Abstract
Introduction
We examined two genetic markers established early in colorectal tumor development, microsatellite instability (MSI) and mutation of the KRAS proto-oncogene, to see if these genetic changes influence metastatic disease progression and survival.
Patients and methods
MSI and KRAS mutation status were assessed in 532 primary adenocarcinomas (stage I–IV) from patients treated by colon resection. Median follow-up was 4.1 years (range 0–13.3 years) overall, 5.4 years for survivors.
Results
MSI and KRAS mutation were detected in 12 and 36% of cases, respectively. MSI was more common in early-stage disease (I, 15%; II, 21%; III, 10%; IV, 2%; P = 0.0001). Prevalence of KRAS mutation did not vary with stage (I, 36%; II, 34%; III, 35%; IV, 40%; P = ns). Disease-specific survival was far superior for MSI tumors than for microsatellite stability (MSS) tumors (5-year survival 92 vs. 59%, P < 0.0001). KRAS mutation was a marker of poor survival (5-year survival 55 vs. 68%, P = 0.0002). Using Cox regression analysis MSI, KRAS mutation, and stage were strong independent predictors of survival in the entire patient population. A high-mortality group with MSS/KRAS-mutant tumors was identified within the stage I and II cohort.
Conclusions
MSI and KRAS mutation provide fundamental genetic signatures influencing tumor behavior across patient subsets and stages of tumor development.
The variable cure rate of colorectal cancer offers an excellent clinical model for studying factors influencing metastatic progression. Following surgical resection of the colon, local tumor relapse is rare.1 Cancer recurrence and death from disease are nearly always due to distant metastases, and are thus determined by tumor biology rather than by variations in presentation or local therapy.
Extent of disease at the time of diagnosis has a major impact on surgical cure rate, which varies from 90 to 50% for cancers that are clinically localized (stages I–III, respectively) to less than 10% for cancers that have metastasized to distant organs (stage IV).2–5 These data support a developmental model in which distant metastases are generally established only after a substantial period of local growth and invasion. Thus the determinants of surgical cure are, first, the time elapsed from cancer initiation to surgical treatment and, second, the speed with which a cancer establishes viable micrometastases in distant organs. The discovery and validation of genetic markers determining the efficiency of metastatic progression of colon cancer is therefore an important area of research, with potential value in disease management and basic investigation.
Previous studies have evaluated a variety of genetic changes that appear to influence prognosis, including microsatellite instability, p53 mutation, KRAS mutation, aneuploidy, 17p loss, 18q loss, 8p loss, and, more recently, patterns of global gene expression and sensitivity to chemotherapeutic agents.6–13 Because of the complexity of both tumor biology and clinical management, study of a large number of cases is essential to successful evaluation of even a single prognostic marker. Other important elements include quality control of the genetic analyses, accuracy of clinical staging, quality of surgical treatment, and adequacy of patient follow-up. Furthermore, when evaluating the impact of tumor biology on prognosis, use of disease recurrence or disease-specific survival as a primary endpoint is preferable to use of overall survival. Because of these numerous pitfalls, published studies have presented uncertain, and at times conflicting, messages about the value of genetic markers in determining prognosis in colorectal cancer.14–16 Accordingly, genetic markers have yet to penetrate clinical management of primary colorectal cancer despite widespread acknowledgment of their potential value.17–19
We have examined two prevalent and well-studied genetic markers that are acquired very early in the development of colorectal neoplasia: microsatellite instability (MSI) and KRAS mutation. MSI defines a class of colon cancers with a high rate of mutations in repeat sequences due to a defect in DNA mismatch repair.20,21 The onset of MSI happens very early in colon cancer development; once established, MSI has a dominant effect on cancer phenotype.22–24 Three large studies have demonstrated the favorable prognosis associated with MSI in colorectal cancers.8,9,25 Though there is a five-microsatellite marker assay, initially recommended by the National Cancer Institute in 1997 (NCI assay), data from our and other laboratories have shown that more specific identification of MSI can be achieved with the use of assays focusing on mononucleotide markers.26–29 In this series we used our previously validated three-marker assay.26 This three-marker assay utilizes two mononucleotide markers (BAT25 and BAT26) and one dinucleotide tie-breaker (D2S123).
