Abstract
The identification of an agent effective for the treatment of intestinal and bone marrow injury following radiation exposure remains a major issue in radiological medicine. In this study, we evaluated the therapeutic impact of single agent or combination treatments with 2-(3-aminopropylamino) ethylsulphanyl phosphonic acid (WR-2721) and peptidoglycan (PGN, a toll-like receptor 2 (TLR-2) agonist) on radiation-induced injury of the intestine and bone marrow in lethally irradiated male C57BL/6 mice. A dose of 3 mg of WR-2721 per mouse (167 mg/kg, intraperitoneally) was given 30 min before irradiation, and 30 μg of PGN per mouse (1.7 mg/kg) was injected intraperitoneally 24 h after 10 Gy irradiation. Bone marrow cluster of differentiation (CD)45+ and CD34+ markers of multiple haematopoietic lineages, number of granulocyte–erythroid–macrophage–megakaryocyte (GEMM) progenitor colonies, bone marrow histopathology, leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) expression in the intestines, xylose absorption and intestinal histopathology were all assessed at various time-points after irradiation. Furthermore, nuclear factor kappa B (NF-κB) p65 protein in the ileum was stained by immunofluorescent labelling. PGN-treated irradiated mice showed an increase in CD45+CD34+ cells compared with untreated mice 1.25 days after 10 Gy ionizing radiation (IR) (P < 0.05). Furthermore, combined PGN and WR-2721 treatment had an obviously synergistic radio-protective effect in nucleated cells in the bone marrow, including GEMM progenitors and CD45+CD34+ cells 4 days after 10 Gy IR. Single agent PGN or WR-2721 treatment after 10 Gy IR clearly increased Lgr5-positive pit cells (P < 0.05) and xylose absorption (P < 0.05). However only PGN and WR-2721 combination treatment markedly increased villus height (P < 0.05), number of crypts (P < 0.05) and whole-body weights after 10 Gy whole-body irradiation (WBI). The NF-κB p65 subunit was translocated to the nucleus, and phosphate-IκBα (Ser32/Ser36) was detected after stimulation with either PGN or WR-2721, which indicates that these two agents act synergistically through the activation of the NF-κB pathway. Administration of PGN in combination with WR-2721 was demonstrated to have a synergistic effect on the increase in haematopoietic cells and intestinal reconstitution, as well as improved survival in lethally irradiated mice, but resulted in some degree of an immune disorder.
Keywords: WR-2721, peptidoglycan, radioprotection, bone marrow, intestine
INTRODUCTION
Although radiation therapy remains one of the most effective modalities for neoplastic disease, the damage caused by ionizing radiation (IR) in the small intestine and bone marrow remains a concern. A major goal of radiation oncology is the radioprotection of normal tissue to improve the therapeutic index. In addition, nuclear accidents lead to risk of radiation exposure, which can cause radiation-induced injury. Therefore, effective therapeutic remedies are urgently needed, and identifying effective and useful substances for the prevention or treatment of intestinal and bone marrow injury due to radiation exposure is critical.
WR-2721 (Amifostine, 2-(3-aminopropylamino) ethylsulphanyl phosphonic acid; marketed as Ethyol® by MedImmune, Gaithersburg, MD) is an organic thiophosphate cytoprotective agent and was the first radio-protective drug to enter routine clinical practice. It has been demonstrated to be effective in protecting the salivary glands in head and neck cancer patients during radiotherapy []. Although Amifostine is currently administered clinically, its radio-protective effects in normal tissue (especially at high radiation doses) are modest [–], which limits its use [5, 6].
Recently, there have been an increasing number of studies devoted to identifying radio-protective drugs or remedies that can be used clinically. Burdelya et al. [7] determined that a single injection of a toll-like receptor (TLR) 5 agonist (CBLB502, a polypeptide drug derived from Salmonella flagellin) before lethal whole-body irradiation (WBI) protects mice against gastrointestinal and haematopoietic acute radiation syndromes and results in survival by activating nuclear factor-kappa B (NF-κB) signalling. CBLB502 injected after irradiation also enhanced survival, but at lower radiation doses.
Ciorba et al. [8] reported that Lactobacillus rhamnosus GG reduced radiation-induced epithelial injury and improved crypt survival via TLR2; this effect was observed when administered before, but not after, radiation.
Intestinal homeostasis is dependent on the proper activity of intestinal stem cells (ISCs). The intestinal epithelium is continually self-renewing and can rapidly regenerate after damage. The leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) is a marker of mitotically active ISCs [9]. The depletion of Lgr5+ cells during radiation-induced damage and subsequent repair has been shown to result in catastrophic crypt loss and the deterioration of crypt–villus architecture. Lgr5+ cells are therefore crucial for robust intestinal regeneration following radiation exposure [10].
In this study, we evaluated the therapeutic effect of TLR2 agonist peptidoglycan (PGN) [11] alone or in combination with WR-2721 on intestinal or haematopoietic injury of mice after 10 Gy WBI. Our results demonstrate that this combination treatment may have a potentially therapeutic effect on lethal dose irradiation injury.
