Abstract
The recent rapid development of electronic communication techniques is resulting in a marked increase in exposure of humans to electromagnetic fields (EMFs). This has raised public concerns about the health hazards of long-term environmental EMF exposure for fetuses and children. Some studies have suggested EMF exposure in children could induce nervous system disorders. However, gender-dependent effects of microwave radiation exposure on cognitive dysfunction have not previously been reported. Here we investigated whether in utero exposure to 9.417-GHz microwave throughout gestation (Days 3.5–18) affected behavior, using the open field test (OFT), elevated-plus maze (EPM), tail suspension test (TST), forced swimming test (FST) and Morris water maze (MWM). We found that mice showed less movement in the center of an open field (using the OFT) and in an open arm (using the EPM) after in utero exposure to 9.417-GHz radiation, which suggested that the mice had increased anxiety-related behavior. Mice demonstrated reduced immobility in TST and FST after in utero exposure to 9.417-GHz radiation, which suggested that the mice had decreased depression-related behavior. From the MWM test, we observed that male offspring demonstrated decreased learning and memory, while females were not affected in learning and memory, which suggested that microwaves had gender-dependent effects. In summary, we have provided the first experimental evidence of microwaves inducing gender-dependent effects.
Keywords: microwave radiation, anxiety, depression, learning and memory, gender-specific
INTRODUCTION
With the rapid development of electronic information and communication techniques, exposure to electromagnetic fields (EMFs) has increased dramatically. Some studies have focused on the biological effects of electromagnetic radiation. Microwave radiation has been reported as producing adverse effects in the central nervous system (CNS), including headache, sleep disorders, anxiety, cognitive dysfunction and neurogenesis impairment in both humans and animals [–6]. However, the direct effects of microwave radiation on neurodevelopment and the underlying mechanisms for any such effects remain unknown.
Some epidemiological investigations have shown that women and children, especially pregnant women and fetuses, are particularly sensitive to EMF exposure [7–9], and neurobehavioral disorders are increasingly prevalent in children [10, 11]. In the developing nervous system, the brain tissue is more conductive than that of adults because it has a higher water content and a higher ion concentration, and young children have greater absorption of microwave frequency energy in the tissue of the head [12]. Therefore, the CNS of the fetus is considered to be potentially susceptible. Even low doses of microwave exposures during fetal life would have a more profound and long-lasting effect than exposure as an adult. Thus, exposure to microwave radiation in utero may have a neurodevelopmentally toxic effect on the fetus. This situation has caused concern about a possible association between prenatal radiation exposure and cognitive dysfunction in children. Mice exposed in utero to 800–1900-MHz-rated cellular telephone radiation (specific absorption rate (SAR) = 1.6 W/kg) were reported to have impaired memory and decreased anxiety, which may have been caused by altered neuronal developmental programming [9]. In addition, extremely low-frequency magnetic radiation (ELF-MF) had significant effects on basic neuronal functions and synaptic plasticity in brain slice preparations of rats exposed either in the fetal or in the newborn period [8]. Thus, previous studies have demonstrated that prenatal EMF exposure will induce behavior and neurodevelopmental injury in offspring; however, gender-specific effects have not been reported. Therefore, gender-dependent effects of EMF were a particular focus of our study.
The 9.417-GHz microwaves used in this experiment belong to X-band electromagnetic radiation, which is widely used in space research, satellite broadcasts, communication satellites, meteorology satellites, and especially radar detection. We also investigated the operators' knowledge of electromagnetic radiation for those working in radar troops via means of a questionnaire about the hazards of and protection against electromagnetic radiation [13]. They generally displayed a state of subhealth, especially in the nervous system and reproductive system, exhibiting well-being issues such as headache, insomnia, drowsiness (sleep disorders) and fatigue; other effects included abortion, infertility, a high proportion of female children, and so on. In addition, extremely low frequency (ELF)-pulse-modulated X-band microwave exposure has been found to increase the proliferation of human astrocytoma cells [14]. It has been suggested that 10-GHz EMF microwave exposure could adversely affect male rat fertility by reducing the availability of malondialdehyde (MDA), melatonin and creatine kinase for sperm production [15]. Accordingly, it has been reported that X-band (8–12-GHz) electromagnetic waves affect the nervous and reproductive systems. Consequently, we experimented on an in utero exposure model using 9.417-GHz microwaves to investigate the effects of EMF on the behavior of sensitive and gender-specific targets.
