Abstract
The present study investigated the ability of carboplatin and paclitaxel to sensitize human non-small-cell lung cancer (NSCLC) cells to carbon-ion beam irradiation. NSCLC H460 cells treated with carboplatin or paclitaxel were irradiated with X-rays or carbon-ion beams, and radiosensitivity was evaluated by clonogenic survival assay. Cell proliferation was determined by counting the number of viable cells using Trypan blue. Apoptosis and senescence were evaluated by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining and senescence-associated β-galactosidase (SA-β-gal) staining, respectively. The expression of cleaved caspase-3, Bax, p53 and p21 was analyzed by western blotting. Clonogenic survival assays demonstrated a synergistic radiosensitizing effect of carboplatin and paclitaxel with carbon-ion beams; the sensitizer enhancement ratios (SERs) at the dose giving a 10% survival fraction (D10) were 1.21 and 1.22, respectively. Similarly, carboplatin and paclitaxel showed a radiosensitizing effect with X-rays; the SERs were 1.41 and 1.29, respectively. Cell proliferation assays validated the radiosensitizing effect of carboplatin and paclitaxel with both carbon-ion beam and X-ray irradiation. Carboplatin and paclitaxel treatment combined with carbon-ion beams increased TUNEL-positive cells and the expression of cleaved caspase-3 and Bax, indicating the enhancement of apoptosis. The combined treatment also increased SA-β-gal-positive cells and the expression of p53 and p21, indicating the enhancement of senescence. In summary, carboplatin and paclitaxel radiosensitized H460 cells to carbon-ion beam irradiation by enhancing irradiation-induced apoptosis and senescence.
Keywords: carbon-ion beams, lung cancer, radiosensitization, paclitaxel, carboplatin
INTRODUCTION
The standard treatment for patients with unresectable locally advanced non-small-cell lung cancer (NSCLC) is combined treatment with radiotherapy and chemotherapy. In the combined treatment, radiotherapy plays a local role by targeting the primary disease site. Meanwhile, chemotherapy serves as a radiosensitizer to increase the intensity of local therapy, and also functions as a systemic therapy targeting micrometastasis throughout the body. To date, the clinical outcome from chemoradiotherapy for locally advanced NSCLC is far from satisfactory because the 5-year overall survival rate is 15–20%. Importantly, in this population, the local recurrence rate accounts for ∼30% of patients [–], highlighting the necessity of increasing the efficacy of local therapy. To this end, X-ray dose-escalation studies have been conducted for locally advanced NSCLC; however, they have not resulted in significant clinical benefit [, 5]. The limitation of the X-ray dose-escalation strategy in locally advanced NSCLC is attributed to the characteristics of the dose distribution achieved by X-rays; i.e. the curative dose cannot be delivered to tumors without exceeding the tolerance of the surrounding organs. Therefore, an alternative method of improving the efficacy of local therapy for locally advanced NSCLC is required.
Recently, carbon-ion beam radiotherapy has generated a great deal of interest as a highly intensive local therapy. Carbon-ion beams have a Bragg peak that results in distal tail-off and a sharp penumbra [6]. This property of carbon-ion beams allows for a highly conformal dose distribution, such that a high dose can be delivered to tumors while maintaining a tolerable dose in the surrounding normal organs, resulting in highly intensive radiotherapy. In early NSCLC, carbon-ion beam radiotherapy has shown a 5-year local control rate of 90–95%, indicating that it can be applied to locally advanced NSCLC. However, of note, subgroup analysis has also shown that the local recurrence rate is negatively correlated with the diameter of the tumors (i.e. T2 tumors versus T1 tumors) [7, 8], suggesting that further enhancement of carbon-ion beam radiotherapy is required to control the primary disease site in locally advanced NSCLC cases.
The addition of chemotherapeutic drugs to carbon-ion radiotherapy could be a possible way to enhance its efficacy. A radiosensitizing effect of various chemotherapeutic drugs to carbon-ion beam irradiation has been shown in various human cancer types in vitro, e.g. docetaxel in esophageal cancer cells [9], temozolomide in glioblastoma cells [10], and camptothecin, cisplatin, gemcitabine and paclitaxel in colon cancer cells [11]. On the other hand, in X-ray chemoradiotherapy for locally advanced NSCLC, the standard chemotherapeutic regimen is the ‘platinum-doublet’, the combination of a platinum drug with a drug of a different class. One of the most common combinations of drugs in platinum-doublet chemotherapy is carboplatin and paclitaxel. Carboplatin is a platinum drug that generates intra- and inter-strand crosslinks in DNA [12], while paclitaxel is a microtubule-stabilizing agent that disturbs mitosis by suppressing spindle microtubule dynamics at metaphase [13]. In the clinical setting of X-ray radiotherapy, concomitant chemoradiotherapy using carboplatin plus paclitaxel has shown favorable outcomes [14, 15] compared with radiotherapy alone [, 16]. In fact, there have been no clinical studies directly comparing chemoradiotherapy using carboplatin plus paclitaxel with radiotherapy alone. Nevertheless, comparison of the outcomes of Phase II and III clinical trials demonstrates that the median survival of chemoradiotherapy using carboplatin plus paclitaxel (22.0–26.9 months) is superior to that of radiotherapy alone (9.7–11.4 months). These data suggest that carboplatin and paclitaxel could be applied to combination use with carbon-ion beam radiotherapy to improve the efficacy of carbon-ion beam radiotherapy in locally advanced NSCLC; however, the combined effect of carboplatin or paclitaxel with carbon-ion beam irradiation in NSCLC cells has not been investigated. In the present study, to provide a biological basis for the combined use of carboplatin and paclitaxel with carbon-ion beam radiotherapy, we investigated the ability of carboplatin and paclitaxel to sensitize a human NSCLC cell line to carbon-ion beam irradiation in vitro.
