Abstract
Which physical parameter of vibrissa deflections is extracted by the rodent tactile system for discrimination? Particularly, it remains unclear whether perception has access to instantaneous kinematic parameters (i.e., the details of the trajectory) or relies on temporally integration of the movement trajectory such as frequency (e.g., spectral information) and intensity (e.g., mean speed). Here, we use a novel detection of change paradigm in head-fixed rats, which presents pulsatile vibrissa stimuli in seamless sequence for discrimination. This procedure ensures that processes of decision making can directly tap into sensory signals (no memory functions involved). We find that discrimination performance based on instantaneous kinematic cues far exceeds the ones provided by frequency and intensity. Neuronal modeling based on barrel cortex single units shows that small populations of sensitive neurons provide a transient signal that optimally fits the characteristic of the subject's perception. The present study is the first to show that perceptual read-out is superior in situations allowing the subject to base perception on detailed trajectory cues, that is, instantaneous kinematic variables. A possible impact of this finding on tactile systems of other species is suggested by evidence for instantaneous coding also in primates.
Keywords: head-fixed rat, neuronal coding, primary somatosensory cortex, psychophysics, tactile perception
Introduction
Rodent whisker-related tactile discrimination is based on a time series of whisker positions (deflections) leading to mechanical strain of the whiskers base, called the vibrotactile signal. An important question in tactile sensing is whether subjects can make use of the full information in the vibrotactile signal, that is, identifying individual precise features, or if they integrate over the vibrotactile signal to reduce its complexity. Electrophysiological evidence in the whisker system suggests that fast kinematic signatures are coded in the tactile system, from primary afferents to primary sensory cortex (Jones et al. 2004; Arabzadeh et al. 2005; Petersen et al. 2008; Jadhav et al. 2009), but time-integrated variables (e.g., variables based on frequency decomposition or averages of kinematic variables across extended time periods) hold substantial information about the vibrotactile signal as well (Arabzadeh et al. 2003; Hipp et al. 2006). On the behavioral level, there is evidence that time-integrated variables can be read out for perception (Gerdjikov et al. 2010). However, psychometric performance fell short of the one expected if information about instantaneous trajectory characteristics present in individual primary afferents were fully accessible (Gerdjikov et al. 2010), prompting the question whether the tasks used so far have been appropriate to reveal the usage of fully detailed trajectory information. Furthermore, the generality of the statement that rats use time-integrated vibrotactile signals has been questioned by the finding that the probability of detection of multipulse whisker deflections stays far below the one expected from the performance with single pulses (Stüttgen and Schwarz 2010).
In view of the missing evidence on whether instantaneous characteristics of the vibrotactile signals can be used for perception, and the well-corroborated fact that they are represented in the ascending tactile system, we set out to revisit this question. We hypothesized that previous task designs entailed process models (i.e., the spatio-temporal description of which brain systems contribute and are critical for performance) that made the read out of instantaneous features impossible or impeded them. For instance, in the Gerdjikov et al. (2010) study , animals had to store vibrotactile information in memory to do the task—discriminanda were presented isolated from each other, such that, in a single trial, the incoming tactile information had to be compared with memory contents. It is intuitive to assume that detailed trajectory information due to capacity limits of the stores cannot be stored in memory, while a time-integrated (i.e., compressed) signal may well offer a less memory-consuming alternative. In a subsequent study, Adibi et al. (2012) used simultaneous bilateral presentation of sinusoidal whisker deflections as discriminanda. The authors interpreted the failure of rats to discriminate sinusoids with high frequency and low amplitude from those with low frequency and high amplitudes as evidence that “intensity,” for example, the product of amplitude and frequency is used for perception. However, the distributions of some instantaneous kinematic parameters, like velocity, are identical in the pairs of discriminanda confused by the animals. Thus, those experiments do not exclude the possibility that rats use detailed kinematic features for perception.
In the present study, we aimed at improving the task characteristics of previous attempts by a behavioral paradigm which allowed the animals to compare discriminanda using sensory representations (not involving memory) and using pulsatile stimuli, in which the parameters frequency, intensity, and instantaneous kinematic features can be readily disentangled (Salinas et al. 2000). We use mean speed as the measure for “intensity” as perceptual analysis is consistent with this parameterization (while classical measure like, e.g. power, are not; Gerdjikov et al. 2010), and “frequency” is measured as interpulse frequency. The third parameter “instantaneous kinematic cues or features” is given by the pulse amplitude (which is equivalent to using the time series of position and its derivatives velocity, acceleration, etc., because manipulation of pulse amplitude, changes the time series of these kinematic parameters in proportion). We established a novel task, the “detection of change” (DOC) psychophysical paradigm, which presents S− (NoGo stimuli predicting no reward) and S+ (Go stimuli predicting reward) in seamless sequence. To compare the discriminanda, the subjects do not need to store stimuli in neither working nor long-term memory (Stüttgen et al. 2011). This enabled us to perform 3 sets of psychophysical experiments, each of them keeping 1 of the 3 parameters (pulse frequency, intensity, and instantaneous kinematic cues) constant in the presented discriminanda and thus allowed us to disentangle them. We found that the animals, first were able to use instantaneous kinematic features for discrimination performance, and, second, performed much better using those compared with situations in which exclusively intensity and frequency cues were available.
Materials and Methods
Animals, Surgery, and General Procedures for Behavioral Testing
All experimental and surgical procedures were carried out in accordance with standards of the Society of Neuroscience and the German Law for the Protection of Animals. Subjects were 6 female Sprague–Dawley rats (Charles River, Germany), aged 12–16 weeks at the time of implantation. The basic procedures of head-cap surgery, habituation for head-fixation, and behavioral training followed the ones published in a technical review (Schwarz et al. 2010). In the following only procedures pertaining to the special paradigm established here are described in detail.
Oral antibiotics (Baytril; Bayer HealthCare, Leverkusen Germany, 2.5% in 100-mL drinking water) were provided for 3 days before surgery and 1 week postoperatively. The animals were anesthetized using ketamine and xylazine (100 and 15 mg/kg body weight, respectively) and chronic electrode arrays (Haiss et al. 2010) were implanted. Barrels were located by mapping the cortex with a single intracerebral microelectrode. Unit and field potential responses to a brief manual whisker flick were monitored until a site maximally responsive to flicks of a single whisker with lower activation of adjacent whiskers was found. Across the 6 animals, columns A3, C1, C2, D1, and D2 were implanted. Movable multi-electrode arrays (2 × 2; electrode distance, 250–375 µm) were centered over the mapped location and slowly inserted into the cortex at a speed of 1.25 µm s–1 until all electrodes had penetrated the dura (usually 300–800 µm). The electrodes were then slowly retracted to a depth of ∼250 µm relative to the cortical surface and fixed to the skullcap with dental cement so that the mobility of the array was still guaranteed. The wound was treated with antibiotic ointment and sutured. Analgesia and warmth were provided after surgery. Rats were allowed to recover for at least 10 days before habituation training. Groups of 3 rats were housed together and kept under a 12/12 h inverted light/dark cycle. Water control was performed exactly as described by Schwarz et al. (2010). During testing, water intake was restricted to the apparatus where animals were given the opportunity to earn water to satiety. Testing was paused and water was available ad lib during 2 days a week. Body weight was monitored daily and typically increased during training. No animal in this study needed supplementary water delivery outside training sessions to keep its weight. The first step of behavioral training was systematic habituation to head-fixation lasting about 2 weeks (Schwarz et al. 2010). After another week of habituation inside the experimental setup, 2 trainings-/recording-sessions were usually conducted per day, each lasting for 15–30 min resulting in 100–200 trials depending on the impulsivity and motivation of the animal. During behavioral testing a constant white background noise (70 dB) was produced by an arbitrary waveform generator (W&R Systems, Vienna, Austria) to mask any sound emission of the piezo benders (see below). All animals underwent a control session performed in the way described above for the first half followed by disconnecting the vibrissa from the piezo actuator for the second (by retracting it a few millimeters). In all animals, discrimination performance broke down after whisker disconnection assuring that the animals were using exclusively tactile cues to perform the task.
Electrophysiology
The movable multielectrode arrays used here are described in Haiss et al. (2010). Voltage traces picked up by the electrodes were band-pass-filtered (200–5000 Hz) and recorded at a sampling rate of 20 kHz using a multichannel extracellular amplifier (Multi Channel Systems, Reutlingen, Germany). Spikes from arrays were detected using amplitude thresholds. Two-millisecond cutouts centered on the time bin in which the voltage trace first traversed the amplitude threshold were recorded and sorted offline using a laboratory-written software package (Hermle et al. 2004). Artifacts were removed and neurons sorted to yield either single-unit or multiunit spike trains. Criteria for classification as a single unit were conservative and have been described in an earlier study (Möck et al. 2006).
Firing rates of all units in the 1-s interval preceding the onset of S+ stimuli were compared between short periods at the start and the end of each behavioral session to clarify whether there were long-term adaptations in the neurons responses. We did not find any statistical significant difference. This was particularly true for those single units that were above the 75th percentile in the ranking by sensitivity (eqs. 2 and 3). On average, the firing rate (in spike/s) during the first 5 trials of a session was 6.6 (standard deviation (SD) 4.1) compared with 6.3 (SD 3.8) during the last 5 trials (Student's t-test, P = 0.83, n = 19). We can thus rule out that systematic long-term run-down of firing rates were present in our experiments.