MATERIALS AND METHODS
Animals and treatments
For the study, 5–7-week-old male C57BL/6 mice (SLACCAS, Shanghai, China) were kept in animal maintenance facilities under conditions of controlled illumination (12:12 h light/dark cycle), humidity (30–50%) and temperature (18–22°C) and were fed a normal rodent laboratory diet and water. All animal studies were performed in accordance with the guidelines and protocols of the Institutional Animal Care and Use Committee of Soochow University. WR-2721 and PGN were purchased from Sigma (St Louis, MO). A dose of 3 mg of WR-2721 per mouse (167 mg/kg) was administered 30 min before irradiation, while PGN at 30 μg per mouse (1.7 mg/kg) was administered 24 h after irradiation. Unless otherwise stated, 10 mice were used per group in each experiment.
Irradiation procedure
WBI was performed on anaesthetised mice (intraperitoneal administration of 0.36% chloral hydrate at 0.8 ml/100 g body weight) using a Philips SL18 X-ray system (Redhill, UK) at a dose rate of 200 cGy/min, following the biosafety guidelines observed in China. After irradiation, the mice were returned to cages (three to four mice per cage) and were given free access to food and water.
Survival fraction and body weight
The survival status and body weight of each mouse was observed and measured daily.
Intestinal absorption
The functional regeneration of irradiated intestines was determined by measuring intestinal absorption on Days 3, 7, 15 and 35 by xylose uptake assay. Briefly, 5% (w/v) d-xylose solution was administered orally by feeding tube (100 µl/mouse; n = 10/cohort) and urine samples were collected 2 h post feeding. Xylose levels were measured using a modified micro-method [12].
Haematoxylin and eosin staining and immunohistochemistry
Tissues (femora and ileum) from live mice were collected at 1.25, 4, 9, 16, 25 and 40 days post IR and fixed in formalin solution. Tissue alterations were evaluated by histological analysis using haematoxylin and eosin (H&E) staining and immunohistochemical staining. Femora were decalcified in 5% nitric acid for 3 h. Each decalcified femur and ileum was then routinely embedded. Paraffin blocks were cut into 4-μm sections, which were mounted, deparaffinized in xylene, and rehydrated in decreasing concentrations of ethanol. Antigen retrieval was performed using citrate buffer, heating sections in a pressure cooker for 5 min and subsequently cooling to room temperature. Blocking of endogenous peroxidases was accomplished by incubating sections in 3% hydrogen peroxide for 5 min. Lgr5 polyclonal antibodies (1:100 dilution; Epitomics Co., Burlingame, CA) were incubated with sections overnight at 4°C. Immunostaining was performed using an Envision System and diaminobenzidine visualization (Dako, Carpinteria, CA) according to the manufacturer's instructions. Sections were counterstained with haematoxylin for 1 min, rinsed in water, dehydrated in increasing concentrations of ethanol followed by clearance with xylene, and cover-slipped permanently for light microscopy. Bone marrow pathology was examined on days 1.25 and 4 post IR; intestinal pathology was examined on day 4. Villus height was measured on intestinal samples from days 1.25, 4, 9, 16, 25 and 40 post IR, but the number of crypts was only counted on days 1.25 and 4.
Immunofluorescent staining
Up to the antibody incubation step, all procedures were performed in the same way as for the immunohistochemistry. After antigen retrieval, tissue sections (1.25 days post 10 Gy IR) were incubated overnight with primary antibodies against NF-κB p65 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with the secondary antibody for 1 h at 23°C. The secondary antibody was fluorescein isothiocyanate (FITC)-conjugated mouse anti-goat immunoglobulin (IgG, 1:1000), obtained from Molecular Probes (Eugene, OR). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Eugene). Photomicrographs were obtained using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Clonogenic assays for myeloid haematopoietic progenitor colony-forming units
Live mice at 4, 9, 16, 25 and 40 days post IR were killed by an overdose of anaesthetic, and marrow cells were obtained from the femora (by flushing with a 22-gauge needle) and resuspended in serum-free medium. After being washed by centrifugation, cells were resuspended in α-minimum essential medium (α-MEM) with 20% foetal bovine serum. Cell number was determined after red blood cells were removed with Zapoglobin (Beckman Coulter, Miami, FL). Cells (105 per well) were cultured in MethoCult® GF M3434 medium (StemCell, Vancouver, BC, Canada) in six-well plates. MethoCult® GF M3434 medium contains interleukin-3 (IL-3), stem cell factor (SCF), interleukin-6 (IL-6) and erythropoietin (EPO) and is optimized for the detection and quantification of mouse haematopoietic progenitors such as granulocyte–macrophage progenitors and granulocyte–erythroid–macrophage–megakaryocytes (GEMMs) in bone marrow using a colony-forming cell assay. Only aggregates with more than 500 cells and a highly dense core were considered colony-forming unit (CFU)-GEMM colonies, and they were scored under a microscope 12–14 days after incubation at 37°C in a 5% CO2 humidified atmosphere. The number of CFU-GEMM colonies was determined per 105 marrow cells plated.
Fluorescence-activated cell sorting analysis
Cell aliquots were obtained from the femora of mice 1.25 and 4 days post IR by flushing with serum-free medium. After red blood cells were removed with Zapoglobin, cells were incubated for 20 min at 4°C with antibodies conjugated with phycoerythrin and fluorescein isothiocyanate against the mouse cluster of differentiation (CD)34 and CD45 (CD34+CD45+, multiple haematopoietic lineages, eBioscience, San Diego, CA). Acquisition was conducted on a FACSAria sorter (BD Biosciences, San Jose, CA), and analysis was performed using FACS DIVE Version 6.1.3 (BD Biosciences).
