Abstract
Cisplatin-based concurrent chemoradiotherapy (CCRT) is a standard treatment for cervical cancer, but nedaplatin-based CCRT is not routinely administered. We evaluated the efficacy and safety of nedaplatin-based CCRT (35 mg/m2 weekly) and analyzed prognostic factors for survival among 52 patients with International Federation of Gynecology and Obstetrics (FIGO) Stage IB2–IVA cervical cancer treated from 1999 to 2009. Patients were treated with a combination of external beam radiotherapy of 40–56 Gy (in 20–28 fractions) and 13.6–28.8 Gy (in 2–4 fractions) of high-dose-rate (HDR) intracavitary brachytherapy or 18 Gy (in 3 fractions) of HDR interstitial brachytherapy. Overall survival (OS), progression-free survival (PFS), and local control (LC) were estimated using the Kaplan–Meier method. The Cox proportional hazard model was used for multivariate analysis. Acute and late toxicities were evaluated using the Common Terminology Criteria for Adverse Events version 4.0. The median follow-up period was 52 months. The median patient age was 63 years. The 5-year OS, PFS and LC rates were 78%, 57% and 73%, respectively. Multivariate analysis showed that histologic type, maximum tumor diameter, and pretreatment hemoglobin level were independent risk factors for PFS. Regarding adverse effects, 24 patients (46%) had acute Grade 3–4 leukopenia and 5 (10%) had late Grade 3 gastrointestinal toxicities. No patient experienced renal toxicity. Nedaplatin-based CCRT for FIGO Stage IB2–IVA cervical cancer was efficacious and safe, with no renal toxicity. Histologic type, maximum tumor diameter, and pretreatment hemoglobin level were statistically significant prognostic factors for PFS.
Keywords: cervical cancer, nedaplatin, chemoradiotherapy, chemoradiation therapy
INTRODUCTION
Cisplatin-based concurrent chemoradiotherapy (CCRT) is a standard treatment for locally advanced cervical cancer. Although many combinations of cisplatin-based chemotherapy have been tested and reported in randomized trials [, ], weekly cisplatin (40 mg/m2) remains the standard treatment in daily practice and current clinical trials in the USA.
In a multicenter Phase II trial, the Japanese Gynecologic Oncology Group (JGOG) study 1066 demonstrated that cisplatin-based CCRT using high-dose-rate (HDR) intracavitary brachytherapy (ICBT) achieved comparable outcomes with global dose schedules, despite Japanese centers adopting lower cumulative radiation dose schedules at Point A than those in the USA and Europe []. These centers reported that HDR ICBT was feasible, with acceptable toxicity compared with previous clinical studies of cisplatin-based CCRT []. However, hematologic and renal toxicities, which may lead to treatment delay or dose reduction, remained. Therefore, less toxic platinum agents with a similar effectiveness to that of cisplatin should be established for patients suffering from uterine cervical cancer.
Nedaplatin (cis-diammine-glycoplatinum), a derivative of cisplatin, was developed in Japan with the aim of producing a treatment with a similar effectiveness to cisplatin, but with decreased renal and gastrointestinal toxicities [5–9]. A previous Phase II study conducted in Japan suggested that nedaplatin had a favorable clinical efficacy, comparable with that of cisplatin [10]. In our facility, locally advanced cervical cancer has been treated with CCRT using nedaplatin since December 1999. We previously reported the clinical treatment outcomes of clinical International Federation of Gynecology and Obstetrics (FIGO) Stage IIIB disease and demonstrated that nedaplatin-based CCRT improved treatment outcome compared with radiotherapy alone with an efficacy similar to that of cisplatin-based CCRT [11]. However, the clinical outcome of nedaplatin-based CCRT for FIGO Stage IB2–IVA has not yet been reported, and the estimation of toxicity remains insufficient because of the short follow-up period in these studies.
This study aimed to retrospectively evaluate the efficacy and safety of nedaplatin-based CCRT and to analyze prognostic factors for survival among patients with FIGO Stage IB2–IVA cervical cancer.
MATERIALS AND METHODS
Patients
Overall, 178 patients with histologically proven FIGO IB2–IVA cervical cancer were treated at Osaka University Hospital (Osaka, Japan) between December 1999 and December 2009. Of these, 126 patients were excluded from this study because of previous radiation therapy (RT) alone, lack of HDR brachytherapy in the treatment schedule, or distant metastases. Thus, a total of 52 patients with FIGO Stage IB2–IVA cervical cancer treated with radical CCRT with nedaplatin were included for analysis. Of a total of 52 patients, 20 were treated within the context of a previous clinical study [11].
Characteristics of the included patients are shown in Table 1. Tumor diameter and pelvic nodal status were diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI) and reviewed by radiologists. No patient underwent biopsy to confirm metastasis of the enlarged nodes (minimum diameter ≥ 10 mm).
Table 1.
