Abstract
The aim of this study was to evaluate the role of radiotherapy in the treatment of hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVT) and to determine the prognostic factors for overall survival. Altogether, 106 patients with HCC and PVT referred for radiotherapy between 2004 and 2009 were retrospectively reviewed. A total of 60 Gy in 30 daily fractions was delivered with intensity-modulated radiotherapy techniques. Patient-related and treatment-related factors were analyzed to evaluate their prognostic significance for the overall survival rate. Complete response was noted in 10 patients and partial response in 55 patients. The liver lesions had become resectable after the completion of radiotherapy in 12 patients, and surgery was subsequently performed. One or two courses of transarterial chemoembolization (TACE) were administered to 19 patients following radiotherapy. The 1-year and 2-year overall survival rates were 34.7% and 11%, respectively, and the median survival was 7 months for the entire cohort of patients. Post-radiotherapy treatment modality, response to radiotherapy and JIS score were demonstrated as independent prognostic factors for overall survival (P = 0.003, P < 0.001, P < 0.001, respectively). For patients who received surgical intervention after radiotherapy, the median survival was 30 months and the 2-year overall survival rate was 67%. Radiotherapy achieved a 61.5% objective response rate and prolonged survival in patients with PVT. The liver tumors had become resectable after radiotherapy in 11% of patients. Our results suggested that radiotherapy could offer survival benefits and should be considered as a treatment option for patients with PVT. Radiotherapy could also be considered as a preoperative treatment modality in patients with HCC and PVT.
Keywords: preoperative, radiotherapy, hepatocellular carcinoma, portal vein
INTRODUCTION
Hepatocellular carcinoma (HCC) remains a common and highly lethal disease worldwide. The incidence of HCC is increasing in the world []. The treatment of choice for hepatocellular carcinoma is surgical resection whenever possible. However, only a minority of patients present with resectable tumors. Most patients have locally advanced disease at diagnosis and are only candidates for palliative treatment. The usual comorbidity of underlying liver diseases might also preclude the surgery or make the surgical exploration dangerous. For locally advanced HCC, portal vein tumor thrombosis (PVT) can be noted in more than 40% of patients and is often associated with extremely poor prognosis [, ].
There is no standard treatment strategy for patients with HCC and PVT. The role of transarterial chemoembolization (TACE) in patients with PVT has previously been considered contraindicated due to the potential risk of liver failure. Recently, some authors have proposed that TACE can be performed for patients with PVT as long as there is sufficient collateral hepatopetal flow and good hepatic reserve []. Historically, radiotherapy has played a minor role in the management of HCC with PVT because of the poor tolerance of the whole liver to radiation and the risk of radiation-induced liver disease (RILD) [5–7]. Liver motion caused by breathing could also make high-precision radiotherapy a challenge. With growing knowledge of normal liver tolerance and the advances in radiotherapy techniques, partial liver irradiation has yielded some promising results in patients with unresectable HCC [8–11]. The tolerance radiation dose for 30% liver irradiation has been demonstrated to be as high as 70 Gy [10]. It has also been hypothesized that high-dose radiotherapy might lead to sustained local control and possible cure of localized HCC [8, 12]. Promising outcomes have also been observed in patients with PVT treated with radiotherapy [13–17].
In this study, we evaluated our experience in patients with HCC and PVT treated with radiotherapy and discussed the role of radiotherapy in the treatment strategy.