Western blotting
Total cell protein from ilea 1.25 days post IR was separated by SDS-10% PAGE, transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA), blocked in 5% non-fat dried milk, and incubated with primary rabbit polyclonal anti-IκBα (Ser32/Ser36) antibody (Millipore, Bedford, MA, diluted 1:500 in 0.1% milk powder in TBS-T) or GAPDH (Cellsignal, Danvers, MA, diluted 1:1000 in 0.1% milk powder in TBS-T) for 3 h at 23°C or overnight at 4°C. The blot was washed and then incubated with anti-rabbit secondary antibody conjugated with horseradish peroxidase (HRP) (Dako, 1 mg/ml; diluted 1:1000 in 0.1% milk powder in TBS-T) at room temperature for 2 h. The blots were visualized by SuperSignal West Pico Luminol/Enhancer solution (Pierce, Rockford, IL).
Measurement of plasma pro-inflammatory molecules
Six mice were used per group in this experiment. An assay using bead technology (0.025–0.05 ml per assay for several cytokines) was adopted to determine the levels of multiple cytokines in mouse plasma 1.25, 4 and 9 days post IR. Mouse Th1/Th2 10plex FlowCytomix for GM-CSF, IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17, TNF-α and Mouse IL-23 FlowCytomix Simplex were purchased from Bender Medsystems (eBioscience). Mouse plasma from different treatment groups was assessed using a flow cytometer (BD FACSCalibur, BD Biosciences).
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Differences between groups were determined using one-way ANOVA. Survival data were assessed using Kaplan–Meier analysis. P < 0.05 was considered to be statistically significant.
RESULTS
WR-2721 and PGN sustained weight and improved survival after 10 Gy WBI
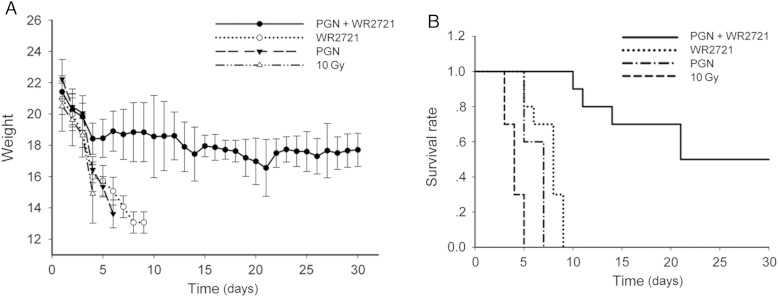
Irradiated C57BL/6 mice treated with WR-2721 or PGN alone lost weight rapidly. In contrast, mice treated with WR-2721 and PGN in combination maintained their weight (∼18 g) (Fig. 1A). The median survival time of the 10 Gy IR group was 3 days, with a 95% confidence interval (CI) of 2–4 days. All C57BL/6 mice pre-treated with WR-2721 died within the 9 days following irradiation, (mean survival 7 days (95% CI: 4–8 days), P < 0.0001 compared with IR group); whereas C57BL/6 mice treated with the TLR2 agonist PGN exhibited worse survival outcomes after 10 Gy WBI (mean survival 6 days (95% CI: 4–6 days), P = 0.0003 compared with the IR group). In contrast, half of the C57BL/6 mice pretreated with WR-2721 and treated with PGN 24 h after 10 Gy WBI as a combination therapy survived for 30 days after 10 Gy WBI (mean survival 20 days (95% CI: 9–undetermined days), P < 0.0001 compared with the IR group) (Fig. 1B).
Fig. 1.
WR-2721 and PGN in combination sustained body weight and improved survival after 10 Gy WBI. (A) All mice except irradiated C57BL/6 mice treated with WR-2721 and PGN in combination lost weight quickly prior to death. (B) Survival of C57BL/6 mice (n = 40) exposed to 10 Gy radiation. The mice were divided into groups of 10, and one group received WR-2721 before IR (3 mg per mouse), another 10 received PGN 24 h post-IR (30 μg per mouse), and the remaining 10 received combination treatment. Survival among the groups was ranked in the following order: W + P group > W group > P group > IR group. Half of the irradiated C57BL/6 mice treated with WR-2721 and PGN survived for more than 30 days. Error bars indicate the SD of the mean for n = 10 mice.