Mutated KRAS is a powerful transforming oncogene that activates a multitude of specific effector molecules such as Raf, PI3 Kinase, Phospholipase C, and Ral, disrupting many cell functions including cell proliferation, cytoskeletal organization, motility, and apoptosis.30,31 KRAS remains among the most common mutations found in human cancers.32 These mutations have been detected in the earliest neoplastic lesions found in colonic mucosa, and appear to exert a strong influence on the growth of polyps and early cancers.33,34 Furthermore, KRAS mutations have been correlated with methylation phenotype and inversely with MSI, and thus may indirectly identify tumors with distinct forms of genetic instability.35 The importance of mutated KRAS as a prognostic marker is controversial. Several large studies have demonstrated that particular KRAS mutations impact survival, though none have demonstrated prognostic value independent of stage.14,36,37 The association between KRAS mutation and the absence of response to cetuximab is now well documented. However, the patients in this study were accrued prior to the clinical use of cetuximab; thus there will be no interaction between KRAS mutation, cetuximab exposure, and survival.
We reasoned that, if the genetic basis for colon tumor progression is established early and sustained through tumor development, these two markers would likely demonstrate an independent and measurable correlation with the late stages of cancer progression. Therefore, we studied MSI and KRAS status in a large series of colon cancer patients treated in the 1990s at one specialty center where staging, surgical resection, and adjuvant therapy were highly consistent. Our aim was to define the relationship of these genetic markers to metastatic disease progression and survival.
PATIENTS AND METHODS
Patients and Samples
Tumor and normal tissue samples were collected under Institutional Review Board protocol from patients undergoing elective surgery for colorectal cancer at Memorial Sloan–Kettering Cancer Center. The operations were performed between January 1990 and December 1997. Colon cancer had greater representation in this series compared with rectal cancer: 400 and 132, respectively. Rectal cancers that received preoperative radiotherapy were excluded. There were 54 patients with distal rectal cancer (within 6 cm of the anal verge). All patients were staged preoperatively with computed tomography (CT) scans and chest X-rays, and all underwent colon resection as their initial cancer treatment. Final tumor–node–metastasis (TNM) stage was assigned using the American Joint Committee on Cancer staging manual and was based on CT findings, intraoperative findings, and final pathology reports. All patients underwent radical resection of the primary tumor. The stage IV group was a combination of patients undergoing palliative colon resection (73%) or potentially curative metastasis resection (27%). The majority of patients with stage III (77%) and stage IV (88%) cancers received 5-flourouracil (5-FU)-based chemotherapy post-operatively. Chemotherapy was not used for stage I cancer and rarely for stage II (11%).
Colon tumor tissue and normal mucosa were obtained at time of surgical resection from 532 patients and snap-frozen in liquid nitrogen. DNA was first extracted and purified using a proteinase potassium/lithium chloride/ethanol (K/ LiCl/EtOH) protocol, then quantified using OD260/280 with a GeneQuant proTM DNA calculator. Median patient age was 67 years (range 23–93 years). Two hundred seventy-three patients were male and 259 were female. Median follow-up after colon resection was 4.1 years (range 0–13.3 years). Survivors were followed for a median of 5.4 years (range 0–13.3 years).
Microsatellite Instability (MSI)
MSI analysis was performed on matched tumor and normal tissue (100 ng DNA per reaction) using a previously published multiplex protocol.26 Oligonucleotide primers for BAT25, BAT26, and D2S123 were fluorescently labeled and amplified simultaneously using AmpliTaqGold® DNA polymerase (Applied Biosystems, Foster City, CA).38 The polymerase chain reaction (PCR) products were resolved in an ABI PRISMTM 377 DNA Sequencer. MSI was defined as two or three PCR products demonstrating instability consistent with the MSI-H genotype.
Polymerase Chain Reaction/Ligase Detection Reaction (PCR/LDR)
KRAS mutation status in tumors was assessed using a previously published polymerase chain reaction and ligase detection reaction (PCR/LDR) that can detect 1 mutant KRAS allele among 200 wild-type alleles and that, in our hands, is slightly more accurate than DNA sequencing.39 Oligonucleotide primers and Taq DNA polymerase were used to amplify KRAS exon 1. Wild-type primers for KRAS exon 30 and mutation-specific primers for codons 12 and 13 were used in the LDR.39 The LDR products were resolved on 12.5% polyacrylamide gel in an ABI PRISMTM 377 DNA sequencer. Specific mutations in codons 12 and 13 were identified by their corresponding ligation products.
Statistics
Pearson’s chi-squared and Fisher’s exact tests were applied to the results, as appropriate. Analysis of variance (ANOVA) was used to analyze MSI prevalence by stage. Survival curves were generated by the Kaplan–Maier method and subjected to the log-rank test. Multivariable analysis was performed using Cox regression. All reported P values are two-sided, and P values of < 0.05 were considered significant.