METHODS
From October 2004 to October 2009, 130 consecutive patients with unresectable HCC complicated with PVT in the main trunk or first branches were referred to our department. With informed patient consent and the approval of the Institutional Review Board, the medical records of these patients were reviewed. All patients were evaluated by a multidisciplinary team. Neither TACE nor surgery was recommended for these patients due to concern about poor hepatic reserve, poor efficacy, and high complication rates. Patients with the following characteristics were excluded from receiving radiotherapy: previously treated for primary HCC, Eastern Cooperative Oncology Group (ECOG) performance status of 3 or more, liver function of Child–Pugh class C, and extrahepatic metastasis. A total of 106 patients received radiotherapy and were evaluated in this retrospective study, comprised of 85 men and 21 women, and the median age was 57 years (range 29–79). The diagnosis of HCC was based on the American Association for the Study of Liver Diseases (AASLD) guidelines [18]. All patients had a pretreatment evaluation including complete history, physical examination, hematology and biochemistry profiles, hepatitis screening, chest radiographs, abdominal sonography and computed tomography (CT) scan of the abdomen. It was noted that 59 patients had chronic hepatitis B (serologically positive for hepatitis B surface antigen, HBsAg) and 37 patients had chronic hepatitis C (serologically positive for anti-hepatitis C virus, anti-HCV).
PVT (main: 30, right first branch: 71, left first branch: 37) was confirmed by sonography, CT scan or angiography. All patients were staged according to the following models: Child–Pugh classification [19], 2010 American Joint Committee on Cancer staging system (AJCC) [20], Okuda staging system [21], Cancer of the Liver Italian Program (CLIP) score [22], Barcelona Clinic Liver Cancer (BCLC) staging system [23], Chinese University Prognostic Index (CUPI) score [24], and Japan Integrated Staging (JIS) score [25]. Due to portal vein invasion, all patients were categorized as AJCC Stage IIIB and BCLC Stage C. Patient characteristics are listed in Table 1.
Table 1.
Characteristics of 106 patients with hepatocellular carcinoma
| Characteristics | No. of patients | Percentage % |
|---|---|---|
| Gender | ||
| Male | 85 | 80 |
| Female | 21 | 20 |
| Age | ||
| <60 | 63 | 59.4 |
| ≥60 | 43 | 40.6 |
| Child–Pugh class | ||
| A | 83 | 78.3 |
| B | 23 | 21.7 |
| PVT | ||
| Main | 30 | 28 |
| Right | 71 | 67 |
| Left | 37 | 35 |
| Chronic hepatitis B | ||
| With | 59 | 56 |
| Without | 47 | 44 |
| Chronic hepatitis C | ||
| With | 37 | 35 |
| Without | 69 | 65 |
| Child–Pugh class | ||
| A | 83 | 78.3 |
| B | 23 | 21.7 |
| Okuda stage | ||
| I | 61 | 57.5 |
| II | 42 | 39.6 |
| III | 3 | 2.9 |
| CLIP score | ||
| 1–2 | 34 | 32 |
| 3–6 | 72 | 68 |
| CUPI score | ||
| ≤1 | 6 | 5.6 |
| 2–7 | 90 | 84.9 |
| ≥8 | 10 | 9.5 |
| JIS Score | ||
| 2 | 18 | 16.9 |
| 3 | 68 | 64.2 |
| 4 | 20 | 18.9 |
Radiotherapy techniques
All patients were immobilized using a customized thermoplastic cast in the supine treatment position with both arms raised above the head. CT scans with contrast enhancement were acquired in helical mode using a 3-mm slice thickness. An attempt was made to acquire the entire scan in one breath hold. Targets and normal tissues were contoured on axial planning CT images. The spinal cord, bilateral kidney, normal liver, stomach and small intestine were delineated as organs at risk. The recommendations for specifying gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) for treatment planning followed the International Commission on Radiation Units (ICRU) Report No. 50 [26]. The GTV was defined as the hypodense intraluminal filling defect area within the portal vein. The CTV was defined as the GTV plus 1-cm uniform 3D margins. If the primary tumors were contiguous with the PVT area, the partial primary tumor volume might be included within the CTV. The PTV included the CTV plus a non-uniform 3D expansion, 0.5-cm radial margins and 1.5-cm craniocaudal margins to compensate for setup uncertainty and respiratory movement of the liver. An intensity-modulated radiotherapy treatment plan was generated and evaluated using the Pinnacle treatment planning system (Ver. 8.0, ADAC, Milpitas, CA). Four to six coplanar beams were most often required to conform the high-dose volume to the PTV and to meet the dose constraints. The fraction volume of the normal liver receiving 30 Gy or more (V30 Gy) was kept less than 30%. Normal liver was quantified as the total liver minus the liver tumor and PVT area. The mean dose delivered to each kidney was kept less than 20 Gy. The maximal dose to the pinal cord was kept less than 45 Gy. The volume of stomach and small intestine receiving more than 50 Gy was kept less than 1 ml. A total of 60 Gy in 30 daily fractions, 5 fractions per week, was delivered to the PTV. The planning goal was to deliver more than 95% of the prescription dose to encompass at least 95% of the PTV. During radiation delivery, all patients were asked to breath shallowly to minimize respiratory motion.