Combination treatment with WR-2721 and PGN reduced bone marrow injury
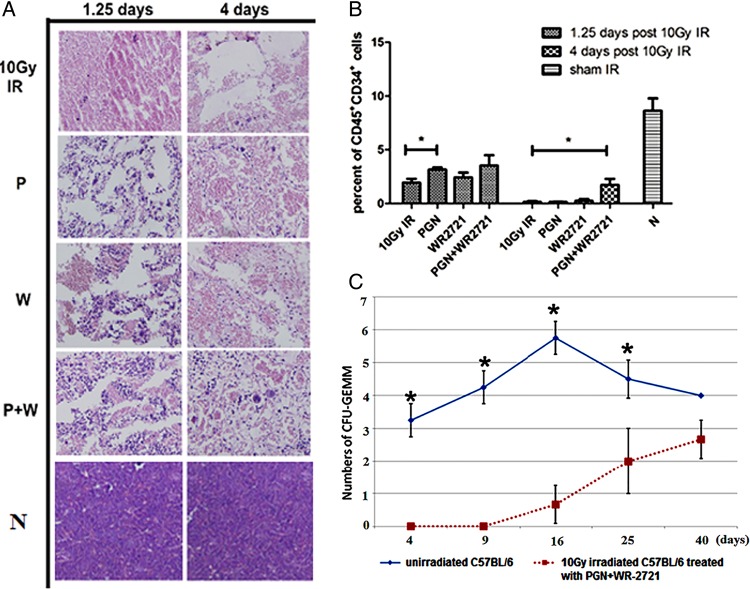
The bone marrow of unirradiated normal mice (N group) was highly cellular. After 10 Gy irradiation, mice exhibited a considerable decrease in the number of bone marrow cells and instead their bone marrow was full of red blood cells, especially four days post IR (IR group). Treatment with PGN (P group) or WR-2721 (W group) alleviated the bone marrow depletion by causing an increase in myelogenic cells, but WR-2721-treated mice exhibited haemorrhages (1.25 days post 10 Gy IR). In contrast, 4 days after irradiation, mice in the WR-2721 + PGN (W + P) group showed the presence of newly formed microvessels lined with endothelial cells, and small round haematopoietic cells were also apparent around the microvessels (Fig. 2A). The cell surface protein CD34 is frequently used as a marker for positive selection of haematopoietic progenitor cells in mouse bone marrow, both in research and in transplantation [13]. At 1.25 days post IR, CD45+CD34+ cells were not drastically depleted. The proportion of CD45+CD34+ cells was evidently higher in the bone marrow of mice that received PGN treatment compared with untreated 10 Gy irradiated mice. On Day 4, CD45+CD34+ cells were drastically depleted, and the combination of WR2721 and PGN treatment was the most effective in restoring numbers of CD45+CD34+ cells. These results highlight the fact that WR-2721 + PGN treatment stimulated haematopoietic recovery, partially because it maintained a population of CD45+CD34+ haematopoietic cells (Fig. 2B).
Fig. 2.
WR-2721 + PGN combined treatment decreased bone marrow damage and increased numbers of CFU-GEMM and CD45+CD34+ cells. (A) Representative histological sections of the femur from mice in each of the 5 treatment groups at 1.25 and 4 days after 10 Gy WBI. N = unirradiated mice, IR = 10 Gy WBI mice, P = 10 Gy WBI mice treated with PGN, W = WR-2721-treated 10 Gy WBI mice, P + W = PGN and WR-2721 combination treated 10 Gy WBI mice (original magnification ×200). After 10 Gy irradiation, C57BL/6 mice could be seen to have lost most white cells in their bone marrow, and cavities in the bone marrow appeared. After single agent treatment with PGN or WR-2721, the numbers of nucleated cells increased, but haemorrhages appeared in the W group at 1.25 days and cavities still remained. On Day 4 post-IR, marrow microvessels appeared in femora of the P + W group and haematopoiesis commenced. (B) Number of CD45+CD34+ cells per 10 000 total cells from femoral marrow detected after red blood cells were removed using Zapoglobin, as analysed by fluorescence-activated cell sorting (FACS). On Day 1.25, only PGN-treated 10 Gy irradiated mice showed a marked increase in the proportion of CD34+CD45+ cells. On Day 4, the number of CD45+CD34+ cells was higher in the WR-2721 + PGN group than in other 10 Gy irradiated mice. (C) Only WR-2721 + PGN combined treatment induced growth of mouse CFU-GEMM haematopoietic progenitors following 10 Gy irradiation, and that growth was considerably subnormal on Days 4, 9, 16 and 25 days post IR. However, on Day 40, no marked difference between the W + P group and the N group was observed. Error bars indicate the SD for n = 10 mice (*P < 0.05).
No CFU-GEMM clones were found in the bone marrow of irradiated mice 16 days after 10 Gy IR, even after PGN or WR2721 single treatment. The clones only appeared after treatment with the combination of PGN and WR-2721. The number of CFU-GEMM clones observed in WR-2721 + PGN mice 40 days post-IR did not markedly differ from the number observed in unirradiated mice (Fig. 2C).
Taken together, the data from bone marrow H&E staining, the proportion of CD45+CD34+ cells, and the CFU-GEMM of mouse bone marrow indicate that PGN treatment could boost the population of CD45+CD34+ cells more than WR-2721, and that the combined use of PGN and WR-2721 further protected haematopoietic stem/progenitor cells and stimulated the structural recovery of bone marrow.
WR-2721 + PGN decreased pathological changes of the intestine
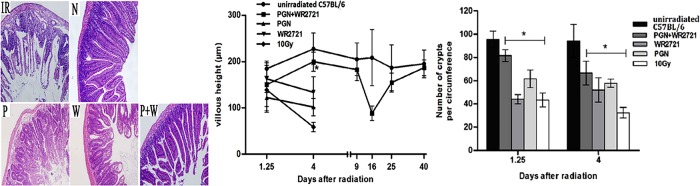
Histological changes in the ilea were characterized by the adherence of submucosal layers and sloughing of crypts, as well as ulcers and ruptured villi in 10 Gy irradiated mice (Fig. 3). Irradiated animals that received either WR-2721 or PGN evidently had longer villi and more crypts compared with untreated irradiated mice four days after 10 Gy IR (Fig. 3). Furthermore, irradiated mice treated with a combination of PGN and WR2721 had normal mucosal thickness, minimal inflammatory changes, and preserved tissue architecture.