Whisker Stimulation
The whisker stimulator was identical to the one used by Stüttgen et al. (2006). The stimuli consisted of brief pulsatile deflections (one single pulse corresponding to a single-period sine wave of frequency 100 Hz; starting from the negative maximum, thus yielding a bell-shaped pulse with smooth on- and offsets; duration 10 ms) presented to one single whisker on the left whisker pad. To manipulate pulsatile stimuli, exclusively 2 basic parameters, “interpulse frequency” and “pulse amplitude,” were changed. Interpulse frequency is defined as the reciprocal of the inter-pulse interval, that is, the time elapsed between the onsets of 2 sequential pulses in seconds. Pulse amplitude is defined by the height of a pulse and is changed by multiplying the signal with a constant. Thus, a change in pulse amplitude leaves the width of the pulse untouched. In fact, pulse-width was fixed at 10 ms in all stimuli used in the present study.
Using these 2 basic parameters, 3 classical vibrotactile parameters as used in similar ways by a large number of previous publications (e.g., LaMotte and Mountcastle 1975; Arabzadeh et al. 2005) were manipulated: 1) instantaneous kinematic cues, 2) frequency, and 3) intensity. With our pulsatile stimuli, numbers 1 and 2 of these correspond simply to the 2 basic parameters interpulse frequency and pulse amplitude. The last one, intensity, corresponds to mean speed of the stimulus (Gerdjikov et al. 2010), and can be manipulated by both of the basic parameters. It is important to note that a balanced change of the 2 basic parameters interpulse frequency and pulse amplitude in opposite direction leaves intensity constant while changing frequency and instantaneous kinematic cues, a feat used systematically in the present study.
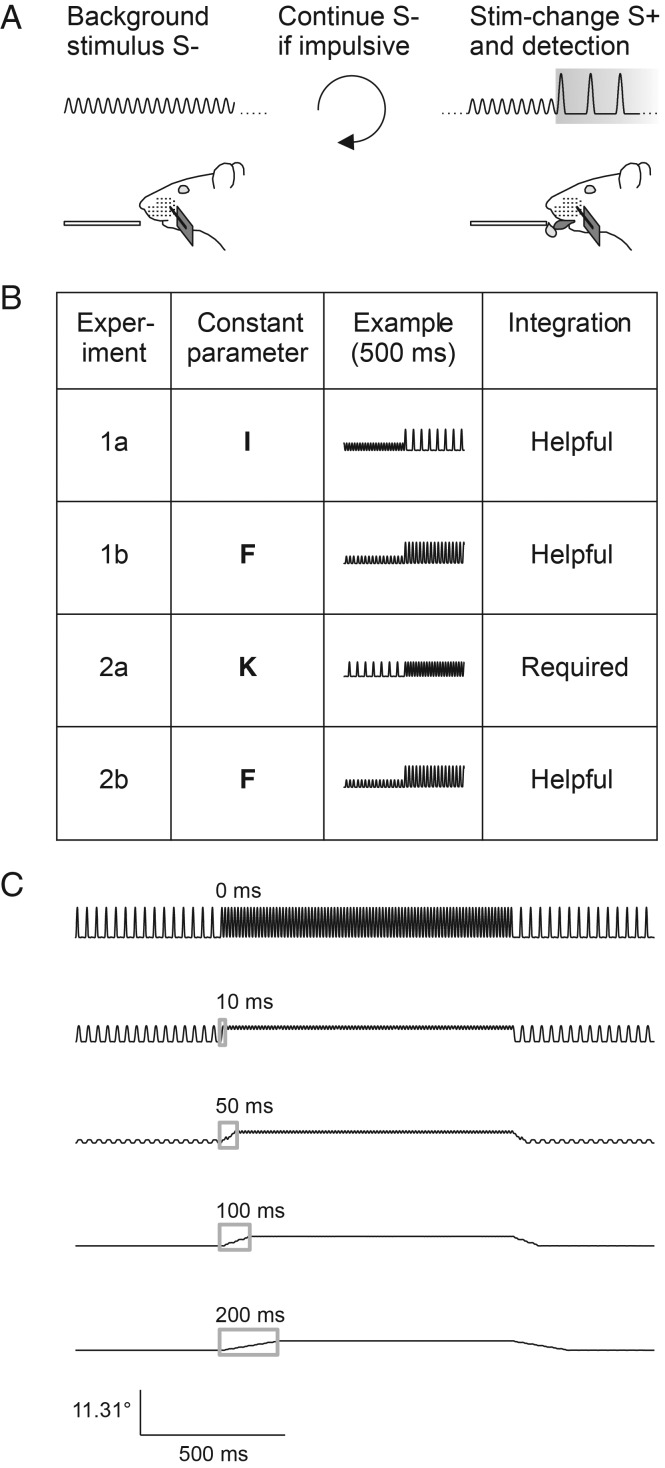
Three different manipulations were carried out to distinguish S+ (rewarded) from S− (nonrewarded) stimuli (Experiments 1a, b, and 2a). The first set of stimuli (used in Experiment 1a) varied frequency, and kinematic variables, but kept intensity constant (balanced change of the 2 basic parameters in opposite direction). The second set (used in Experiments 1b and 2b) varied intensity and instantaneous kinematic cues but kept frequency constant (exclusive manipulation of pulse amplitude). Finally, the third set (used in Experiment 2a) varied frequency and intensity, and kept instantaneous kinematic cues constant (exclusive manipulation of interpulse frequency) (cf. Fig. 1).
Figure 1.
Experimental strategy. (A) Head-fixed rats were trained on a detection of change (DOC) task. Constant pulsatile stimuli were applied to a single whisker and the animals had to detect a 1-s change (S+, gray box) with a lick response in order to get a water reward. No change served as catch trial (S−). Impulsive licks triggered extra time of background stimulation. (B) Overview of the stimulus sets applied in the different experiments of this study (first column). The general idea was to keep one of the 3 vibrotactile parameters intensity (I), frequency (F), or instantaneous kinematic cues (K) constant between S− and S+ (second column) and only vary the other 2. Schematic stimulus waveforms at the time of stimulus transitions are shown in the third column. Using pulsatile stimuli, an increase of instantaneous kinematic cues (i.e., increase of pulse amplitude) is correlated with intensity but not pulse frequency. Column 4 denotes the role played for temporal integration for successful discrimination in each experiment. (C) In Experiment 2a, the instantaneous kinematic cues are constant; therefore, the observer has to integrate the running stimulus with a minimal time window comprising more than one pulse (>10 ms). Stimulus transitions filtered with integration windows of different size (gray boxes = moving average) demonstrate that perfect discrimination of these stimuli can theoretically be performed very soon after the stimulus onset.
Interpulse intervals ranged from 11.1 to 33.25 ms corresponding to interpulse frequencies of 30–90 Hz (for the stimulus set applied in Experiment 2a, we also tried frequencies down to 10 Hz to improve performance, see Results). These frequencies cover the main frequency range carrying texture information (Hipp et al. 2006). Deflection amplitudes ranged from 3.9 to 11.3° (equivalent to 0.35–1 mm deflections at 5 mm distance from the whisker base). The stimulator was calibrated using a modified phototransistor with resolution of 20 µs and 1 µm (HLC1395, Honeywell, Morristown, NJ, USA) and an optoelectronic measuring device with a resolution of 1.4 ms and 11 µm (laser emitter and detector; PAS 11 MH; Hama Laboratories, Redwood City, CA, USA) (Stüttgen et al. 2006). The length of the glass capillary and point of attachment of the piezo element were adjusted such that the ringing of the stimulator was minimal between pulses (<0.1° at frequencies around 1 kHz). The capillary tip was positioned 5 mm away from the skin and tilted at an angle of 155°–175° such that the vibrissa rested against the inside wall of the capillary, ensuring that the stimulator immediately engaged the whisker. Stimulation was delivered in the rostro-caudal direction. The chosen frequencies and amplitudes of the pulsatile stimuli used here gave intensities ranging from 482 to 1240°/s (cf. Table 1). The kinematic, frequency, and intensity values were assessed based on the measured trajectory (i.e., the output of the phototransistor tracking the whisker).
Table 1.
Stimuli and psychophysical experiments
| Extra stimulus | Intensity (°/s) | Pulse-frequency (Hz) | Pulse-amplitude (°) | Trials |
||||
|---|---|---|---|---|---|---|---|---|
| Rat 1 | 2 | 3 | ||||||
| Experiment 1a | S− | 646 | 90 | 4.14 | 536 | 605 | 617 | |
| S+ − S− | 0 | −12 | +0.33 | 586 | 596 | 616 | ||
| 0 | −24 | +1.14 | 586 | 649 | 553 | |||
| 0 | −36 | +2.20 | 579 | 609 | 642 | |||
| 0 | −48 | +3.86 | 567 | 631 | 648 | |||
| 0 | −60 | +7.17 | 576 | 615 | 624 | |||
| S+ − S− | + | +64 | −24 | +1.66 | 366 | 425 | 471 | |
| + | −64 | −24 | +0.62 | 424 | 507 | 444 | ||
| + | −128 | −24 | +0.09 | 452 | 513 | 455 | ||
| Experiment 1b | S− | 507 | 66 | 4.14 | 626 | 489 | 454 | |
| S+− S− | +41 | 0 | +0.33 | 637 | 502 | 461 | ||
| +140 | 0 | +1.14 | 636 | 509 | 447 | |||
| +268 | 0 | +2.20 | 567 | 525 | 468 | |||
| +464 | 0 | +3.86 | 676 | 531 | 471 | |||
| +844 | 0 | +7.17 | 630 | 475 | 513 | |||
| S+ − S− | + | +140 | +24 | 0 | 630 | 508 | 445 | |
| Rat 4 | 5 | 6 | ||||||
| Experiment 2a | S− | 1240a | 90a | 7.76 | 272 | 136 | 49 | |
| S+ − S− | −131a | −12a | 0 | 303 | 190 | 69 | ||
| −291a | −24a | 0 | 316 | 159 | 51 | |||
| −451a | −36a | 0 | 287 | 159 | 63 | |||
| −602a | −48a | 0 | 282 | 184 | 70 | |||
| −759a | −60a | 0 | 314 | 193 | 49 | |||
| Experiment 2b | S− | 482 | 66 | 3.90 | 319 | 271 | 302 | |
| S+ − S− | +157 | 0 | +1.29 | 299 | 252 | 297 | ||
| +307 | 0 | +2.53 | 305 | 251 | 317 | |||
| +468 | 0 | +3.85 | 363 | 295 | 297 | |||
| +628 | 0 | +5.17 | 366 | 283 | 298 | |||
| +759 | 0 | +6.34 | 345 | 244 | 304 | |||
In rat 5, stimulus order was reversed: the absolute intensity and frequency of S− was 482 °/s and 30 Hz, respectively, the difference S+ − S− was in this case always positive (same value). Data with a ‘+’ in the column ‘extra stimulus’ describe added stimuli that deliberately varied the parameter that was else kept constant in the respective experiment (cf. Fig. 2C,D).