MATERIALS AND METHODS
Cell line
The human NSCLC cell line H460 was obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured at 37°C in a humidified 5% CO2 atmosphere in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Carlsbad, CA).
Irradiation
Carbon-ion beam irradiation (290 MeV/nucleon) was performed at Gunma University Heavy Ion Medical Center (Gunma, Japan) [17]. Cells cultured on 60-mm dishes were irradiated with carbon-ion beams at the center of a 6-cm spread-out Bragg peak (SOBP) in the vertical direction. The linear energy transfer (LET) at the center of the SOBP was ∼50 keV/μm.
X-ray irradiation was performed using a Faxitron RX-650 (Faxitron Bioptics, LCC, Tucson, AZ) operated at 100 kVp with a dose rate of 1.14 Gy/min.
Chemotherapeutic drugs
Carboplatin and paclitaxel (Wako Pure Chemical Industries, Tokyo, Japan) were solubilized in dimethyl sulfoxide (DMSO) and prepared as stock solutions at 100 mM and 1 mM, respectively. The drugs were diluted to their final concentrations with culture medium, resulting in a DMSO concentration of less than 0.01% by weight/volume. Therefore, DMSO was not added to the control samples.
Treatments
Cells were plated on culture dishes in media containing carboplatin or paclitaxel for 24 h. The drug-containing media was replaced with drug-free media immediately prior to irradiation with X-rays or carbon-ion beams.
Clonogenic survival assay
Nine days after irradiation, cells were fixed with 100% ethanol and stained with 2% crystal violet. Colonies consisting of >50 cells were counted. The plating efficiency of untreated cells was 83.3 ± 7.7%. The surviving fractions of cells treated with irradiation plus a single drug were normalized to the surviving fractions of cells treated with that drug alone. Survival curves were obtained by fitting the surviving fractions to a linear–quadratic model expressed by the following formula: SF = exp − (αD + βD2), where SF is the surviving fraction and D is the dose. The dose resulting in a surviving fraction of 10% (D10) was calculated from the survival curves. The relative biological effectiveness (RBE) of carbon-ion beams compared with X-rays was calculated at the D10. The sensitizer enhancement ratio (SER), an indicator of the radiosensitizing effect of a drug of interest, was calculated as the ratio of the D10 of cells treated with irradiation alone to the D10 of cells treated with irradiation plus the drug.
Cell proliferation assay
Three days after irradiation, the cells were harvested and centrifuged. The pellets were resuspended in PBS containing Trypan blue (Bio-Rad Laboratories, Tokyo, Japan). Viable cells negative for Trypan blue staining [18] were counted using a TC20 Automated Cell Counter (Bio-Rad Laboratories). Cell proliferation was calculated as the ratio of the number of viable cells in the treated group to that in the untreated control group.
TUNEL staining
Apoptosis was evaluated by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining using the ApopTag Fluorescein In Situ Apoptosis Detection Kit S7100 (Millipore, Billerica, MA). Three days after irradiation, cells were subjected to dual staining with TUNEL and 4,6-diamino-2-phenylindole (DAPI) (Life Technologies) according to the manufacturer's protocol. TUNEL staining positivity was calculated as the ratio of the number of cells positive for TUNEL staining to the number of cells positive for DAPI staining. For each experimental condition, at least 300 cells were scored.
Senescence-associated β-galactosidase staining
Senescence was evaluated by senescence-associated β-galactosidase (SA-β-gal) staining using the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology, Tokyo, Japan) according to the manufacturer's instructions. Three days after irradiation, cells were subjected to SA-β-gal staining. Senescent cells were identified (by blue staining under light microscopy) and counted. For each experimental condition, at least 300 cells were scored.
Western blotting
Three days after irradiation, cells were harvested, lysed with Cell Lysis Buffer (Millipore) containing phosphatase inhibitor cocktails 1 and 2 (Sigma–Aldrich, St Louis, MO) and protease inhibitor cocktail 3 (Calbiochem, San Diego, CA), and centrifuged at 15 000 g. Protein concentrations of the lysates were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL) and the samples were subjected to western blot analysis. The samples were resolved by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. Bax, p53, p21 and cleaved caspase-3 (Asp175) were evaluated using the corresponding primary antibodies (Cell Signaling Technology, Danvers, MA). Actin (Sigma–Aldrich) was used as a loading control. The primary antibodies were labeled with a horseradish peroxidase-conjugated secondary antibody, and the proteins were visualized using the electrochemiluminescent detection system (GE Healthcare, Tokyo, Japan).
Statistics
The data from three independent experiments were expressed as the mean values with standard deviations (SDs). Statistical significance was determined by Student's t-test using IBM SPSS Statistics 21.0 software (IBM, Armonk, NY). A P-value < 0.05 was considered to be statistically significant.