Fig. 3.
Treatment with PGN and WR-2721 in combination normalized ileum villus height and number of crypts after 10 Gy WBI. N = unirradiated mice, IR = 10 Gy WBI mice, P = 10 Gy WBI mice treated with PGN, W = WR-2721-treated 10 Gy WBI mice, P + W = PGN and WR-2721 combination-treated 10 Gy WBI mice (original magnification ×200). After 10 Gy WBI the small intestine became vacuolated, villus height was clearly shortened, and half or fewer of the crypts remained. On Day 1.25 post IR, villus height and number of crypts in the P and W groups group had not changed obviously compared with the IR group, and marked differences could be observed in comparison with the N group. However, on Day 4 post-IR, the P + W group had longer villi and more crypts compared with the IR group (*P < 0.05), while the P and W groups were unchanged. Error bars indicate the SD for n = 10 mice.
WR-2721 and PGN promoted recovery of intestinal absorption in irradiated mice
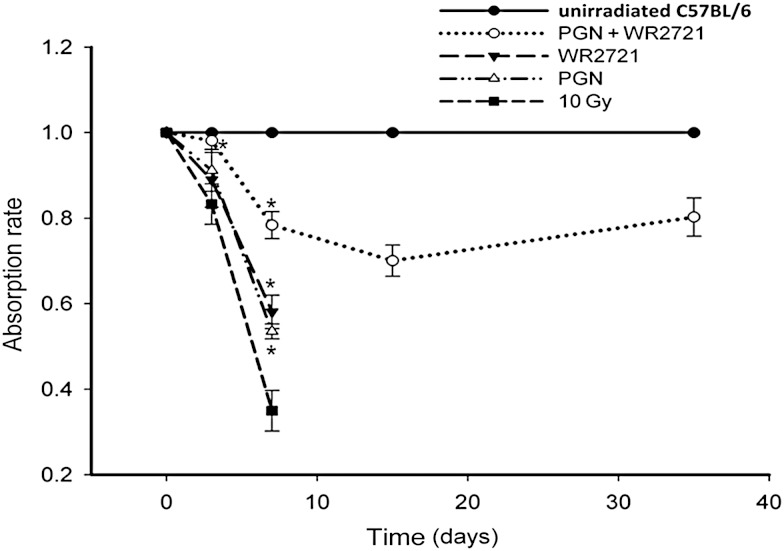
As xylose is not metabolised in the body, the d-xylose absorption test is used to measure the level of d-xylose in blood or urine samples to help diagnose problems that prevent the small intestine from absorbing nutrients in food. In this study, urine xylose levels were tested 2 h after a test feeding dose at 3, 7, 15 and 35 days after 10 Gy IR. Normal unirradiated mice demonstrated 100% xylose absorption. As expected, a progressive reduction in xylose absorption rates was observed in irradiated mice. However, intestinal absorptive function was elevated in the combined treatment group compared with the IR group (Fig. 4), indicating that the combination of WR-2721 and PGN best restituted the intestinal villi damaged by IR compared with WR-2721 or PGN treatment alone.
Fig. 4.
WR-2721 and PGN promoted recovery of intestinal absorption in irradiated mice. Xylose uptake assay of 10 mice per group was performed 1 day before mice were euthanized. Samples of urine were collected, and xylose levels were measured using a modified micro-method and normalized to data from unirradiated mice at the same time-points. On Day 3 after 10 Gy IR, the absorption rate of mice treated with WR-2721 + PGN demonstrated a clear difference from that of mice exposed to 10 Gy irradiation (P < 0.01). On Day 7, the absorption rate of mice treated with WR-2721 or PGN alone, or with WR-2721 + PGN, was obviously higher than that of the 10 Gy irradiated mice. Error bars indicate the SD for n = 10 mice (*P < 0.05).
WR-2721 and PGN promoted translocation of the NF-κB p65 subunit and augmented intestinal regeneration following irradiation
Lgr5 has recently been identified as a murine marker of intestinal stem cells [14]. Data from C57BL/6 mice showed that the number of Lgr5+ cells was lowest in irradiated mice and highest in unirradiated mice, while mice treated with WR-2721 in combination with PGN showed median levels of expression. Irradiation with 10 Gy badly damaged intestinal stem cells, but WR-2721 and PGN evidently protected Lgr5+ cells from damage (Fig. 5A).
Fig. 5.