RESULTS
Frequency
MSI status was evaluated in 478 cases with matched tumor and normal tissue. The other 54 cases had insufficient normal tissue for MSI analysis. MSI was detected in 58 cases (12%). KRAS mutation status was documented in 531 cases. The KRAS gene failed to PCR amplify in one tumor due to insufficient tumor DNA. One or more KRAS mutations were detected in 190 tumors (36%). Of these, 157 tumors had codon 12 mutations (67 were aspartate-12, 47 were valine-12, 15 were alanine-12, 15 were cysteine-12, 7 were serine-12, 3 were arginine-12), and 33 had codon 13 mutations (all were aspartate-13).
Patient Characteristics
Neither MSI nor KRAS mutations were associated with patient age or gender (data not shown).
Tumor Characteristics
MSI was more common in early-stage cancers (P < 0.0001), whereas prevalence of KRAS mutation did not vary with stage (Fig. 1a). Consistent with prior studies, MSI was strongly associated with tumor location proximal to the splenic flexure (38 of 58 cases, P < 0.0001), poorly differentiated cancers (18 of 58 cases, P < 0.001), and mucinous cancers (38 of 58, P < 0.0001), and was inversely correlated with presence of KRAS mutation (Fig. 1b). Cancers with KRAS mutation had a higher likelihood of mucinous histology (78 of 190, P = 0.003). Neither KRAS mutation nor MSI were associated with location in the rectum (P [ 0.40).
FIG. 1.
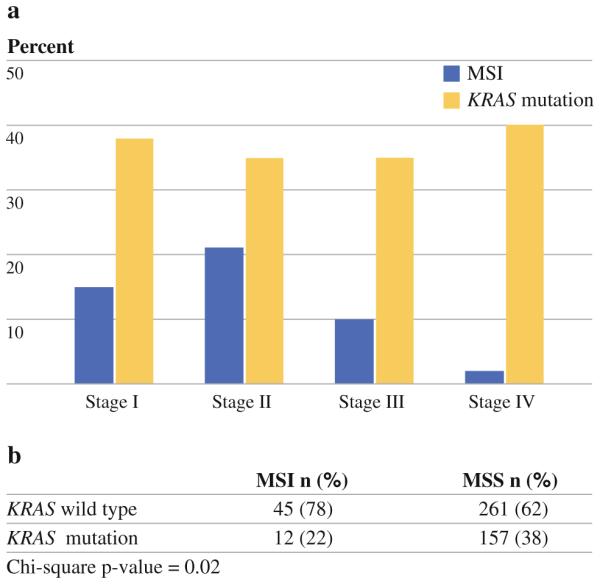
a Stage distribution of MSI (P < 0.0001) and KRAS. b Chisquare table of MSI and KRAS prevalence
Survival
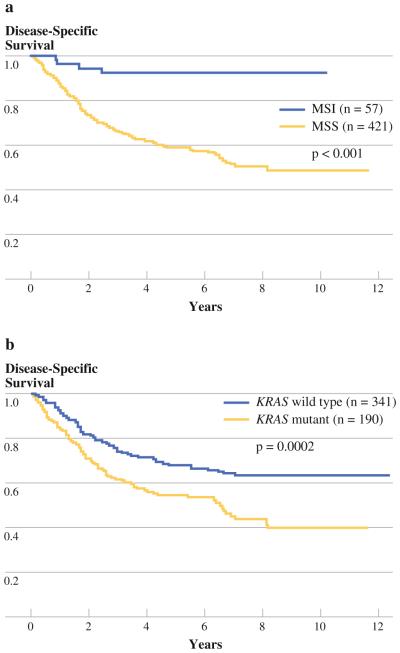
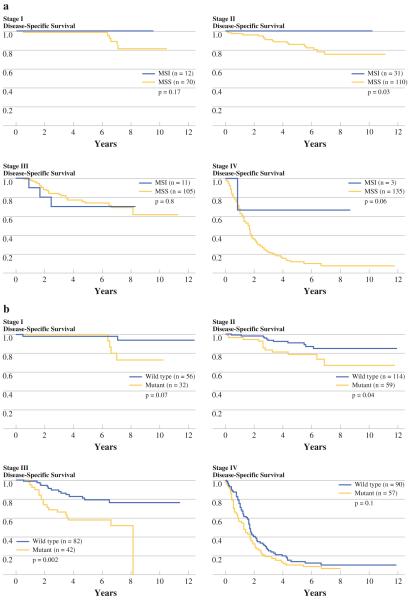
MSI was a favorable marker of survival compared with those tumors that had MSS (5-year survival 92 vs. 59%, P < 0.0001) (Fig. 2a). KRAS was an unfavorable marker of survival (5-year survival 55 vs. 68%, P = 0.0002) (Fig. 2b). Among patients with MSI tumors there was a trend towards better survival in stages I and IV, and a significant survival difference in stage II (Fig. 3a). Among patients with KRAS mutant tumors, there was a strong trend towards worse survival in stages I and IV, and a significant survival difference in stages II and III (Fig. 3b). Survival analyses were performed on the individual codon 12 and 13 mutations. There were no significant survival differences seen between the individual mutations.