RESULTS
Radiosensitizing effect of carboplatin and paclitaxel to carbon-ion beams
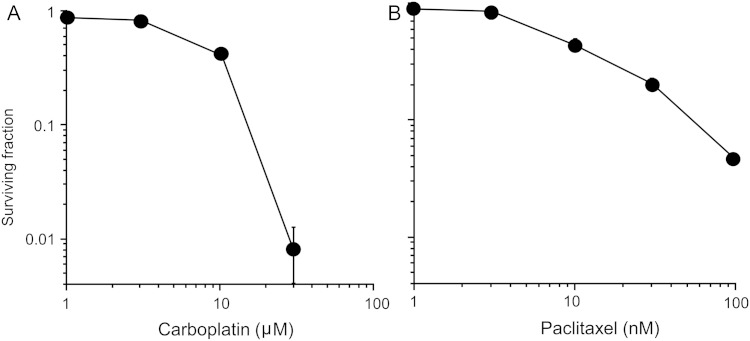
The cytotoxicity of carboplatin and paclitaxel in H460 cells was assessed by clonogenic survival assay (Fig. 1), and both drugs showed a dose-responsive relationship to survival. The median effective doses (IC50) of carboplatin and paclitaxel were 7.9 μM and 8.3 nM, respectively; these IC50 values were employed in subsequent experiments, based on previous studies investigating the radiosensitizing effects of chemotherapeutic drugs [9, 11].
Fig. 1.
Cytotoxicity of carboplatin and paclitaxel in H460 cells assessed by clonogenic survival assay. (A) Carboplatin. (B) Paclitaxel. The mean ± SD is shown.
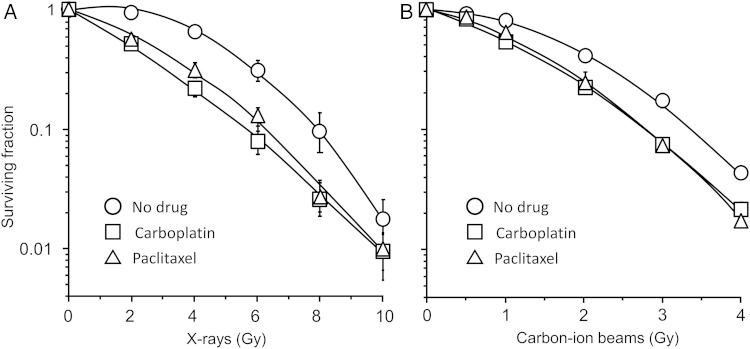
The survival of H460 cells irradiated with X-rays or carbon-ion beams was assessed by clonogenic survival assay (Fig. 2). The RBE of carbon-ion beams to X-rays was calculated as 2.3. Both carboplatin and paclitaxel showed a radiosensitizing effect to X-rays (Fig. 2A) and carbon-ion beam irradiation (Fig. 2B). The SER of carboplatin was 1.41 for X-ray irradiation and 1.21 for carbon-ion beam irradiation, which was significantly higher for X-rays than for carbon-ion beams (P = 0.03) (Table 1). The SER of paclitaxel was 1.29 for X-ray and 1.22 for carbon-ion beam irradiation, which was not a significant difference (P = 0.09) (Table 1).
Fig. 2.
Effects of carboplatin and paclitaxel on the survival of H460 cells irradiated with X-rays or carbon-ion beams. Survival curves of cells receiving X-ray (A) and carbon-ion beam (B) irradiation. Carboplatin and paclitaxel were used at each respective IC50 (7.9 μM and 8.3 nM). Datapoints were fitted to the linear–quadratic model. The mean ± SD is shown.
Table 1.
D10 values from clonogenic assays and calculated sensitizer enhancement ratios
| X-rays |
Carbon-ion beams |
|||||
|---|---|---|---|---|---|---|
| No drug | CBDCA | PTX | No drug | CBDCA | PTX | |
| D10 (Gy) | 7.99 ± 0.40 | 5.68 ± 0.40 | 6.20 ± 0.21 | 3.42 ± 0.07 | 2.82 ± 0.05 | 2.82 ± 0.08 |
| SER | 1.41 ± 0.10 (P = 0.003) |
1.29 ± 0.04 (P = 0.002) |
1.21 ± 0.02 (P < 0.001) |
1.22 ± 0.04 (P < 0.001) |
||
D10 = the dose leading to a survival rate of 10%, CBDCA = carboplatin, PTX = paclitaxel, SER = sensitizer enhancement ratio.