Experimental Paradigm
Rats were trained on a novel DOC psychophysical task. In this task, the whisker is continuously vibrated, but vibration parameters change once in a while, an event that is to be detected and indicated by the animal to gain a reward (intertrial interval 4–10 s drawn from a flat probability distribution). The change in stimulus properties is called “stimulus” (S+) from here on although background stimulation (S−) certainly continued between trials. Catch trials contained a continuation of S− instead of presenting an S+. Special care was taken to assure that the change from S− to S+ (and back) occurred within 1 interpulse-interval, that is, we made sure that the succession of interpulse-intervals was a clean step function (i.e., no other interpulse interval than the ones defining S− and S+ occurred). In a first step, a clearly suprathreshold S+ lasting for 1 s was automatically accompanied by the delivery of a water drop to condition the consummatory response (licking) upon the stimulus. The water drop was released 500 ms after S+ onset to give the animal enough time to feel the stimulus and to use any temporal integration. The intertrial interval ranged between 4 and 10 s. Once the animal regularly licked off the water, the task was switched from classical to operant conditioning, that is, the reward delivery was made contingent on an operant lick during the S+ (Fig. 1A). Now, the rats were able to retrieve a water reward by licking immediately after they detected the onset of S+. Licking during a “no-lick-interval” that spanned the last 2 s before the scheduled S+ presentation was punished by resetting time and starting a new intertrial interval of 4–10 s duration, drawn at random from a flat probability distribution. Psychophysical testing was conducted using the method of constant stimuli with a randomized stimulus order. Experiment 1a presented 5 intensity-matched stimuli (S+), 1 catch trial (S−), and 3 additional stimuli that modulated intensity cues (S+). Experiment 1b presented 5 frequency-matched stimuli (S+), 1 catch trial (S−), and 1 additional stimulus that modulated frequency cues. Experiment 2a presented 5 kinematics-matched stimuli (S+) and 1 catch trial (S−). Experiment 2b was very similar to Experiment 1b but presented no additional stimuli. See Figure 1B and Table 1 for overview. Animals 1–3 were used in Experiments 1a and b, whereas animals 4–6 were exclusively used in Experiments 2a and 2b. Both experiments were conducted in the order listed in Table 1.
Data Analysis and Statistics
Psychophysical data assessed as response probabilities was converted into sensitivity d′ using the following equation:
| (1) |
where phit signifies the probability of correct responses, pFA the probability of false alarms, and Φ−1 is the probit function. In order to compare psychometric with neurometric sensitivities, d′ values were converted to area under the receiver operating curve (AUROC) (Stanislaw and Todorov 1999) by
| (2) |
(for the correction term see ref. Stüttgen et al. 2011); note that despite the typing error in their equation (3), this is identical to what has been done by Gerdjikov et al. (2010). The psychometric curves in this study are Weibull fits estimated from a maximum likelihood estimator (Wichmann and Hill 2001a 2001b). Error bars of psychometric data signify 95% confidence intervals calculated from a binomial model setting the animal's response probability to the probability of a Bernoulli trial.
To give a rough estimate of psychophysical performance across sessions (Figs 2B and 3B), we calculated a simple discrimination index
| (3) |
where is the response probability to presentation of the strongest S+ and is the response probability to S−.
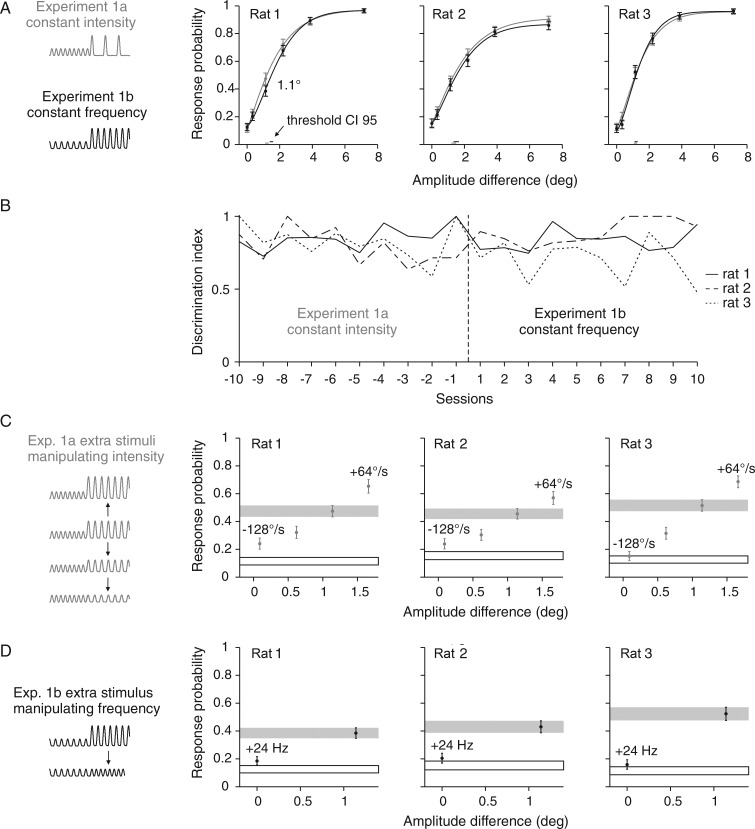
Figure 2.
Psychometric performance with 2 stimulus sets that both employed kinematics as discriminative cues. (A) Response probabilities of 3 rats are depicted as a function of amplitude differences in gray for Experiment 1a and in black for Experiment 1b. Data points represent means and are based on 447–676 trials. Smooth lines are Weibull fits estimated from a maximum likelihood estimator. Vertical error bars represent 95% confidence intervals. Horizontal bars at the bottom represent 95% confidence intervals of the thresholds. (B) Discrimination index (eq. 3) achieved in the last 10 sessions of the first block of Experiment 1a and the first 10 sessions after switching to Experiment 1b. (C) Additional stimuli with manipulated intensities were introduced to test the animals' sensitivity compared with a stimulus with matched intensity from the psychometric curve (gray bar). (D) Same as in C but additional stimuli that modulated frequency. Performance to catch trials is shown as black outlined box. Schematics of stimulus traces used in each experiment are shown on the left side.
Neuronal sensitivities were computed from distributions of spike counts calculated as the difference of the spike counts found in intervals of equal length just before and after each stimulus onset (negative spike counts indicate a suppressed response under S+ relative to background). A criterion shifted in steps of 1 spike across the 2 distributions was used to determine the hits and false alarms of the neuron, and thus the ROC curves (Britten et al. 1992). Sensitivities for all S+ are expressed as AUROC.
Neurometric sensitivities of pools of neurons were fitted to the psychometric one by applying a Monte Carlo maximum likelihood procedure (Stüttgen and Schwarz 2008). The database included the probability of spike counts in a variable window after stimulus onset (as calculated from 50 single-unit trains recorded in Experiment 1a and 24 single-unit trains recorded in Experiment 1b). The fit was performed for all combinations of 4 parameters: 1) the number of most sensitive neurons accepted into the pool (3, 5, 10, and 15), 2) the pool size (5, 10, 20, 40, 100, 200, and 500), 3) the duration of the time window in which the spikes were counted (12.5, 25, 50, 100, 200, 400, and 800 ms), and 4) the prior expectation of the animal of receiving a stimulus that predicts reward, that is, the probability of the occurrence or absence of a certain stimulus given the subject's knowledge about stimulus ratios. This prior ratio was varied between the extremes (number of catch trials divided by the number of S+ in Experiment 1a) and (time of background stimulation divided by the time of S+ presentation, average from all sessions that entered the dataset). To reliably account for neuronal responses despite the limited number of spikes that could be sampled in the course of one session, we focused exclusively on spike counts (thus ignoring information potentially included in temporal spike patterns, cf. Stüttgen and Schwarz et al. 2008).
In each resampling step, the selection of pool units was performed by a random pick (with return) from a subset of neurons taken from the top of a ranked list according to their sensitivity (assessed from responses to S+ with a maximum of 7.2° amplitude difference to the background stimulus using eq.2). Based on the measured response probabilities, the responses of each pool member to all stimuli were determined by a random pick. These resulted in the likelihood function of each neuron. Assuming independence of neuronal responses, the pool's response was then found by summing the logarithmized likelihood functions of all pool members. The decision was formed by comparing the likelihoods for each stimulus (S+) versus no stimulus (S−). From Bayes' rule, one can derive that it is optimal to decide for hypothesis h1 versus the alternative h2 if
| (4) |
that is, if the likelihood ratio of h1 and h2 given the pool response exceeds the inverse ratio of the respective prior probabilities . The optimal criterion (converted to log space) to decide about the presence of a stimulus then is
| (5) |
On the left side of the inequality, is accounted for by taking the logarithm and subtracting from the log likelihood of the stimulus (). The right side holds the log likelihood of the catch trial given r. The pool's decision was set to 1 (stimulus present) if any comparison favored the presence of a S+, otherwise it was set to 0. The pool's neurometric sensitivity was calculated based on its decisions exactly as done with the behavioral data gained from the rat (see above), and compared with these psychometric data using the Euclidean distance.
