 200 μm of the surface to ensure that all recorded units were within the superficial, retinorecipient layers. The V1 recordings were made within the area defined as the V1 in a hamster brain atlas (Morin and Wood 2001). Signals were amplified, filtered (10 000x; 0.5–3 kHz; Bak Electronics A-1), and digitized using the Spike2 software and CED hardware (20 kHz; Micro 1401; Cambridge Electronic Design).
Visual Stimulus Presentation
Visual stimuli used in both V1 and SC recordings were delivered to a CRT monitor positioned 40 cm from the left eye. For recordings made in V1, 2 different simple visual stimuli were presented: a flashing square and a moving bar. The 2° white square on a black background was presented for 300 ms, followed by a 1-s interstimulus interval, and repeated at 2° intervals across the horizontal meridian of the screen. This stimulus was chosen because it activated the majority of units and is not susceptible to potential errors caused by activity that can continue after a moving stimulus leaves the RF. The 2° wide, 20° long, vertically oriented, white moving bar stimulus on a black background was drifted across the screen in one and then in the opposite direction at 20°/s, with a 3-s interstimulus interval. This stimulus was added because it is commonly used to activate V1 neurons, facilitating comparison with previous work. For recordings made in the SC, a 1° square was drifted at 14°/s from the top to the bottom of the screen at successive nasotemporal locations, shifting 2° each presentation, as in a previous study (Carrasco et al. 2005).
Analysis of RFs
Single units were isolated by offline spike sorting using Wave_clus (Quiroga et al. 2004) and analyzed with Matlab. Analyses were conducted blind to the condition of the animal to prevent experimenter bias.
The flashing square data obtained from V1 were analyzed by plotting the location at which responses were produced as the square was progressively repositioned across the horizontal meridian of the visual field. A uniform fraction of the peak response (20%) was defined as the minimum stimulus-evoked response threshold, as in a previous study of SC (Carrasco et al. 2005). The horizontal extent of the locations within which the stimulus elicited an above threshold response was defined as the RF width. This method of RF width measurement is not affected by differences in spontaneous activity, such as may occur after DR.
The moving bar data obtained from V1 were analyzed by plotting peri-stimulus time histograms (PSTHs) with 50 ms time bins, and again using a uniform fraction of the peak response (20%) as the threshold. SC RF widths were calculated as above, using the horizontal extent of the region within which the drifting square elicited a response at least 20% of the peak response.
For the SC, in addition to the RF analysis methods outlined above, we also estimated RF areas by fitting a 2-dimensional Gaussian function to reconstructions of the spatial area within which the drifting square visual stimulus elicited responses (Tavazoie and Reid 2000; Chandrasekaran et al. 2005). Only RFs with high quality Gaussian fits were used [defined as having a coefficient of determination (R2) values of ≥0.6]. We estimated the RF area as an ellipse using the thresholds calculated as described above. To provide a measure of the shape of RFs, we used the ratio of the width of the RF to the height of the RF.
Putative Excitatory and Inhibitory Unit Classification
V1 units were classified as broad-spiking (putative excitatory) or narrow-spiking (putative inhibitory) units using 2 discrimination parameters calculated from the average waveform of each extracellularly isolated unit: The ratio of the peak height to the trough depth, and the time from the peak to its return to zero voltage (Mitchell et al. 2007; Niell and Stryker 2008; Mruczek and Sheinberg 2012). Waveforms of each unit in V1 were averaged, interpolated, normalized, and separated by k-means clustering (Matlab clustering toolbox). Units from recordings made with the flashing square and moving bar were analyzed separately, because in some cases the same units responded to both stimuli. Using other discrimination parameters including the slope after the peak, or the time between the trough and following peak, in place of or in addition to the return to zero time parameter, resulted in similar clusters. Some units that were used in the cluster analysis were not used for RF width analyses because they did not have a delimited RF, but they were used to estimate the number of putative inhibitory and excitatory units. RFs of putative excitatory and inhibitory V1 neurons from the groups that had enlarged RFs (P90DR and P37–40 light) were combined and compared with groups that had refined RFs (P90 normal, P60DR, and P33–40 light). RF widths were normalized to the mean of the refined RF group for each visual stimulus in order to combine both data sets. This was necessary to improve statistical power, because we expected to encounter relatively few narrow-spiking units. The majority of V1 neurons are excitatory (broad-spiking) neurons.
Statistical Analysis
Student's t-tests or 1-way analysis of variance (ANOVA), followed by Tukey's post hoc tests, were used for normally distributed data with equal variance between groups. These data are presented as mean ± standard error of the mean (SEM). For data that did not meet these requirements, Mann–Whitney rank sum tests or Kruskal–Wallis 1-way ANOVA on ranks were used, followed by Dunn's post hoc tests and presented as median ± interquartile range (IQR).
Results
To investigate the role of visual experience in RF refinement, 6 experimental groups were used for recordings in V1 (Fig. 1B): Normally reared P25–35 (“P30 normal,” n = 4): Normally reared adults >P90 (“P90 normal,” n = 5), DR P55–65 (“P60DR,” n = 8), DR adults >P90DR (“P90DR,” n = 5); DR adults that received light from P33 to 40 (“P33–40 light,” n = 4), or from P37 to 40 (“P37–40 light,” n = 5). Another 6 groups were used for SC recordings (Fig. 1C): Normally reared adults >P90 (“P90 normal,” n = 4), adults that received light from P8 to 21 (“P8–21 light,” n = 5), P60 hamsters that received light from P8 to 21 and were recorded at P60 (“P8–21 light,” n = 5), and DR adults >P90 that had received light from P22 to 40 (“P22–40 light,” n = 4), from P33 to 40 (“P33–40 light,” n = 4), or from P37 to 40 (“P37–40 light,” n = 4).
Dark Rearing to P60 Does not Prevent RF Refinement in V1
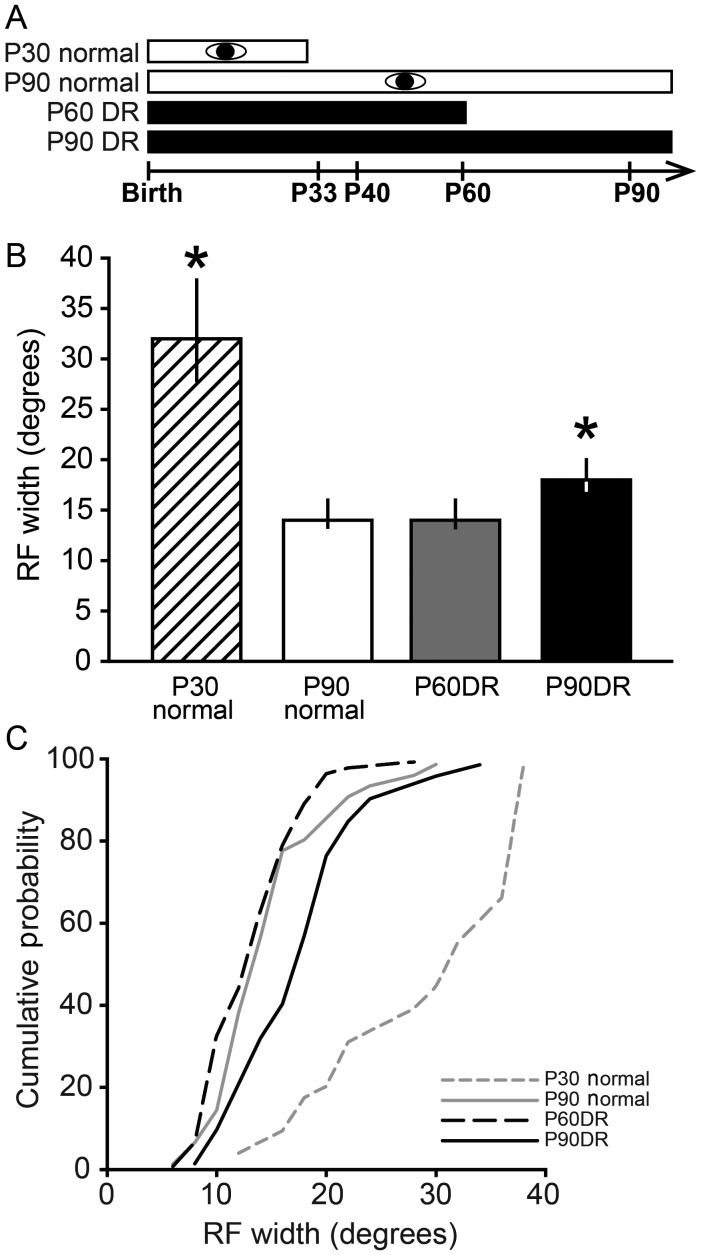
Visual cortical RFs refine during development through activity-dependent mechanisms, but the roles of visual experience versus spontaneous activity are unclear. We used in vivo extracellular electrophysiology to test whether V1 RFs require visual experience for refinement. RF widths were measured by flashing a 2° square of light in different locations across the visual field in young (P30) and adult (P90) hamsters (Fig. 2A). This type of stimulus was used for the determination of RF width because it effectively elicited neuronal responses, but did not evoke the prolonged discharges that can occur after a moving stimulus leaves the RF (Hensch et al. 1998). We found that cortical RFs of young hamsters were markedly larger than RFs in adult hamsters (Kruskal–Wallis 1-way ANOVA on ranks, n = 182, P < 0.001; Dunn's post hoc test for P90 normal: 14 ± 4°, n = 40 vs. P30 normal: 32 ± 16°, n = 37, P < 0.05; Fig. 2B,C). This suggests that RFs refine from a very large size to a much smaller size during development, and that even 2–3 weeks after eye opening, the RFs remain approximately twice as large as in adults.