MATERIALS AND METHODS
Ethics statement
The experimental procedures used and care of the animals observed the Principles of Laboratory Animal Care (NIH Publication No. 85–23, revised 1985) [16]. The OPRR Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 1986) and the US Animal Welfare Act, as amended, were also followed, as well as the requirements of the Animal Care Committee and Ethics Committee of the Animal Center Laboratory (Beijing) of the Academy of Military Medical Sciences (AMMS).
Animal preparation
A total of 24 CD1 male mice and 12 female mice aged 6 weeks were obtained from the Animal Center Laboratory (Beijing) of the AMMS. Mice were housed with four animals per cage and were exposed to a 12-h light, 12-h dark cycle (lights were activated each day at 7:00 am) with ad libitum access to food and water. After 5 days of recovery period, 12 breeding cages were set up, each containing one CD1 female mouse and two CD1 male mice at 17:00; pregnancy/no pregnancy was determined for the female mice at 8:30 am the next day.
Radiation procedure
Twelve CD1 pregnant mice were divided into three groups: Control, Sham-control and Radiation. The pregnant mice in the Radiation group received a 9.417-GHz irradiation with intensity of 200 V/m for 12 h from 9:00 am to 9:00 pm per day (during Pregnancy Days 3.5–18) in a shielded room and were treated normally from 9:00 pm to 9:00 am. The radiation source was placed over the cage at a distance of 10 cm from the mice. The average power of the radiation source was 1.93 W, and the SAR was 2.0 W/kg at the location of the mice. Mice in the Sham-control group were housed and treated with the same procedures as the exposed group, except with the radiation source turn off from 9:00 am to 9:00 pm. The Sham-control group mice were treated normally from 9:00 pm to 9:00 am. Mice in the Control group were housed outside the shielded room for the radiation period (9:00 am to 9:00 pm) and were treated normally between 9:00 pm and 9:00 am. Every group was placed in a tailor-made polypropylene cage during the radiation period each day. Each mouse was placed in separate cells measuring 6 × 3 × 8 cm (height). Mice could move freely in the cell. The pregnant mice were kept alone following the last day of radiation. The offspring were raised normally after birth, and separated from their mothers when 3 weeks old. Ethology tests were performed at 5 weeks of age. The litters of pregnant mice were randomly mixed to exclude any maternal effects.
Behavioral paradigms
Behavioral tests started at 5 weeks after birth of the offspring as follows: anxiety-related behavior (open field test (OFT) and elevated-plus maze (EPM)), depression-related behavior (tail suspension test (TST) and forced swimming test (FST)) and learning and memory behavior (Morris water maze (MWM)). The behavioral tests were performed between 8:00 am and 12:00 am.
Open field test
Spontaneous exploratory behavior was assessed in an automated OFT following previously described procedures [17]. The open field was a square arena (60 cm × 60 cm) 25 cm in height, with gray ligneous walls. The apparatus was set up under a digital camera, which was connected to a video recorder and a computer under the control of the EthoVisionver 8.0 tracking system. This system was also used in other behavioral experiments. The mice were individually placed in the center of the open field and left to explore freely for 5 min. The total distance moved in the arena in 5 min was recorded as a measure of locomotor distance. Frequency and duration of entering into a central square (20 cm × 20 cm) of the open field during the 5 min were automatically recorded and were used as measures of anxiety-related behavior.
Elevated-plus maze
EPM was performed immediately after the OFT. The apparatus consisted of an elevated plus made up of opaque Plexiglas, of which the four arms were each 30 cm long and 5 cm wide. It had a central area of 5 × 5 cm connecting the arms. Two opposite ‘closed’ arms were enclosed by a 10-cm high wall, while the other two ‘open’ arms had no enclosing walls. The whole apparatus was elevated at a height of 50 cm from the ground. The closed arms provided a safe zone for the animals. The EPM test was carried out according to previously described methods [18, 19]. Animals were placed on the central area of the maze facing the open arm and allowed to explore freely for 5 min. Frequency and time in the closed arms and in the open arms were manually recorded. Percent frequency and percent time in the open arms were the evaluation indexes of anxiety-related behavior and were calculated as follows:
Tail suspension test
Briefly, mice undertook the TST in an individual cubicle while suspended from a tail hanger with adhesive tape wrapped around the tail (1.5–2 cm from the tip) 35 cm above the floor [20]. The trial was conducted for a period of 6 min and the period of immobility during the last 4 min was manually measured by a blinded observer. Mice were considered immobile when they hung passively and motionless.