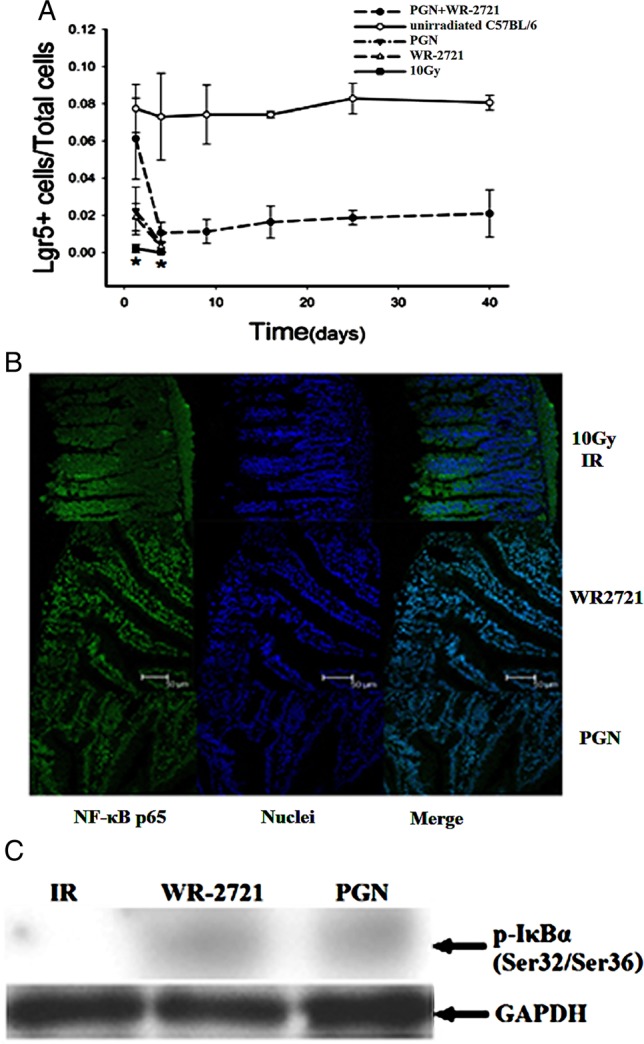
Both single treatment with WR-2721 or PGN and combination treatment augmented intestinal Lgr5 expression and activated NF-κB following irradiation. (A) The percentage of Lgr5+ cells in the ilea of mice treated with WR-2721 and PGN was higher than that in the ilea of mice irradiated with 10 Gy, but lower than the percentage in the ilea of normal mice (*P < 0.05). (B) The NF-κB p65 subunit is located in the cytoplasm 1.25 days post 10 Gy irradiation. Both WR-2721 and PGN treatment may activate NF-κB and induce it to move into the nuclei of such cells as enterocytes and smooth muscle cells. (C) Western blot data of phospho-IκBα in the ileum 1.25 days post 10 Gy irradiation.
Most NF-κB p65 protein was located in the cytoplasm 1.25 days after IR, showing that IR did not activate NF-κB. Both PGN and WR-2721 did activate NF-κB and caused NF-κB to translocate to the nuclei (Fig. 5B).
Ilea from irradiated mice (1.25 days post 10 Gy WBI) and irradiated mice treated with PGN or WR-2721 were collected, then p-IκBα and GAPDH proteins were examined by western blotting. In the irradiated mice, no p-IκBα protein was detected, while positive bands were detected in samples from irradiated mice treated with PGN or WR-2721 (Fig. 5C).
Serum cytokine measurement
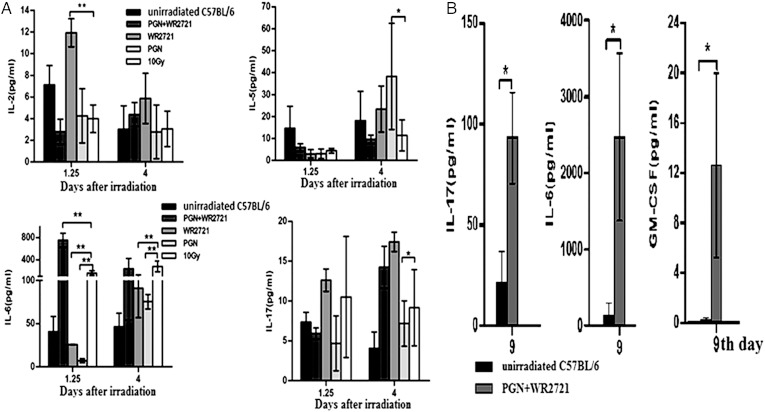
As cytokines modulate the immune response, this study tested 11 cytokines (GM-CSF, IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17, TNF-α and IL-23) in the sera of five experimental groups: unirradiated C57BL/6 mice, irradiated C57BL/6 mice, and irradiated C57BL/6 mice treated with WR-2721, PGN or both. IL-4, IL-5, IL-6 and IL-10 were quantified as markers of the Th2 pathway (humoral immune response), whereas IL-2, TNF-α and IFN-γ were used as markers of the Th1 pathway (cell-mediated immune response) [15–17]. The data showed that the immune response following IR was both humoral and cytotoxic. TNF-α was detected near the quantitation limit of the kit in five groups (data not shown). No significant changes were observed in GM-CSF, IFN-γ, IL-1α, IL-4, IL-10 or IL-23 at 1.25 days and 4 days post 10 Gy IR (data not shown). In contrast IL-2, IL-5, IL-6 and IL-17 were significantly changed at certain time-points in untreated irradiated mice in comparison with treated mice. The level of IL-2 was higher, but IL-6 was lower, in WR-2721 treated mice compared with untreated irradiated mice (1.25 days post IR). The level of IL-5 was significantly higher, but IL-6 and IL-17 were obviously lower, in PGN-treated mice compared with the untreated IR group (4 days post IR). In the W + P treatment group, the level of IL-6 was restored to normal (Fig. 6A). Further, in serum samples from mice of the W + P group 9 days post IR, GM-CSF, IL-6 and IL-17 were all significantly higher than in samples from unirradiated mice (Fig. 6B). Treatment with PGN or WR-2721 alone was able to significantly reduce IL-6 at 1.25 and 4 days post IR compared with the unirradiated group, but PGN and WR-2721 co-treatment increased these levels. These results indicate that irradiation induces immune response disorders and that WR-2721 and PGN were not able to protect against them completely (Fig. 6).