FIG. 2.
Disease-specific survival for stage I–IV patients: a MSI versus MSS, b KRAS mutant versus wild type
FIG. 3.
Disease-specific survival by stage: a MSI versus MSS, b KRAS mutant (mut) versus Wild type (wt)
Combining MSI and KRAS
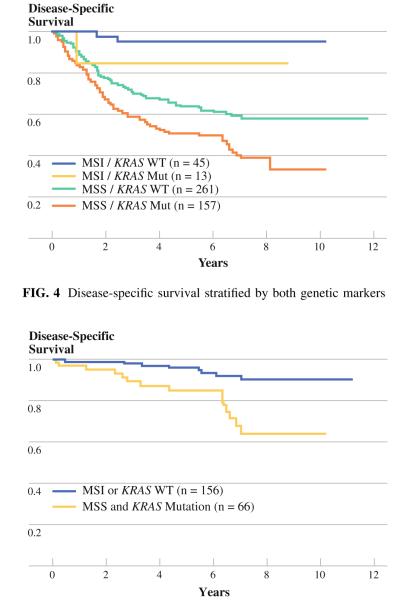
Survival was analyzed for the four groups identified by these two genetic markers (Fig. 4). Each marker was found to exert a consistent impact on prognosis irrespective of the status of the other marker. The group with both markers favorable (MSI and wild-type KRAS, n = 45) had the best survival (95% at 5 years), whereas the group with both markers unfavorable (MSS and mutant KRAS, n = 157) had the worst survival (51% at 5 years). Among the earlystage cancers (stages I and II, n = 222) expected to have an excellent prognosis, those patients with MSS and mutant KRAS identified in their tumors (n = 66) had significantly worse survival compared with all other stage I and II patients (85 vs. 96% at 5 years, 64 vs. 92% at 7 years, P = 0.0005) (Fig. 5).
FIG. 4.
Disease-specific survival stratified by both genetic markers
FIG. 5.
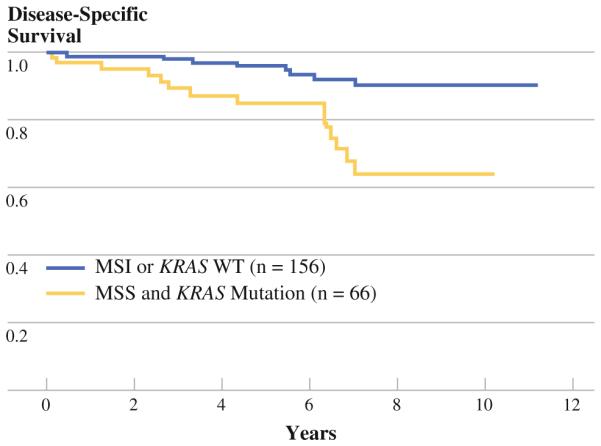
Early-stage cancer stratified by MSS and mutant KRAS
Multivariable Regression Analysis
To determine their prognostic value independent of disease stage, MSI and KRAS mutation were entered into a Cox regression model (Table 1). TNM stage, KRAS mutation, and MSI were found to be independently associated with disease-specific survival. MSI was associated with a fivefold reduction in risk of cancer death, whereas KRAS was associated with a 1.75-fold increase in risk of cancer death.
TABLE 1.