Figure 2.
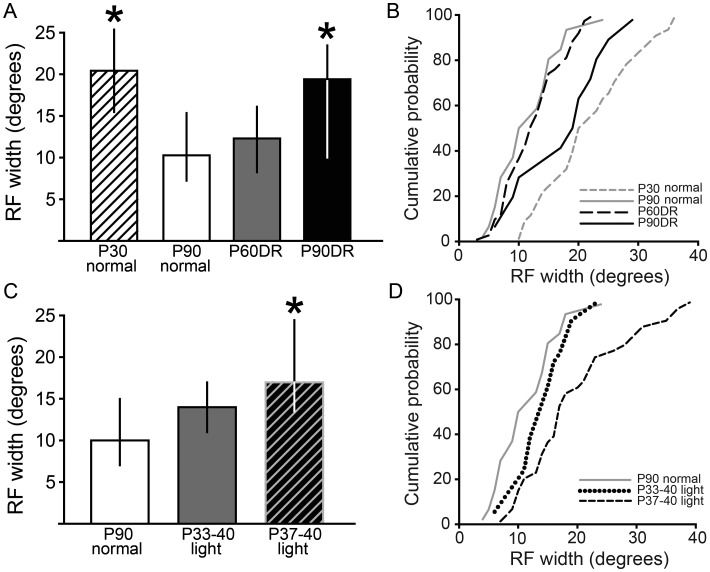
Visual experience is necessary for adult maintenance, but not developmental refinement, of RFs in V1. (A) Experimental design for V1 experiments. Conventions as in Figure 1. (B) To test whether visual experience is necessary for refinement and maintenance of RFs in V1, a flashing square stimulus was presented in different locations across the visual field to plot the RFs. RFs were larger at P30 than at P90 (*P < 0.05), showing that refinement of cortical RFs occurs in the hamster cortex. DR from birth to P60 did not alter the width of RFs compared with normally reared animals, suggesting that visual experience is not necessary for refinement of visual cortical RFs during development. In contrast, DR until P90 caused an expansion of RFs (*P < 0.05) compared with the P90 normal and P60 DR groups, indicating that DR causes enlargement of previously refined RFs. Data are presented as median ± IQR. (C) Cumulative probability plots showing the distribution of data in B.
To determine whether visual experience is necessary for this developmental refinement of RFs in V1, we measured RFs in hamsters that were DR until P60. We chose P60 because a previous study showed that RFs in the visual midbrain SC refine by P60 in both normally reared and DR hamsters (Carrasco et al. 2005). Large, unrefined RFs in DR animals would support the hypothesis that visual experience is necessary for refinement. Alternatively, RF refinement in V1 might occur under the influence of spontaneous retinal activity, independent of visual experience, as in the SC. In the latter case, animals that are visually deprived until adulthood should have refined, adult-size RFs. We found that the P60DR hamsters had RF widths that were not significantly different from those in the normally reared adult hamsters (P90 normal: 14 ± 4°, n = 40 vs. P60DR: 14 ± 6°, n = 69; Dunn's post hoc test, P > 0.05; Fig. 2B,C). These results support the interpretation that visual activity is not necessary for developmental refinement of RFs in hamster V1.
Dark Rearing to P90 Caused a Failure to Maintain RF Refinement in V1
In the SC, the RFs that have been refined during development require visual input during a late juvenile critical period in order to maintain the refined state (Carrasco et al. 2005). To determine whether this is also true in V1, hamsters were DR from birth until P90, and RF widths were compared with those measured in normally reared animals (Fig. 2A, P90 normal and P90DR). Chronic DR to P90 led to significantly larger RFs than in normally reared animals (P90 Normal: 14 ± 4°, n = 40 vs. P90DR: 18 ± 6°, n = 36; Dunn's post hoc test, P < 0.05; Fig. 2B,C). These data suggest that, as in the SC, visual experience is necessary to maintain the refined RFs of V1 neurons into adulthood, even though it is not necessary for the initial refinement process during postnatal development.
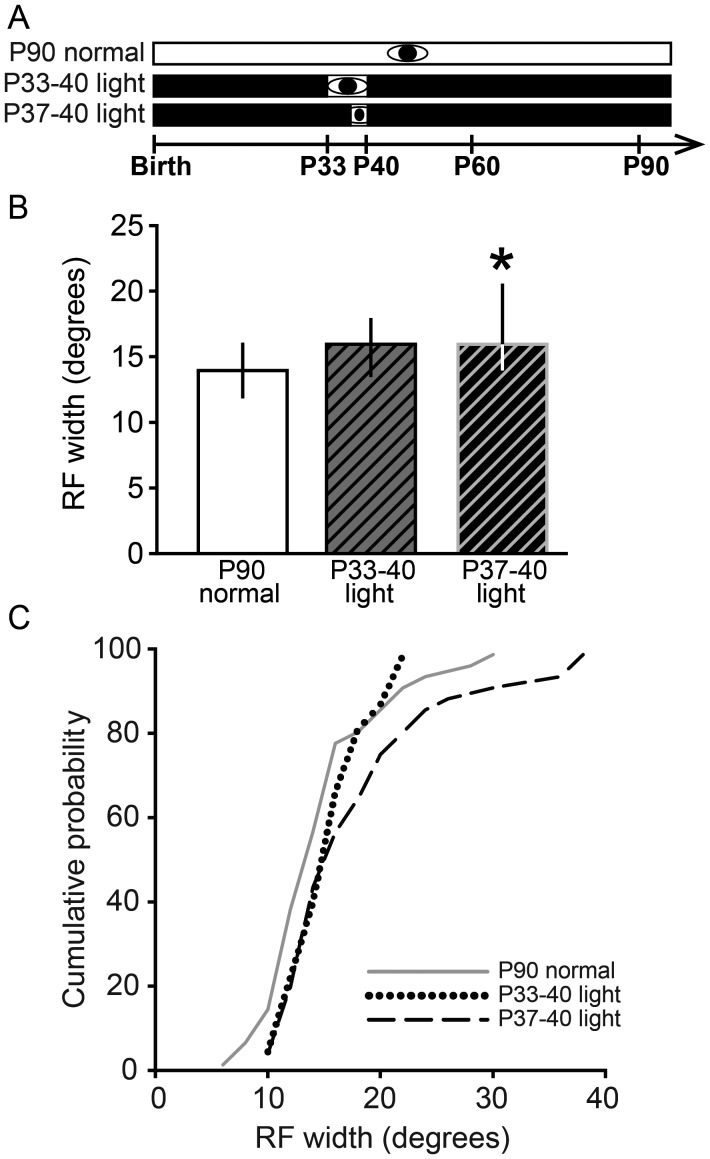
One Week but Not 3 Days of Late Juvenile Visual Experience Was Sufficient to Maintain Refined RFs in V1
After finding that visual experience is necessary for maintenance of refined RFs in V1, the duration of visual experience sufficient to maintain RFs was determined by exposing animals to 3 or 7 days of visual experience, preceded and followed by DR (Fig. 3A). It was previously reported that visual experience from P21 to 40 was sufficient to maintain RF sizes in SC despite DR (Carrasco and Pallas 2006). We found that 1 week (P33–40) of late juvenile visual experience followed by DR to P90 was sufficient to maintain V1 RFs in their refined state, although 3 days from P37 to 40 was not sufficient (Kruskal–Wallis 1-way ANOVA on ranks, n = 112, P < 0.05, P90 Normal: 14 ± 4°, n = 40; P33–40 light: 16 ± 4°, n = 34; P37–40 light: 16 ± 6°, n = 38; Dunn's post hoc test, P90 Normal vs. P37–40 light: P < 0.05; Fig. 3B,C). Note that we report the median and IQR of these data because they are not normally distributed. Although the medians for both the P33–40 light and P37–40 light groups were 16°, there was a higher proportion of very large RFs in the P37–40 light group (see distribution in Fig. 3C), which accounts for the significant difference between that group and the P90 Normal group. The means and SEM of these groups are as follows: Normally reared: 14.3 ± 0.95°; P33–40 light: 15.8 ± 0.59°; P37–40 light: 18.2 ± 1.18°. Thus, as in SC, late juvenile visual experience prevents DR-induced expansion of RFs in adulthood, suggesting that a similar mechanism may underlie experience-independent RF refinement and experience-dependent RF maintenance in both visual areas.
Figure 3.
One week of late juvenile visual experience prevents deprivation-induced RF enlargement in adulthood. (A) Experimental design. Conventions as in Figure 1. (B) Effect of a brief period of normal light cycle from P33 to 40 or P37 to 40 on DR-induced RF enlargement. The P33–40 light group had RFs that were normal in width. However, the P37–40 light group had significantly enlarged RFs (*P < 0.05, compared with P90 Normal). Data are presented as median ± IQR. (C) Cumulative probability plots showing the distribution of data in B. Note that the difference between groups is due to an increased proportion of large RFs.