Forced swimming test
For the FST, mice were placed individually in clear Plexiglas cylinders (height = 25 cm; diameter = 10 cm) containing 15 cm depth of water at 23 ± 1°C, and the trial was conducted for 6 min [21]. The total period of immobility during the last 4 min was manually recorded. A mouse was considered to be immobile when it remained floating in an upright position, making only slight movements to keep its head above the water [22].
Morris water maze
The procedure for the MWM was performed as reported in our previous study [23]. Mice were trained in a MWM for five consecutive days (four trials per day). A platform of 6 cm in diameter was placed 1.5 cm below the surface of the water in the center of the southwest quadrant of a circular pool 60 cm in height and 100 cm in diameter. The water was maintained at 22–23°C throughout the training and testing. For a given training trial, a mouse was introduced into the pool at one of four possible starting points (north, east, northwest and southeast) and was allowed to find the platform during a period of 60 s. The order of starting points for each mouse varied in a pseudorandom manner every day. If the mouse failed to find the platform within 60 s, it was directly placed on the platform by the experimenter. After staying on the platform for 20 s, the mouse was then placed into a holding cage for 10 min as an inter-trial interval. At 24 h after the last training session, the mice were tested for reference memory by a probe test. For this, the hidden platform was removed. Mice started at a northeast location, 180° from the original hidden platform position, and were allowed to swim for 60 s. The period(s) and frequency when a mouse stayed in or entered the goal quadrant (where the hidden platform was previously located), respectively, were recorded by the computerized swimming tracking system (EthoVisionver 8.0, Netherlands).
Statistical analysis
The results of the behavioral tests were expressed as mean ± SEM. To compare the escape latency in the five initial training days for the different groups, the data were analyzed by two-way, one-repeated measures analysis of variance (ANOVA) using treatment and training days as two between-subject factors. Other data were analyzed by one-way ANOVA. All post hoc tests were performed by Fisher's LSD multiple comparison tests. Statistical significance was accepted at P < 0.05 (* or #) and P < 0.01 (**).
RESULTS
Microwave radiation in utero increased the anxiety-related behavior of mice
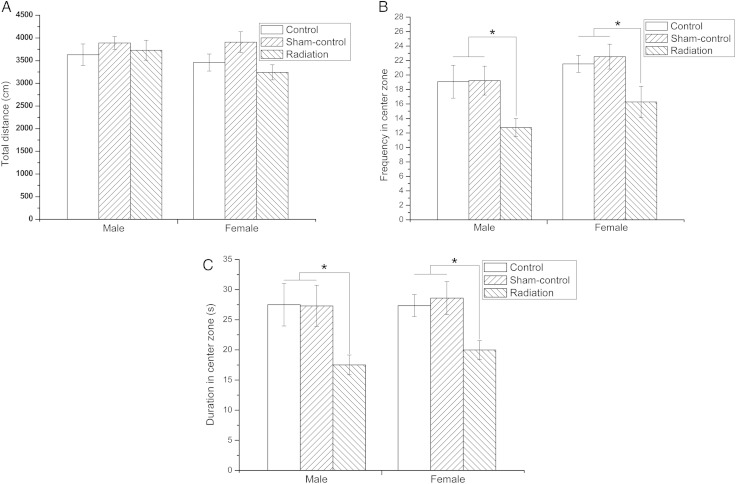
The OFT and EPM were performed to evaluate the anxiety-related behavior of offspring. The results of the OFT indicated that the total distance covered by the offspring of the various study groups did not differ significantly. This suggested that the mice did not differ in motor ability (Fig. 1A). However, frequency of entries into and duration of time spent in the center zone for the Radiation group of mice were lower compared with the Control and Sham-control mice (P < 0.05), suggesting an increase in the anxiety-related behavior of exposed mice (for both male and female mice) (Fig. 1B and C).
Fig. 1.
The effect of fetal microwave exposure on anxiety as evaluated by the OFT. The total distance moved by offspring among the study groups was no different (A). However, the frequency and duration of entry into the center zone of the exposed mice was lower than that of the Control and Sham-control mice (B, C), indicating anxiety behavior. Data are means ± SEM, 12 animals per group; *P < 0.05, versus corresponding values in Control and Sham-control groups.