Evaluation of treatment response and follow-up
All patients were followed up every month by means of physical examination and biochemistry after completion of radiotherapy. The treatment response was evaluated with CT scans and abdominal sonography performed 4–8 weeks after the completion of radiotherapy. According to the World Health Organization criteria [27], treatment response was divided into: complete response (complete disappearance of PVT), partial response (more than 50% of PVT regression), stable disease (less than 50% of PVT regression or less than 25% of PVT progression), and progressive disease (more than 25% of PVT progression). Objective response rates were defined as the sum of the complete response and partial response rates.
Evaluation of liver toxicity
Blood chemistry tests and complete blood cell counts were monitored weekly during the radiotherapy course and then monthly after the completion of radiotherapy. Radiation-induced liver toxicities were defined according to the common toxicity criteria [28]. RILD was defined as anicteric non-malignant ascites and elevation of alkaline phosphatase levels to more than twice the pretreatment values.
Statistics
The endpoints of this study were overall survival rates. Treatment outcomes were analyzed in relation to patient-related and treatment-related factors using univariate and multivariate analyses. Survival was calculated from the date of commencement of radiotherapy.
The probability of survival was calculated actuarially using Kaplan–Meier methods [29]. Differences between potential prognostic subgroups were tested for statistical significance by the log-rank test, P < 0.05 as the significance limit [30]. All statistically significant prognostic variables in univariate analysis were considered in the multivariate analysis. Multivariate analysis was performed with the Cox regression model to identify independent prognostic factors [31].
RESULTS
Two patients were lost to follow-up, with a median follow-up duration of 10 months. Ten patients did not complete their prescribed course of treatment (median dose: 52 Gy). Complete response was noted in 10 patients (9.5%), partial response in 55 patients (52%), stable disease in 35 patients (33%), and progressive disease in 6 patients (5.5%). The objective response rate was 61.5%. The liver lesions were converted to resectable after the completion of radiotherapy in 12 patients and surgery was subsequently performed. Liver lesions were considered as resectable in patients with the following characteristics: liver function of Child–Pugh class A, unilobar tumor location, unilateral PVT with main portal vein or contralateral portal vein involvement within 2 cm of portal vein confluence, estimated remnant liver volume >40% of total liver volume or 1% of patient body weight, indo-cyanide green 15 min retention test <15% and platelet count >100 000/μl. Most patients who fulfilled these criteria were patients with the tumor downsized and hypertrophy of the contralateral lobe. The interval between completion of radiotherapy and surgical intervention ranged from 1.2 to 5 months (median: 1.9 months). One or two courses of TACE were administered to 19 patients after radiotherapy.