Type of Visual Stimulus Did Not Influence RF Width Measurements in V1
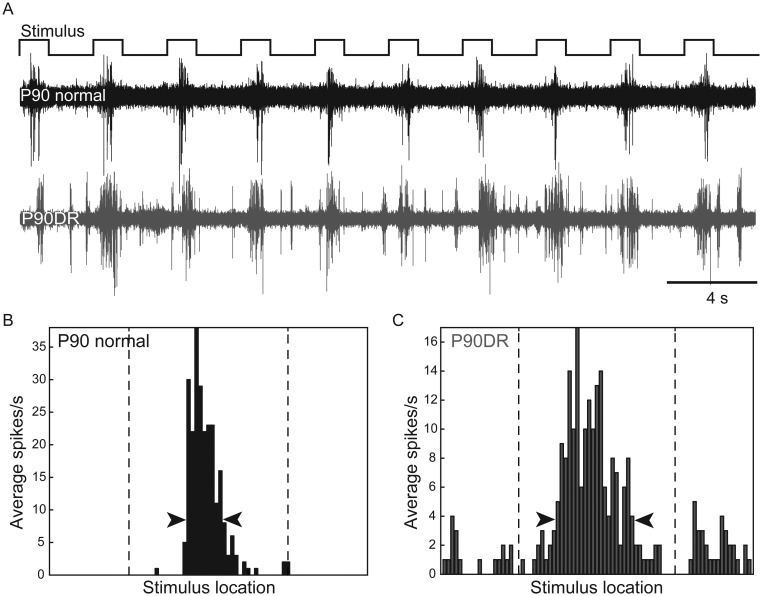
The difference in our results compared with previous reports (Fagiolini et al. 1994; Gianfranceschi et al. 2003) on the relationship between visual experience and RF development could potentially be explained by methodological differences. One difference is that we used a flashing square stimulus instead of moving bars of light as an assay for RF size. Thus, to determine whether stimulus shape and motion could influence the results, we repeated the experiments above with a moving bar stimulus. The bars were moved slowly across the visual field to reduce the prolonged discharges that can be elicited by rapidly moving stimuli. Examples of RF responses to this stimulus in Figure 4 show responses from a P90 Normal unit and a P90DR unit and how PSTHs were measured to calculate RF width.
Figure 4.
Examples of extracellular recordings and RF plots from P90 normal and P90DR V1. (A) Typical responses to a moving bar. The visual receptive field (RF) of a neuron in the P90 normal case (black trace) is narrower compared with that of a neuron in the P90DR case (gray trace). RF PSTHs of the P90 normal (B) and P90DR (C) shown in A, after single-unit isolation. The dashed vertical lines represent onset and offset of the moving bar stimulus. The arrowheads indicate the 20% peak threshold used to calculate RF width.
As with the flashing square stimulus, we observed a significant effect of the duration of DR on RF width using a moving bar (Kruskal–Wallis 1-way ANOVA on ranks, n = 141, P < 0.001). The RF width measured using the moving bar stimulus was similar in the P90 Normal and P60DR hamsters (P90 Normal: 10 ± 8°, n = 23; P60DR: 12 ± 8°, n = 56; Dunn's post hoc test, P > 0.05; Fig. 5A,B), but as expected, RFs in the P30 Normal group were significantly larger than those in both the P90 normally reared group (P30 Normal: 20 ± 13°, n = 39; P90 normal: 10 ± 8°, n = 23; Dunn's post hoc test, P < 0.05; Fig. 5A,B) and the group that was DR until after P90 (P90DR: 19 ± 13°, n = 23; Dunn's post hoc test, P < 0.05; Fig. 5A,B).
Figure 5.
Type of visual stimulus did not influence RF width measurements in V1. To test whether the motion or shape of the visual stimulus used to plot the RF had an effect on the RF width estimates, we repeated the experiment using a moving light bar stimulus. (A) As with the flashing square stimulus, RF width estimates using the moving bar stimulus were significantly larger in the P30 normal and P90DR groups (*P < 0.05) compared with the P90 normal group. These experiments suggest that visual experience is necessary for adult maintenance, but not for developmental refinement, of RFs. (B) Cumulative probability plots showing the distribution of data in A. (C) As with the flashing square stimulus, RF width estimates were significantly larger in the P37–40 light group. These experiments suggest that the type of visual stimulus used to estimate RF width did not affect our results, and support the conclusion that 7 days of visual experience beginning at P33 is sufficient to maintain V1 RFs in a refined state despite chronic DR into adulthood, but 3 days beginning at P37 is not. (D) Cumulative probability plots showing the distribution of data in C. Data are presented as median ± IQR.
We also used the moving bar stimulus to assay RF widths in adult DR animals that had experienced light from either P33 to 40 or from P37 to 40, and the results were again similar to those obtained with the flashing square stimulus. The group that experienced light for 1 week had RFs that were similar in width to those in the normally reared group, but the group that experienced light for 3 days had RFs that were significantly larger than those in the normally reared group (P90 normal: 10 ± 8°, n = 23; P33–40 light: 14 ± 6°, n = 27; P37–40 light: 17 ± 10°, n = 37; Kruskal–Wallis 1-way ANOVA on ranks, n = 87, P < 0.001; Dunn's post hoc tests, P90 normal vs. P33–40 light: P > 0.05; P90 Normal vs. P37–40: P < 0.05; Fig. 5C,D). These results suggest that postnatal visual experience is, indeed, necessary for maintenance, but not for refinement, of RFs, and that this result is not due to the type of stimulus (flashed square or moving bar) used to measure the RFs.
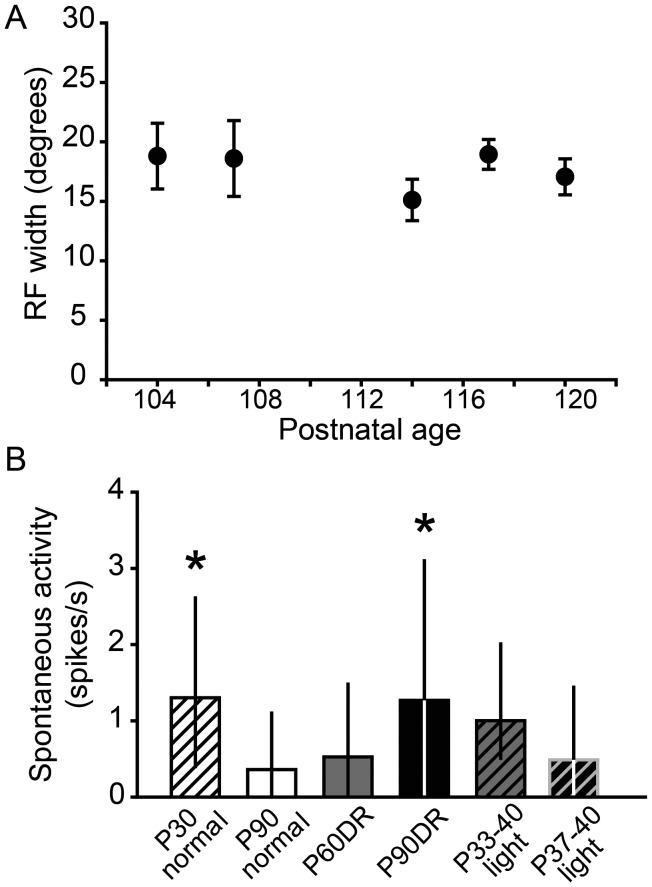
To test whether RFs continue expanding after P90 in DR animals, we tested for a correlation between age and RF width within the >P90DR group. The animals in the >P90 group ranged in age from P104 to 120. RF widths measured using both stimulus types were combined, because there was no significant difference in their means (flashing square: 17.94 ± 0.98° vs. moving bar: 17.48 ± 1.49°, t-test, P = 0.786). There was no significant correlation between age and RF width (P = 0.119, Fig. 6A), suggesting that RFs do not continue to expand after P104, at least not by P120.
Figure 6.
(A) There was no correlation between age after P90DR and RF width, suggesting that RFs do not continue to enlarge after P90. Data are presented as mean ± SEM. (B) Spontaneous activity was higher in P30 normal and P90DR animals compared with P90 normal animals (*P < 0.05). Data are presented as median ± IQR.
DR Increased Spontaneous Activity in V1
Spontaneous activity was quantified for each unit as the spike rate during a 1- to 5-s period before the first stimulus presentation. We found that spontaneous activity was higher in the P30 Normal and P90DR groups compared with the P90 Normal group (P30 normal: 1.29 ± 2.24, n = 65; P90 normal: 0.36 ± 1.12, n = 50; P60DR: 0.52 ± 1.49, n = 106; P90DR: 1.26 ± 3.10, n = 52; P33–40 light: 1.00 ± 1.54, n = 55; P37–40 light: 0.49 ± 1.45, n = 72; Kruskal–Wallis 1-way ANOVA on ranks, n = 400, P < 0.001; Fig. 6B). Note that our method of RF determination is independent of the level of spontaneous firing. The finding that these groups have the largest RFs suggests a relationship between the lack of refinement and levels of spontaneous activity, which could result from a loss of inhibition.