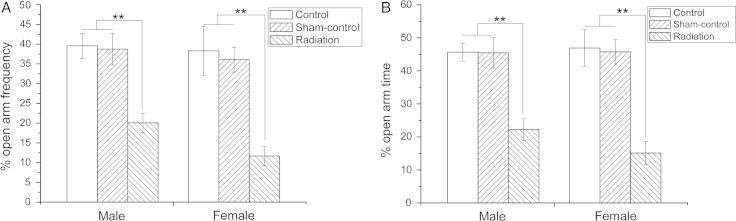
Similarly, the EPM indicated that both the percent frequency of entering the open arms and the percent time spent in the open arms for the radiation-exposed mice were lower compared with the Control and Sham-control mice (P < 0.01) (Fig. 2). This suggested that exposed mice had increased anxiety-related behavior (for both male and female mice), which is in accordance with the OFT results.
Fig. 2.
The effect of fetal microwave exposure on anxiety as determined using EPM. Compared with the Control and Sham-control mice, the offspring of exposed mice had a lower percent open arm frequency (A) and percent open arm time (B), suggesting anxiety-related behavior, in accordance with the OFT. Data are means ± SEM, 12 animals per group; **P < 0.01, vs corresponding values in Control and Sham-control groups.
Microwave radiation in utero decreased the depression-related behavior of mice
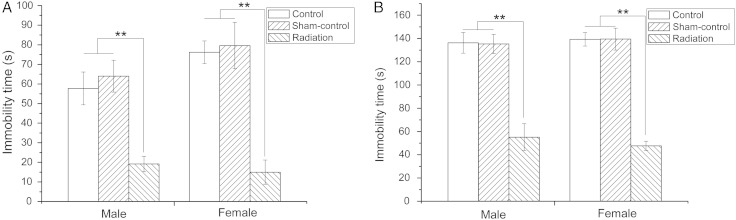
The TST and the FST were used to assess the effects of microwave radiation on the depression-related behavior of offspring [24, 25]. Post hoc analysis for the TST indicated that microwave exposure significantly decreased immobility time compared with the Control and Sham-control group (P < 0.01) (Fig. 3A). This suggested that the offspring of exposed mice had decreased depression-related behavior. In accordance with the results obtained in TST, post hoc analysis in the FST also revealed significant effects of microwave radiation on immobility time (P < 0.01) (Fig. 3B). Thus, these results also suggested that radiation exposure in utero decreased depression-related behavior in the mice.
Fig. 3.
The effect of fetal microwave exposure on depression based on the TST and FST. Radiation exposure decreased the immobility time compared with the Control and Sham-control mice in TST (A) and FST (B), illustrating the decreased depression-related behavior. Data are means ± SEM, 12 animals per group; **P < 0.01, versus corresponding values in Control and Sham-Control groups.
Only the males had impaired memory after fetal exposure to microwave radiation
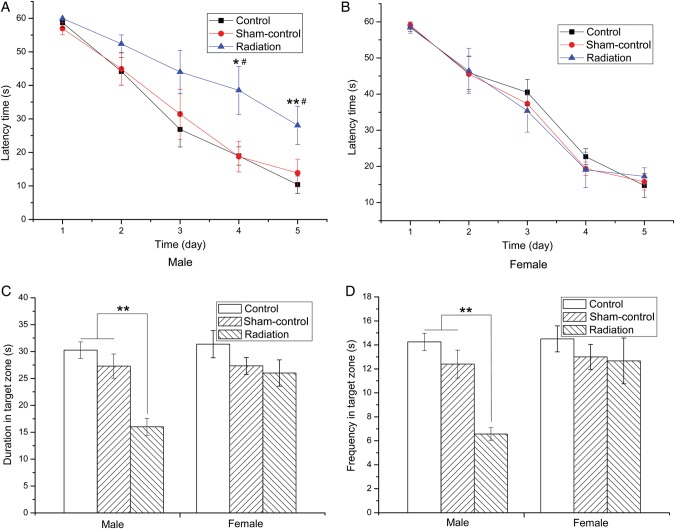
To investigate the effects of microwave exposure on spatial learning and memory in offspring, we performed the MWM task in offspring. All of the irradiated mice showed a progressive decline in escape latency (the time it takes to find the platform) during the five training days. On the fourth and fifth days of MWM, male mice in Radiation group spent more time trying to find the platform (Fig. 4A), indicating reduced spatial learning ability. In contrast, there were no differences in the latency time for female mice between any of the study groups (Fig. 4B). The Sham-control group did not show a significant difference compared with Control group. The probe test (after removal of the platform) indicated that male mice in Radiation group showed a reduced time and frequency in the quadrant of the original platform location (Fig. 4C), i.e. reduced memory retention, whereas the female mice did not differ in performance between the groups (Fig. 4D). Both of these results suggested that microwave irradiation induced gender-specific effects.