At the time of this analysis, seven patients were alive. The 1-year and 2-year overall survival rates were 34.7% and 11%, respectively, and the median survival was 7 months for the entire cohort of patients. On univariate analysis, post-radiotherapy treatment modality, response to radiotherapy, multiple lesions, bilateral lesions, main portal vein invasion, Okuda stage, CLIP score, CUPI score, JIS score and Child–Pugh classification were revealed as significant prognostic factors for overall survival. Multivariate analysis further confirmed that post-radiotherapy treatment modality, response to radiotherapy and JIS score were independent prognostic factors for overall survival. For patients who received surgery after radiotherapy, the 2-year overall survival rate was 66.7% and the median survival was 30 months (Fig. 1). For those with a response to radiotherapy, the 1-year survival rate was 48.7%, compared with 12% for those without response (P < 0.0001). The results of the univariate and multivariate analyses are listed in Tables 2 and 3.
Fig. 1.
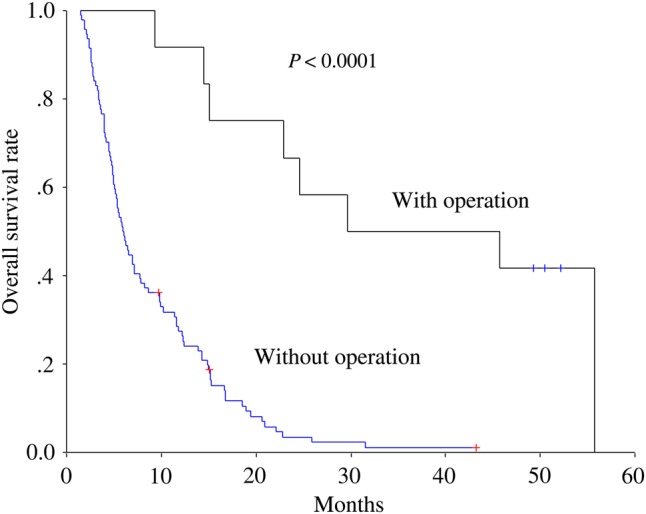
Overall survival rates for patient with and without operation after radiotherapy.
Table 2.
Univariate analysis of prognostic factors
| No. | 1-year OS (%) | 2-year OS (%) | |
|---|---|---|---|
| Post-RT treatment | P < 0.0001 | ||
| Operation | 12 | 91.7 | 66.7 |
| TACE | 19 | 57.9 | 15.8 |
| Without | 75 | 19.6 | 0 |
| Response to RT | P < 0.0001 | ||
| With | 65 | 48.7 | 17 |
| Without | 41 | 12 | 0 |
| No. of tumors | P = 0.0014 | ||
| Single | 21 | 61.9 | 25.8 |
| Multiple | 85 | 28 | 6.5 |
| Laterality | P = 0.0040 | ||
| Unilateral | 63 | 42.6 | 16.2 |
| Bilateral | 43 | 23.3 | 2.3 |
| Location of PVT | P = 0.0053 | ||
| Main | 30 | 26.7 | 0 |
| First branch | 76 | 37.8 | 14.7 |
| Okuda stage | P = 0.0050 | ||
| I | 61 | 42.3 | 17.9 |
| II | 42 | 26.2 | 2.4 |
| III | 3 | 0 | 0 |
| CLIP score | P = 0.0006 | ||
| 1–2 | 34 | 58.8 | 18.6 |
| 3–6 | 72 | 23.2 | 6.4 |
| CUPI score | P = 0.0043 | ||
| ≤ 3 | 34 | 52.8 | 18.6 |
| > 3 | 72 | 26.1 | 6.4 |
| Child-Pugh class | P < 0.0001 | ||
| A | 83 | 42 | 14.1 |
| B | 23 | 8.7 | 0 |
| JIS score | P < 0.0001 | ||
| 2 | 18 | 66.7 | 33.3 |
| 3 | 68 | 35.0 | 8.1 |
| 4 | 20 | 5 | 0 |
OS = overall survival, RT = radiotherapy.
Table 3.
Multivariate analysis of prognostic factors
| Parameter | P value | Hazard ratio | 95% CI |
|---|---|---|---|
| Post-RT Treatment | 0.003 | 0.622 | 0.456–0.846 |
| Response | <0.001 | 0.346 | 0.224–0.536 |
| JIS score | <0.001 | 2.327 | 1.618–3.347 |
CI = confidence interval, RT = radiotherapy.