Both Putative Excitatory and Putative Inhibitory V1 Units Fail to Maintain Refined RFs
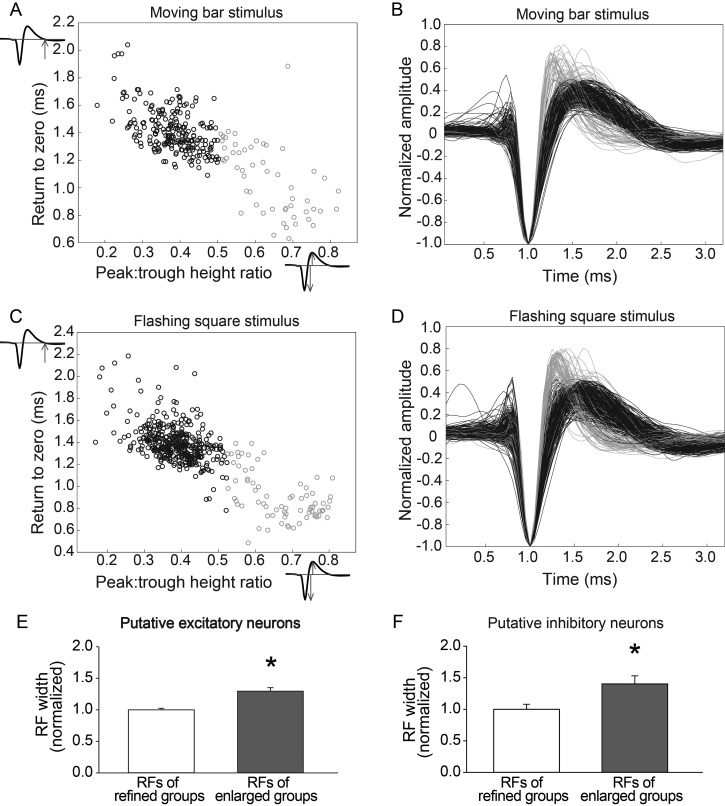
Inhibitory neurons provide lateral inhibition that keeps the excitatory center of RFs constrained (Sengpiel et al. 1997). The mean increase in the RF size of the population of units we studied in DR animals may be caused by an increase in RFs of excitatory neurons, which constitute the majority of neurons in V1. In addition, a decrease in the RF size or strength of inhibitory neurons could contribute to the expanded RFs of the majority of neurons recorded, due to a reduction of lateral inhibition. To investigate which type of neurons exhibit enlarged RFs after DR, units were classified as being either broad-spiking or narrow-spiking (Fig. 7A–D). Narrow-spiking neurons are likely to be inhibitory neurons because their action potentials are brief compared with action potentials of excitatory neurons (McCormick et al. 1985). This difference in spike duration can be utilized to identify putative excitatory and inhibitory neurons (Mitchell et al. 2007; Niell and Stryker 2008; Durand et al. 2012; Mruczek and Sheinberg 2012). In this study, 17% of the units were classified as putative inhibitory neurons, which is similar to proportions reported in the visual cortex of rat (15%) (Lin et al. 1986), mouse (19%) (Tamamaki et al. 2003; Niell and Stryker 2008), cat (21%) (Gabbott and Somogyi 1986), and monkey (20%) (Hendry et al. 1987). RFs of putative excitatory and inhibitory neurons from the groups that had enlarged RFs (P90DR and P37–40 light) were combined to improve statistical power and compared with groups that had refined RFs (P90 normal, P60DR, and P33–40 light). RFs of putative excitatory units were significantly enlarged (refined group: 100.0 ± 2.4%, n = 162; enlarged group: 129.5 ± 5.6%, n = 96; Mann–Whitney rank sum test, P < 0.001; Fig. 7E). Those of putative inhibitory units were also significantly enlarged (refined group: 100.0 ± 7.9%, n = 26; enlarged group: 140.0 ± 12.8%, n = 22; t-test, P = 0.008; Fig. 7F). These data argue that enlargement of RFs in both excitatory and inhibitory neurons contributes to the overall enlarged RFs in groups with insufficient visual experience to maintain them in a refined state.
Figure 7.
Both putative excitatory and putative inhibitory units have expanded RFs in the absence of sufficient postnatal visual experience. Scatter plots of waveform discrimination parameters for moving bar data (A) and flashing square data (C) demonstrate categorization of broad-spiking (putative excitatory units, black) and narrow-spiking (putative inhibitory units, gray) neurons. Normalized waveforms of all units using moving bar (B) or flashing square (D) stimuli. RFs of both putative excitatory units (E) and putative inhibitory units (F) contribute to enlarged RFs in the P90DR and P37–40 light groups compared with groups with refined RFs (P90 normal, P60DR, and P33–40 light groups, *P < 0.05). Data are presented as mean ± SEM.
Late but Not Early Juvenile Visual Experience Was Sufficient to Maintain Refined RFs in SC
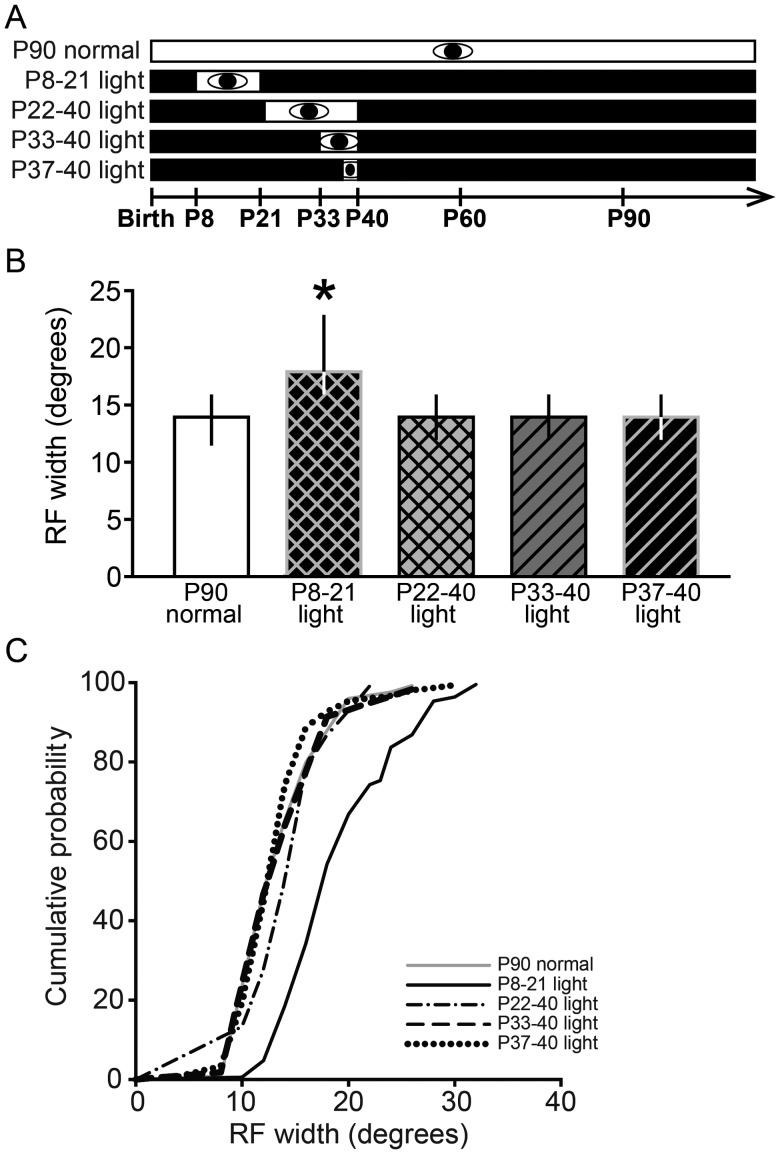
Given the finding that RF maintenance in V1 was achieved with 7 but not 3 days of late juvenile visual experience during visual deprivation, we investigated whether this requirement was similar in the SC. The SC is essential for visual orientation in rodents (Schneider 1969; Carman and Schneider 1992). Both the retina and V1 send strong inputs directly to the SC. In a previous study, we demonstrated that visual experience from P8 to 40 is sufficient to maintain SC RFs, but P8–21 is not a sufficient exposure time (Carrasco and Pallas 2006). Thus, in a related set of experiments, we tested the duration of visual experience necessary to maintain SC RF size and whether it is similar to or different from the duration of experience necessary for RF maintenance in V1. We generated 4 groups of hamsters that received different durations of visual experience: 13 early days (P8–21), 18 late days (P22–40), 7 late days (P33–40), or 3 late days (P37–40), that were preceded and followed by DR (Fig. 8A). Extracellular electrophysiological recordings in superficial SC were performed to estimate RF size after P90, which is the point when RFs would have expanded in DR animals.
Figure 8.
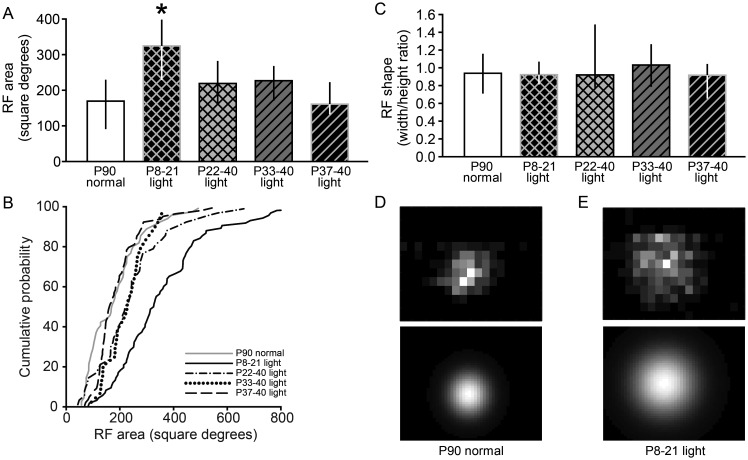
Late but not early juvenile visual experience was sufficient to maintain refined RFs in SC. (A) Experimental design for the SC study. Conventions as in Figure 1. (B) Maintenance of RF width in the SC during DR requires precise timing of visual experience. Animals were DR from before birth and exposed to light from P8 to 21, P22 to 40, or P33 to 40, followed by DR until >P90, when RFs would become enlarged during continuous DR. Late visual experience from P22 to 40, P33 to 40, or P37 to 40 was sufficient to maintain RF width in adulthood (>P90), but early visual experience from P8 to 21 was not sufficient, compared with the P90 normal group (*P < 0.05). Data are presented as median ± IQR. (C) Cumulative probability plots showing the distribution of data in B.