Univariable (log rank) and multivariable (Cox regression) analysis of clinical, pathological, and molecular variables
| Variable | Univariable analysisa |
Multivariable analysisb |
||||
|---|---|---|---|---|---|---|
| n | 7-year DSS (%) | P-Value | Hazard ratio | 95% CI | P-Value | |
| Men | 111 | 54 | 0.07 | |||
| Women | 262 | 61 | ||||
| Age ≤ 67 years | 251 | 55 | 0.17 | |||
| Age ≥ 67 years | 283 | 61 | ||||
| Proximal to splenic flexure | 198 | 60 | 0.87 | |||
| Distal to splenic flexure | 336 | 57 | ||||
| Tumor grade I | 25 | 60 | 0.75 | |||
| Tumor grade II | 432 | 58 | ||||
| Tumor grade III | 77 | 58 | ||||
| Mucinous cell type | 76 | 59 | 0.78 | |||
| Nonmucinous cell type | 458 | 57 | ||||
| PNI present | 41 | 48 | 0.0004 | 1.38 | 0.71–2.70 | 0.34 |
| PNI absent | 349 | 63 | ||||
| LVI present | 109 | 46 | 0.0004 | 1.07 | 0.64–1.77 | 0.80 |
| LVI absent | 406 | 60 | ||||
| Stage IV versus I | 147 | 8 | <0.0001 | 25.6 | 9.1–71.4 | <0.0001 |
| Stage III versus I | 124 | 67 | 9.4 | 5.2–17.2 | ||
| Stage II versus I | 171 | 80 | 6.2 | 3.7–10.5 | ||
| Stage I | 92 | 92 | ||||
| Chemotherapy | 178 | 44 | <0.0001 | 1.04 | 0.55–1.98 | 0.91 |
| No chemotherapy | 212 | 78 | ||||
| Preop CEA > 5.0 ng/ml | 179 | 42 | 0.001 | 1.22 | 0.73–2.04 | 0.46 |
| Preop CEA normal | 252 | 80 | ||||
| MSI | 58 | 92 | <0.0001 | 0.18 | 0.06–0.60 | 0.005 |
| MSS | 420 | 53 | ||||
| KRAS mutant | 190 | 46 | 0.0002 | 1.75 | 1.15–2.67 | 0.009 |
| KRAS wild type | 342 | 64 | ||||
LVI lymphovascular invasion, PNI perineural invasion, CEA carcinoembryonic antigen, Preop preoperative
Kaplan–Meier with P-values calculated by log-rank test
Cox regression (backwards stepwise)
DISCUSSION
Our data demonstrate that genetic events established early in tumor development have a powerful effect on metastatic progression of colorectal cancer. In our large series of surgical patients with primary adenocarcinoma of the colon or rectum, 37% died of cancer. MSI was linked to only 2% of cancer deaths, and multivariable models including stage of disease predicted a fourfold reduction in actuarial risk of cancer mortality. Conversely, KRAS mutation was linked to 47% of all cancer deaths and predicted a nearly twofold increase in cancer mortality risk. In every stage of disease and in nearly every patient stratum, one or both of these markers identified patients with better (MSI) or worse (KRAS mutation) prognosis. These data indicate that MSI and KRAS mutation are biomarkers which distinguish genetic subsets of colorectal cancer that differ in speed and efficiency of metastatic spread. MSI tumors rarely progress to metastasis, whereas MSS tumors with KRAS mutation progress to metastasis in greater than 50% of cases.
Evidence from other investigators supports the idea that MSI and KRAS mutation are genetic markers that are established early and remain biologically relevant throughout all stages of tumor development. Both MSI and KRAS mutation have been found in aberrant crypt foci, the earliest neoplastic lesions that can be identified in the colon.40,41 Topographic sampling of colon cancers arising within colon adenomas has shown that, when present in an adenoma, both MSI and KRAS mutation are stable and clonally expanded within the cancer.34,42 In addition, KRAS mutations found in primary colon cancers are preserved in recurrences and metastases.43
Our data convincingly show that MSI and KRAS status each provide unique and complementary information about prognosis (Fig. 4). We believe that, when used in combination, these markers constitute a starting point for developing a molecular prognostic scoring system for early-stage colorectal cancer. The multivariable model demonstrates that both markers are predictors of outcome independent of stage. However, when stratifying by individual stage, some statistical power is lost due to smaller sample size (Figs. 3, 4). Nevertheless, in this series, stage I and II patients (n = 222) had an overall 7-year disease-specific survival of 84%. Because of this overall good prognosis, high-risk patients were hard to identify on clinical grounds and adjuvant chemotherapy was rarely used. In this population, MSI and KRAS mutation are common (stage I: 15 and 36%, respectively; stage II: 22 and 34%, respectively). In combination, the markers were capable of identifying a high-risk subset within the stage I and II cohort (MSS/KRAS mutation, n = 66) representing only 30% of the early-stage patients but 14 of 25 (56%) of the cancer deaths. These two markers provided excellent stratification of prognosis in stage I and II patients: 64% survival at 7 years for the high-risk group versus 92% for the low-risk group. Useful prognostic information about both groups can be provided by these markers (Fig. 5). The clinical utility of these markers was further supported by multivariate analysis of the entire cohort showing that MSI and KRAS have prognostic power similar to that of lymph node status, which is currently the standard used to select patients for adjuvant chemotherapy (Table 1).