Dose–volume histogram parameters
The median values of V20 Gy and V30 Gy of the normal liver were 40% (29% to 43%) and 28% (21% to 30%), respectively. The mean dose to the normal liver ranged from 15.3 to 23.1 Gy (median: 20.1 Gy). The mean dose to the kidney ranged from 0.6 to 14 Gy (median: 10.3 Gy).
Treatment-related complications
Acute radiation-related toxicities of Grade 1 or 2 were noted in 88% of patients. No acute radiation-related toxicity ≥ Grade 3 was noted. Three patients (2.8%) developed RILD. These patients presented with anicteric ascites, fatigue, body weight gain, vague discomfort over the right upper quadrant of the abdomen, elevation of alkaline phosphatase levels to more than three times the pretreatment values, and elevation of transaminase levels to more than twice the normal values. No mortality was directly related to the occurrence of RILD.
DISCUSSION
PVT is often associated with subsequent development of liver function impairment, extrahepatic tumor spread, and portal vein hypertension. For patients with HCC and PVT left untreated or treated with only symptomatic management, the median survival is reported to be <3 months [, 16]. Proton beam radiotherapy has been employed in the treatment of HCC with PVT, and Sugahara et al. reported an excellent result with a median local progression-free survival of 21 months [32]. For patients treated with 3D or intensity-modulated radiotherapy techniques, median survival ranges from 3.8 to 9.7 months, and 1-year overall survival rates ranging from 16.7% to 45.1% have been reported [15–17, 33]. In our study, the median survival was 7 months, and the 1-year overall survival rate was 34.7%. Our results showed that aggressive treatment did offer survival benefits compared with symptomatic management.
The role of radiotherapy in the treatment of HCC complicated with PVT is still controversial. The tolerance dose of the entire liver was thought to be much lower than the tumoricidal dose [5–7]. The antitumor effect of radiation was often offset by its adverse effect on liver function. However, the great technologic advances of radiotherapy in recent years have provided the means to deliver tumoricidal radiation doses to the partial liver, and objective tumor response rates ranging from 25.2% to 56.6% have been reported [17].
The optimal radiation dose for HCC remains a subject of debate. A radiation dose–response relationship has been observed in patients with unresectable HCC [8, 12]. In the literature, the dose of liver irradiation ranges from 30–72 Gy [12, 17, 34, 35]. Local radiotherapy applying 50–70 Gy was reported to achieve some clinical benefit, but was not capable of curing HCC [36].
With respect to radiobiology, HCC was considered to be a relatively radiosensitive tumor with an α/β ratio > 10 Gy [37]. Sinusoidal congestion, hyperemia, loss of central hepatic cells, loss of endothelium and thickening of intima have been noted in the irradiated liver. Hepatocytes are long-lived cells, dividing irregularly and rarely. However, after cell loss, the hepatocytes regenerate rapidly. Compensatory enlargement of non-irradiated liver after radiotherapy has been observed, and this was considered to contribute to improvement of the liver reserve [38]. Considering the physical characteristics of photon beams and the toxicities of the normal liver and surrounding normal tissues, it is still difficult for radiotherapy to serve as a definitive, curative treatment modality for locally advanced HCC with PVT, even with the aid of modern radiotherapy techniques. However, radiotherapy might serve as a neoadjuvant treatment modality for patients with HCC and PVT. In the literature, radiotherapy has been used for many years as a neoadjuvant treatment modality in malignancies of other anatomic sites, such as the rectum and esophagus. For liver cancer, little attention has been paid to the neoadjuvant role of radiotherapy. Besides reducing the portal vein occlusion by irradiation, the unique characteristic of compensatory liver enlargement after irradiation could increase the hepatic reserve and make subsequent surgery or TACE feasible. Our study offered some promising data in that 12 patients received post-RT surgery and 19 patients received post-RT TACE; these patients did have better overall survival rates than those treated with radiotherapy alone.