Patient characteristics
| Number of patients (%)a | ||
|---|---|---|
| Age | Median (years) (range) | 63 (25–73) |
| Performance status | 0 | 46 (88) |
| 1 | 5 (10) | |
| 2 | 1 (2) | |
| FIGO stage | IB2 | 3 (6) |
| IIA2 | 2 (4) | |
| IIB | 16 (30) | |
| IIIA | 1 (2) | |
| IIIB | 27 (52) | |
| IVA | 3 (6) | |
| Hydronephrosis | Yes | 6 (11) |
| No | 46 (89) | |
| Histology | Squamous cell carcinoma | 44 (85) |
| Adenocarcinoma | 8 (15) | |
| Pretreatment Hbb | Median (mg/dl) (range) | 12.0 (6.1–13.8) |
| Pretreatment Crc | Median (mg/dl) (range) | 0.6 (0.3–1.4) |
| Maximum tumor diameter | Median (mm) (range) | 46 (30–100) |
| >40 mm | 36 (60) | |
| ≤40 mm | 16 (40) | |
| PLNd metastases (≥10 mm) | Negative | 32 (62) |
| Positive | 20 (38) | |
| Duration of follow-up (for all patients) | Median (months) (range) | 52 (4–123) |
aValues are presented as number (%) or median (range), bHb = hemoglobin, cCr = creatinine, dPLN = pelvic lymph node.
This study was performed according to the guidelines approved by the institutional review board of our institution. Written informed consent was obtained from each patient before receiving treatment.
External beam radiotherapy
External beam radiotherapy (EBRT) was performed using a 10-MV X-ray machine. Anterior–posterior parallel-opposed portals or 4-field orthogonal portals were used to administer a single 2-Gy dose in five fractions per week. The upper margin of the radiation field for the whole pelvis was taken as the upper border of the fifth lumbar vertebra, the lower margin was the inferior border of the obturator foramen, and the lateral margin was 1.5–2 cm lateral to the bony pelvis. Since April 2008, all patients have been treated with 3D conformal radiotherapy. A whole pelvic field with midline block was defined as the whole pelvic field, with a 4-cm-wide midline block at the isocenter plane at the lower two-thirds length of the field. An additional boost of irradiation at a total dose of 6 Gy in three fractions, using opposing anterior–posterior beams, was delivered to the enlarged nodes in one patient.
For HDR brachytherapy, HDR ICBT or HDR interstitial brachytherapy (HDR ISBT) was performed. HDR ICBT was performed once per week during the course of EBRT with a midline block field. Otherwise, HDR ISBT was performed in the period between the course of EBRT and that of EBRT with a midline block field. EBRT was skipped on the day that HDR ICBT or HDR ISBT was performed.
Different radiotherapy schedules and doses were administered for each FIGO stage and tumor size because of differences in extension of tumors in the parametrium and vagina. Details of our RT schedule are described in Table 2. The equivalent dose in 2-Gy fractions (EQD2) administered to Point A, defined as 2 cm above the cervical os and 2 cm perpendicular to the uterine axis along the plane of the uterus, was calculated using the linear quadratic (LQ) equation for each schedule. The equation used to calculate the EQD2 was as follows:
where N is the fraction number of EBRT (before central shielding), d is the fractional dose of EBRT, NB is the fraction number of HDR ICBT or HDR ISBT, and dB is the fractional dose of HDR ICBT or HDR ISBT. For the LQ calculation, a value of α/β = 10 was assumed for tumors.
Table 2.
Treatment schedule
| EBRT (Gy) |
HDR |
EQD2 (Gy) | No. of pts. | |||
|---|---|---|---|---|---|---|
| WP | WP with MB | ICBT (Gy/fr.) | ISBT (Gy/fr.) | |||
| 0 | 40 | 28.8/4 | 41 | 1 | ||
| 20 | 30 | 28.8/4 | 61 | 11 | ||
| 30 | 20 | 27.2/4 | 68 | 29 | ||
| 40 | 10 | 20.4/3 | 69 | 6 | ||
| 50 | 0 | 13.6/2 | 69 | 2 | ||
| 50 | 0 | 18/3 | 74 | 3 | ||
EBRT = external beam radiotherapy, WP = whole pelvis, MB = midline block, HDR = high-dose rate, ICBT = intracavitary brachytherapy, fr. = fraction, ISBT = interstitial brachytherapy, EQD2 = equivalent dose in 2 Gy fractions, pts. = patients.
Intracavitary brachytherapy
ICBT was administered to patients using the microSelectron Digital (HDR-V3) Brachytherapy Afterloader (Elekta Inc., Atlanta, GA, USA) with Fletcher-type (Fletcher–Williamson Asia Pacific) metal applicators (Elekta Inc.) comprising one curved central tandem and two non-shielded ovoids without general anesthesia [12]. For patients with vaginal infiltration or a narrow vagina, a tandem with a vaginal cylinder was used. The treatment unit houses an iridium (192Ir) source of 370 GBq at maximum activity. After insertion of the applicators, the vaginal wall was kept distant from the applicator with packing gauze to protect the rectum and bladder from exposure to the high-dose area. Next, a flexible lead bead wire was inserted into the rectum to sense the rectal lumen. Each prescribed ICBT dose was 6.8 or 7.2 Gy per fraction at Point A. The rectal dose was estimated using the points of the lead bead wire that was inserted into the rectum and calculated using a treatment-planning system (PLATO; Elekta Inc.). In the early period, only radiolucent gauze was used for vaginal packing; therefore, it was impossible to calculate the dose to the rectal reference point as defined in Report 38 from the International Commission on Radiation Units and Measurements.