The link between favorable prognosis and MSI status is well supported in the literature.7,8,25 However, previously published data on KRAS mutation is inadequate for drawing conclusions about prognosis. Most studies evaluating KRAS mutation as a prognostic marker have been severely underpowered.44,45 A meta-analysis of data from 22 centers published in 1998 and a follow-up meta-analysis of data from 35 centers published in 2001 concluded that KRAS mutation predicts poor survival in colorectal cancer patients, although the most recent study limited this conclusion to the valine-12 mutation.14,15 Unfortunately, both meta-analyses were limited by data that was heterogenous with regard to patient accrual, experimental method, marker prevalence, and clinical follow-up.
Only three groups have previously reported data on the prognostic implications of both markers simultaneously in colorectal cancer.46–48 None demonstrated meaningful relationships between the markers and survival. Our series is the largest study of MSI and KRAS mutation in the same cohort, and we were able to perform an adequately powered analysis. Additionally we used well-validated assays for MSI and KRAS mutation, and marker prevalence was highly consistent with that demonstrated by other well-controlled studies.26,39
There are data that show MSI or KRAS mutation is associated with in vitro and in vivo variation in response to chemotherapy.49–53 However, this retrospective study is not designed to address the interaction between these molecular changes, chemotherapy, and outcome. The finding by univariable analysis that patients who were exposed to chemotherapy had significantly worse survival should be considered in conjunction with the fact that patients with stage III and IV colorectal cancer (CRC) were routinely treated with postoperative chemotherapy and those with stage I and II CRC were routine treated with surgery alone. This difference disappeared in the multivariable model. However, confounding by indication would obviously occur and therefore we cannot comment on possible effect modification, the greater or lesser impact of KRAS mutation or MSI in the chemotherapy and non-chemotherapy groups. Nevertheless, given that none of these patients received cetuximab during the study period one may exclude the possibility that the differences in survival are due to KRAS-mutant tumor resistance to cetuximab.
Our study provides strong evidence that likelihood of metastatic progression in colorectal cancer can be estimated based on biomarkers present in the primary tumor. Knowledge of MSI and KRAS status may enhance clinicopathologic staging in colorectal cancer patients who are staged and treated in a consistent manner. Validation of additional prognostic markers promises to provide a panel of genetic markers that will help refine management decisions for individual patients based on tumor biology.
ACKNOWLEDGMENT
This study was supported by a grant from the National Cancer Institute (2 P01 CA65930-05A2) awarded to P.B.P., and by the philanthropy of Marie and William Bianco honoring Cathy Kiley.
REFERENCES
- 1.Skibber J, Minsky B, Hoff P. Cancer of the colon. In: DeVita VT, Rosenberg SA, editors. Principles and practice of oncology. Lippincott Williams & Wilkins; Philadelphia: 2001. [Google Scholar]
- 2.Chapuis PH, Fisher R, Dent OF, Newland RC, Pheils MT. The relationship between different staging methods and survival in colorectal carcinoma. Dis Colon Rectum. 1985;28:158–61. doi: 10.1007/BF02554232. [DOI] [PubMed] [Google Scholar]
- 3.Barillari P, de Angelis R, Valabrega S, Indinnimeo M, Gozzo P, Ramacciato G, et al. Relationship of symptom duration and survival in patients with colorectal carcinoma. Eur J Surg Oncol. 1989;15:441–5. [PubMed] [Google Scholar]
- 4.Robinson M, Hardcastle J. Should we be screening for colorectal cancer? Br Med Bull. 1998;54:807–821. doi: 10.1093/oxfordjournals.bmb.a011731. [DOI] [PubMed] [Google Scholar]
- 5.Culliford A, Paty P. Surgery of colon cancer. In: Saltz L, editor. Colorectal cancer: multimodality management. Blackwell; Oxford, UK: 2002. [Google Scholar]
- 6.Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA. Genetic tumor markers with prognostic impact in Dukes’ stages B and C colorectal cancer patients. J Clin Oncol. 2003;21:820–9. doi: 10.1200/JCO.2003.05.190. [DOI] [PubMed] [Google Scholar]