RILD is one of the most common radiation-related complications. RILD typically occurs 4–8 weeks after the completion of radiotherapy and limits the size of radiation doses [13]. RILD has been reported to be associated with a mortality rate of 84% [39]. With advanced radiotherapy techniques, the incidence and severity of RILD can be reduced if carefully selected dose–volumetric parameters are used for treatment planning. It has been reported that RILD can be prevented if the mean dose to the normal liver is <30 Gy [40, 41] or if V30 Gy is <33% [42]. In our study, V30 Gy of the normal liver was kept to <30%. High-dose radiation was delivered to the targets, but we simultaneously kept the radiation dose to the normal liver below the constraint. The incidence of RILD was <3%, and no mortality was directly associated with RILD (similar to previous studies). Our study indicated that high-dose radiotherapy for PVT is feasible as long as a substantial portion of the normal liver is spared.
In the literature, the treatment response rates to radiotherapy for patients with HCC and PVT range from 39–57% [17]. Our study showed a response rate of 61.5%, and treatment response to radiotherapy was demonstrated as an independent prognostic factor for overall survival. Improvement in the PVT might be a prerequisite for improved overall survival. Patients with a response to radiotherapy may have the chance to receive subsequent TACE or surgery. Both TACE and surgery have a positive impact on overall survival rates, and prolongation of survival is feasible.
An optimal staging system plays an important role in predicting the prognosis and directing the treatment strategy. To best assess the prognosis of HCC, it has been recommended that an ideal staging system should take tumor stage, liver function and physical status into account. Currently, there is no agreement on which staging systems would be optimal for patients with HCC and PVT. The Okuda staging system is the first staging system integrating tumor status and liver function reserve. The Child–Pugh classification is widely used to evaluate the liver damage in cirrhotic patients and has been demonstrated to have predictive significance in HCC patients [19]. The AJCC staging system only includes variables reflecting the tumor morphology and is less frequently surveyed in HCC patients. The CLIP score is a simple scoring system that accounts for liver function, tumor characteristics, presence of PVT, and serum level of alfa-fetoprotein. The BCLC classification has ever been demonstrated to offer peculiar prognostic ability [43]. The JIS score is the sum of simplified Tumor Node Metastasis (TNM) score and the Child–Pugh score. Neither the AJCC nor the BCLC staging systems could serve as significant prognostic predictors for patients with PVT because all patients were categorized as AJCC Stage IIIB and BCLC Stage C. In our study, the JIS score was found to be an independent prognostic factor for overall survival in patients with PVT. The Okuda, CLIP, Child–Pugh and CUPI systems were noted as significant prognostic factors for overall survival with univariate analysis, but failed to show independent prognostic significance with multivariate analysis.
CONCLUSIONS
Our study offers some promising results for modern radiotherapy to be able to serve as a neoadjuvant, especially preoperative, treatment modality in patients with HCC and PVT. Prospective randomized trial is desirable to assess the potential benefits and hazards of radiotherapy as a preoperative treatment modality.