Interstitial brachytherapy
ISBT was also administered using the microSelectron Digital (HDR-V3) Brachytherapy Afterloader with an 192Ir source [13]. For implantation, interstitial stainless steel needles were inserted using the Martinez Universal Perineal Interstitial Template. The template and vaginal cylinder were inserted into the vagina under guidance by transrectal ultrasound imaging and sutured to the perineal skin. Under real-time transrectal ultrasound monitoring of the largest cross-section of the tumor, the applicators were placed at 1-cm intervals along a line covering the tumor and the vaginal cylinder. On the rectal side, the applicators were positioned 0–3 mm inside the tumor border. The top 1-cm bands of the applicators were inserted into the peritoneum beyond the tumor. HDR ISBT was performed using 3D image-based planning with CT scans (slice thickness, 2.5 mm) after the needles were inserted. The treatment-planning system (PLATO; Elekta Inc.) was then used to contour the target volume based on the CT-standardized contour guidelines [14]. Patients underwent diagnostic MRI before ISBT administration to obtain scans for use as references when the gross tumor volume (GTV) and organs at risk (OARs; rectum and bladder) were contoured during the planning CT scan (CT–MRI fusion was not used). GTV was determined based on the tumor dimensions detected by T2-weighted MRI. The clinical target volume (CTV) consisted of GTV plus the upper third of the vagina. During the CT-based dose prescription, the treatment-planning system was used in combination with manual modification to ensure that 100% of the isodose line encompassed the CTV on every slice. We ensured that the doses delivered to OARs were <100% of the prescribed dose, except in cases where the OARs adhered to or were invaded by the tumor. The first fraction of irradiation (prescribed dose, 6 Gy) was administered during the afternoon on the day of the implant procedure. Beginning on the following day, 6 Gy of irradiation was administered twice a day with at least 6 h between each treatment session (total dose, 18 Gy).
Chemotherapy
For CCRT, chemotherapy consisted of weekly nedaplatin intravenously administered at a dose of 35 mg/m2 in a 1-h infusion. Hydration was not required before or after drug administration. The first cycle of nedaplatin was initiated during the first round of EBRT. Drug administration was repeated weekly during the course of EBRT and HDR brachytherapy, except on the same day that HDR brachytherapy was performed. A median of five cycles (range, 1–6) was administered per patient. Two patients (4%) received six cycles of chemotherapy, 32 (62%) received five cycles, 10 (19%) received four cycles, 4 (8%) received three cycles, 3 (6%) received two cycles, and one patient received only one cycle because of anaphylaxis. Renal function and blood counts were estimated before each cycle. Drug administration was withheld if the granulocyte count was <1500/μl or the platelet count was <100 000/μl. Drug administration was also withheld if the patient could not tolerate acute gastrointestinal toxicity during the treatment course. Even if chemotherapy was suspended, radiation therapy was continued as long as the white blood cell count was >2000/μl and the platelet count was >50 000/μl.
Follow-up and treatment assessment
During treatment, patients were evaluated at least weekly by clinical assessment, pelvic examination, and complete blood count. MRI examinations were performed at 20–40 Gy of EBRT to assess treatment response and ICBT applicability.
From 2–4 weeks after completion of treatment we evaluated the response during regular follow-up examinations every 1–2 months for the first year and then every 3 months thereafter. A pelvic examination was performed during each follow-up visit, and tumor markers were checked every 3–6 months. Three months after completion of treatment, we evaluated initial treatment response by radiologic pelvic examinations and smears. If residual disease was suspected, a biopsy was performed. Additionally, radiographic examinations (chest X-ray, CT scan, and positron emission tomography CT) were conducted yearly. Local and pelvic recurrences were confirmed if disease was detected in the irradiated field. Distant metastases were defined by tumor growth outside of the pelvis. When pelvic recurrence was noted at follow-up, salvage surgery was performed whenever possible. Otherwise, chemotherapy or palliative RT was administered for treatment of recurrent tumors. Acute and late adverse effects were evaluated according to the Common Terminology Criteria for Adverse Events (ver. 4.0) guidelines.