- 7.Elsaleh H, Powell B, McCaul K, Grieu F, Grant R, Joseph D, et al. P53 alteration and microsatellite instability have predictive value for survival benefit from chemotherapy in stage III colorectal carcinoma. Clin Cancer Res. 2001;7:1343–9. [PubMed] [Google Scholar]
- 8.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 9.Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 10.Peltomäki P, Lothe RA, Aaltonen LA, Pylkkänen L, Nyström-Lahti M, Seruca R, et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993;53:5853–5. [PubMed] [Google Scholar]
- 11.Slattery ML, Curtin K, Schaffer D, Anderson K, Samowitz W. Associations between family history of colorectal cancer and genetic alterations in tumors. Int J Cancer. 2002;97:823–7. doi: 10.1002/ijc.10148. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liévre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 14.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer. 2001;85:692–6. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter ‘‘RASCAL’’ study. J Natl Cancer Inst. 1998;90:675–84. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 16.Klump B, Hehls O, Okech T, Hsieh CJ, Gaco V, Gittinger FS, et al. Molecular lesions in colorectal cancer: impact on prognosis? Original data and review of the literature. Int J Colorectal Dis. 2004;19:23–42. doi: 10.1007/s00384-003-0499-7. [DOI] [PubMed] [Google Scholar]
- 17.Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348–60. doi: 10.1016/j.ejca.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003;39:718–27. doi: 10.1016/s0959-8049(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 19.Graziano F, Cascinu S. Prognostic molecular markers for planning adjuvant chemotherapy trials in Dukes’ B colorectal cancer patients: how much evidence is enough? Ann Oncol. 2003;14:1026–38. doi: 10.1093/annonc/mdg284. [DOI] [PubMed] [Google Scholar]
- 20.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–61. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 21.Kolodner RD, Marsischky GT. Eukaryotic DNA mismatch repair. Curr Opin Genet Dev. 1999;9:89–96. doi: 10.1016/s0959-437x(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 22.Pedroni M, Sala E, Scarselli A, Borghi F, Menigatti M, Benatti P, et al. Microsatellite instability and mismatch-repair protein expression in hereditary and sporadic colorectal carcinogenesis. Cancer Res. 2001;61:896–9. [PubMed] [Google Scholar]
- 23.Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C. The significance of unstable chromosomes in colorectal cancer. Nat Rev Cancer. 2003;3:695–701. doi: 10.1038/nrc1165. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita K, Dai T, Dai Y, Yamamoto F, Perucho M. Genetics supersedes epigenetics in colon cancer phenotype. Cancer Cell. 2003;4:121–31. doi: 10.1016/s1535-6108(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 25.Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, Leppert M, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–23. [PubMed] [Google Scholar]
- 26.Nash GM, Gimbel M, Shia J, Culliford AT, Nathanson DR, Ndubuisi M, et al. Automated, multiplex assay for high-frequency microsatellite instability in colorectal cancer. J Clin Oncol. 2003;21:3105–12. doi: 10.1200/JCO.2003.11.133. [DOI] [PubMed] [Google Scholar]
- 27.Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K, et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123:1804–11. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- 28.Xicola RM, Llor X, Pons E, Castells A, Alenda C, Piñol V, et al. Performance of different microsatellite marker panels for detection of mismatch repair-deficient colorectal tumors. J Natl Cancer Inst. 2007;99:244–52. doi: 10.1093/jnci/djk033. [DOI] [PubMed] [Google Scholar]
- 29.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox AD, Der CJ. Ras family signaling: therapeutic targeting. Cancer Biol Ther. 2002;1:599–606. doi: 10.4161/cbt.306. [DOI] [PubMed] [Google Scholar]
- 31.Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–66. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porta M, Ayude D, Alguacil J, Jariod M. Exploring environmental causes of altered ras effects: fragmentation plus integration? Mol Carcinog. 2003;36:45–52. doi: 10.1002/mc.10093. [DOI] [PubMed] [Google Scholar]