Fig. 4.
The effect of fetal microwave exposure on learning and memory assessed using the MWM. All mice showed a progressive decline in escape latency during the five training days. Microwave exposure induced significant prolongation in latency time on the fourth and fifth days for male offspring mice (A), while there was no difference for the female offspring mice (B). In the probe test, the time spent in the quadrant of the original platform location, and the frequency with which animals crossed the original platform location for male mice in the Radiation group was significantly reduced compared with the Control and Sham-control groups (C), whereas the females did not differ among the groups (D), suggesting impairment in learning and memory for male mice, but no effect in the female. Data are means ± SEM, eight animals per group, *P < 0.05, **P < 0.01, vs corresponding values in the Control group, #P < 0.05 vs corresponding values in Sham-control group.
DISCUSSION
Possible harmful effects of EMF on cognitive and behavioral aspects of humans and rodents have been considered controversial [, 26–28]. Here we demonstrated that 9.417-GHz microwave exposure to pregnant mice caused behavioral alterations and gender-specific learning and memory deficits in offspring. Both male and female mice demonstrated increased anxiety-related and decreased depression-related behavior. The male mice displayed impaired learning and memory, whereas the female mice were not affected. These results were novel in that previous research has not distinguished any gender-specific effects. Our research will provide an important reference in relation to the protection of a fetus during pregnancy from EMFs.
It has been reported that microwave radiation in adults has been known to cause anxiety. Anxiety-related symptoms, including sleep disorders (58%), headaches (41%), nervousness or distress (19%), fatigue (18%) and concentration difficulties (16%) were found in the crowd exposed to EMF [29]. Fetal radiofrequency exposure from 800–1900-MHz cellular telephones, however, did not induce anxiety behavior in the F1 generation [9]. In our study, on the other hand, mice exposed in utero to 9.417-GHz radiation did demonstrate increased anxiety behavior. This conflict might be a result of diverse experimental designs and methods (in relation to frequency, power density, radiation devices, orientation, exposure duration, or even the different maze apparatus). Depression-related behavior was found following long-term extremely-low-frequency EMF exposure in rats [30]. People exposed to an EMF environment were also reported as having depression-related symptoms [31, 32]. However, there has been limited research on depression induced by EMFs in rodents, especially with in utero exposure. We found that mice showed decreased depression-related behavior after in utero exposure to 9.417-GHz radiation. Therefore, further research is needed to reach a definite conclusion about the behavioral effects of microwave exposure.
There have been some reports about the effects of EMF exposure on cognition. Universal Mobile Telecommunications System (UMTS) exposure with a SAR in the range of 2 W/kg was not found to be harmful to critical markers for memory storage and memory consolidation, however, an influence from UMTS at high energy absorption rates (10 W/kg) cannot be excluded [33]. The hippocampus was revealed to be injured by long-term microwave exposure, which could result in impairment of cognitive function as a consequence of neurotransmitter disruption [34]. Mice exposed to microwave radiation in utero demonstrated impaired memory in the research of Aldad et al. [9], which is partly accordant with our results. However, a gender-dependent aspect of microwave radiation effects on cognitive function was revealed in our study. Our results demonstrated that only male mice were affected in learning and memory, whereas female mice were not affected. This indicated that the sensitivity of male and female mice to microwave radiation in utero was different, which is a novel finding.
The gender-dependent effects of microwaves on learning and memory were properly due to hormones. It has been reported estrogen provides a measure of protection against radiation. The phytoestrogen isoflavonoid equol is known to protect against solar-simulated UV radiation-induced inflammation, immunosuppression, and skin carcinogenesis [35–37]. Sitarina et al. demonstrated that estrogen receptor (ER) and 17-β estradiol had a natural role in photoimmune protection, but antogonist could exacerbate immunosuppression resulting from UV irradiation [38]. Equol, one member of the estrogen family is not only a radical scavenger but also enhances the induction of a relevant cutaneous antioxidant, metallothionein. Thus the protection mechanism may involve antioxidant actions [39–41]. Electromagnetic radiation induced the formation of reactive oxygen species (ROS) in mice by affecting the activity of superoxide dismutase (SOD), catalase (CAT), glutathion peroxidase (GPx) and malondialdehyde (MDA) content, a potential mediator of the alterations caused by electromagnetic radiation [42–45]. Neurogenesis in the adult hippocampus is correlated with the neuronal plasticity of learning and memory, which is exquisitely sensitive to changes in redox balance [46]. Consequently, in this study equol may be protecting female mice against EMF-induced impaired memory via regulating the oxidative stress level. Therefore, ER signaling may underlie significant gender-specific differences in susceptibility to radiation, and reflect different needs for environmental photoprotection in males and females.