Consistent with previous results (Carrasco and Pallas 2006), we found an effect of the duration of visual experience on RF width in SC (Kruskal–Wallis 1-way ANOVA on ranks, n = 310, P < 0.001). Early visual experience from P8 to 21, preceded and followed by DR, was not sufficient to maintain refined SC RF widths beyond P90 (P8–21 light:18 ± 6.75°, n = 95 vs. P90 normal: 14 ± 4°, n = 62; Dunn's post hoc test, P < 0.05; Fig. 8B,C). However, either 18 (P22–40), 7 (P33–40), or 3 (P37–40) days of late visual experience was sufficient to maintain adult-size RF width beyond P90 during DR (P22–40 light: 14 ± 4°, n = 49; P33–40 light: 14 ± 4°, n = 29; P37–40 light: 14 ± 4, n = 75; Dunn's post hoc tests, P > 0.05; Fig. 8B,C). Analysis of RF areas was consistent with the analysis of RF widths (Fig. 9A,B). As with the width analysis, there was also an effect of visual experience on RF area (Kruskal–Wallis 1-way ANOVA on ranks, n = 275, P < 0.001). Early visual experience from P8 to 21 was not sufficient to maintain refined RF areas beyond P90 (P8–21 light: 326 ± 214°, n = 79 vs. P90 normal: 169 ± 140°, n = 58; Dunn's post hoc test, P < 0.05; Fig. 9A,B). However, the other 3 groups that received later visual experience had SC RF areas that were not significantly different from those of the normally reared animals (P22–40 light: 219 ± 121°, n = 45; P33–40 light: 227 ± 98°, n = 22; P37–40 light: 161 ± 91°, n = 71; Dunn's post hoc test, P90 normal vs. P22–40 light, P33–40 light, P37–40 light: P > 0.05, Fig. 9A,B).
Figure 9.
Maintenance of RF area in the SC during DR requires late visual experience. (A) Late juvenile visual experience from P22 to 40, P33 to 40, or P37 to 40 was sufficient to maintain the RF area in adulthood (>P90) despite DR, but early visual experience from P8 to 21 was not sufficient (compared with the P90 normal group; *P < 0.05). (B) Cumulative probability plots showing the distribution of data in A. (C) The shape of RFs is maintained in groups that have expanded RFs. The width/height ratios of the RFs were not affected, even though the RF sizes were larger in the group that received light from P8 to 21, compared with all other groups. This suggests that RFs expanded equally in horizontal and vertical directions. (D and E) Example RF area plots: refined RF of a neuron from a normal adult (D, upper panel) and enlarged RF of a neuron from a P8–21 light case (E, upper panel) and their fitted 2D Gaussians used to calculate the area and width/height ratio (D and E, lower panels). Gray level represents the response level, with white corresponding to peak response and black corresponding to zero. Data are presented as median ± IQR.
A change in RF shape could indicate a rearrangement of retinocollicular inputs as seen under the condition of disrupted retinal waves (Chandrasekaran et al. 2005; Mrsic-Flogel et al. 2005). Using the RF area estimates obtained from the SC, we found that the width and height of the RFs changed in parallel, such that the enlarged RFs were expanded in both the horizontal and vertical axes (Fig. 9C). Although RF area was enlarged in the group that received light from P8 to 21, there were no differences between the width/height ratios in that or any other group (Kruskal–Wallis 1-way ANOVA on ranks, n = 275, P = 0.282; P90 normal: 0.94 ± 0.43, n = 58; P8–21 light: 0.92 ± 0.25, n = 79; P22–40 light: 0.92 ± 0.70, n = 45; P33–40 light: 1.03 ± 0.48, n = 22; P37–40 light: 0.92 ± 0.38, n = 71; Fig. 9C). Examples of refined and expanded RFs are shown in Figure 9D,E.
In summary, these data suggest that there is a sensitive period after P21 when as little as 3 days of visual experience are sufficient to prevent DR-induced RF enlargement and thus, maintain a refined, adult RF size in the SC.
Early Juvenile Visual Experience That is Not Sufficient to Maintain RFs Does not Prevent RF Refinement in the SC
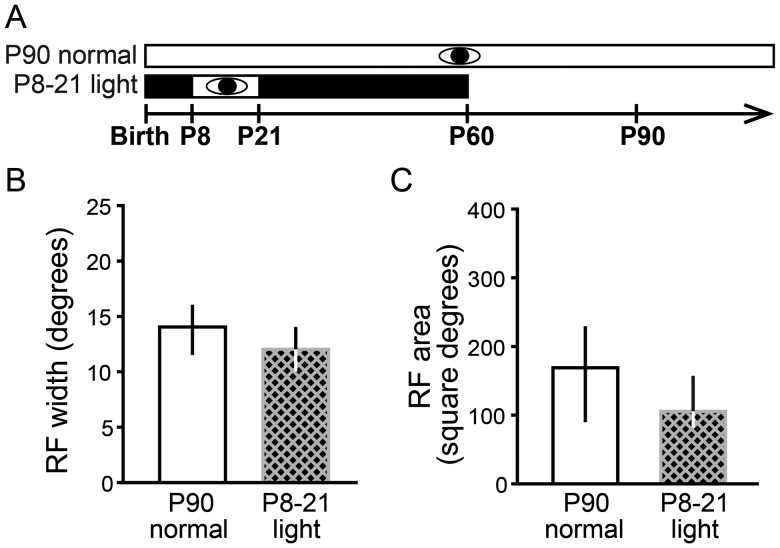
Animals that are either normally reared or DR have refined RFs at P60 in the SC (Carrasco et al. 2005; Wang et al. 2010). Our findings that visual experience from P8 to 21 resulted in enlarged RFs after P90 might also be explained by a failure to maintain refined RFs, as in animals that have been DR from birth until P90. Alternatively, the enlarged RFs could be caused by RFs failing to refine. To distinguish between these hypotheses, we measured RF sizes in the P8–21 light group at P60 when normal or DR animals already have refined RFs. We found that the RFs of neurons from the P8 to 21 DR group were refined at P60 (P8–21 light width at P60: 12 ± 4°, n = 62 vs. P90 normal: 14 ± 4°, n = 62; Dunn's post hoc test, P > 0.05; P8–21 light area at P60: 106 ± 75°, n = 66 vs. P90 normal: 169 ± 140°, n = 58; Dunn's post hoc test, P > 0.05; Fig. 10). These results rule out the alternative hypothesis that early visual experience prevents RF refinement in the SC but is insufficient to forestall RF enlargement in adulthood.
Figure 10.
Early visual experience does not prevent the refinement of RFs in the SC. This experiment tested the alternative hypothesis that enlarged RFs in animals that received early visual experience from P8 to 21 were caused by a failure of RFs to refine rather than a failure to maintain their refined state. Single-unit RFs were measured at P60 in animals that had visual experience from P8 to 21 preceded and followed by DR. (A) Experimental design. Conventions as in Figure 1. We found that RF width (B) and RF area (C) were not different than in P90 normal controls, refuting the alternate hypothesis that early light exposure prevented RF refinement. Data are presented as median ± IQR.
Discussion
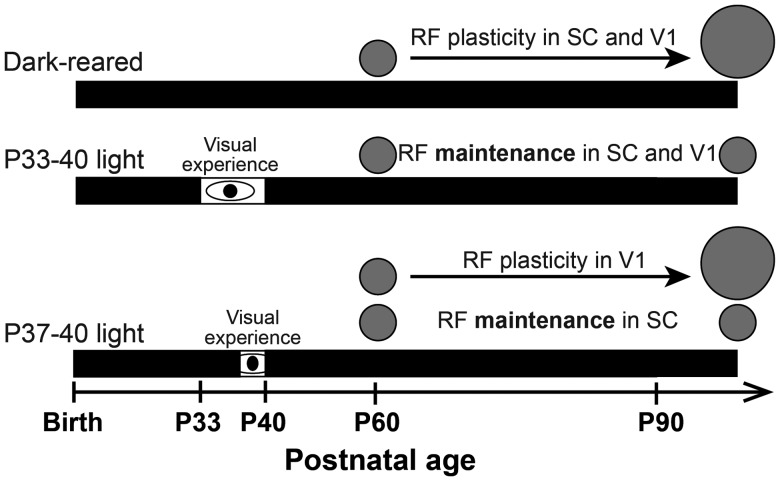
The experiments described in this study demonstrate that RF refinement in V1 of Syrian hamsters occurs without visual experience, arguing that spontaneous activity is sufficient for developmental refinement of RFs in both SC and V1. One week of late juvenile visual experience from P33 to 40 was sufficient to prevent both SC and V1 RFs from becoming enlarged at P90, whereas 3 days from P37 to 40 was sufficient to protect SC but insufficient to maintain V1 RFs. These data argue that visual experience must occur during a late juvenile sensitive period in order to forestall deprivation-induced adult RF plasticity in both the form vision (retinogeniculocortical) and spatial orientation (retinocollicular) pathways, but that V1 requires a longer duration of exposure than SC (Fig. 11).