Statistical analysis
Patient survival was measured from the date of therapy initiation to the date of the last follow-up examination. Overall survival (OS), progression-free survival (PFS), and local control (LC) were estimated using the Kaplan–Meier method. The statistical significance of various parameters for PFS was analyzed using the Cox proportional hazards model. The logistic regression method was used for analysis of factors affecting local recurrence after achieving a complete response (CR), acute Grade 3–4 hematologic toxicities, and late Grade 3 gastrointestinal toxicities. Comparison of categorical variables was performed using the chi-square test. A probability (P) value of <0.05 was considered statistically significant. All analyses were performed using JMP ver. 9.0.2 statistical software (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Treatment outcomes
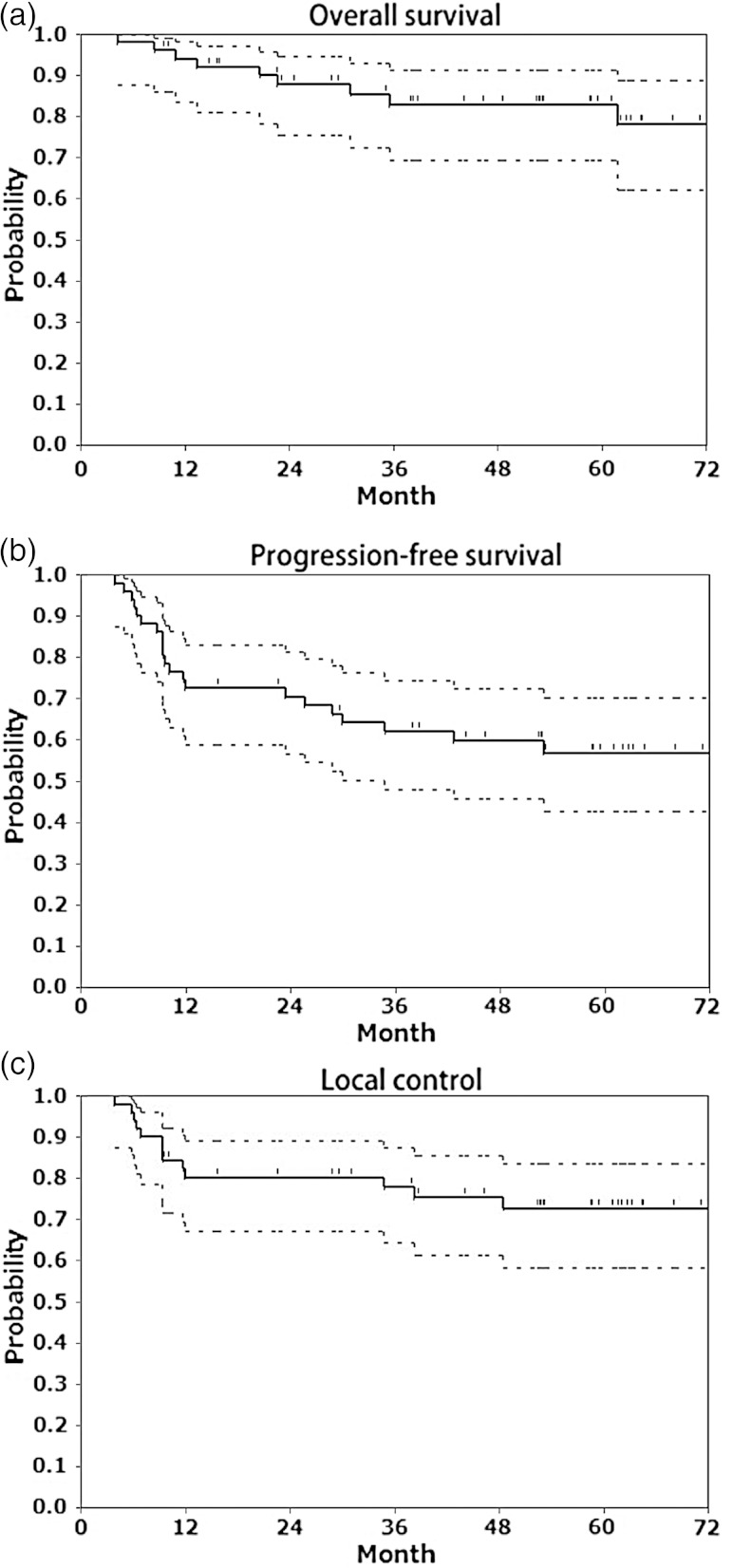
Three months after completion of CCRT, 44 patients (85%) achieved CR within the pelvic lesion and 8 (15%) exhibited a partial response (PR). CR of the primary tumor was achieved by 46 patients (88%), but nine developed local recurrence. Of the 52 patients, 20 experienced treatment failure: 15 (29%) had locoregional failure, and 5 (10%) experienced only distant metastases. The estimated 5-year OS, PFS and LC rates were 78% [95% confidence interval (CI), 69–91%], 57% (95% CI, 42–70%) and 73% (95% CI, 58–84%), respectively (Fig. 1).
Fig. 1.
(a) Overall survival, (b) progression-free survival, and (c) local control of cervical cancer by treatment modality. Kaplan–Meier estimates of overall survival, progression-free survival, and local control in 52 patients with cervical cancer treated with nedaplatin-based concurrent chemoradiotherapy using high-dose-rate intracavitary brachytherapy. Dashed lines represent 95% confidence intervals.
Acute adverse effects
The most severe toxicities experienced by the patients are listed in Table 3. There were no treatment-related deaths. Hematologic toxicity was the most common acute toxicity. Otherwise, there was no incident of acute kidney toxicity, as evaluated by an increase in serum creatinine.
Table 3.
Acute adverse effects
| Adverse effects | Grade |
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ≥3 (%)a | |
| Leukopenia | 11 | 4 | 13 | 23 | 1 | 24 (46) |
| Anemia | 8 | 19 | 18 | 7 | 0 | 7 (13) |
| Neutropenia | 14 | 2 | 21 | 14 | 1 | 15 (29) |
| Thrombocytopenia | 34 | 10 | 4 | 4 | 0 | 5 (10) |
| Diarrhea | 19 | 25 | 6 | 2 | 0 | 2 (4) |
| Acute kidney toxicity | 55 | 0 | 0 | 0 | 0 | 0 (0) |
aValues are presented as patient number (%).
Late adverse effects
Major late adverse effects are described in Table 4. There were no Grade 4 complications, whereas Grade 3 complications occurred in 7 patients (14%). Five patients (10%) developed Grade 3 gastrointestinal toxicities diagnosed as intestinal obstruction. Two patients (4%) developed Grade 3 rectovaginal fistulas and all received a colostomy. Regarding renal and urinary disorders, there was no instance of renal toxicity.
Table 4.