Heat effects from microwave irradiation were also observed in our study. As a result of the long-term and high-frequency EMF exposure, mice in the radiation group were sweating after exposure. It has been reported that exposure to high temperatures during early pregnancy induces impaired learning and memory, which is correlated with mRNA expression of c-fos and NR1 in the hippocampus [47]. Therefore, the behavior effects of microwave exposure in utero were probably partly due to the thermal effects of microwave irradiation.
In summary, our research demonstrated that fetal exposure to 9.417-GHz microwave radiation led to behavioral alterations that persisted into adulthood. We found that fetal microwave radiation caused neurobehavioral disorders and gender-specific learning and memory deficits in mice, and provided significant targets for neurobehavioral detection. These findings will be beneficial in relation to the perinatal care of pregnant women and improve our understanding of the etiology of neurobehavioral disorders. The increasing rate of behavioral disorders in children may be, at least in part, due to fetal microwave radiation exposure. Further testing is warranted in non-human primates and humans to determine EMF health hazards and to establish safe limits during pregnancy.
FUNDING
This work was supported by the Special Key Program for Science and Technology of China (2012ZX09102301-016, 2012ZX09J12107-03C, 2012AA022402), the General Program of the Natural Science Foundation of China (31101049, 31300703, 81170558), the General Program of the Key Laboratory of Tropical Marine Bio-resources and Ecology (LMB111001), and the Program of the State Key Laboratory of Proteomics (SKLP-O201207-WTN). Funding to pay the Open Access publication charges for this article was provided by the General Program of the Natural Science Foundation of China (31101049).
ACKNOWLEDGEMENTS
We would like to thank to Nan Zhao of the Beijing Institute of Pharmacology and Toxicology for providing access to the OFT and the EPM. This work was presented in part at the 7th International EMF Seminar in China, 2013, Beijing, China.
REFERENCES
- 1.Nittby H, Grafstrom G, Tian DP, et al. Cognitive impairment in rats after long-term exposure to GSM-900 mobile phone radiation. Bioelectromagnetics. 2008;29:219–32. doi: 10.1002/bem.20386. [DOI] [PubMed] [Google Scholar]
- 2.Hossmann KA, Hermann DM. Effects of electromagnetic radiation of mobile phones on the central nervous system. Bioelectromagnetics. 2002;24:49–62. doi: 10.1002/bem.10068. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Xu S, He M, et al. Glucose administration attenuates spatial memory deficits induced by chronic low-power-density microwave exposure. Physiol Behav. 2012;106:631–7. doi: 10.1016/j.physbeh.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Valentini E, Curcio G, Moroni F, et al. Neurophysiological effects of mobile phone electromagnetic fields on humans: a comprehensive review. Bioelectromagnetics. 2007;28:415–32. doi: 10.1002/bem.20323. [DOI] [PubMed] [Google Scholar]
- 5.Salford LG, Brun AE, Eberhardt JL, et al. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ Health Perspect. 2003;111:881–3. doi: 10.1289/ehp.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grafstrom G, Nittby H, Brun A, et al. Histopathological examinations of rat brains after long-term exposure to GSM-900 mobile phone radiation. Brain Res Bull. 2010;77:257–63. doi: 10.1016/j.brainresbull.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Ahlbom IC, Cardis E, Green A, et al. Review of the epidemiologic literature on EMF and health. Environ Health Perspect. 2001;109(Suppl 6):911–33. doi: 10.1289/ehp.109-1240626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balassa T, Varro P, Elek S, et al. Changes in synaptic efficacy in rat brain slices following extremely low-frequency magnetic field exposure at embryonic and early postnatal age. Int J Dev Neurosci. 2013;31:724–30. doi: 10.1016/j.ijdevneu.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Aldad TS, Gan G, Gao XB, et al. Fetal radiofrequency radiation exposure from 800–1900 MHz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep. 2012;2:312–8. doi: 10.1038/srep00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 11.Rappley MD. Clinical practice. Attention deficit–hyperactivity disorder. N Engl J Med. 2005;352:165–73. doi: 10.1056/NEJMcp032387. [DOI] [PubMed] [Google Scholar]