Effects of carboplatin and paclitaxel on proliferation of cells irradiated by carbon-ion beams
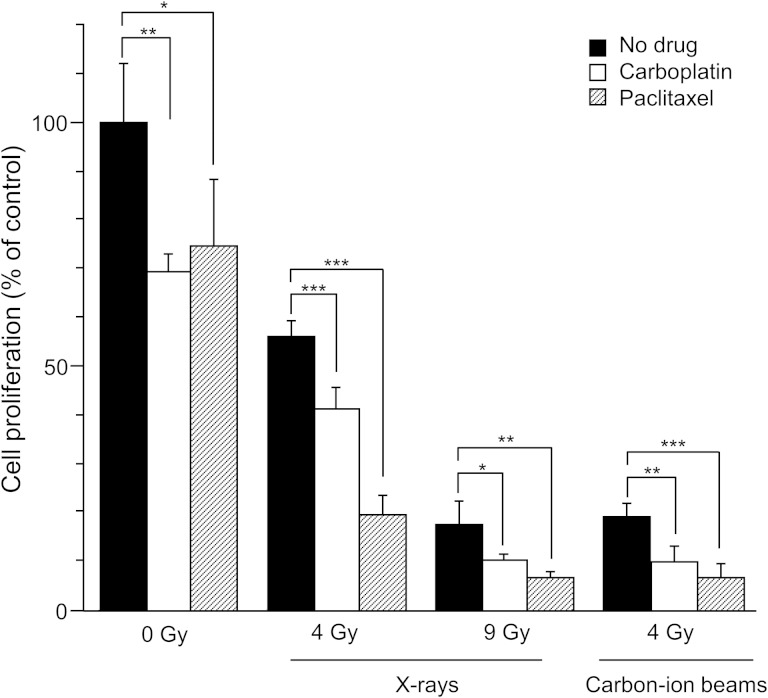
Next, a cell proliferation assay was performed to validate the radiosensitizing effect that was observed in the clonogenic survival assay (Fig. 3). As expected, at a dose of 4 Gy, carbon-ion beam irradiation suppressed cell proliferation more effectively than X-ray irradiation (18.9% vs 56.1%, P < 0.001). The experiment was next repeated using the iso-survival doses obtained from the clonogenic survival assay (i.e. 9 Gy with X-ray and 4 Gy with carbon-ion beams, which resulted in survival fractions of 4.6% and 4.5%, respectively) (Fig. 1). Irradiation with X-rays or carbon-ion beams at these doses suppressed cell proliferation to a similar extent (17.3% with X-ray and 18.9% with carbon-ion beams; P = 0.57), which was consistent with the results of the clonogenic survival assay. At the iso-survival doses, the addition of carboplatin significantly reduced the proliferation of cells irradiated with either X-ray or carbon-ion beams (10.1% and 9.8%, respectively) compared with cells treated with irradiation alone. The radiosensitizing effect of carboplatin was comparable between X-rays and carbon-ion beams (P = 0.87). Similarly, at the iso-survival doses, the addition of paclitaxel significantly reduced the proliferation of cells irradiated with either X-ray or carbon-ion beams (6.3% and 6.4%, respectively) compared with cells treated with irradiation alone. The radiosensitizing effect of paclitaxel was also comparable between X-ray and carbon-ion beams (P = 0.95). These data validated the radiosensitizing effect of carboplatin and paclitaxel to both X-ray and carbon-ion beam irradiation.
Fig. 3.
Effects of carboplatin and paclitaxel on the inhibition of H460 cell proliferation by X-ray or carbon-ion beam irradiation. Cell proliferation was calculated as the ratio of the number of viable cells in a treated group to that in the untreated control group 3 days after irradiation. Viable cells were determined by negativity for Trypan blue. Carboplatin and paclitaxel were used at each respective IC50 (7.9 μM and 8.3 nM). The mean ± SD is shown. A single asterisk indicates P < 0.05; two asterisks indicate P < 0.01; three asterisks indicate P < 0.001.
Carboplatin and paclitaxel enhanced apoptosis induced by carbon-ion beam irradiation
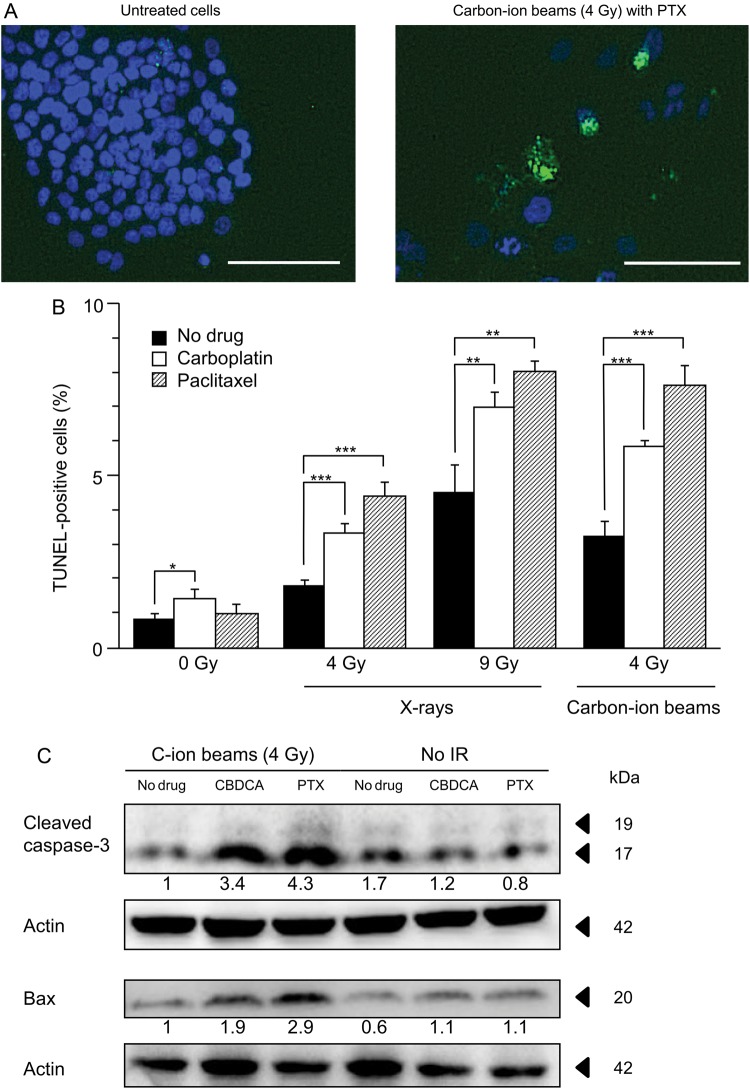
Next, TUNEL staining was used to determine whether the induction of apoptosis was enhanced by carboplatin or paclitaxel in cells irradiated with X-rays or carbon-ion beams (Fig. 4). A representative micrograph of apoptotic cells is shown in Fig. 4A. X-ray irradiation induced apoptosis in a dose-dependent manner (0, 4 and 9 Gy) (Fig. 4B). At both 4 Gy and 9 Gy, the addition of carboplatin or paclitaxel significantly enhanced apoptosis in cells irradiated by X-rays. Carbon-ion beam irradiation at 4 Gy also induced apoptosis. At 4 Gy, the addition of carboplatin or paclitaxel significantly enhanced apoptosis in cells treated with carbon-ion beam irradiation. Irradiation by X-ray or carbon-ion beam at the iso-survival doses obtained from the clonogenic survival assay (Fig. 1) resulted in a comparable induction of apoptosis (4.5% with X-rays vs 3.2% with carbon-ion beams, P = 0.07). At the iso-survival doses, the additional effect of carboplatin or paclitaxel on the induction of apoptosis was comparable between X-rays and carbon-ion beams (2.5% for X-rays and carboplatin vs 2.6% for carbon-ion beams and carboplatin, P = 0.82; 3.5% for X-rays and paclitaxel vs 4.4% for carbon-ion beams and paclitaxel, P = 0.30).