Late adverse effects
| Adverse effects | Grade |
|||
|---|---|---|---|---|
| 2 | 3 | 4 | ≥3 (%)a | |
| Enterocolitis | 15 | 5 | 0 | 5 (10) |
| Intestinal obstruction | 4 | 0 | 0 | 0 (0) |
| Rectovaginal fistula | 0 | 2 | 0 | 2 (4) |
| Vesicovaginal fistula | 0 | 0 | 0 | 0 (0) |
| Creatinine increase | 0 | 0 | 0 | 0 (0) |
| Urine output decrease | 0 | 0 | 0 | 0 (0) |
aValues are presented as patient number (%).
Statistical analysis
Table 5 shows the results of the Cox proportional hazards regression model for predicting PFS in cervical cancer. Univariate analysis revealed that histologic type [squamous cell carcinoma (SCC) vs adenocarcinoma] (P = 0.03), maximum tumor diameter (≤40 vs >40 mm; P = 0.01), and pretreatment hemoglobin level (≥11 vs <11 g/dl; P = 0.03) were significantly associated with PFS. Multivariate analysis revealed that histologic type (SCC vs adenocarcinoma; P = 0.02), maximum tumor diameter (≤40 vs >40 mm; P < 0.01), and pretreatment hemoglobin level (≥11 vs <11 g/dl; P = 0.01) remained as significant prognostic factors.
Table 5.
Univariate and multivariate Cox proportional hazards model to predict progression-free survival in cervical cancer treated with CCRT
| Variables | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age (as continuous variable) | 0.99 (0.96–1.03) | 0.51 | |||
| BMI (as continuous variable) | 0.95 (0.82–1.08) | 0.42 | |||
| Tobacco use | No vs Yes | 0.57 (0.13–1.69) | 0.33 | ||
| Alcohol use | No vs Yes | 0.87 (0.26–2.48) | 0.87 | ||
| Diabetes | No vs Yes | 1.54 (0.44–4.18) | 0.46 | ||
| FIGO stage | IB2, IIA2 vs IIB, IIIA | 4.30 (0.82–79.07) | 0.09 | ||
| vs IIIB, IVA | 2.31 (0.44–42.52) | 0.37 | |||
| Hydronephrosis | No vs Yes | 1.37 (0.31–3.93) | 0.67 | ||
| Histologic type | SCC vs Adenocarcinoma | 3.32 (1.12–8.44) | 0.03 | 3.55 (1.22–9.23) | 0.02 |
| Maximum tumor diameter (as continuous variable) | 1.49 (1.17–1.84) | <0.01 | |||
| Maximum tumor diameter | ≤40 vs >40 mm | 3.82 (1.29–16.38) | 0.01 | 4.93 (1.60–21.72) | <0.01 |
| Pretreatment hemoglobin (as continuous variable) | 0.70 (0.52–0.93) | 0.01 | |||
| Pretreatment hemoglobin | ≥11 vs <11 g/dl | 2.72 (1.12–6.49) | 0.03 | 3.22 (1.34–8.13) | 0.01 |
| Pelvic lymph node metastases | Negative vs Positive | 2.22 (0.93–5.35) | 0.07 | ||
| Pretreatment SCC Ag (as continuous variable) | 1.01 (0.99–1.02) | 0.18 | |||
| Pretreatment CEA (as continuous variable) | 1.02 (0.97–1.06) | 0.43 | |||
| Overall treatment time (as continuous variable) | 1.00 (0.95–1.04) | 0.91 | |||
CCRT = concurrent chemoradiotherapy, BMI = body mass index, FIGO = International Federation of Gynecology and Obstetrics, SCC = squamous cell carcinoma, SCC Ag = squamous cell carcinoma antigen, CEA = carcinoembryonic antigen, HR = hazard risk, CI = confidence interval.
Comparisons of categorical variables between cases with and without evidence of local disease after CR using the chi-square test are shown in Table 6. There were significant differences between histologic type (P < 0.01), maximum tumor diameter (P = 0.03), and pretreatment hemoglobin level (P = 0.04). However, in this case, the cut-off values of maximum tumor diameter of 4.0 cm and pretreatment hemoglobin level of 11.0 g/dl were not significant.
Table 6.
Comparison between local CR group and local recurrence after CR groupa
| Characteristic | NED after CR group | Recurrence after CR group | Chi-square test |
|---|---|---|---|
| No. of pts. = 37 (80%) | 9 (20%) | P-value | |
| Age; Median (years) (range) | 63 (25–73) | 64 (47–71) | 0.83 |
| FIGO Stage (IB2/II vs III/IVA) | 0.59 | ||
| IB2/II | 16 (43) | 3 (33) | |
| III/IVA | 21 (57) | 6 (64) | |
| Histology (SCC vs Adenocarcinoma) | <0.01 | ||
| SCC | 34 (92) | 5 (56) | |
| Adenocarcinoma | 3 (8) | 4 (44) | |
| Maximum tumor diameter; Median (mm) (range) | 4.2 (3–8.5) | 5.8 (3.8–7.5) | 0.03 |
| ≤40 vs >40 mm | 0.09 | ||
| ≤40 mm | 14 (38) | 1 (11) | |
| >40 mm | 23 (62) | 8 (89) | |
| Pretreatment hemoglobin; Median (mg/dl) (range) | 12.4 (8.7–13.8) | 11.0 (6.1–13.7) | 0.04 |
| Pretreatment SCC Ag; Median (ng/ml) (range) | 13 (<1–66) | 3 (<1–193) | 0.31 |
| Pretreatment CEA; Median (ng/ml) (range) | 3 (<1–43) | 7 (<1–27) | 0.18 |
| EQD2; Median (Gy) (range) | 68 (41–74) | 68 (59–68) | 0.31 |
| Overall treatment time; Median (days) (range) | 44 (28–70) | 49 (41–87) | 0.07 |
aValues are presented as number (%) or median (range). CR = complete response, NED = no evidence of disease, EQD2 = equivalent dose in 2 Gy fractions.