- 12.Kheifets L, Repacholi M, Saunders R, et al. The sensitivity of children to electromagnetic fields. Pediatrics. 2005;116:e303–13. doi: 10.1542/peds.2004-2541. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YC, Chao XH, Geng DJ, et al. Analysis of operators knowledge of electromagnet in complex electromagnetic environments: a preliminary investigation. Mil Med Sci. 2014;38:57–61. [Google Scholar]
- 14.Perez-Castejon C, Perez-Bruzon RN, Llorente M, et al. Exposure to ELF-pulse modulated X band microwaves increases in vitro human astrocytoma cell proliferation. Histol Histopathol. 2009;24:1551–61. doi: 10.14670/HH-24.1551. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Behari J, Sisodia R. Impact of microwave at X-band in the aetiology of male infertility. Electromagn Biol Med. 2012;31:223–32. doi: 10.3109/15368378.2012.700293. [DOI] [PubMed] [Google Scholar]
- 16.Gottenbos B, van der Mei HC, Klatter F, et al. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coating on silicone rubber. Biomaterials. 2002;23:1417–23. doi: 10.1016/s0142-9612(01)00263-0. [DOI] [PubMed] [Google Scholar]
- 17.Paylor R, Nguyen M, Crawley JN, et al. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1999;5:302–16. [PMC free article] [PubMed] [Google Scholar]
- 18.Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–47. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 19.Cecchi M, Khoshbouei H, Javors M, et al. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 20.Svenningsson P, Tzavara ET, Qi H, et al. Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J Neurosci. 2007;27:4201–9. doi: 10.1523/JNEUROSCI.3110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vry J, Maurel S, Schreiber R, et al. Comparison of hypericum extracts with imipramine and fluoxetine in animal models of depression and alcoholism. Eur Neuropsychopharmacol. 2000;9:461–8. doi: 10.1016/s0924-977x(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 22.Pechnick RN, Chesnokova VM, Kariagina A, et al. Reduced immobility in the forced swim test in mice with a targeted deletion of the leukemia inhibitory factor (LIF) gene. Neuropsychopharmacology. 2004;29:770–6. doi: 10.1038/sj.npp.1300402. [DOI] [PubMed] [Google Scholar]
- 23.Hou B, Xu ZW, Yang CW, et al. Protective effects of inosine on mice subjected to lethal total-body ionizing irradiation. J Radiat Res. 2007;48:57–62. doi: 10.1269/jrr.06067. [DOI] [PubMed] [Google Scholar]
- 24.Heurteaux C, Lucas G, Guy N, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–41. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 25.Holick KA, Lee DC, Hen R, et al. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2007;33:406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 26.Kesari KK, Behari J. Fifty-gigahertz microwave exposure effect of radiations on rat brain. Appl Biochem Biotechnol. 2009;158:126–39. doi: 10.1007/s12010-008-8469-8. [DOI] [PubMed] [Google Scholar]
- 27.Ning W, Xu SJ, Chiang H, et al. Effects of GSM 1800MHz on dendritic development of cultured hippocampal neurons. Acta Pharmacol Sin. 2007;28:1873–80. doi: 10.1111/j.1745-7254.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Wang Y, Zhang Y, et al. Elevation of plasma corticosterone levels and hippocampal glucocorticoid receptor translocation in rats: a potential mechanism for cognition impairment following chronic low-power-density microwave exposure. J Radiat Res. 2008;49:163–70. doi: 10.1269/jrr.07063. [DOI] [PubMed] [Google Scholar]
- 29.Röösli M, Moser M, Baldinini Y, et al. Symptoms of ill health ascribed to electromagnetic field exposure – a questionnaire survey. Int J Hyg Environ Health. 2004;207:141–50. doi: 10.1078/1438-4639-00269. [DOI] [PubMed] [Google Scholar]