- 33.Cheng L, Lai MD. Aberrant crypt foci as microscopic precursors of colorectal cancer. World J Gastroenterol. 2003;9:2642–9. doi: 10.3748/wjg.v9.i12.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata D, Schaeffer J, Li ZH, Capella G, Perucho M. Genetic heterogeneity of the c-K-ras locus in colorectal adenomas but not in adenocarcinomas. J Natl Cancer Inst. 1993;85:1058–63. doi: 10.1093/jnci/85.13.1058. [DOI] [PubMed] [Google Scholar]
- 35.Kondo Y, Issa JP. Epigenetic changes in colorectal cancer. Cancer Metastasis Rev. 2004;23:29–39. doi: 10.1023/a:1025806911782. [DOI] [PubMed] [Google Scholar]
- 36.Ahnen DJ, Feigl P, Quan G, Fenoglio-Preiser C, Lovato LC, Bunn PA, Jr., et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res. 1998;58:1149–58. [PubMed] [Google Scholar]
- 37.Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193–7. [PubMed] [Google Scholar]
- 38.Umetani N, Sasaki S, Watanabe T, Ishigami H, Ueda E, Nagawa H. Diagnostic primer sets for microsatellite instability optimized for a minimal amount of damaged DNA from colorectal tissue samples. Ann Surg Oncol. 2000;7:276–80. doi: 10.1007/s10434-000-0276-6. [DOI] [PubMed] [Google Scholar]
- 39.Khanna M, Park P, Zirvi M, Cao W, Picon A, Day J, et al. Multiplex PCR/LDR for detection of K-ras mutations in primary colon tumors. Oncogene. 1999;18:27–38. doi: 10.1038/sj.onc.1202291. [DOI] [PubMed] [Google Scholar]
- 40.Alrawi SJ, Schiff M, Carroll RE, Dayton M, Gibbs JF, Kulavlat M, et al. Aberrant crypt foci. Anticancer Res. 2006;26:107–19. [PubMed] [Google Scholar]
- 41.Pretlow TP, Brasitus TA, Fulton NC, Cheyer C, Kaplan EL. K-ras mutations in putative preneoplastic lesions in human colon. J Natl Cancer Inst. 1993;85:2004–7. doi: 10.1093/jnci/85.24.2004. [DOI] [PubMed] [Google Scholar]
- 42.Shibata D, Peinado MA, Ionov Y, Malkhosyan S, Perucho M. Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nat Genet. 1994;6:273–81. doi: 10.1038/ng0394-273. [DOI] [PubMed] [Google Scholar]
- 43.Losi L, Benhattar J, Costa J. Stability of K-ras mutations throughout the natural history of human colorectal cancer. Eur J Cancer. 1992;28A:1115–20. doi: 10.1016/0959-8049(92)90468-h. [DOI] [PubMed] [Google Scholar]
- 44.Finkelstein SD, Sayegh R, Christensen S, Swalsky PA. Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation type. Cancer. 1993;71:3827–38. doi: 10.1002/1097-0142(19930615)71:12<3827::aid-cncr2820711207>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 45.Vogelstein B, Fearon ER, Kern SE, Hamilton SR, Preisinger AC, Nakamura Y, et al. Allelotype of colorectal carcinomas. Science. 1989;244:207–11. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- 46.Curran B, Lenehan K, Mulcahy H, Tighe O, Bennett MA, Kay EW, et al. Replication error phenotype, clinicopathological variables, and patient outcome in Dukes’ B stage II (T3,N0,M0) colorectal cancer. Gut. 2000;46:200–4. doi: 10.1136/gut.46.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gnanasampanthan G, Elsaleh H, McCaul K, Iacopetta B. Ki-ras mutation type and the survival benefit from adjuvant chemotherapy in Dukes’ C colorectal cancer. J Pathol. 2001;195:543–8. doi: 10.1002/path.990. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, van Rijnsoever M, Grieu F, Bydder S, Elsaleh H, Joseph D, et al. Prognostic significance of microsatellite instability and Ki-ras mutation type in stage II colorectal cancer. Oncology. 2003;64:259–65. doi: 10.1159/000069311. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez R, Hansen LT, Phear G, Scorah J, Spang-Thomsen M, Cox A, et al. Thymidine selectively enhances growth suppressive effects of camptothecin/irinotecan in MSI? cells and tumors containing a mutation of MRE11. Clin Cancer Res. 2008;14:5476–83. doi: 10.1158/1078-0432.CCR-08-0274. [DOI] [PubMed] [Google Scholar]
- 50.Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- 51.Liévre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 52.Fallik D, Borrini F, Boige V, Viguier J, Jacob S, Miquel C, et al. Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res. 2003;63:5738–44. [PubMed] [Google Scholar]
- 53.Zhu CQ, da Cunha Santos G, Ding K, Sakurada A, Cutz JC, Liu N, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR 21. J Clin Oncol. 2008;26:4268–75. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]