Fig. 4.
Effects of carboplatin and paclitaxel on apoptosis induction in H460 cells irradiated with X-rays or carbon-ion beams. (A) Representative micrograph of cells negative (left panel) and positive (right panel) for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining. TUNEL and 4,6-diamino-2-phenylindole (DAPI) staining are shown by green and blue fluorescence, respectively. Bars, 100 μm. (B) Percentages of TUNEL-positive cells. The mean ± SD is shown. A single asterisk indicates P < 0.05; two asterisks indicate P < 0.01; three asterisks indicate P < 0.001. (C) Western blotting for cleaved caspase-3 and Bax. Actin was used as a loading control. Levels of cleaved caspase-3 and Bax are shown as ratios to their levels in cells treated with carbon-ion beam irradiation alone, after normalization to loading controls. TUNEL staining and Western blotting were performed 3 days after irradiation. Carboplatin and paclitaxel were used at each respective IC50 (7.9 μM and 8.3 nM). C-ion = carbon-ion, CBDCA = carboplatin, PTX = paclitaxel, IR = irradiation.
The enhancement of apoptosis by carboplatin or paclitaxel in carbon-ion beam-irradiated cells was further investigated by western blotting. The addition of carboplatin or paclitaxel to cells irradiated with carbon-ion beams resulted in an increase in the expression of cleaved caspase-3 and Bax, which are involved in the activation of the apoptosis pathway [19, 20] (Fig. 4C). Together, these results indicate that carboplatin and paclitaxel enhanced apoptosis induced by carbon-ion beam irradiation.
Carboplatin and paclitaxel enhanced senescence induced by carbon-ion beam irradiation
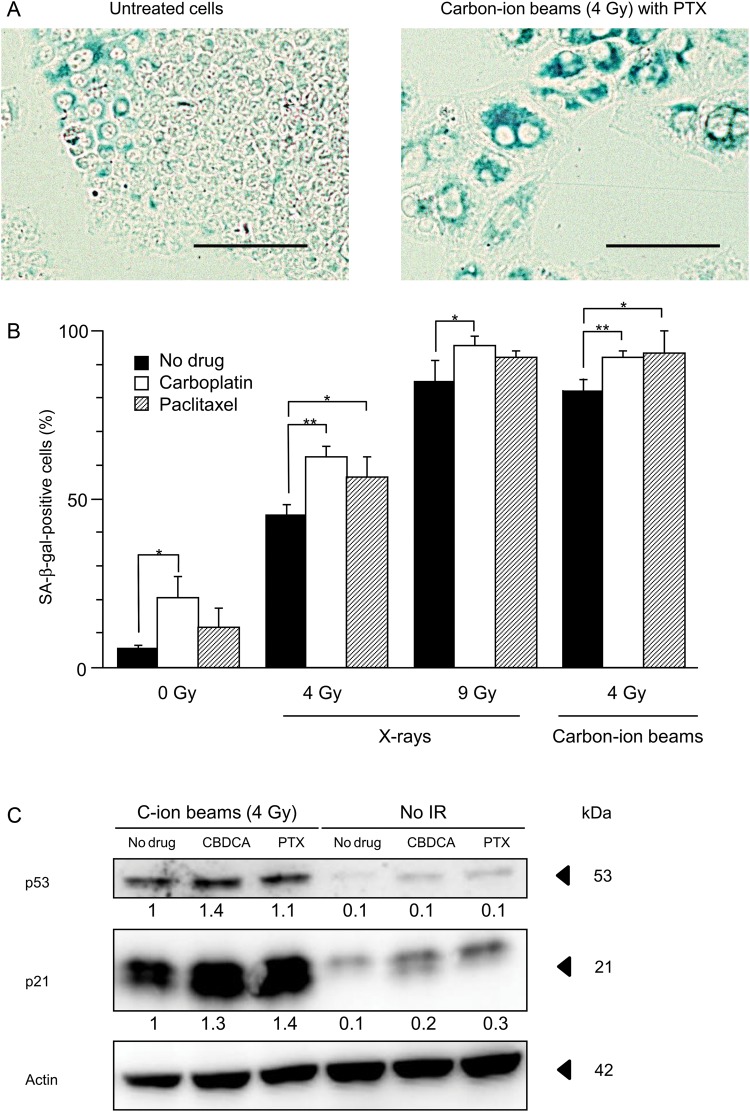
Finally, SA-β-gal staining was used to examine whether the induction of senescence is enhanced by carboplatin or paclitaxel in cells irradiated with X-rays or carbon-ion beams (5). A representative micrograph of senescent cells is shown in Fig. 5A. As shown in the right panel of Fig. 5A, senescent cells not only stained blue but also showed an enlarged and flattened morphology [21]. X-ray irradiation induced senescence in a dose-dependent manner (0, 4 and 9 Gy) (Fig. 5B). At 4 Gy and 9 Gy, the addition of carboplatin significantly enhanced senescence in cells irradiated by X-rays, and at 4 Gy, the addition of paclitaxel significantly enhanced senescence in cells irradiated by X-rays. Carbon-ion beam irradiation at 4 Gy also induced senescence. At 4 Gy, the addition of carboplatin or paclitaxel significantly enhanced senescence in carbon-ion beam-irradiated cells. Irradiation by X-ray or carbon-ion beam at the iso-survival doses obtained from the clonogenic survival assay (Fig. 1) resulted in a comparable induction of senescence (85.0% in cells irradiated by X-rays vs 82.2% in those irradiated by carbon-ion beams, P = 0.52). At the iso-survival doses, the additional effect of carboplatin or paclitaxel on the induction of senescence was comparable in cells irradiated by X-rays or carbon-ion beams (10.6% with X-rays and carboplatin vs 10.0% with carbon-ion beams and carboplatin, P = 0.82; 8.4% with X-rays and paclitaxel vs 11.3% with carbon-ion beams and paclitaxel, P = 0.48).