- 30.Szemerszky R, Zelena D, Barna I, et al. Stress-related endocrinological and psychopathological effects of short- and long-term 50 Hz electromagnetic field exposure in rats. Brain Res Bull. 2009;81:92–9. doi: 10.1016/j.brainresbull.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Kheifets L, Bowman JD, Checkoway H, et al. Future needs of occupational epidemiology of extremely low frequency electric and magnetic fields: review and recommendations. Occup Environ Med. 2009;66:72–80. doi: 10.1136/oem.2007.037994. [DOI] [PubMed] [Google Scholar]
- 32.Johansson A, Nordin S, Heiden M, et al. Symptoms, personality traits, and stress in people with mobile phone-related symptoms and electromagnetic hypersensitivity. J Psychosom Res. 2009;68:37–45. doi: 10.1016/j.jpsychores.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Prochnow N, Gebing T, Ladage K, et al. Electromagnetic field effect or simply stress? Effects of UMTS exposure on hippocampal longterm plasticity in the context of procedure related hormone release. PLoS One. 2011;6:e19437. doi: 10.1371/journal.pone.0019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L, Peng RY, Wang SM, et al. Relationship between cognition function and hippocampus structure after long-term microwave exposure. Biomed Environ Sci. 2012;25:182–8. doi: 10.3967/0895-3988.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Widyarini S, Spinks N, Husband AJ, et al. Isoflavonoid compounds from red clover (Trifolium pratense) protect from inflammation and immune suppression induced by UV radiation. Photochem Photobiol. 2001;74:465–70. doi: 10.1562/0031-8655(2001)074<0465:icfrct>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Friedmann AC, Halliday GM, Barnetson RS, et al. The topical isoflavonoid NV-07alpha reduces solar-simulated UV-induced suppression of Mantoux reactions in humans. Photochem Photobiol. 2004;80:416–21. doi: 10.1562/0031-8655(2004)080<0416:TTINRS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Widyarini S, Husband AJ, Reeve VE. Protective effect of the isoflavonoid equol against hairless mouse skin carcinogenesis induced by UV radiation alone or with a chemical cocarcinogen. Photochem Photobiol. 2005;81:32–7. doi: 10.1562/2004-06-02-RA-183. [DOI] [PubMed] [Google Scholar]
- 38.Widyarini S, Domanski D, Painter N, et al. Estrogen receptor signaling protects against immune suppression by UV radiation exposure. Proc Natl Acad Sci U S A. 2006;103:12837–42. doi: 10.1073/pnas.0603642103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys. 1998;356:133–41. doi: 10.1006/abbi.1998.0783. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell JH, Gardner PT, McPhail DB, et al. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch Biochem Biophys. 1998;360:142–8. doi: 10.1006/abbi.1998.0951. [DOI] [PubMed] [Google Scholar]
- 41.Sierens J, Hartley JA, Campbell MJ, et al. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen. 2002;22:227–34. doi: 10.1002/tcm.10015. [DOI] [PubMed] [Google Scholar]
- 42.Zmyslony M, Politanski P, Rajkowska E, et al. Acute exposure to 930 MHz CW electromagnetic radiation in vitro affects reactive oxygen species level in rat lymphocytes treated by iron ions. Bioelectromagnetics. 2004;25:324–8. doi: 10.1002/bem.10191. [DOI] [PubMed] [Google Scholar]
- 43.Aydin B, Akar A. Effects of a 900-MHz electromagnetic field on oxidative stress parameters in rat lymphoid organs, polymorphonuclear leukocytes and plasma. Arch Med Res. 2011;42:261–7. doi: 10.1016/j.arcmed.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Irmak Mk, Fadillioglu E, Gulec M, et al. Effects of electromagnetic radiation from a cellular telephone on the oxidant and antioxidant levels in rabbits. Cell Biochem Funct. 2002;20:279–83. doi: 10.1002/cbf.976. [DOI] [PubMed] [Google Scholar]
- 45.Cao Y, Zhang W, Lu Mx, et al. 900-MHz microwave radiation enhances gamma-ray adverse effects on SHG44 cells. J Toxicol Environ Health A. 2009;72:727–32. doi: 10.1080/15287390902841466. [DOI] [PubMed] [Google Scholar]
- 46.Huang TT. Redox balance- and radiation-mediated alteration in hippocampal neurogenesis. Free Radic Res. 2012;46:951–8. doi: 10.3109/10715762.2012.664770. [DOI] [PubMed] [Google Scholar]
- 47.Dou C, Shuling Wang, Jinliang Zhang, et al. Effect of exposure to high temperatures during early pregnancy on hippocampal-dependent learning and memory integrity in neonatal rats. Toxicol Ind Health. 2011;27:431–5. doi: 10.1177/0748233710387968. [DOI] [PubMed] [Google Scholar]