Figure 11.
Summary of findings: Precise timing of visual experience prevents deprivation-induced RF plasticity. Our results show that DR does not prevent RF refinement, but rather causes RFs to expand after P90 in V1, as in the SC. This RF plasticity can be prevented by visual experience from P33 to 40 in both SC and V1 and from P37 to 40 in SC only.
Spontaneous Retinal Activity Is Sufficient to Refine SC and V1 RFs
The present study refutes the alternative hypothesis that the vision independence of RF refinement in the SC (Carrasco et al. 2005; Carrasco and Pallas 2006; Wang et al. 2010) is a subcortical area-specific phenomenon. Both SC and V1 neurons can refine their RFs without visual experience, likely through spontaneous activity (Pfeiffenberger et al. 2006). This finding is consistent with reports that the lateral geniculate nucleus (LGN) exhibits normal physiological responses after prolonged DR (Hendrickson and Boothe 1976; Mower, Burchfiel, et al. 1981), and that spontaneous activity, rather than visual activity, is necessary for initial retinogeniculate refinement (Hooks and Chen 2006). Thus, there may be a common, vision-independent mechanism for RF refinement throughout the visual pathway. Current models of visual experience-dependent refinement of RFs may need to be modified to incorporate these findings.
V1 Requires Different Timing of Light Exposure Than SC to Prevent Deprivation-Induced Plasticity
The finding that 3 days of exposure to a normal light cycle after P21 was sufficient to maintain SC but not V1 RFs during later DR is interesting, because the 2 areas are heavily interconnected through the cortico-collicular projection (Rhoades and Chalupa 1978b; Pallas et al. 1988). This may indicate that visual experience stabilizes circuitry in SC earlier than in V1, or V1 may be more plastic in response to long-term visual deprivation than SC. Although it seems unlikely given that SC matures earlier than V1 (Clancy et al. 2001), we cannot rule out that 3 days of exposure may be sufficient for V1 if it occurs from P33 to 36, earlier than SC. Further studies are needed to explore the basis for the difference.
In the LGN, DR beginning at P20 causes weakening and an increase in the number of retinal inputs to geniculate neurons; more so than DR from birth or from P15 (Hooks and Chen 2008). This suggests that several days of visual experience are necessary for DR-induced retinogeniculate plasticity. In our study of V1, DR animals that experienced light from P37 to 40 did not have larger RFs than animals that were DR without exposure to light. Whether earlier visual experience (P20–30) promotes RF plasticity in V1 or whether retinogeniculate plasticity underlies these potential changes are attractive avenues for future study.
The Shape of Enlarged RFs Was Maintained in the SC
The shape of SC RFs was not affected by DR-induced expansion of the RF areas. The initial formation of the retinocollicular map is under the control of molecular guidance cues and spontaneous activity. The anteroposterior axis is organized by graded interactions between ephrinAs and their EphA receptors (Feldheim et al. 2000) and the dorsoventral axis depends on ephrinB/EphB gradients (Hindges et al. 2002). Disruption of retinal waves during early development results in the development of elongated RFs (Chandrasekaran et al. 2005; Mrsic-Flogel et al. 2005). However, spontaneous retinal waves are not affected by DR. For this reason, we did not expect to see changes in RF shape.
Comparison with Other Species
In some studies, DR has been reported to delay or prevent refinement of RFs in the visual cortex of rats (Fagiolini et al. 1994) and mice (Gianfranceschi et al. 2003), and to reduce visual acuity of cats (Timney et al. 1978) and visual responsiveness of monkeys (Regal et al. 1976). This is contrary to our results, which suggest that RF refinement is largely determined by spontaneous activity. As a burrowing rodent species, Syrian hamster pups may not experience light until later than carnivore or primate species. Indeed, Syrian hamsters develop normal circadian systems without visual experience (Kampf-Lassin et al. 2011). Alternatively, the RFs of cats, rats, and mice in previous studies may have refined normally during development but enlarged by the time of recording. Some investigators reported that RFs in V1 can be normal in size at early ages in cats (Buisseret and Imbert 1976; Fregnac and Imbert 1978; Braastad and Heggelund 1985), rats (Fortin et al. 1999), and rabbits (Mathers et al. 1974; but see Fagiolini et al. 1994; Gianfranceschi et al. 2003). Thus, we argue that it is unlikely that our results are specific to hamsters.
The Mechanism Underlying RF Refinement May Differ From Mechanisms Underlying the Development of Other RF Properties
The refinement of RF size differs in several ways from maturation of other RF properties (for review see Daw 2006). The initial formation of ocular dominance columns in V1 occurs prenatally (Rakic 1976; Horton and Hocking 1996; Hevner 2000), despite binocular lid suture (Sherk and Stryker 1976; Crair et al. 1998), dark rearing (Mower, Berry, et al. 1981; Stryker and Harris 1986), or enucleation (Crowley and Katz 1999). Orientation-specific responses and orientation maps in V1 also begin to form before eye opening (Hubel and Wiesel 1963; Chapman and Stryker 1993), despite dark rearing (Singer et al. 1981) or binocular lid suture (Crair et al. 1998), but adult levels of selectivity develop over the course of several weeks and require visual experience (Bonds 1979; Leventhal and Hirsch 1980; Crair et al. 1998). Direction-specific responses in V1 require visual experience for normal development in ferrets (Li et al. 2006), cats (Leventhal and Hirsch 1980), and rats (Fagiolini et al. 1994), but not mice (Rochefort et al. 2011). Spatial frequency selectivity increases independently of visual experience up to 3 weeks postnatal in cats, but requires visual experience to improve further (Derrington and Fuchs 1981; Derrington 1984). Sensitivity to binocular disparity, a measure of depth perception, increases from birth, but does not develop during binocular eyelid suture in cats (Pettigrew 1974). Our findings that RFs refine to an adult state without light-evoked activity suggest that RF refinement may involve different developmental mechanisms than some of these other RF properties.
It Is Unlikely That the Effects of DR on SC and V1 RF Size Are Due to Changes in the Retina
Dark rearing has little effect on retinal activity measured by electroretinogram (Reuter 1976), or synaptic input to retinal ganglion cells (RGCs) and amacrine cells (He et al. 2011). Velocity tuning and direction selectivity of RGCs develop during DR (Chan and Chiao 2008; Elstrott et al. 2008), although segregation into ON and OFF pathways is disrupted (Tian and Copenhagen 2003). DR in turtles causes spontaneous RGC bursting, which appears to cause enlarged RFs (Sernagor and Grzywacz 1996). In contrast, DR in rats is reported to strengthen RGC surround inhibition (Chan and Chiao 2008) and decrease RF size of RGCs (Di Marco et al. 2009). The effects of DR on hamster retina are unknown, and we cannot rule out the possibility that changes in the retina play a part in the observed changes in SC and V1 RF size. However, it is unlikely that DR increases RGC RFs in hamsters and decreases RGC RFs in rats.
What Mechanism Underlies Visual Deprivation-Induced Enlargement of RFs?
Experience-dependent changes in inhibition are thought to be necessary for plastic changes in the visual system. Reducing inhibition in adult V1 permits ocular dominance plasticity (Maya-Vetencourt et al. 2008; Baroncelli et al. 2010; Harauzov et al. 2010; Heimel et al. 2011; Spolidoro et al. 2011; Maya-Vetencourt, Baroncelli, et al. 2012). Because lateral inhibition defines the periphery of RFs, any change in inhibition could affect RF size directly (Ramoa et al. 1988). DR reduces inhibition in the SC, which likely underlies DR-induced RF enlargement (Carrasco et al. 2011). It is possible that in V1, as in SC, RF enlargement during DR results from loss of inhibition (Bakkum et al. 1991; Benevento et al. 1992, 1995). The finding that insufficient visual experience results in enlarged RFs of putative excitatory and inhibitory neurons suggests that lateral inhibition may be weakened in both classes of neurons.
What Mechanism Underlies Visual Experience-Dependent Prevention of RF Plasticity?
Visual experience during postnatal development prevents RF expansion by forestalling RF plasticity in adulthood. Similarly, the critical period for ocular dominance plasticity is prolonged by DR, closed by visual experience (Cynader and Mitchell 1980; Mower et al. 1983; Fagiolini et al. 1994) and increased by prior monocular deprivation (Hofer et al. 2006, 2009). Because reducing inhibition in adulthood appears to reactivate cortical plasticity mechanisms (Heimel et al. 2011), visual experience-dependent strengthening of inhibition may prevent it. In V1, visual experience is necessary for developmental strengthening of inhibition (Morales et al. 2002; Katagiri et al. 2007) and for maturation of inhibitory basket cells (Sugiyama et al. 2008). The rise in inhibition initiates ocular dominance plasticity and then closes the critical period through a consolidation of inhibitory synapses (Heimel et al. 2011). Prevention of RF plasticity may also require visual experience-dependent enhancement of inhibition.
What Is Special About Visual Experience That Spontaneous Retinal Activity Does not Provide?