Fig. 5.
Effects of carboplatin and paclitaxel on senescence induction in H460 cells irradiated with X-rays or carbon-ion beams. (A) Representative micrograph of cells negative (left panel) and positive (right panel) for SA-β-gal staining. Cells positive for SA-β-gal staining are shown in blue. Bars, 100 μm. (B) Percentages of SA-β-gal-positive cells. The mean ± SD is shown. A single asterisk indicates P < 0.05; two asterisks indicate P < 0.01. (C) Western blotting against p53 and p21. Actin was used as a loading control. Levels of p53 and p21 are shown as ratios to their levels in cells treated with carbon-ion beam irradiation alone, after normalization to loading controls. SA-β-gal staining was performed 3 days after irradiation. Carboplatin and paclitaxel were used at each respective IC50 (7.9 μM and 8.3 nM). C-ion = carbon-ion, CBDCA = carboplatin, PTX = paclitaxel, IR = irradiation.
We further investigated the enhancement of senescence by carboplatin or paclitaxel in carbon-ion beam-irradiated cells by western blotting for p21 and p53, because p21 is involved in the induction of senescence and p53 is a positive regulator of p21 [22]. The addition of carboplatin or paclitaxel increased the expression of p21 and p53 in cells irradiated by carbon-ion beams (Fig. 5C). Together, these results indicate that carboplatin and paclitaxel enhanced the induction of senescence by carbon-ion beam irradiation.
DISCUSSION
The present study showed that carboplatin and paclitaxel sensitized NSCLC H460 cells to carbon-ion beam irradiation by enhancing irradiation-induced apoptosis and senescence.
The cytotoxic effect of ionizing irradiation is mainly based on the generation of DNA double-strand breaks (DSBs). When cells receive ionizing irradiation, DNA damage response (DDR) signaling is transduced from the generated DSBs to various downstream substrates via ataxia telangiectasia mutated (ATM) protein. Among the substrates of ATM, p53 plays a central role in inducing anti-tumor effects, including apoptotic cell death and senescence [23]. Meanwhile, carboplatin is known to sensitize cells to X-rays by enhancing the generation of DSBs [24] and persistent single-strand breaks (SSBs) [25], which can progress to DSBs through replication fork collapse in the S phase. In the present study, carboplatin enhanced the expression of p53 (5C) and the induction of apoptosis (Fig. 4B and C) and senescence (Fig. 5B and C) in response to carbon-ion beam irradiation. Taken together, these results suggest that carboplatin sensitizes cells to carbon-ion beam irradiation by enhancing the generation of DSBs and consequently activating the DSBs–ATM–p53 axis. Evidence that carboplatin might exert its radiosensitizing effect via the DSB–ATM–p53 axis by enhancing radiation-induced DSBs, combined with our data showing that apoptosis and senescence induced by irradiation alone were comparable between X-rays and carbon-ion beams at their respective iso-survival doses, may provide a rationale for the observation that carboplatin had a similar effect on apoptosis and senescence induced by either beam source (Figs 4B and C, and 5B and C).