Comparisons of categorical variables between cases with and without the development of acute Grade 3–4 hematologic toxicities, including leucopenia, anemia, neutropenia and thrombocytopenia (using the chi-square test) are shown in Table 7. There was no statistically significant difference. Additionally, we analyzed comparisons of categorical variables between cases with and without the development of late Grade 3 gastrointestinal toxicities with respect to the same factors on Table 7 [age (P = 0.56), BMI (P = 0.81), tobacco use (P = 0.22), alcohol use (P = 0.48), diabetes (P = 0.35), FIGO Stage (IB2/II vs III/IVA) (P = 0.91) and total nedaplatin dose (P = 0.08)]. No statistically significant differences were found.
Table 7.
Univariate analysis for the development of Grade 3–4 hematologic toxicitiesa,b
| Characteristic | Grade 0–2 | Grade 3–4 | Chi-square test |
|---|---|---|---|
| No. of pts. = 21 (40%) | 31 (60%) | P-value | |
| Age; Median (years) (range) | 64 (25–71) | 62 (38–73) | 0.68 |
| BMI; Median (kg/m2) (range) | 20 (16–30) | 21 (16–26) | 0.59 |
| Tobacco use | 0.70 | ||
| Yes | 5 (24) | 6 (19) | |
| No | 16 (76) | 25 (81) | |
| Alcohol use | 0.28 | ||
| Yes | 6 (29) | 5 (16) | |
| No | 15 (71) | 26 (84) | |
| Diabetes | 0.13 | ||
| Yes | 1 (5) | 6 (19) | |
| No | 20 (95) | 25 (81) | |
| FIGO Stage (IB2/II vs III/IVA) | 0.07 | ||
| IB2/II | 12 (57) | 10 (32) | |
| III/IVA | 9 (43) | 21 (68) | |
| Total nedaplatin dose; Median (mg) (range) | 200 (50–300) | 228 (71–325) | 0.45 |
aHematologic toxicities including leucopenia, anemia, neutropenia, thrombocytopenia. bValues are presented as number (%) or median (range).
DISCUSSION
Since the 1999 National Cancer Institute (NCI) Clinical alert was issued, chemoradiotherapy has become widely used in the treatment of cervical cancer. Various chemotherapeutic regimens have been investigated for CCRT in cervical cancer. Currently, weekly administration of cisplatin at a dose of 40 mg/m2 has become a standard regimen [15–20]. The Phase II JGOG 1066 study demonstrated that CCRT with a weekly cisplatin dose of 40 mg/m2 for locally advanced cervical cancer was available in Japan, despite using lower cumulative radiation dose schedules at Point A []. Comparisons of the clinical outcome and toxicities of cisplatin- or nedaplatin-based CCRT are indicated in Table 8. The 3-year OS rate of 83% and the 3-year PFS rate of 35% found in our study are comparable with those demonstrated in previous clinical studies of cisplatin- and nedaplatin-based CCRT.
Table 8.
Literature review: survival and complications
| Author (Reference) | Year | Study type | Chemo | FIGO stage | 3-y OS (%) | 3-y PFS (%) | Leukopenia G3–4 (%) | Thrombocytopenia (G3–4) (%) | Renal toxicity (G1–2) (%) |
|---|---|---|---|---|---|---|---|---|---|
| M Morris [15] | 1999 | RCT | Cisplatin | III–IVA | 75 | ||||
| PG Rose [16] | 1999 | RCT | Cisplatin | II–IVA | 66 | 62 | 23 | 2 | |
| T Toita [17] | 2005 | Retro | Cisplatin | IB2–III | 79 | 82 | 26 | 10 | |
| YL Chung [18] | 2005 | Phase I/II | Cisplatin | IIB–IVA | 83 | 10 | 3 | ||
| SW Chen [19] | 2006 | Retro | Cisplatin | IIB–III | 80 | 24 | 4 | ||
| R Potter [20] | 2006 | Retro | Cisplatin | IB–IV | 61 | 51 | 23 | 10 | |
| K Ushijima [21] | 2013 | Retro | Cisplatin | IB2–II | 72 | 58 | 61 | ||
| III–IVA | 52 | 40 | |||||||
| Y Yokoyama [10] | 2008 | Phase I/II | Nedaplatin | IB2–IVA | 78 | 59 | 45 | 4 | |
| Present study | Retro | Nedaplatin | IB2–IVA | 83 | 62 | 46 | 10 | 0 |
Chemo = chemotherapy, 3-y OS = 3-year Overall Survival, 3-y PFS = 3-year Progression-free Survival, G = grade, RCT = randomized clinical trial.
Regarding acute toxicities, the hematologic toxicity of nedaplatin-based CCRT was comparable with that of cisplatin-based CCRT. There was no statistically significant association with Grade 3–4 hematologic toxicities found in this study.