Results
Psychometrics
The present psychophysical data were sampled from 2 groups of 3 rats each subjected to a DOC paradigm (Fig. 1A). The DOC paradigm is a trial based Go/NoGo task where a continuous “background” stimulation is interspersed with a target stimulus predicting reward (Go, S+). When the animals detected the S+ and reported it by a lick during the 1-s presentation time, they obtained a water reward. The animals were allowed to immediately report the change after it had occurred. Catch trials consisted of a continuation of the background stimulus (NoGo, S−) instead of switching to an S+ stimulus. Rats were head-fixed and whisker stimulation was applied to the base of one whisker (5 mm from the base) with a piezo bender. One pulse consisted of a single period of a sinusoid (starting from the negative peak to allow smooth pulse on- and offsets) and was separated from following pulses by whisker rest (Fig. 1B). We tested whether rats used one of 3 vibrotactile stimulus parameters for discrimination: 1) “instantaneous kinematic cues” which can be used to detect so-called kinematic events, or extremes in amplitude or velocity, 2) “pulse frequency,” and 3) “intensity” as measured by mean speed in an interval minimally encompassing one stimulus period (interval between 2 sequential pulse onsets). The choice to measure intensity as mean speed was based on psychophysical results showing that rats confound stimuli matched in mean speed (Gerdjikov et al. 2010). To change the instantaneous kinematic cues, we multiplied the pulses with a constant factor resulting in pulses with different amplitudes and maximal velocities. Changes in frequency were introduced by manipulating interpulse intervals. By applying 1 of these 2 manipulations, instantaneous kinematic cues and frequency can be changed independently. However, the intensity of the stimulus is duly affected by both manipulations. Therefore, to keep intensity constant, the pulse amplitude and the interpulse interval had to be changed in reverse directions. Three sets of switches from S− to S+ could be constructed by manipulating pulse amplitude and interpulse interval in a systematic way, each keeping one of the vibrotactile stimulus parameters constant while changing the other 2 (Fig. 1B). This strategy is not possible with the classically applied sinusoid waveforms where changes in frequency necessarily cause changes in both the remaining parameters as well. Experiment 1, performed with the first group of rats, was designed to test the contribution of instantaneous kinematic cues, and therefore, held first the intensity (Experiment 1a) and then the frequency (Experiment 1b) constant between S− and S+, while the pulse amplitudes changed. Experiment 2a then tested if rats temporally integrate the stimulus to extract frequency or intensity information from stimuli that did not offer any differences in pulse waveforms (i.e., the kinematic events that could be extracted were identical). We wish to stress that for Experiment 2, a second group of naïve rats were used (rather than testing the animals that already learned the tasks in Experiment 1). This was done to exclude the possibility that subjects may have learned an inappropriate strategy due to their prior experience (in Experiment 1) with stimuli that differed in amplitude. This strategy assured that in case of minor performance on the task of Experiment 2a, we can exclude the possibility that rats did not perform well because their learning ability was impaired by whatever they had learned before. In order to investigate how the temporal evolution of such an integration compares to the duration of the stimuli, running averages were computed from windows of different length (10–200 ms) and are shown for one stimulus used in Experiment 2a (Fig. 1C). Assuming an optimal discriminator, minimal integration time needed to discriminate our stimuli is one period (33 ms for pulsatile stimuli at the lowest frequency of 30 Hz). Although we do not know the properties of the hypothesized integrator, with this analysis, we can be fairly confident that the integration time needed to discriminate the stimuli is going to be a small fraction of a second, very likely below 100 ms, which will give the rat ample time to respond during stimulus duration (typically 1 s, some sessions of Experiment 2a used 1.5 s).
Experiment 1a used stimuli that displayed constant intensity, which could be detected either by monitoring instantaneous kinematic cues or by integrating the signal for frequency decomposition. Previous results using a standard trial-based Go/NoGo psychophysical task without background stimulation and similar stimuli, predicted that the animals should have difficulties to discriminate these stimuli, as intensity was found to be the decisive cue in the earlier task (Gerdjikov et al. 2010). In contrast to this expectation, all 3 rats working on the DOC paradigm could readily discriminate the stimuli, indicating intensity coding is not necessary in the present context (Fig. 2A, gray psychometric curves). Experiment 1b used the same amplitude differences between S− and S+ but the pulse frequency of 66 Hz was kept constant (Table 1, Fig. 1B, and Fig. 2A, black psychometric curves). Switched to the new stimulus set, the animals immediately performed well. Within the first session their response was nearly as good as with the old stimulus set (Fig. 2B). The presentations of stimulus sets 1a and b were then alternated in blocks of 10 sessions, and performance never dropped when switching between the experimental blocks. Consequently, the psychophysical curves shown in Figure 2A have been constructed from all sessions including the ones right after switching from one stimulus set to the next. Plotted across the instantaneous kinematic cues (the shared parameter between stimulus sets 1a and b), the 2 psychometric curves were nearly identical for all 3 rats (black and gray curves in Fig. 2A). The confidence limits of the 2 curves overlap for all the stimuli except for a slight deviation of the response to stimuli at amplitude difference 1.1° in rat 1. This demonstrates that stimuli lacking either intensity or frequency cues were detected equally well, suggesting that the instantaneous kinematic features are the relevant cue used by the animals during discrimination.
This conclusion is supported further by the analysis of additional stimuli that have been presented together with the core stimulus sets in Experiments 1a and b reported so far. These additional stimuli (inserted into the sequence of core stimuli in due pseudorandom order) deviated from the equal intensity (1a), or frequency (1b) rule respectively. We asked if discrimination improved if some intensity cue (1a) or frequency cue (1b) were added to a stimulus close to perceptual threshold (1.1° amplitude difference). Experiment 1a contained 3 additional stimuli, 2 deviating downward and 1 deviating upward from the intensity of the original 1.1° stimulus (see schematic in Fig. 2C). This was done by manipulating the pulse amplitude of the stimulus; thus, it was a co-manipulation of intensity and instantaneous kinematic cues. In the downward direction, the intensity cue increased (because it deviated more and more from that of the S−) but, at the same time, we drew the amplitude closer to the one of S−, in fact reducing the instantaneous kinematic cue. The expectation then was that the animals should show better discrimination whenever they used the intensity cue while they should show the opposite if they use the instantaneous kinematic cues. Clearly, in all animals, the latter was the case supporting the hypothesis that the animals used dominantly the instantaneous kinematic cues to solve the task (Fig. 2C). Note that the performance of all rats on the stimuli with negligible amplitude differences (−128°/s in Fig. 2C, +24 Hz in Fig. 2D; significantly so in rats 1 and 2) was small but better than catch performance (black outlined box). This indicates the remaining ability to discriminate the stimuli after abolishing all instantaneous kinematic cues. In Experiment 1b, we added one stimulus of increased frequency while reducing the pulse amplitude to match the intensity of the original stimulus (see schematic in Fig. 2D). The same logic applies here. The animals should discriminate this stimulus better if they used the introduced frequency cue but they should perform worse if they used the abolished instantaneous kinematic cue. Again, the latter was clearly the case in all animals (Fig. 2D).
The results presented so far strongly argue in favor of the hypothesis that the animals used mainly the instantaneous kinematic cues of the pulses as the cue to perform the discrimination. Regarding our previous study (Gerdjikov et al. 2010), this fits the hypothesis that the preference for intensity only emerges if instantaneous kinematic features are not available as discriminant cue. However, it is also possible that in principle rats can use all parameters of vibrotactile stimuli to do the discrimination, but they stick to the cue presented when learning the task for the first time and refuse (or are unable) to relearn if the cues change later on. Particularly, we were concerned that in Experiments 1a and b, the animals adopted the strategy to watch out for the first different pulse (with respect to the background S− pulses) to perform the task. Such a strategy could possibly divert them away from integrating the stimulus (and the use of intensity or frequency cues). In order to address this point, we designed an experiment to investigate how a second set of naïve rats learned the discrimination task if the instantaneous kinematic cue (i.e., a divergent waveform or “oddball” pulse) was absent from the start. Experiment 2a held the instantaneous kinematic cue (i.e., the pulse waveform) constant across stimuli and thus forced the subjects to use temporal integration, if they could. Later, a stimulus set very similar to the one in Experiment 1b was presented in Experiment 2b, to check if the animals would switch to instantaneous kinematic cues when present or stay with whatever they learned in Experiment 2a (Figs 1B and 3A).
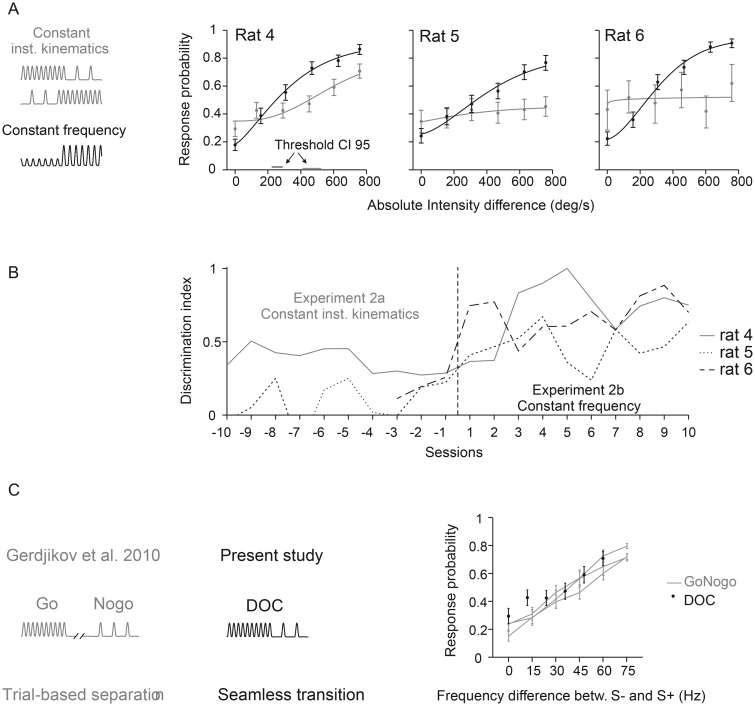
Figure 3.