REFERENCES
- 1.El Serag H-B, Mason A-C. Rising incidence of hepatocellular carcinoma in the United States. N Eng J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J-M, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 3.Pirisi M, Avellini C, Fabris C, et al. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397–400. doi: 10.1007/s004320050189. [DOI] [PubMed] [Google Scholar]
- 4.Lee H-S, Kim J-S, Choi I-J, et al. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–94. [PubMed] [Google Scholar]
- 5.Stillwagon G-B, Order S-E, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group study. Int J Radiat Oncol Biol Phys. 1989;17:1223–9. doi: 10.1016/0360-3016(89)90530-0. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence T-S, Robertson J-M, Anscher M-S, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–48. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 7.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 8.Dawson L-A, McGinn C-J, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–8. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 9.Cheng S-H, Lin Y-M, Chuang V-P, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–33. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence T-S, Ten Haken R-K, Kessler M-L, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–8. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 11.Seong J, Park H-C, Han K-H, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331–5. doi: 10.1016/s0360-3016(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 12.Park H-C, Seong J, Han K-H, et al. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–5. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombosis in patient with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–9. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 14.Ishikura S, Ogino T, Furuse J, et al. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–93. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Yamashita H, Shiraishi K, et al. Radiation therapy for portal venous invasion by hepatocellular carcinoma. World J Gastroenterol. 2005;11:7237–41. doi: 10.3748/wjg.v11.i46.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Z-C, Fan J, Tang Z-Y, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–43. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Kim J-Y, Chung S-M, Choi B-O, et al. Hepatocellular carcinoma with portal vein tumor thrombosis: improved treatment outcomes with external beam radiation therapy. Hepatol Res. 2011;41:813–24. doi: 10.1111/j.1872-034X.2011.00826.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 19.Pugh R-N-H, Murry-Lyon I-M, Dawson J-L, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 20.Edge S-B, Byrd D-R, Compton C-C. 7th ed. New York: Springer-Verlag Publishers; 2010. American Joint Committee on Cancer. Cancer Staging Manual. [Google Scholar]
- 21.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.The Cancer of the Liver Italian Program (CLIP) Investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28:751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 23.Llovet J-M, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 24.Leung T-W, Tang A-M, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–9. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–15. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 26.Bethesda, MD: ICRU; 1993. International Commission on Radiation Units and Measurements: ICRU Report No. 50: Prescribing, recording, and reporting photon beam therapy. [Google Scholar]
- 27.Miller A-B, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Trotti A, Colevas A-D, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan E-L, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 30.Peto R, Pike M-C, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox D-R. Regression models and life tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- 32.Sugahara S, Nakayama H, Fukuda K, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–8. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y-J, Hsu H-C, Wang C-Y, et al. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–63. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 34.Pattaranutaporn P, Chansilpa Y, Ieumwananonthachai N, et al. Three-dimensional conformal radiation therapy and periodic irradiation with the deep inspiration breath-hold technique for hepatocellular carcinoma. J Med Assoc Thai. 2001;84:1692–700. [PubMed] [Google Scholar]
- 35.Gunderson L-L, Haddock M-G, Foo M-L, et al. Conformal irradiation for hepatobiliary malignancies. Ann Oncol. 1999;10(Suppl 4):221–5. [PubMed] [Google Scholar]
- 36.Aoki K, Okazaki N, Okada S, et al. Radiotherapy for hepatocellular carcinoma: clinicopathological study of seven autopsy cases. Hepatogastroenterology. 1994;41:427–31. [PubMed] [Google Scholar]
- 37.Zeng Z-C, Jiang G-L, Wang G-M, et al. DNA-PKcs subunits in radiosensitization by hyperthermia on hepatocellular carcinoma hepG2 cell line. World J Gastroenterol. 2002;8:797–803. doi: 10.3748/wjg.v8.i5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imada H, Kato H, Yasuda S, et al. Compensatory enlargement of the liver after treatment of hepatocellular carcinoma with carbon ion radiotherapy—relation to prognosis and liver function. Radiother Oncol. 2010;96:236–42. doi: 10.1016/j.radonc.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 39.Liang S-X, Zhu X-D, Lu H-J, et al. Hypofractionated three-dimensional conformal radiation therapy for primary liver carcinoma. Cancer. 2005;103:2181–8. doi: 10.1002/cncr.21012. [DOI] [PubMed] [Google Scholar]
- 40.Liang S-X, Zhu X-D, Xu Z-Y, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–34. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Kim T-H, Kim D-Y, Park J-W, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225–31. doi: 10.1016/j.ijrobp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Cheng J-C, Wu J-K, Huang C-M, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156–62. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- 43.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–31. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]