Nevertheless, the mechanisms underlying the observation that X-rays and carbon-ion beams had a similar effect on apoptosis and senescence at their iso-survival doses are largely unknown because the characteristics of the DDR after carbon-ion beam irradiation have not been well elucidated. The difficulty in investigating the DDR in carbon-ion beam irradiation is partially due to the complexity of the DNA damage generated by carbon-ion beams. It is thought that high-LET carbon-ion beams generate ‘clustered DNA damage’ characterized by a complex DNA lesion consisting of multiple DSBs and/or other forms of DNA damage, such as SSBs and base damage [26]. To date, efforts to identify the specific forms of DNA damage present in a lesion using molecular markers have been limited. For example, there is controversy as to whether a single molecule of γH2AX (a marker for DNA DSBs) corresponds to a single DSB generated by carbon-ion beam irradiation or whether it reflects a cluster of DSBs in close proximity. Recently, high-resolution imaging technology has emerged, with which a single molecule involved in DDR, such as Ku, can be detected [27]. We are currently preparing for the examination of the DDR in NSCLC cells using this high-resolution imaging technology. Furthermore, recent reports indicate that indirect action plays a role in cell killing in high-LET radiation (including carbon-ion beams), where direct action had hitherto been thought to be predominant [28, 29]. The similar phenotype in the induction of apoptosis and senescence between X-ray and carbon-ion beams observed in the present study should be further pursued in light of these findings.
In contrast to carboplatin, the mechanisms underlying the radiosensitizing effect of paclitaxel are not well understood, even with respect to X-ray irradiation. Accumulation of cells in the G2/M phase, which is highly radiosensitive, has been proposed as a candidate mechanism for the ability of paclitaxel to sensitize cells to X-rays [30]; however, this hypothesis remains controversial [31, 32]. Meanwhile, previous studies have demonstrated that high-LET beam irradiation has anti-tumor effects regardless of the cell cycle phase [33], indicating that paclitaxel may sensitize cells to carbon-ion beam irradiation through mechanisms other than G2/M arrest. Further research on the mechanisms through which paclitaxel sensitizes cells to carbon-ion beam irradiation is warranted.
The present study indicated that the radiosensitizing effect of carboplatin and paclitaxel to carbon-ion beams is comparable to the radiosensitizing effect of carboplatin and paclitaxel to X-rays at their respective iso-survival doses. It is noteworthy that carbon-ion beam radiotherapy can achieve highly conformal dose distribution (i.e. a high dose delivered to tumors while maintaining a tolerable dose in the surrounding normal tissue) compared with X-rays. These results suggest that the combination of carboplatin or paclitaxel with carbon-ion beam radiotherapy would be more clinically beneficial than combination with X-ray radiotherapy, leading to enhanced efficacy of the local therapy at the primary disease site.
The present study has limitations. First, the radiosensitizing effects of carboplatin and paclitaxel were examined separately because of the difficulty in determining the optimal balance of the doses of the two drugs that shows maximal SER. However, the two drugs are used concomitantly as platinum-doublet chemotherapy with X-rays in the clinic [14, 15] and thus the radiosensitizing effect of combined carboplatin and paclitaxel should be investigated in the future for the clinical application of platinum-doublet chemotherapy in combination with carbon-ion radiotherapy. Secondly, in the present study, only apoptosis and senescence were examined as endpoints for the anti-tumor effect; however, there are modes of reproductive death other than apoptosis and senescence [34]. In fact, Jinno-Oue et al. reported that carbon-ion beam irradiation induced autophagy along with apoptosis and senescence in glioma-derived cell lines [35]. Autophagy induced by ionizing irradiation can result in both cell survival and cell death (autophagic survival and autophagic cell death, respectively) [34, 36, 37]; however, to date, there are no established molecular markers that can distinguish autophagic cell death from autophagic cell survival and, therefore, in the present study, we did not evaluate autophagy as the mode of reproductive death induced by irradiation. In addition, the present study showed that in the particular cell line H460, senescence was the predominant mode of reproductive death induced by the combined treatment, suggesting that autophagic cell death may not be a major mode of reproductive death underlying the radiosensitizing effect of carboplatin and paclitaxel. Nevertheless, comprehensive research covering various modes of reproductive death including apoptosis, senescence, autophagy and necrosis should be conducted to fully elucidate the mechanisms underlying the radiosensitizing effect of carboplatin and paclitaxel. Lastly, the present study examined only one cell line and thus these results may not be generally applicable to human NSCLC. Further studies using multiple cell lines will be required to generalize the ability of carboplatin and paclitaxel to sensitize tumor NSCLC cells to carbon-ion beam irradiation.
In summary, the present study showed that carboplatin and paclitaxel sensitized NSCLC H460 cells to carbon-ion beam irradiation by enhancing irradiation-induced apoptosis and senescence. These findings are a first step in providing a biological basis for the clinical use of combined therapy with carboplatin and/or paclitaxel and carbon-ion beam radiotherapy, although further efforts should be made to elucidate the underlying mechanism of the radiosensitizing effect and to generalize these findings to multiple cell lines.
FUNDING
This work was supported by JSPS KAKENHI Grant Number 24591834. Funding to pay the Open Access publication charges for this article was provided by JSPS KAKENHI Grant Number 24591834.