Psychometric performance without kinematic cues. (A) Response probabilities of 3 rats are depicted as a function of intensity differences in gray for Experiment 2a and in black for Experiment 2b. Data points represent means and are based on 49–316 trials. Weibull fits and statistics are the same as in Figure 2. 95% confidence intervals of the thresholds (horizontal bars) are only shown if the psychometric curve surpasses the threshold. Rats 4 and 6 experienced negative change (intensity and frequency of S+ was lower than that of S−, upper gray icon), rat 5 worked on the inverted stimulus relationship (intensity/frequency S+ >intensity/frequency S−, lower gray icon, see details in Table 1). Rats were naïve before being trained on stimulus set 2a. (B) Discrimination index (eq. 3) achieved in the last 10 sessions of the first block of Experiment 2a (no kinematic cue) and the first 10 sessions after switching to Experiment 2b (almost identical to 1b, see Table 1). Note that the index in sessions 1–7 before switching to 2b was not plotted for rat 6. This individual was frustrated by stimulus set 2a and thus was presented only with extreme stimuli (S− and strongest S+) in these sessions in an attempt to keep up its motivation. (C) Comparison of psychometric results with an earlier study using the same type of stimuli (Gerdjikov et al. 2010). The earlier study (left) used a classical Go/NoGo paradigm with stimuli separated in time; S+ was the highest frequency stimulus (90 Hz). The present study used a DOC paradigm with seamless presentation of S− and S+ (center). The psychophysical results of rat 4 (the one successfully trained on the DOC task) was obtained with S− being the highest frequency stimulus (90 Hz). Despite the differences in psychophysical design and in stimulus-reward association, the psychophysical results of the 2 experiments are comparable (right). To allow direct comparison of the curves, the absolute stimulus frequencies used in the earlier study, were converted into frequency differences between S+ and S−.
The hypothesis that animals learn the task equally well using any of the 3 parameters was rejected by Experiment 2a. A second naïve set of 3 rats showed great difficulties learning the task when instantaneous kinematic cues were absent (Fig. 3A, gray curves). An extension of S+ duration to 1.5 s and the use of larger frequency differences, 90 versus 10 Hz (data not shown) in some training sessions did not help. The resulting psychometric curves indicated hit rates between 0.45 and 0.71 during presentation of the strongest stimulus change and false alarm rates between 0.29 and 0.43 during catch trials (with n = 49–316 trials per stimulus). Rat 5 experienced positive changes in intensity (intensity S− < intensity S+) while rats 4 and 6 were confronted with negative changes (intensity S− > intensity S+; see Table 1 for details). Only rat 4 was able to perform reasonably well (hit rate 0.71, false alarm rate = 0.29), but never reached the performance achieved by the first set of animals that experienced changes in kinematic cues. Moreover, rat 6 refused to work on the task at one point, forcing us to terminate this part of the experiment at a lower number of sampled trials than intended (cf. Table 1). Finally, when we switched to a stimulus set that contained instantaneous kinematic cues (but held the frequency constant, Experiment 2b), all 3 rats readily learned the task (Fig. 3A, black curves). The transition of the general discrimination index after the first switch to Experiment 2b is shown in Figure 3B. After learning to discriminate stimulus set 2b, the rats were repeatedly switched back to Experiment 2a but never showed any improvement in discrimination performance on this stimulus set. As shown in Figure 3C, the performance of the successful rat 4 was roughly in the range of performances observed using a quite different psychophysical design but similar stimuli (no instantaneous kinematic cues) (Gerdjikov et al. 2010). We wish to stress that this comparison must be viewed with caution—not only because the data were obtained with different psychometric paradigms—but also because, in the previous study, the S+ were the higher frequency stimuli while our present rat 4 was confronted with S+ that were of lower frequency than S−. Nevertheless, we find the comparison instructive as it shows that the exclusive presence of intensity and frequency cues does not abolish discrimination performance but rather is able to grant a minor amount of discrimination ability consistent with the previous results using a quite different task design. In summary, these experiments conclusively show that the usage of a certain type of cue is not dependent on the sequence of learning. Furthermore, they show that it is also not due to a bias generated by the structure of the psychophysical Experiment 1ab, which potentially led the animals' to use the strategy to detect oddball pulses. Rather, the results point to a limitation of the perceptual capabilities of the rats: they show superior performance when instantaneous kinematic cues are present, and their ability to discriminate vibrotactile stimuli using frequency or intensity cues is minor.
Neurometrics
Barrel cortex activity has been shown to be critical for whisker-based passive detection and discrimination (Miyashita and Feldman 2012). We, therefore, investigated how barrel cortex unit activity represents the present stimuli and explore possibilities for perceptional read-out. During the behavioral session of rats 1–3 (Experiment 1), we recorded a total of 74 single units and 91 multiunits (Experiment 1a: 50 SU, 61 MU; Experiment 1b: 24 SU, 30 MU) in the barrel column associated with the stimulated whisker. By gradually moving the electrodes, neurons were found between 400 and 1700 µm. Figure 4 shows representative neuronal responses to different S+ and catch trial (the 3 units shown were recorded in the same session of Experiment 1b). All units generated a phasic response in response to the first few pulses (best seen with the highest amplitude difference). This pattern is indicative of cortical (re-) adaptation with fairly short time constants kicking in after the stimulus switch. Similar observations have been made with pulsatile stimuli engaging the cortex in the nonadapted (idle) state (Stüttgen and Schwarz 2010). After the transient response at S+ onset, the firing rate settled at a new level for the remainder of the S+ presentation. With respect to S−, this sustained firing during S+ was elevated in part of the cells (Fig. 4, bottom), and it was reduced in very few (Fig. 4, top). In many cases, however, sustained firing rate appeared the same in both conditions, and the only visible signs of S+ presence were the transient ON and OFF responses (Fig. 4, center).
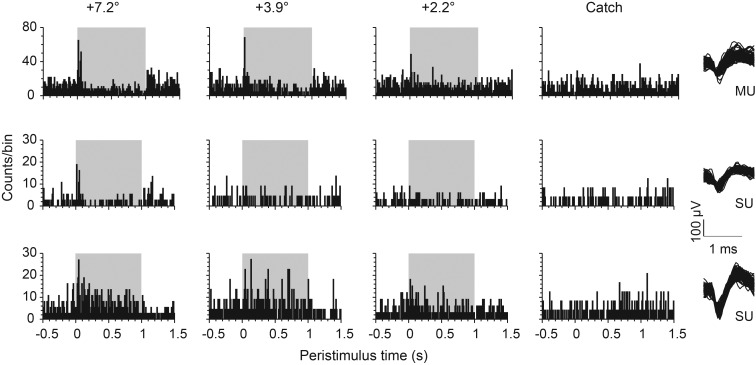
Figure 4.
Example responses to DOC stimuli (Experiment 1b). PSTHs of representative barrel cortex units recorded from one animal in one session of Experiment 1b. Spike counts across time (bin width 10 ms) are plotted for 2 single units (SU) and 1 multiunit (MU) (the right column plots a random selection of 100 waveforms for each unit). The other columns plot the response to 3 different S+ amplitudes (label indicates the difference of S+ and S− pulse amplitudes) and the catch trials. The gray area represents the time of stimulus change (S+).
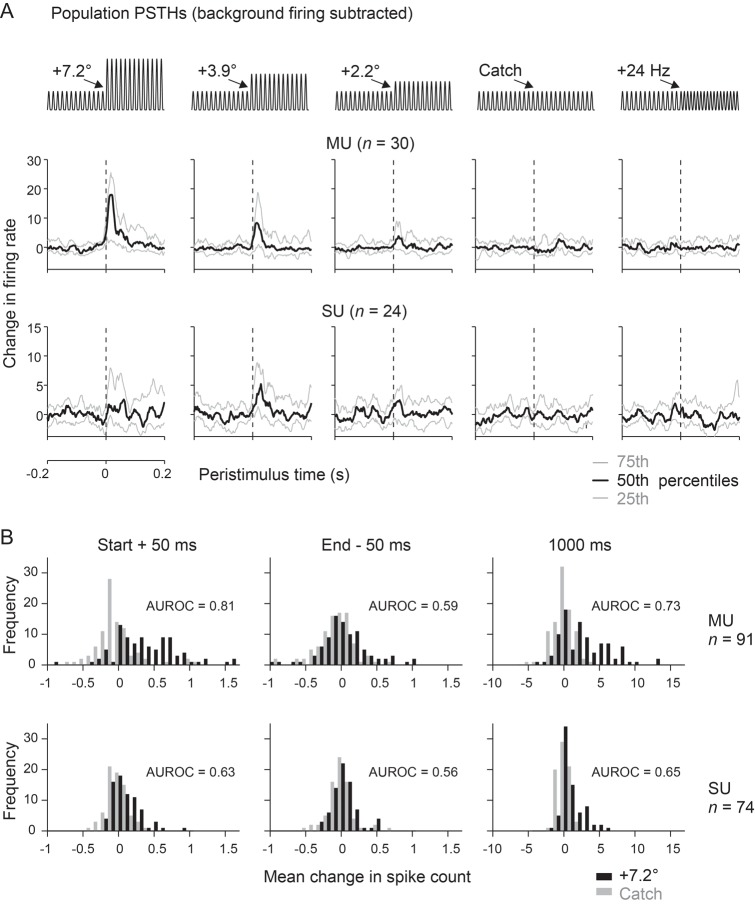
In Figure 5A the distribution of PSTHs obtained from the whole sample are shown for 4 different stimuli and catch trial used in Experiment 1b. Median firing rate changes (black) and 25% and 75% percentile levels (gray) are depicted for the 30 multiunits (top) and 24 single units (bottom) recorded in this experiment. The sustained firing rate to S− was subtracted, so that positive/negative firing rates would indicate a higher/lower sustained response to S+ as compared with S−. The median firing rate of multiunits was clearly modulated by the first pulses with amplitude changes down to +2.2°. Inspection of different percentile levels reveals that more than 75% of the multiunits showed an excitatory response to amplitude changes of 7.2° and 3.9°. Owing to very low firing rates, single-unit population activity appeared noisier but generally matched the observation from multiunits. Interestingly, in experiments which kept the pulse kinematics constant but varied the frequency (labeled “+24 Hz”), all recorded neurons showed flat PSTHs varying around zero change in firing rate. This result reveals the near complete absence of neuronal responses to an isolated switch in stimulus frequency.