Fig. 6.
GM-CSF, IL-2, IL-5, IL-6 and IL-17 showed significant changes over the course of the experiments. WR-2721 reversed the radiation-induced changes in IL-2 and IL-6. In the PGN group, IL-5 and IL-6 levels were similar to those in the IR group. The combination of WR-2721 and PGN was unable to reverse the increase in IL-6 observed following IR (A). IL-6, IL-17 and GM-CSF in the serum of mice treated with WR-2721 and PGN were all significantly higher than in the unirradiated mice on Day 9 (B) (*P < 0.05).
DISCUSSION
In this study, we observed that WR-2721 and PGN improved survival of irradiated mice following 10 Gy WBI by improving the condition of their bone marrow (H&E stain, CD45+CD34+ and CFU-GEMM) and intestines (H&E stain, xylose absorption and Lgr5+ cells). These data are consistent with other studies that have demonstrated that WR-2721 stimulates formation of haematopoietic progenitors [18–21]. Our results suggest that PGN may protect haematopoietic progenitor cells better than WR-2721. Both WR-2721 and PGN protected the population of Lgr5+ intestinal stem cells, while the combination of WR-2721 and PGN markedly extended the survival of irradiated mice. TLR2 protein is expressed in intestinal epithelial cells and lamina propria mononuclear cells in the GI mucosa of healthy mice. To maintain commensal and mucosal homeostasis, mucosal TLR2 exhibits several cell-type-specific features of barrier protection. In the intestinal epithelia, TLR2 critically controls mucosal inflammation by directly preserving tight junction-associated barrier integrity that is at the frontline of host defences [22]. In addition, in dendritic cells, TLR2 engagement promotes antigen capture and processing [22]. TLR2 also modulates regulatory T cells by controlling adaptive immune responses [22]. Wang et al. [23] reported that PGN induces TLR2-dependent activation of the myeloid differentiation primary response protein 88 (MyD88), interleukin receptor associated kinase (IRAK), TNF Receptor-Associated Factor (TRAF), NF-κB-inducing kinase (NIK), IκB kinase (IKK), and NF-κB. The data in this study also demonstrated that WR-2721 induces NF-κB activation. Shen et al. [24] reported that N-(2-mercaptoethyl)-1,3-propanediamine (WR-1065, the active form of WR-2721) bound directly to the transcription factor NF-κB p50 subunit and activated NF-κB. NF-κB activation has been observed to provide radioprotection to the intestinal epithelium [25]. Murley et al. [26] reported that WR-1065 activated NF-κB and enhanced expression of the intracellular antioxidant enzyme manganese superoxide dismutase (MnSOD) gene. Taken together, these data show that WR-2721 and PGN activate NF-κB by separate mechanisms, at least in part, illustrating their protective effect against intestinal damage induced by radiation. Perhaps this effect is the reason that PGN significantly prolongs survival when combined with WR-2721.
Adverse effects such as nausea, vomiting and hypotension have been reported for WR-2721. The human equivalent dose (910 mg/m2) of the mouse 0.5 maximum tolerated dose (MTD, 400 μg/g) is the highest WR-2721 dose used clinically [27]. Here, we used WR-2721 at a dose of 167 μg/g in mice, which is safe when scaling from the mouse to the human.
However, even with combined PGN and WR-2721 treatment, half of the irradiated mice did not survive for 30 days, but began to die at 10 days post 10 Gy IR. This is most likely due to dyshaematopoiesis, incomplete intestinal injury recovery, and immune response disorders. We hypothesize that immune response disorders may have contributed to the cause of death in half of the 10 Gy WBI mice. Researchers from the Kimmel Cancer Center at Jefferson [28] found that direct inhibition of the activity of NF-κB, a key protein mediator of inflammation, reduces radiation toxicity in zebrafish embryos and may ultimately be of help to patients receiving radiation therapy. PGN and WR-2721 activated NF-κB, which protects the intestine from IR-induced damage, but NF-κB also results in an immune disorder, which evidently reduces the radio-protective effect of PGN and WR-2721.
In summary, combination treatment with WR-2721 and PGN protects irradiated mice against bone marrow and intestinal tract damage and prolongs their survival. Our findings suggest that post-WBI survival might be further increased with the use of immunomodulatory agents in the treatment of acute severe radiation sickness.
FUNDING
This work was supported by the Jiangsu Provincial Key Laboratory of Radiation Medicine and Protection, the National Natural Science Foundation of China (Grant Nos 81001317, 81172597 and 81372920), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant Nos 09KJD310007 and 11KJA31001), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Program for Changjiang Scholars and Innovative Research Team in University (IRT0849), and Defense basic research projects. Funding to pay the Open Access publication charges for this article was provided by the National Natural Science Foundation of China.