It is, however, remarkable that there was no renal toxicity found in our study in contrast to other studies of cisplatin-based CCRT [10, 15–21]. Serkies et al. [22] reported that the most common side effects causing discontinuation of cisplatin-based chemotherapy included gastrointestinal toxicity and impaired renal function. Chen et al. [19] also reported discontinuation of chemotherapy in three (4%) patients because of renal toxicity. Additionally, in the JGOG 1066 study, acute toxicity of increased serum creatinine occurred in 14 patients (19%), including Grade 3 toxicity in two patients. Although there was no discontinuation of chemotherapy, chemotherapy delay or dose reduction was necessary in four patients (6%) because of elevated serum creatinine. In contrast, renal toxicity did not affect the chemotherapy schedule in our study.
Regarding late complications, Grade 3 gastrointestinal toxicities (diagnosed as intestinal obstruction) occurred in five patients (10%) in this study. Some studies reported that severe late gastrointestinal toxicities (≥Grade 3) were observed in 3–9% of patients treated with cisplatin-based CCRT [16, 17, 19]. The frequency of Grade 3 gastrointestinal toxicities in our study was almost the same as that found in these previous studies. And there was no significant statistic factor by our result in this study. In regards to late renal toxity, although Chen et al. reported that five patients (6.2%) developed renal insufficiency as an irreversible late adverse effect [19], none of the patients developed this complication in our study.
In our study population, histologic type (SCC vs adenocarcinoma), maximum tumor diameter (≤40 vs >40 mm), and pretreatment hemoglobin level (≥11 vs <11 g/dl) were significant prognostic factors for PFS. Additionally, these parameters were reported as prognostic factors for cisplatin-based CCRT in other studies [10, 23–26]. With nedaplatin-based CCRT, we should consider the prognostic factors found for cisplatin-based CCRT.
Regarding local tumor control, 46 patients in this study achieved local CR, although nine experienced local recurrence after CR, and five of these patients later developed distant metastases. Our study showed that histologic type had the most significant association with local recurrence after CR. Adenocarcinoma occurred most often as the local recurrence after CR. Katanyoo et al. reported that adenocarcinoma developed significantly more often as residual disease after treatment than did SCC and also had a significantly lower CR rate [27]. Our study demonstrated that adenocarcinoma requires careful follow-up, even if local CR is achieved.
Currently, there are a range of approaches to improving PFS and LC. A meta-analysis has revealed that the benefit associated with chemoradiotherapy might be obtained not only with the use of platinum but also with the use of non-platinum regimens as an additional option []. Moreover, that study suggests that additional chemotherapy after chemoradiotherapy might offer even greater benefits. Furthermore, consolidated CCRT regimens using a combination of platinum and non-platinum agents are available. Conversely, based on recommendations of the Groupe Européen de Curiethérapie/European Society for Therapeutic Radiation and Oncology working group [28, 29], 3D imaging (MRI or CT)-based treatment planning for cervical cancer brachytherapy, known as image-guided brachytherapy (IGBT), has become more widely used than traditional 2D brachytherapy. Hence, IGBT may achieve appropriate target coverage and local tumor control [30].
In conclusion, our data showed that nedaplatin-based CCRT for cervical cancer was feasible and efficacious and resulted in less toxicity compared with cisplatin-based CCRT, especially with respect to the lack of renal toxicity. Statistically, histologic type, maximum tumor diameter, and pretreatment hemoglobin level were significant prognostic factors for PFS. In the case of adenocarcinoma there was a greater incidence of local recurrence after CR. To improve treatment outcomes in patients exhibiting these factors, we should carefully note them and select higher intensity modalities, such as consolidation CCRT and IGBT.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant No. 25861097.
ACKNOWLEDGEMENTS
The results of this study were presented at the Radiological Society of North America 99th Scientific Assembly and Annual Meeting (RSNA 2013), 1–6 December 2013, Chicago, IL, USA.
REFERENCES
- 1.Lanciano R, Calkins A, Bundy BN, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J Clin Oncol. 2005;23:8289–95. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 2.Vale CL. Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–12. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toita T, Kitagawa R, Hamano T, et al. Phase II study of concurrent chemoradiotherapy with high-dose-rate intracavitary brachytherapy in patients with locally advanced uterine cervical cancer: efficacy and toxicity of a low cumulative radiation dose schedule. Gynecol Oncol. 2012;126:211–6. doi: 10.1016/j.ygyno.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Toita T, Kitagawa R, Hamano T, et al. Feasibility and acute toxicity of concurrent chemoradiotherapy (CCRT) with high-dose rate intracavitary brachytherapy (HDR-ICBT) and 40-mg/m2 weekly cisplatin for Japanese patients with cervical cancer: results of a Multi-Institutional Phase 2 Study (JGOG1066) Int J Gynecol Cancer. 2012;22:1420–6. doi: 10.1097/IGC.0b013e3182647265. [DOI] [PubMed] [Google Scholar]
- 5.Kanzawa F, Matsushima Y, Nakano H, et al. Antitumor activity of a new platinum compound (glycolate o,o') diammineplatinum (II) (254-S), against non-small cell lung carcinoma grown in a human tumor clonogenic assay system. Anticancer Res. 1988;8:323–7. [PubMed] [Google Scholar]