Figure 5.
Population analyses of responses to DOC stimuli. (A) Distributions of evoked activity across time (bin width 1 ms) for the whole population of units (multiunits, n = 30; single units, n = 24) recorded in Experiment 1b. (Note that the ordinate scales responses as “changes of firing rate” because the sustained firing rate to the S− background stimulus has been subtracted). Each subplot shows the unitary responses to a different S+ stimulus or catch trial (schematized on top). The curves indicate the median (black) and the 75th (top) and 25th (bottom) percentile (gray). Note that the firing rate distributions labeled “+24 Hz” were the ones obtained with the extra stimuli of Experiment 1b, in which frequency was manipulated and waveform kept constant (cf. Fig. 2D). These responses, although obtained in Experiment 1b, in fact belong to the same category of stimuli as the ones used in Experiment 2a. (B) Distributions of neuronal activity evoked by the strongest amplitude change (7.2°, black bars) and catch trials (gray bars) as observed in Experiments 1a and b. The abscissa scales response as spike counts in 3 different intervals. Left: immediately after stimulus onset (0–50 ms peristimulus time). Center: before the stimulus offset (950–1000 ms peristimulus time). Right: the whole stimulus period (0–1000 ms peristimulus time). Multi- (MU, n = 91, top) and single-unit data (SU, n = 74, bottom) are shown.
Nevertheless, as mentioned above, rats display the low but consistent ability to discriminate even when instantaneous kinematic cues were absent (cf. Figs 2C,D and 3C). To examine more closely the sustained firing rates that might give rise this accomplishment, we compared the firing rate response shortly after stimulus onset, at the end of the stimulus presentation, and for the entire stimulus period for the total sample of single units (n = 74) and multiunits (n = 91). At stimulus onset, the distribution of spike numbers evoked by catch versus highest amplitude difference differed clearly in both single- and multiunit data. However, at the end of the stimulus or taking the whole stimulus period into account, the difference in response to S− and the strongest S+ was mainly visible in multiunit recordings (Fig. 5B). The information about the stimulus in the steady state as revealed by the MU data are likely the neuronal correlate of the minor psychometric performance when only intensity and frequency cues are present (Figs 2C,D and 3C). [The detailed effect sizes of spike count distributions obtained comparing 0 (catch) and 7.2° difference amplitude (the strongest S+) and using different durations of integration windows are the following (effect size is expressed as AUROC, i.e., values of 0 and 1 indicate completely separated distributions and a value of 0.5 indicates identical distributions; values in-between describe distributions overlapping to different degrees). Below, the first number depicts the effect size obtained with single unit trains (n = 74) and the second the one obtained with multiunit trains (n = 91) from animals working on Experiments 1a and b: stimulus onset: window 50 ms: 0.63, 0.81, window 100 ms: 0.63, 0.76, and window 200 ms: 0.68, 0.73. Stimulus end: window 50 ms: 0.56, 0.59; window 100 ms: 0.56, 0.62; window 200 ms: 0.60, 0.65. Entire stimulus period: 0.65, 0.73.]
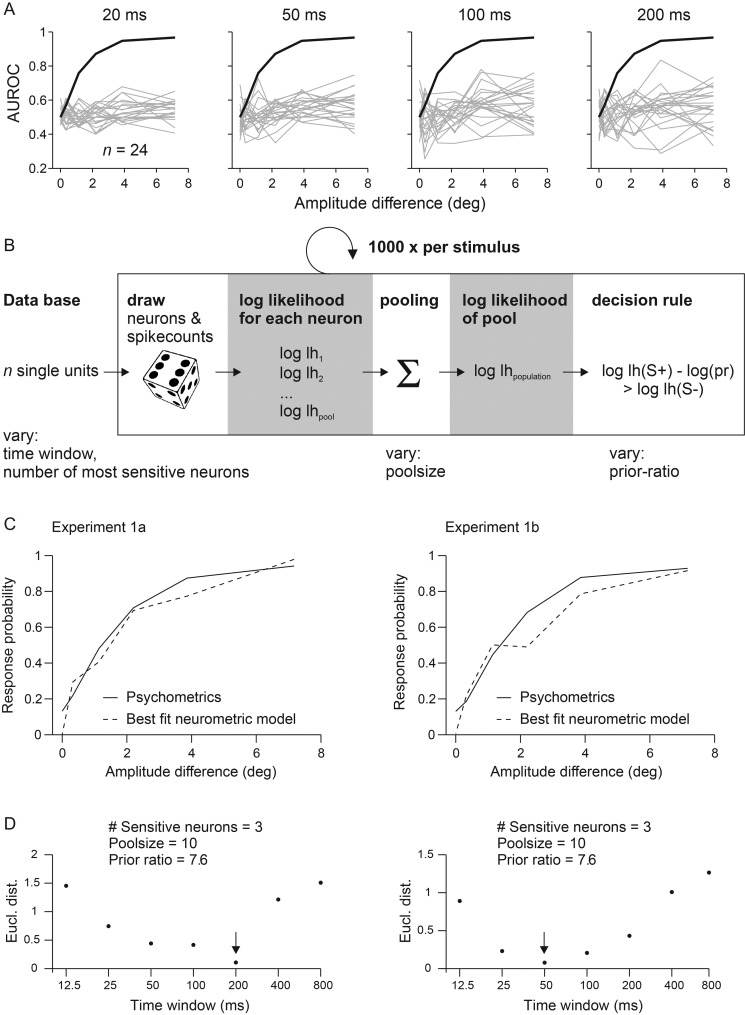
Our next aim was to estimate the integration interval employed by the perceptual read-out using quantitative comparison of the neurometric with the psychometric data. The hypothesis that instantaneous kinematic cues are the main basis of perception would be strengthened by the finding of short optimal integration windows. To this end, we converted the distributions of single-trial spike counts to neurometric sensitivities, again by applying ROC analysis (see Materials and Methods; note that for the present purpose of calculating sensitivities, AUROC values are used to compare single units' ability to discriminate a stimulus pair on the basis of single trials while, in the section before, we used AUROC as a measure of effect size for the comparison of mean responses measured across our sample of units). Neuronal sensitivities with which each S+ could be discriminated from S− were then compared with the respective psychometric sensitivities calculated from the rat's lick responses in the same sessions. Panel A of Figure 6 plots AUROC values for all single units (gray) recorded in Experiment 1b (AUROC values of 0.5–1 scale chance to perfect discrimination if cells are excited, while values of 0.5–0 scale the same in the case of inhibitory responses). The psychometric curve shown in black (identical for all subplots) is the average across 3 rats. Each subplot illustrates the neurometric performance based on spike counts taken from integration intervals starting at the stimulus onset but with different durations. The few responding neurons showed a tendency to increase sensitivity with increasing amplitudes, but did so in highly variable ways. Some of them showed low, others rather high amplitude thresholds to reach elevated sensitivity. However, even those who did respond stayed far below the performance of the rat. One reason for the poor neuronal sensitivity was their low spike number per trial, especially when using small encoding windows. In a 20-ms window, for example, the spike count was mostly zero and virtually did not exceed one or 2 spikes. In addition, neuronal sensitivity did not vary significantly across cortical depth. Units grouped into 3 bins indicating roughly superficial (400–800 µm), middle (800–1200 µm), and deep (1200–1700 µm) locations (as measured by screw turns at the electrode array) showed similar mean AUROC values for the discrimination of the strongest S+ stimulus from S− [mean ± SD; 0.57 ± 0.13 (n = 44), 0.61 ± 0.12 (n = 84), and 0.59 ± 0.10 (n = 37); t-test for independent samples, all 3 pairwise comparisons P > 0.05]. In conclusion, responses of individual barrel cortex neurons contain low sensitivity for the task, and thus, provide a poor tool to estimate the integration interval. Individual neurons are unlikely to be at the basis of the animals' discrimination performance.
Figure 6.