Visual experience evokes higher levels of neural activity than can be provided by spontaneous activity (Crair 1999; Huberman et al. 2008; Chalupa 2009). Higher levels of activity may be required to increase the expression of genes critical for normal maturation (Tropea et al. 2006) and to activate structural changes preventing plasticity in mature circuits (Pizzorusso et al. 2002; Bence and Levelt 2005; Pizzorusso et al. 2006; McRae et al. 2007; Balmer et al. 2009; Carulli et al. 2010; Kind et al. 2013). Visually driven activity increases Npas4, a transcription factor that upregulates plasticity-related genes in V1 such as brain-derived neurotrophic factor (Lin et al. 2008; Maya-Vetencourt, Tiraboschi, et al. 2012), which, in turn, stimulates maturation of inhibitory synapses (Rutherford et al. 1997; Huang et al. 1999) and the development and maintenance of visual acuity (Gianfranceschi et al. 2003; Heimel et al. 2010; Schwartz et al. 2011).
Maladaptive Plasticity and Age-Related Loss of Acuity in Humans
Sensory deprivation occurs in humans with diseases of the eye such as macular degeneration and cataract (Lewis and Maurer 2009), and as in our model, RF expansion and a loss of visual acuity may result. A common mechanism for both RF expansion and loss of sensory acuity may be inhibitory plasticity; reduction of inhibition could enhance the gain of sensory signals, but this comes at the expense of discriminative ability. Indeed, our result that DR until >P90 increased spontaneous activity could be the result of reduced inhibition. Studying how sensory experience prevents maladaptive plasticity could provide an insight into age-related loss of sensory acuity in humans (Habak and Faubert 2000; Leventhal et al. 2003; Betts et al. 2005). Moreover, sensory circuits may develop to a greater extent under conditions of sensory deprivation than previously appreciated. A focus on blocking plasticity and thus maintaining existing sensory processing capacity, rather than enhancing plasticity, may be a beneficial and safer route to recovery from these disorders. Future work investigating how visual experience forestalls maladaptive plasticity will contribute to our understanding of sensitive periods and how they are regulated.
Funding
This work was supported by grants from the National Science Foundation (IBN-0451018), the National Institutes of Health (EY/MH 12696), the STC Program of the National Science Foundation (IBN-9876754), the Georgia State University Research Foundation, Brains and Behavior Fellowship, Honeycutt Fellowship, and Sigma Xi Grants-in-Aid of Research to T.S.B.
Notes
We thank Profs Paul Katz, Peter Wenner, and Vincent Rehder for providing advice on experimental design, Yuting Mao and members of the Pallas Lab for comments on the manuscript and for technical assistance, the animal care staff at GSU, and Erik Tollerud for help with data analysis. Conflict of Interest: None declared.
References
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman CJ, Smyth D, Thompson ID. Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron. 2002;36:869–879. doi: 10.1016/S0896-6273(02)01010-3. [DOI] [PubMed] [Google Scholar]
- Bakkum BW, Benevento LA, Cohen RS. Effects of light/dark- and dark-rearing on synaptic morphology in the superior colliculus and visual cortex of the postnatal and adult rat. J Neurosci Res. 1991;28:65–80. doi: 10.1002/jnr.490280107. [DOI] [PubMed] [Google Scholar]
- Balmer TS, Carels VM, Frisch JL, Nick TA. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci. 2009;29:12878–12885. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Sale A, Viegi A, Maya-Vetencourt J, De Pasquale R, Baldini S, Maffei L. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp Neurol. 2010;226:100–109. doi: 10.1016/j.expneurol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Bence M, Levelt CN. Structural plasticity in the developing visual system. Prog Brain Res. 2005;147:125–139. doi: 10.1016/S0079-6123(04)47010-1. doi: [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS. gamma-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res. 1995;689:172–182. doi: 10.1016/0006-8993(95)00553-3. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Port JD, Cohen RS. The effects of dark-rearing on the electrophysiology of the rat visual cortex. Brain Res. 1992;572:198–207. doi: 10.1016/0006-8993(92)90470-T. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45:361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Bonds AB. Development of orientation tuning in the visual cortex of kittens. In: Freeman RD, editor. Developmental neurobiology of vision. New York: Plenum Press; 1979. pp. 31–41. [Google Scholar]
- Bourgeois JP, Jastreboff PJ, Rakic P. Synaptogenesis in visual cortex of normal and preterm monkeys: evidence for intrinsic regulation of synaptic overproduction. Proc Natl Acad Sci USA. 1989;86:4297–4301. doi: 10.1073/pnas.86.11.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Synaptogenesis in the occipital cortex of macaque monkey devoid of retinal input from early embryonic stages. Eur J Neurosci. 1996;8:942–950. doi: 10.1111/j.1460-9568.1996.tb01581.x. [DOI] [PubMed] [Google Scholar]
- Braastad BO, Heggelund P. Development of spatial receptive-field organization and orientation selectivity in kitten striate cortex. J Neurophysiol. 1985;53:1158–1178. doi: 10.1152/jn.1985.53.5.1158. [DOI] [PubMed] [Google Scholar]
- Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman LS, Schneider GE. Orienting behavior in hamsters with lesions of superior colliculus, pretectum, and visual cortex. Exp Brain Res. 1992;90:79–91. doi: 10.1007/BF00229259. [DOI] [PubMed] [Google Scholar]
- Carrasco MM, Mao YT, Balmer TS, Pallas SL. Inhibitory plasticity underlies visual deprivation-induced loss of receptive field refinement in the adult superior colliculus. Eur J Neurosci. 2011;33:58–68. doi: 10.1111/j.1460-9568.2010.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MM, Pallas SL. Early visual experience prevents but cannot reverse deprivation-induced loss of refinement in adult superior colliculus. Vis Neurosci. 2006;23:845–852. doi: 10.1017/S0952523806230177. [DOI] [PubMed] [Google Scholar]
- Carrasco MM, Razak KA, Pallas SL. Visual experience is necessary for maintenance but not development of receptive fields in superior colliculus. J Neurophysiol. 2005;94:1962–1970. doi: 10.1152/jn.00166.2005. [DOI] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Chalupa LM. Retinal waves are unlikely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:25. doi: 10.1186/1749-8104-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y-C, Chiao C-C. Effect of visual experience on the maturation of ON-OFF direction selective ganglion cells in the rabbit retina. Vision Res. 2008;48:2466–2475. doi: 10.1016/j.visres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci. 2005;25:6929–6938. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13:5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Weliky M. Spontaneous activity in developing ferret visual cortex in vivo. J Neurosci. 2001;21:8906–8914. doi: 10.1523/JNEUROSCI.21-22-08906.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across species. Neuroscience. 2001;105:7–17. doi: 10.1016/S0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Colonnese M, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, Moriette G, Chiron C, Ben Ari Y, Khazipov R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PM, Prusky G, Ramoa AS. The role of spontaneous retinal activity before eye opening in the maturation of form and function in the retinogeniculate pathway of the ferret. Vis Neurosci. 1999;16:491–501. doi: 10.1017/s0952523899163107. [DOI] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol. 1999;9:88–93. doi: 10.1016/S0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Development of ocular dominance columns in the absence of retinal input. Nat Neurosci. 1999;2:1125–1130. doi: 10.1038/16051. [DOI] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Daw NW. Development of receptive field properties. In: Daw NW, editor. Visual development. New York: Springer; 2006. pp. 91–109. [Google Scholar]
- Derrington AM. Development of spatial frequency selectivity in striate cortex of vision-deprived cats. Exp Brain Res. 1984;55:431–437. doi: 10.1007/BF00235273. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Fuchs AF. The development of spatial-frequency selectivity in kitten striate cortex. J Physiol. 1981;316:1–10. doi: 10.1113/jphysiol.1981.sp013767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco S, Nguyen VA, Bisti S, Protti DA. Permanent functional reorganization of retinal circuits induced by early long-term visual deprivation. J Neurosci. 2009;29:13691–13701. doi: 10.1523/JNEUROSCI.3854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Patrizi A, Quast KB, Hachigian L, Pavlyuk R, Saxena A, Carninci P, Hensch TK, Fagiolini M. NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron. 2012;76:1078–1090. doi: 10.1016/j.neuron.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Anishchenko A, Greschner M, Sher A, Litke A, Chichilnisky EJ, Feller M. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008;58:499–506. doi: 10.1016/j.neuron.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker M. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Kim YI, Bergemann AD, Frisén J, Barbacid M, Flanagan JG. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25:563–574. doi: 10.1016/S0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fortin S, Chabli A, Dumont I, Shumikhina S, Itaya SK, Molotchnikoff S. Maturation of visual receptive field properties in the rat superior colliculus. Brain Res Dev Brain Res. 1999;112:55–64. doi: 10.1016/S0165-3806(98)00157-6. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J Physiol. 1978;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Somogyi P. Quantitative distribution of GABA-immunoreactive neurons in the visual cortex (area 17) of the cat. Exp Brain Res. 1986;61:323–331. doi: 10.1007/BF00239522. [DOI] [PubMed] [Google Scholar]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242:90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci USA. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W. Refinement of the retinogeniculate pathway. J Physiol. 2008;586:4357–4362. doi: 10.1113/jphysiol.2008.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habak C, Faubert J. Larger effect of aging on the perception of higher-order stimuli. Vision Res. 2000;40:943–950. doi: 10.1016/S0042-6989(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30:361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wang P, Tian N. Light-evoked synaptic activity of retinal ganglion and amacrine cells is regulated in developing mouse retina. Eur J Neurosci. 2011;33:36–48. doi: 10.1111/j.1460-9568.2010.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimel JA, Saiepour MH, Chakravarthy S, Hermans JM, Levelt CN. Contrast gain control and cortical TrkB signaling shape visual acuity. Nat Neurosci. 2010;13:642–648. doi: 10.1038/nn.2534. [DOI] [PubMed] [Google Scholar]