REFERENCES
- 1.Shaw L-M, Turrisi A-T, Glover D-J, et al. Human pharmacokinetics of WR-2721. Int J Radiat Oncol Biol Phys. 1986;12:1501–4. doi: 10.1016/0360-3016(86)90203-8. [DOI] [PubMed] [Google Scholar]
- 2.Bisht K-S, Prabhu S, Devi P-U. Modification of radiation induced damage in mouse intestine by WR-2721. Indian J Exp Biol. 2000;38:669–74. [PubMed] [Google Scholar]
- 3.Naruka K, Bhartiya H-C. Protection of bone marrow of Swiss albino mouse against whole body gamma irradiation by WR-2721. Indian J Exp Biol. 1992;30:535–7. [PubMed] [Google Scholar]
- 4.Ma C, Xie J, Jiang Z, et al. Does amifostine have radioprotective effects on salivary glands in high-dose radioactive iodine-treated differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37:1778–85. doi: 10.1007/s00259-009-1368-6. [DOI] [PubMed] [Google Scholar]
- 5.Mettler FA, Jr, Brenner D, Coleman C-N, et al. Can radiation risks to patients be reduced without reducing radiation exposure? The status of chemical radioprotectants. AJR Am J Roentgenol. 2011;196:616–8. doi: 10.2214/AJR.10.4959. [DOI] [PubMed] [Google Scholar]
- 6.Winczura P, Jassem J. Combined treatment with cytoprotective agents and radiotherapy. Cancer Treat Rev. 2010;36:268–75. doi: 10.1016/j.ctrv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Burdelya L-G, Krivokrysenko V-I, Tallant T-C, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–30. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciorba M-A, Riehl T-E, Rao M-S, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829–38. doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan K-S, Chia L-A, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–71. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe C, Kljavin N-M, Ybarra R, et al. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–59. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: a reevaluation. Infect Immun. 2005;73:5212–6. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberts T-J, Sample R-H, Glick M-R, et al. A simplified, colorimetric micromethod for xylose in serum or urine with phloroglucinol. Clin Chem. 1979;25:1440–3. [PubMed] [Google Scholar]
- 13.Street N-E, Schumacher J-H, Fong T-A, et al. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990;144:1629–39. [PubMed] [Google Scholar]
- 14.Firestein G-S, Roeder W-D, Laxer J-A, et al. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989;143:518–25. [PubMed] [Google Scholar]
- 15.Paliard X, de Waal Malefijt R, Yssel H, et al. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988;141:849–55. [PubMed] [Google Scholar]
- 16.Dao M-A, Arevalo J, Nolta J-A. Reversibility of CD34 expression on human hematopoietic stem cells that retain the capacity for secondary reconstitution. Blood. 2003;101:112–8. doi: 10.1182/blood-2002-01-0025. [DOI] [PubMed] [Google Scholar]
- 17.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 18.List A-F, Heaton R, Glinsmann-Gibson B, et al. Amifostine stimulates formation of multipotent and erythroid bone marrow progenitors. Leukemia. 1998;12:1596–602. doi: 10.1038/sj.leu.2401151. [DOI] [PubMed] [Google Scholar]
- 19.Ramdas J, Warrier R-P, Scher C, et al. Effects of amifostine on clonogenic mesenchymal progenitors and hematopoietic progenitors exposed to radiation. Pediatr Hematol Oncol. 2003;25:19–26. doi: 10.1097/00043426-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Momm F, Bechtold C, Fischer K, et al. Alteration of radiation-induced hematotoxicity by amifostine (ethyol) Strahlenther Onkol. 1999;175(Suppl 4):2–5. ; : [PubMed] [Google Scholar]
- 21.Romano M-F, Lamberti A, Bisogni R, et al. Amifostine inhibits hematopoietic progenitor cell apoptosis by activating NF-κB/Rel transcription factors. Blood. 1999;94:4060–2. [PubMed] [Google Scholar]
- 22.Cario E. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 2008;1(Suppl 1):S62–6. doi: 10.1038/mi.2008.47. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Dziarski R, Kirschning C-J, et al. Micrococci and peptidoglycan activate TLR2 → MyD88 → IRAK → TRAF → NIK → IKK → NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect Immun. 2001;69:2270–6. doi: 10.1128/IAI.69.4.2270-2276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H-X, Chen Z-J, Zilfou J-T, et al. Binding of the aminothiol WR-1065 to transcription factors influences cellular response to anticancer drugs. J Pharmacol Exp Ther. 2001;297:1067–73. [PubMed] [Google Scholar]
- 25.Egan L-J, Eckmann L, Greten F-R, et al. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A. 2004;101:2452–7. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murley J-S, Kataoka Y, Hallahan D-E, et al. Activation of NFκB and MnSOD gene expression by free radical scavengers in human microvascular endothelial cells. Free Radic Biol Med. 2001;30:1426–39. doi: 10.1016/s0891-5849(01)00554-8. [DOI] [PubMed] [Google Scholar]
- 27.Soref C-M, Hacker T-A, Fahl W-E. A new orally active, aminothiol radioprotector—free of nausea and hypotension side effects at its highest radioprotective doses. Int J Radiat Oncol Biol Phys. 2012;82:e701–7. doi: 10.1016/j.ijrobp.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 28.Daroczi B, Kari G, Ren Q, et al. NF-κB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol Cancer Ther. 2009;8:2625–34. doi: 10.1158/1535-7163.MCT-09-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]