REFERENCES
- 1.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesin and cisplatin in unresectable stage III non- small-cell lung cancer. J Clin Oncol. 1999;17:2692–9. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739–45. doi: 10.1200/JCO.2009.24.5050. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sause W, Kolesar P, Taylor S, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000;117:358–64. doi: 10.1378/chest.117.2.358. [DOI] [PubMed] [Google Scholar]
- 5.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042–4. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 6.Kanai T, Furusawa Y, Fukutsu K, et al. Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Radiat Res. 1997;147:78–85. [PubMed] [Google Scholar]
- 7.Miyamoto T, Baba M, Sugane T, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2:916–26. doi: 10.1097/JTO.0b013e3181560a68. [DOI] [PubMed] [Google Scholar]
- 8.Sugane T, Baba M, Imai R, et al. Carbon ion radiotherapy for elderly patients 80 years and older with stage I non-small cell lung cancer. Lung Cancer. 2009;64:45–50. doi: 10.1016/j.lungcan.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Kitabayashi H, Shimada H, Yamada S, et al. Synergistic growth suppression induced in esophageal squamous cell carcinoma cells by combined treatment with docetaxel and heavy carbon-ion beam irradiation. Oncol Rep. 2006;15:913–8. [PubMed] [Google Scholar]
- 10.Combs SE, Bohl J, Elsasser T, et al. Radiobiological evaluation and correlation with the local effect model (LEM) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int J Radiat Biol. 2009;85:126–37. doi: 10.1080/09553000802641151. [DOI] [PubMed] [Google Scholar]
- 11.Schlaich F, Brons S, Haberer T, et al. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat Oncol. 2013;8:260. doi: 10.1186/1748-717X-8-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 13.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 14.Choy H, Safran H, Akerley W, et al. Phase II trial of weekly paclitaxel and concurrent radiation therapy for locally advanced non-small cell lung cancer. Clin Cancer Res. 1998;4:1931–6. [PubMed] [Google Scholar]
- 15.Carter DL, Garfield D, Hathorn J, et al. A randomized phase III trial of combined paclitaxel, carboplatin, and radiation therapy followed by weekly paclitaxel or observation for patients with locally advanced inoperable non-small-cell lung cancer. Clin Lung Cancer. 2012;13:205–13. doi: 10.1016/j.cllc.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323:940–5. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 17.Ohno T, Kanai T, Yamada S, et al. Carbon ion radiotherapy at the Gunma University Heavy Ion Medical Center: new facility set-up. Cancers (Basel) 2011;3:4046–60. doi: 10.3390/cancers3044046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tennant JR. Evaluation of the trypan blue technique for determination of cell viability. Transplantation. 1964;2:685–94. doi: 10.1097/00007890-196411000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Mazumder S, Plesca D, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2008;414:13–21. doi: 10.1007/978-1-59745-339-4_2. [DOI] [PubMed] [Google Scholar]
- 20.Adams JM. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 21.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–57. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 23.Cremona CA, Behrens A. ATM signalling and cancer. Oncogene. 2014;33:3351–60. doi: 10.1038/onc.2013.275. [DOI] [PubMed] [Google Scholar]
- 24.Yang LX, Douple EB, O'Hara JA, et al. Production of DNA double-strand breaks by interactions between carboplatin and radiation: a potential mechanism for radiopotentiation. Radiat Res. 1995;143:309–15. [PubMed] [Google Scholar]
- 25.Yang LX, Douple EB, O'Hara JA, et al. Carboplatin enhances the production and persistence of radiation-induced DNA single-strand breaks. Radiat Res. 1995;143:302–8. [PubMed] [Google Scholar]
- 26.Terato H, Ide H. Clustered DNA damage induced by heavy ion particles. Biol Sci Space. 2004;18:206–15. doi: 10.2187/bss.18.206. [DOI] [PubMed] [Google Scholar]
- 27.Britton S, Coates J, Jackson SP. A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol. 2013;202:579–95. doi: 10.1083/jcb.201303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito A, Nakano H, Kusano Y, et al. Contribution of indirect action to radiation-induced mammalian cell inactivation: dependence on photon energy and heavy-ion LET. Radiat Res. 2006;165:703–12. doi: 10.1667/RR3557.1. [DOI] [PubMed] [Google Scholar]
- 29.Hirayama R, Ito A, Tomita M, et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat Res. 2009;171:212–8. doi: 10.1667/RR1490.1. [DOI] [PubMed] [Google Scholar]
- 30.Tishler RB, Schiff PB, Geard CR, et al. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992;22:613–7. doi: 10.1016/0360-3016(92)90888-o. [DOI] [PubMed] [Google Scholar]
- 31.Wenz F, Greiner S, Germa F, et al. Radiochemotherapy with paclitaxel: synchronization effects and the role of p53. Strahlenther Onkol. 1999;175:2–6. doi: 10.1007/BF03215919. [DOI] [PubMed] [Google Scholar]
- 32.Niero A, Emiliani E, Monti G, et al. Paclitaxel and radiotherapy: sequence-dependent efficacy—a preclinical model. Clin Cancer Res. 1999;5:2213–22. [PubMed] [Google Scholar]
- 33.Bird RP, Burki HJ. Survival of synchronized Chinese hamster cells exposed to radiation of different linear-energy transfer. Int J Radiat Biol Relat Stud Phys Chem Med. 1975;27:105–20. doi: 10.1080/09553007514550121. [DOI] [PubMed] [Google Scholar]
- 34.Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinno-Oue A, Shimizu N, Hamada N, et al. Irradiation with carbon ion beams induces apoptosis, autophagy, and cellular senescence in a human glioma-derived cell line. Int J Radiat Oncol Biol Phys. 2010;76:229–41. doi: 10.1016/j.ijrobp.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 36.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–10. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]