- Heimel JA, van Versendaal D, Levelt CN. The role of GABAergic inhibition in ocular dominance plasticity. Neural Plast. 2011;2011:391763. doi: 10.1155/2011/391763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Boothe R. Morphology of the retina and dorsal lateral geniculate nucleus in dark-reared monkeys (Macaca nemestrina) Vision Res. 1976;16:517–521. doi: 10.1016/0042-6989(76)90033-X. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF. Development of connections in the human visual system during fetal mid-gestation: a DiI-tracing study. J Neuropath Exp Neur. 2000;59:385–392. doi: 10.1093/jnen/59.5.385. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O'Leary DDM. Ephb forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/S0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci. 2008;28:4807–4817. doi: 10.1523/JNEUROSCI.4667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. J Neurosci. 1996;16:1791–1807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/S0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Jasnow AM, Sisitsky AK, Albers HE. Glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus of rats housed in constant darkness. Brain Res. 1999;851:266–269. doi: 10.1016/S0006-8993(99)02167-8. [DOI] [PubMed] [Google Scholar]
- Kampf-Lassin A, Wei J, Galang J, Prendergast BJ. Experience-independent development of the hamster circadian visual system. PLoS One. 2011;6:e16048. doi: 10.1371/journal.pone.0016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–812. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Kind PC, Sengpiel F, Beaver CJ, Crocker Buque A, Kelly GM, Matthews RT, Mitchell DE. The development and activity-dependent expression of aggrecan in the cat visual cortex. Cereb Cortex. 2013;23:349–360. doi: 10.1093/cercor/bhs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer S, Mrsic Flogel T. The emergence of functional microcircuits in visual cortex. Nature. 2013;496:96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Hirsch HV. Receptive-field properties of different classes of neurons in visual cortex of normal and dark-reared cats. J Neurophysiol. 1980;43:1111–1132. doi: 10.1152/jn.1980.43.4.1111. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Effects of early pattern deprivation on visual development. Optom Vis Sci. 2009;86:640–646. doi: 10.1097/OPX.0b013e3181a7296b. [DOI] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9:676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- Lin CS, Lu SM, Schmechel DE. Glutamic acid decarboxylase and somatostatin immunoreactivities in rat visual cortex. J Comp Neurol. 1986;244:369–383. doi: 10.1002/cne.902440309. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers LH, Chow KL, Spear PD, Grobstein P. Ontogenesis of receptive fields in the rabbit striate cortex. Exp Brain Res. 1974;19:20–35. doi: 10.1007/BF00233393. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Baroncelli L, Viegi A, Tiraboschi E, Castren E, Cattaneo A, Maffei L. IGF-1 restores visual cortex plasticity in adult life by reducing local GABA levels. Neural Plast. 2012;2012:250421. doi: 10.1155/2012/250421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Tiraboschi E, Greco D, Restani L, Cerri C, Auvinen P, Maffei L, Castren E. Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. J Physiol. 2012;590:4777–4787. doi: 10.1113/jphysiol.2012.234237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RL. Tetrodotoxin inhibits the formation of refined retinotopography in goldfish. Brain Res. 1983;282:293–298. doi: 10.1016/0165-3806(83)90068-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. San Diego: Academic Press; 2001. [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Res. 1981;220:255–267. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Mower GD, Burchfiel JL, Duffy FH. The effects of dark-rearing on the development and plasticity of the lateral geniculate nucleus. Brain Res. 1981;227:418–424. doi: 10.1016/0165-3806(81)90079-1. [DOI] [PubMed] [Google Scholar]
- Mower GD, Christen WG, Caplan CJ. Very brief visual experience eliminates plasticity in the cat visual cortex. Science. 1983;221:178–180. doi: 10.1126/science.6857278. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Creutzfeldt C, Cloez-Tayarani I, Changeux JP, Bonhoeffer T, Hubener M. Altered map of visual space in the superior colliculus of mice lacking early retinal waves. J Neurosci. 2005;25:6921–6928. doi: 10.1523/JNEUROSCI.1555-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruczek RE, Sheinberg DL. Stimulus selectivity and response latency in putative inhibitory and excitatory neurons of the primate inferior temporal cortex. J Neurophysiol. 2012;108:2725–2736. doi: 10.1152/jn.00618.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas SL, Finlay BL. Conservation of receptive-field properties of superior colliculus cells after developmental rearrangements of retinal input. Vis Neurosci. 1989;2:121–135. doi: 10.1017/S0952523800011986. [DOI] [PubMed] [Google Scholar]
- Pallas SL, Gilmour SM, Finlay BL. Control of cell number in the developing neocortex. I. Effects of early tectal ablation. Brain Res. 1988;471:1–11. doi: 10.1016/0165-3806(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974;237:49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci USA. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Paradiso MA, Freeman RD. Blockade of intracortical inhibition in kitten striate cortex: effects on receptive field properties and associated loss of ocular dominance plasticity. Exp Brain Res. 1988;73:285–296. doi: 10.1007/BF00248220. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM, Pallas SL. Developmental plasticity of inhibitory receptive field properties in the auditory and visual systems. In: Pallas SL, editor. Developmental plasticity of inhibitory circuitry. New York: Springer; 2010. pp. 71–89. [Google Scholar]
- Razak KA, Pallas SL. Neural mechanisms of stimulus velocity tuning in the superior colliculus. J Neurophysiol. 2005;94:3573–3589. doi: 10.1152/jn.00816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal DM, Boothe R, Teller DY, Sackett GP. Visual acuity and visual responsiveness in dark-reared monkeys (Macaca nemestrina) Vision Res. 1976;16:523–530. doi: 10.1016/0042-6989(76)90034-1. [DOI] [PubMed] [Google Scholar]
- Reuter JH. The development of the electroretinogram in normal and light-deprived rabbits. Pflugers Arch. 1976;363:7–13. doi: 10.1007/BF00587395. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM. Effects of neonatal cortical lesions upon directional selectivity in the superior colliculus of the golden hamster. Brain Res. 1978a;147:188–193. doi: 10.1016/0006-8993(78)90787-4. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM. Functional properties of the corticotectal projection in the golden hamster. J Comp Neurol. 1978b;180:617–634. doi: 10.1002/cne.901800312. [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of direction selectivity in mouse cortical neurons. Neuron. 2011;71:425–432. doi: 10.1016/j.neuron.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Aizenman CD. Learning to see: patterned visual activity and the development of visual function. Trends Neurosci. 2010;33:183–192. doi: 10.1016/j.tins.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider GE. Two visual systems. Science. 1969;163:895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- Schwartz N, Schohl A, Ruthazer E. Activity-dependent transcription of BDNF enhances visual acuity during development. Neuron. 2011;70:455–467. doi: 10.1016/j.neuron.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Sen A, Blakemore C. Characteristics of surround inhibition in cat area 17. Exp Brain Res. 1997;116:216–228. doi: 10.1007/PL00005751. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM. Influence of spontaneous activity and visual experience on developing retinal receptive fields. Curr Biol. 1996;6:1503–1508. doi: 10.1016/S0960-9822(96)00755-5. [DOI] [PubMed] [Google Scholar]
- Sherk H, Stryker MP. Quantitative study of cortical orientation selectivity in visually inexperienced kitten. J Neurophysiol. 1976;39:63–70. doi: 10.1152/jn.1976.39.1.63. [DOI] [PubMed] [Google Scholar]
- Singer W, Freeman B, Rauschecker J. Restriction of visual experience to a single orientation affects the organization of orientation columns in cat visual cortex. A study with deoxyglucose. Exp Brain Res. 1981;41:199–215. doi: 10.1007/BF00238877. [DOI] [PubMed] [Google Scholar]
- Spolidoro M, Baroncelli L, Putignano E, Maya-Vetencourt JF, Viegi A, Maffei L. Food restriction enhances visual cortex plasticity in adulthood. Nat Commun. 2011;2:320. doi: 10.1038/ncomms1323. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Reid RC. Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nat Neurosci. 2000;3:608–616. doi: 10.1038/75786. [DOI] [PubMed] [Google Scholar]
- Thompson I, Holt C. Effects of intraocular tetrodotoxin on the development of the retinocollicular pathway in the Syrian hamster. J Comp Neurol. 1989;282:371–388. doi: 10.1002/cne.902820305. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/S0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Timney B, Mitchell DE, Giffin F. The development of vision in cats after extended periods of dark-rearing. Exp Brain Res. 1978;31:547–560. doi: 10.1007/BF00239811. [DOI] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci. 2010;30:16573–16584. doi: 10.1523/JNEUROSCI.3305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- Wong ROL. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wong ROL, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Xu H-P, Furman M, Mineur Y, Chen H, King S, Zenisek D, Zhou ZJ, Butts D, Tian N, Picciotto M, et al. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron. 2011;70:1115–1127. doi: 10.1016/j.neuron.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]