Quantitative comparison of neurometric and psychometric curves to estimate the optimal integration interval used to generate a percept. (A) Neurometric sensitivity of single neurons. The graphs show neuronal sensitivities based on spike count distributions observed within integration intervals of different durations starting at stimulus onset (top). The neurometric (gray lines) and psychometric (black line) sensitivities are plotted as the function of changes in stimulus amplitude (pairwise comparisons to catch trials) and are expressed as area under the ROC curve (AUROC). The psychometric sensitivity is calculated as the mean across 3 animals subjected to Experiment 1b. Single neuron’s activity cannot explain the animals' performance as none reached the psychometric sensitivity, irrespective of the integration interval used. (B) Performance of neuronal pools. A Monte Carlo procedure was used to fit the neurometric performance of a population of neurons to the psychometric performance. Random picks from the measured spike count distributions of pool neurons, were converted into log likelihood functions and summed up to yield the pooled log likelihood (lh) function and the pool's decision (eq. 5). Repeating the procedure 1000 times yielded the pool's response probability. Four variables were varied to find the best fit of the pool response to the psychometric performance: 1) the duration of the integration interval, 2) the number of neurons in the pool, 3) the number of sensitive neurons chosen for read-out, and 4) the prior belief of the animal. (C) Optimal model performance was assessed by calculating the Euclidean distance between the outcome of each combination of model variables and the psychometric performance in Experiments 1a and b. The best fits of pool neurometric sensitivity (broken lines) are plotted together with the average psychometric curve (solid lines) of the 3 rats. (D) Model performance as a function of the integration interval. The 3 other variables (listed on top) were fixed to their best fits, which were found consistently in Experiments 1a and b. Smallest Euclidean distances (Eucl. dist.) were found for intervals between 50 and 200 ms. The best fits are indicated by arrows. Note the log scale of the abscissae.
To explore whether the psychometric results can be better explained by population activity, and thus allow us to better estimate the optimal integration interval, we fitted a probabilistic model that computes the likelihood function of neurons within a pool under the assumption of independence (Fig. 6B) as done previously (Jazayeri and Movshon 2006; Stüttgen and Schwarz 2008). The pool neurons were modeled based on our single-unit data obtained in Experiments 1a and b. Using a Monte Carlo procedure, we varied 1) the duration of the spike count window, 2) the number of most sensitive neurons picked to compose the pool, 3) the pool size, and 4) the animal's expectation, that is, the prior ratio. The first parameter, the spike count window, is the most important one for the present purpose, because it gives us an estimate of the optimal integration interval. Parameters 2 and 3 are important as they hint at spatial specifications of optimal readout mechanisms. With low optimal number of neurons selected and used for the pool, read out mechanisms that assess neurons in highly specific ways are better than unspecific ones. Lastly, the fourth parameter addresses the expectation of the animal. In the behavioral sessions, the animals experience favorable times (presence of S+ and availability of reward) and unfavorable times (absence of S+, i.e., presence of S−). The trained animal, thus, forms an expectation of the presence S+ through learning and uses it (e.g., to adjust lick rates) even before perceiving the sensory stimuli. Under the Bayesian framework, incoming actual sensory data are integrated with this prior belief to take a decision (eqs. 4 and 5). A low value of this parameter would indicate that the animals count trial numbers of S− and S+ to calculate their prior belief. A high value speaks in favor of the alternative strategy, to measure presentation times of S+ and S−. Refer to the Materials and Methods for more details and the justification of each parameter's range in which values were varied to fit the model.
The single units in the dataset were ranked according to their sensitivity to discriminate the strongest stimulus changes. In each resampling step, spike responses of randomly chosen units were drawn based on the measured firing probabilities, and the log-likelihood function was computed for each neuron. The log-likelihood function of the whole population was then calculated by summing the contributions of individual neurons. In the last step of each simulation, a decision rule was applied that compared likelihoods of S+ and S− stimuli, taking into account varying prior probabilities of the absence or presence of a stimulus (see Materials and Methods for more details). The goodness of fit of the simulated pool-neurometric curves with the psychometric ones was estimated by calculating their Euclidean distance. Figure 6C shows the best fit results for Experiments 1a and b, respectively. The simulated pool response curve with the best fit is shown as dashed line, whereas psychometric data are shown as solid line. Both, neurometric and psychometric data are shown as a function of amplitude difference for the 6 core stimuli, although the best fit was calculated for the whole stimulus set including additional stimuli that modulated intensity or frequency (cf. Fig. 2C,D). There was a consistent underestimation of the catch trial performance of the neuronal pools, in line with the notion that the neurons were purely sensory driven and did not reflect the (presumptive top-down) neuronal correlate of the animal's impulsivity. Across experimental conditions 1a and b, we found that the model robustly fitted the free parameters at optimal pool size of 10 neurons, the usage of the 3 most sensitive neurons (located at a depth of 930–1325 µm), and a prior ratio of 7.6. A pool consisting of more than 10 neurons led to an increase in neuronal sensitivity, that is, exceeding the performance of the animal, leading to a larger Euclidean distance, and a worse fit of the model. The low number of pool neurons (and the usage of a small number of sensitive neurons) speaks in favor of the notion that very few neurons can carry enough information to explain the performance of the subject as noted before (Stüttgen and Schwarz 2008, 2010). The optimal prior ratio of 7.6 was closer to the value of 10.5 expected if the animals measure durations of S+ versus S− presentations than the one expected from using a number of trials for this calculation (value 0.13 or 0.17 for Experiments 1a and b, respectively). This finding is reasonable, because the trial-based structure of the DOC task is not easily assessable for the subject: S− trials have low probability to be noted consciously (they lead to a longer period of perceived background stimulation), and, therefore, are unlikely to contribute to a generation of a prior belief based on trial numbers. The most important finding of the modeling exercise was that the optimal spike count window was of short duration and ranged between 50 and 200 ms (indicated by small Euclidean distances in Fig. 6D, note that a higher Euclidean distance indicates a mismatch between neurometric and psychometric curves irrespective of which of the 2 is better or worse in absolute terms). The finding of short integration windows might have been expected from the strong response adaptation of firing rates, reported above. It is interesting to note, however, that the best fit interval is somewhat longer than the peaks of spiking activity in the PSTHs, likely reflecting the information contained in tonic increment of the firing rate as observed in some units (cf. Fig. 5B). In summary, these results suggest that the decision of the animals requires a small population of cells and is possible using fast and transient responses. Such a coding scheme seems adequate to process instantaneous kinematic cues which, as shown by our psychophysical analysis, is the essential parameter used for the performance on the DOC task.
Discussion
The present psychophysical and electrophysiological measurements demonstrate, for the first time, that neuronal representations of kinematic events, extracted from the instantaneous kinematic variables of whisker trajectories, play a decisive role for the subject's performance in a tactile discrimination task. Stimulus switches in the DOC format were detected well by animals as well as barrel cortex neurons, given the switch was characterized by a change in instantaneous kinematic cues. In contrast, if the stimulus switch was based exclusively on the classical parameters frequency and intensity, the performance of animals and neurons was minor.
How can our results in the whisker system, clearly favoring instantaneous extraction of kinematic events, be brought into register with previous evidence presented for each of the 3 candidate parameters? The original evidence for the “frequency” and “intensity hypothesis” comes from the primate literature on perception of “flutter.” Classic experiments have shown that the perception of “intensity” and “pitch,” reported by human observers, cannot unequivocally be attributed to single stimulus parameters like amplitude and frequency of a sine wave (LaMotte and Mountcastle 1975). Our perceptual measurements in the whisker system using the DOC task were aimed to disentangle instantaneous and time integrated parameters (i.e., instantaneous kinematic cues and mean speed) which had been conflated by the amplitude changes of sine wave stimuli in the older literature. Our data clearly show a high level of psychophysical performance whenever instantaneous kinematic cues were present and a diminished one whenever these cues were absent. Thus, we could not find strong evidence for dominant perceptual qualities of pitch and intensity as conjectured in the primate hand system. In our view, this does not exclude the possibility that the 2 systems share mechanistic principles of tactile processing. First, the classic studies, unable to separate the contribution of instantaneous versus time integrated parameters, may have ignored the contribution of instantaneous cues. Second, the similarity of instantaneous coding in primate hand and rodent whisker systems found more recently (Mackevicius et al. 2012) suggests a read-out of such information for perception. Finally, the insight that vibrissae and skin represent bioelastic elements, both capable of adding instantaneous kinematic cues about the probed texture to the vibrotactile signal (Scheibert et al. 2009; Jadhav and Feldman 2010), support our expectation that future work may well reveal a significant role of instantaneous kinematic cues for primate vibrotactile perception as well.
The intensity hypothesis has received great biomechanical evidence also in the whisker system: the power of the vibrotactile signal (another possible measure of intensity) has been shown to carry a large amount of information about the roughness of the contacted texture (Hipp et al. 2006). Psychophysical evidence using a trial based Go/No Go paradigm showed that rats used intensity cues while being unable to use frequency and instantaneous kinematic cues offered to them (Gerdjikov et al. 2010). Our present data using the DOC paradigm are in accordance with a role of intensity cues. However, the prominence of the instantaneous kinematic cues for discrimination performance shown here raises the question as to why the importance of this parameter was ignored by the previous study. One possibility is that the cognitive process model (Stüttgen et al. 2011) of the previous task contains a step in which stimulus information must be stored in long-term memory. In each trial, the animal observes only one stimulus which it compares to the S+ stored in long-term memory during the learning of the task (the memory period in the Gerdjikov et al., experiments was the time difference between the current trial and the last S+ trial successfully discriminated, i.e., minimally one interstimulus trial of 15–25 s). Considering the need to limit the size of content stored in memory due to capacity limits, it is conceivable that the memory about a pulsatile stimulus contains some strongly compressed version of the vibrotactile signal. This is exactly what was found: intensity, the average of the speed trajectory rather than the full trajectory, is what the animals used for discrimination (Gerdjikov et al. 2010). Another characteristic possibly favoring the use of instantaneous kinematic cues for discrimination is neuronal adaptation, which has been shown to give rise to higher fidelity representation of instantaneous kinematic cues in barrel cortex (Wang et al. 2010). Thus, cortical adaptation, present before onset of the discriminanda, may well play a role for the amenability of instantaneous kinematic parameters as a basis for discrimination found by the present study.