- 6.Monk BJ, Alberts DS, Burger RA, et al. In vitro phase II comparison of the cytotoxicity of a novel platinum analog, nedaplatin (254-S), with that of cisplatin and carboplatin against fresh, human cervical cancers. Gynecol Oncol. 1998;71:308–12. doi: 10.1006/gyno.1998.5140. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki Y, Shinkai T, Eguchi K, et al. Prediction of the antitumor activity of new platinum analogs based on their ex vivo pharmacodynamics as determined by bioassay. Cancer Chemother Pharmacol. 1991;27:263–70. doi: 10.1007/BF00685110. [DOI] [PubMed] [Google Scholar]
- 8.Uehara T, Watanabe H, Itoh F, et al. Nephrotoxicity of a novel antineoplastic platinum complex, nedaplatin: a comparative study with cisplatin in rats. Arch Toxicol. 2005;79:451–60. doi: 10.1007/s00204-005-0648-6. [DOI] [PubMed] [Google Scholar]
- 9.Kawai Y, Taniuchi S, Okahara S, et al. Gemba. Relationship between cisplatin or nedaplatin-induced nephrotoxicity and renal accumulation. Biol Pharm Bull. 2005;28:1385–8. doi: 10.1248/bpb.28.1385. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama Y, Takano T, Nakahara K, et al. A phase II multicenter trial of concurrent chemoradiotherapy with weekly nedaplatin in advanced uterine cervical carcinoma: Tohoku Gynecologic Cancer Unit Study. Oncol Rep. 2008;19:1551–6. [PubMed] [Google Scholar]
- 11.Mabuchi S, Ugaki H, Isohashi F, et al. Concurrent weekly nedaplatin, external beam radiotherapy and high-dose-rate brachytherapy in patients with FIGO stage IIIb cervical cancer: a comparison with a cohort treated by radiotherapy alone. Gynecol Obstet Invest. 2010;69:224–32. doi: 10.1159/000273207. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki O, Yoshioka Y, Isohashi F, et al. Effect of high-dose-rate 192Ir source activity on late rectal bleeding after intracavitary radiation therapy for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:1329–34. doi: 10.1016/j.ijrobp.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Mabuchi S, Takahashi R, Isohashi F, et al. Reirradiation using high-dose-rate interstitial brachytherapy for locally recurrent cervical cancer: a single institutional experience. Int J Gynecol Cancer. 2014;24:141–8. doi: 10.1097/IGC.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan AN, Dimopoulos J, Kirisits C, et al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–8. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 16.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 17.Toita T, Moromizato H, Ogawa K, et al. Concurrent chemoradiotherapy using high-dose-rate intracavitary brachytherapy for uterine cervical cancer. Gynecol Oncol. 2005;96:665–70. doi: 10.1016/j.ygyno.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Chung YL, Jian JJ, Cheng SH, et al. Extended-field radiotherapy and high-dose-rate brachytherapy with concurrent and adjuvant cisplatin-based chemotherapy for locally advanced cervical cancer: a phase I/II study. Gynecol Oncol. 2005;97:126–35. doi: 10.1016/j.ygyno.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Chen SW, Liang JA, Hung YC, et al. Concurrent weekly cisplatin plus external beam radiotherapy and high-dose rate brachytherapy for advanced cervical cancer: a control cohort comparison with radiation alone on treatment outcome and complications. Int J Radiat Oncol Biol Phys. 2006;66:1370–7. doi: 10.1016/j.ijrobp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Pötter R, Dimopoulos J, Bachtiary B, et al. 3D conformal HDR-brachy- and external beam therapy plus simultaneous cisplatin for high-risk cervical cancer: clinical experience with 3 year follow-up. Radiother Oncol. 2006;79:80–6. doi: 10.1016/j.radonc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Ushijima K, Fujiyoshi K, Kawano K, et al. Concurrent chemoradiotherapy with low-dose daily cisplatin for high risk uterine cervical cancer: a long-term follow-up study. J Gynecol Oncol. 2013;24:108–13. doi: 10.3802/jgo.2013.24.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serkies K, Jassem J. Concurrent weekly cisplatin and radiotherapy in routine management of cervical cancer: a report on patient compliance and acute toxicity. Int J Radiat Oncol Biol Phys. 2004;60:814–21. doi: 10.1016/j.ijrobp.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Nugent E, Case A, Hoff J, et al. Chemoradiation in locally advanced cervical carcinoma: an analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol. 2010;116:438–41. doi: 10.1016/j.ygyno.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Lim A, Sia S. Outcomes of chemoradiotherapy in cervical cancer—the western Australian experience. Int J Radiat Oncol Biol Phys. 2012;82:1431–8. doi: 10.1016/j.ijrobp.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 25.Kudaka W, Nagai Y, Toita T, et al. Long-term results and prognostic factors in patients with stage III–IVA squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy from a single institution study. Int J Clin Oncol. 2013;18:916–21. doi: 10.1007/s10147-012-0457-x. [DOI] [PubMed] [Google Scholar]
- 26.Shim S, Lee S, Park J, et al. Risk assessment model for overall survival in patients with locally advanced cervical cancer treated with definitive concurrent chemoradiotherapy. Gynecol Oncol. 2013;128:54–9. doi: 10.1016/j.ygyno.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012;125:292–6. doi: 10.1016/j.ygyno.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Haie-Meder C, Pötter R, van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2004;74:235–45. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Pötter R, Haie-Meder C, van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:66–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos JC, Lang S, Kirisits C, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:56–63. doi: 10.1016/j.ijrobp.2008.10.033. [DOI] [PubMed] [Google Scholar]