A second line of evidence in favor of intensity is based on recordings in urethane-anesthetized rats (Arabzadeh et al. 2003) which showed that neuronal spike counts in barrel cortex can neither be aligned with frequency nor with the amplitude of sinusoidal stimulation alone. In that study, long intervals of spiking were analyzed ignoring transient responses. In contrast, we show in the present study that information about the awake animal's choice is largely contained within the first 200 ms after stimulus change, that is, in the transient response. We therefore conclude that the analysis of a long stimulus period—at least under the present experimental conditions—does not lead to an adequate description of neuronal activity used for perception. A more recent psychophysical study in freely running rats simultaneously presented sinusoidal whisker deflections on each side of the face, whose frequency (f) and amplitude (A) could be either low (f, A) or of double value (2f, 2A) (Adibi et al. 2012). The main point of that study was that the animals failed to discriminate the stimuli whenever the 2 variables were varied in the opposite direction (i.e., f/2A vs. 2f/A), which led Adibi and coworkers to conclude that discrimination cannot be based on f or A but rather on their product f*A which is proportional to the intensity (mean speed) in sinusoidal stimuli. Importantly, our present results show, that the conclusion of Adibi et al. is not the only valid interpretation of their result. The distributions of instantaneous velocities contained in the 4 stimuli (f/A, 2f/A, f/2A, 2f/2A) are identical (because all are sinusoids), but are differently scaled: f/A is characterized by the slowest maximum velocities, 2f/A and f/2A yield intermediate (but identical) maximal velocities, and 2f/2A gives the highest maximal velocities. It is thus evident, that if the animals used the detection of instantaneous velocities, instead of f*A, the result of the Adibi et al. experiments would have been the same. However, the question remains why the animals did not use the amplitude differences (A vs. 2A) as discriminant cues. One possible answer is that the quite small amplitudes applied in that study (13 vs. 26 µm at the whisker tips, which gave rise to a low discrimination performance of max. ∼75%) activated exclusively rapidly adapting primary afferents, which have been reported to scale their response with velocity and are insensitive to amplitude (Stüttgen et al. 2006). From these considerations, we conclude that the results of Adibi et al. are well compatible with the notion that the animals use instantaneous kinematic cues for vibrotactile discrimination.
In summary, our findings best fit the notion that rat whisker-related tactile perception is based on instantaneous kinematic cues. Intensity cues (average speed) are less powerful and may be the parameter of choice if context demands (Gerdjikov et al. 2010). These results match the previous finding that for detection of repetitive whisker deflections, temporal integration is a minor factor. Temporal integration of short bursts of whisker deflections is limited to small integration windows, and even falls short of what is expected from simple probability summation (i.e., the probability to detect one of a number of single pulses presented in isolation) (Stüttgen and Schwarz 2010). Small integration windows are in line with single unit recordings in anesthetized animals which suggest that barrel cortex responses are transient and are most sensitive to whisker velocity (Pinto et al. 2000), that spiking on the ascending tactile pathway is precise and carries information about detailed features of the trajectory (Jones et al. 2004; Arabzadeh et al. 2006; Petersen et al. 2008), and that such precise spiking is particularly useful to represent so-called whisker slips—high amplitude/velocity/acceleration due to whisker elasticity (Arabzadeh et al. 2005; Ritt et al. 2008; Jadhav et al. 2009). So far, it has been demonstrated that different mean counts of slips can be obtained with different textures (Wolfe et al. 2008). Our present results support the slip hypothesis as a theory of perception, as we show that detailed kinematic features, encoded in the ascending tactile system, are not only read out as a basis for the animals' percept and decisions, but also optimize performance. To consolidate the slip hypothesis further, the next step must be to show how an observer fares when presented with very short and single trial slip sequences sampled from different textures. Our present finding that rats use the barrel cortex response to very few initial pulses of the S+ to perform the DOC task predicts that discrimination may not be based on slip counts, but rather on kinematic differences of a very small number of occurring slips.
Funding
This research was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, SCHW577/10-2) and the German Federal Ministry for Education and Research (BMBF; CRCNS 01GQ1113). Further support was provided by the Hertie Foundation and the Hermann and Lilly Schilling Foundation. Funding to pay the Open Access publication charges for this article was provided by Deutsche Forschungsgemeinschaft (DFG).
Notes
We thank Ursula Pascht for excellent technical assistance and Sinem Erisken to improve our English. Conflict of Interest: None declared.
References
- Adibi M, Diamond ME, Arabzadeh E. Behavioral study of whisker-mediated vibration sensation in rats. Proc Natl Acad Sci USA. 2012;109:971–976. doi: 10.1073/pnas.1116726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Panzeri S, Diamond ME. Deciphering the spike train of a sensory neuron: counts and temporal patterns in the rat whisker pathway. J Neurosci. 2006;26:9216–9226. doi: 10.1523/JNEUROSCI.1491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Petersen RS, Diamond ME. Encoding of whisker vibration by rat barrel cortex neurons: implications for texture discrimination. J Neurosci. 2003;23:9146–9154. doi: 10.1523/JNEUROSCI.23-27-09146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Zorzin E, Diamond ME. Neuronal encoding of texture in the whisker sensory pathway. PLoS Biol. 2005;3:e17. doi: 10.1371/journal.pbio.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdjikov TV, Bergner CG, Stüttgen MC, Waiblinger C, Schwarz C. Discrimination of vibrotactile stimuli in the Rat whisker system: behavior and neurometrics. Neuron. 2010;65:530–540. doi: 10.1016/j.neuron.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Haiss F, Butovas S, Schwarz C. A miniaturized chronic microelectrode drive for awake behaving head restrained mice and rats. J Neurosci Methods. 2010;187:67–72. doi: 10.1016/j.jneumeth.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Hermle T, Schwarz C, Bogdan M. Employing ICA and SOM for spike sorting of multielectrode recordings from CNS. J Physiol Paris. 2004;98:349–356. doi: 10.1016/j.jphysparis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Hipp J, Arabzadeh E, Zorzin E, Conradt J, Kayser C, Diamond ME, König P. Texture signals in whisker vibrations. J Neurophysiol. 2006;95:1792–1799. doi: 10.1152/jn.01104.2005. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Feldman DE. Texture coding in the whisker system. Curr Opin Neurobiol. 2010;20:313–318. doi: 10.1016/j.conb.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Wolfe J, Feldman DE. Sparse temporal coding of elementary tactile features during active whisker sensation. Nat Neurosci. 2009;12:792–800. doi: 10.1038/nn.2328. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, Movshon JA. Optimal representation of sensory information by neural populations. Nat Neurosci. 2006;9:690–696. doi: 10.1038/nn1691. [DOI] [PubMed] [Google Scholar]
- Jones LM, Depireux DA, Simons DJ, Keller A. Robust temporal coding in the trigeminal system. Science. 2004;304:1986–1989. doi: 10.1126/science.1097779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Mountcastle VB. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol. 1975;38:539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- Mackevicius EL, Best MD, Saal HP, Bensmaia SJ. Millisecond precision spike timing shapes tactile perception. J Neurosci. 2012;32:15309–15317. doi: 10.1523/JNEUROSCI.2161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Feldman DE. Behavioral detection of passive whisker stimuli requires somatosensory cortex. Cereb Cortex. 2012;23:1655–1662. doi: 10.1093/cercor/bhs155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möck M, Butovas S, Schwarz C. Functional unity of pontine and cereballar signal processing: evidence that intrapontine communication is mediated by a reciprocal loop with the cerebellar nuclei. J Neurophysiol. 2006;95:3414–3425. doi: 10.1152/jn.01060.2005. [DOI] [PubMed] [Google Scholar]
- Petersen RS, Brambilla M, Bale MR, Alenda A, Panzeri S, Montemurro MA, Maravall M. Diverse and temporally precise kinetic feature selectivity in the VPm thalamic nucleus. Neuron. 2008;60:890–903. doi: 10.1016/j.neuron.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol. 2000;83:1158–1166. doi: 10.1152/jn.2000.83.3.1158. [DOI] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron. 2008;57:599–613. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Hernandez A, Zainos A, Romo R. Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci. 2000;20:5503–5515. doi: 10.1523/JNEUROSCI.20-14-05503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibert J, Leurent S, Prevost A, Debregeas G. The role of fingerprints in the coding of tactile information probed with a biomimetic sensor. Science. 2009;323:1503–1506. doi: 10.1126/science.1166467. [DOI] [PubMed] [Google Scholar]
- Schwarz C, Hentschke H, Butovas S, Haiss F, Stüttgen MC, Gerdjikov T, Bergner C, Waiblinger C. The head-fixed behaving rat—procedures and pitfalls. Somatosens Mot Res. 2010;27:131–148. doi: 10.3109/08990220.2010.513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Stüttgen MC, Rüter J, Schwarz C. Two psychophysical channels of whisker deflection in rats align with two neuronal classes of primary afferents. J Neurosci. 2006;26:7933–7941. doi: 10.1523/JNEUROSCI.1864-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüttgen MC, Schwarz C. Integration of vibrotactile signals for whisker-related perception in rats is governed by short time constants: comparison of neurometric and psychometric detection performance. J Neurosci. 2010;30:2060–2069. doi: 10.1523/JNEUROSCI.3943-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüttgen MC, Schwarz C. Psychophysical and neurometric detection performance under stimulus uncertainty. Nat Neurosci. 2008;11:1091–1099. doi: 10.1038/nn.2162. [DOI] [PubMed] [Google Scholar]
- Stüttgen MC, Schwarz C, Jäkel F. Mapping spikes to sensations. Front Neurosci. 2011;5:125. doi: 10.3389/fnins.2011.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Webber RM, Stanley GB. Thalamic synchrony and the adaptive gating of information flow to cortex. Nat Neurosci. 2010;13:1534–1541. doi: 10.1038/nn.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. fitting, sampling, and goodness of fit. Percept Psychophys. 2001b;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001a;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol. 2008;6:e215. doi: 10.1371/journal.pbio.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]