Abstract
The purpose of this study was to determine the outcomes and optimal practice patterns of definitive radiotherapy for primary vaginal cancer. Between 1993 and 2012, 49 patients were treated with definitive radiotherapy for primary vaginal cancer in three hospitals. Of these, 15 patients (31%) had clinically positive regional lymph node metastasis. A total of 34 patients (70%) received external beam radiotherapy with high-dose-rate brachytherapy (interstitial or intracavitary), and 8 (16%) (with small superficial Stage I tumors) were treated with local radiotherapy. The median follow-up was 33 months (range: 1–169 months). The 3-year overall survival (OS), disease-free survival (DFS), and loco-regional control (LRC) rates were 83%, 59% and 71%, respectively. In multivariate analysis, the histological type (P = 0.044) was significant risk factors for LRC. In Federation of Gynecology and Obstetrics (FIGO) Stage I cases, 3 of 8 patients (38%) who did not undergo prophylactic lymph node irradiation had lymph node recurrence, compared with 2 of 12 patients (17%) who underwent prophylactic pelvic irradiation. For Stage III–IV tumors, the local recurrence rate was 50% and the lymph node recurrence rate was 40%. Patients with FIGO Stage I/II or clinical Stage N1 had a higher recurrence rate with treatment using a single modality compared with the recurrence rate using combined modalities. In conclusion, our treatment outcomes for vaginal cancer were acceptable, but external beam radiotherapy with brachytherapy (interstitial or intracavitary) was needed regardless of FIGO stage. Improvement of treatment outcomes in cases of FIGO Stage III or IV remains a significant challenge.
Keywords: high-dose-rate brachytherapy, prophylactic pelvic irradiation, radiotherapy, vaginal cancer
INTRODUCTION
Vaginal cancer is rare, comprising only about 2% of all gynecologic malignancies. Radiotherapy plays a significant role in the management of primary vaginal cancer, but there have been no prospective randomized trials for this cancer []. Thus, analysis of institutional retrospective data is important for obtaining a better understanding of patterns of failure and for determining optimal treatment regimes for radiotherapy. However, most retrospective studies have had a limited number of patients and have used a variety of modalities, schedules and total doses. Therefore, the optimal dose and combinations of modalities are still poorly understood.
Here, we present a retrospective analysis of definitive radiotherapy for primary vaginal cancer at our hospital (Osaka University Hospital) and related hospitals (Osaka Rosai Hospital and the National Hospital Organization Osaka National Hospital). The goal of this study was to determine the optimal treatment practice patterns for this disease by evaluating the outcomes, toxicities, prognostic factors, and correlations of recurrence rates with the extent of radiotherapy and total dose.
MATERIALS AND METHODS
Patient characteristics
Between May 1993 and July 2012, 49 patients were treated with definitive radiotherapy for primary vaginal cancer at Osaka University Hospital, Osaka Rosai Hospital and the National Hospital Organization Osaka National Hospital. Patient characteristics and outcomes were obtained from hospital records. For patients who were no longer being followed up, we contacted them or their family by telephone. Informed consent was obtained from all patients. The study was approved by the institutional review board of Osaka University Hospital.
Patient and disease characteristics are listed in Table 1. Of the 49 patients, 21 (43%) were treated at Osaka University Hospital, 16 (33%) at Osaka Rosai Hospital, and 12 (24%) at the National Hospital Organization Osaka National Hospital. Vaginal cancer occurring 5 or more years after complete response of uterine cervical cancer was defined as primary vaginal cancer, based on the definition of the International Union Against Cancer. Therefore, four patients with a history of uterine cervical cancer and five with a history of pelvic irradiation were included in the study. Pretreatment abdominal and pelvic computed tomography (CT) scans were obtained for all patients, and a positive diagnosis was made based on a lymph node short axis >10 mm. Of the 49 patients, 15 (31%) had clinically positive regional lymph node metastasis. One patient had distant lymph node metastasis (common iliac lymph node), but was treated with radical intent. Chemotherapy was used for six patients (12%), including concurrently with radiotherapy in two cases. The other four patients received pre- or post-radiotherapy. All cases treated with chemotherapy received platinum-based regimens, including two patients given weekly cisplatin concurrently with radiotherapy and four who received pre- or post-radiotherapy with: (i) carboplatin and peplomycin; (ii) pirarubicin, cisplatin and peplomycin; (iii) nedaplatin and peplomycin; and (v) carboplatin and paclitaxel, respectively.
Table 1.
Patient and disease characteristics
| Characteristics | n | % |
|---|---|---|
| Age, years | ||
| <69 | 25 | 51 |
| ≥70 | 24 | 49 |
| Histological type | ||
| Squamous cell carcinoma | 42 | 86 |
| Adenocarcinoma | 6 | 12 |
| Carcinosarcoma | 1 | 2 |
| FIGO stage | ||
| I | 20 | 41 |
| II | 19 | 39 |
| III | 7 | 14 |
| IV | 3 | 6 |
| Clinical N stage | ||
| N0 | 34 | 69 |
| N1 | 15 | 31 |
| Size, cm | ||
| <4 | 31 | 63 |
| ≥4 | 18 | 37 |
| Location | ||
| Upper 2/3 | 30 | 61 |
| Lower 1/3 | 11 | 22 |
| Whole vagina | 8 | 16 |
| Chemotherapy | ||
| Yes | 6 | 12 |
| No | 43 | 88 |
| Radiotherapy | ||
| EBRT alone | 8 | 16 |
| ICBT alone | 4 | 8 |
| ISBT alone | 3 | 6 |
| EBRT + ICBT | 9 | 19 |
FIGO = International Federation of Gynecology and Obstetrics, EBRT = external beam radiotherapy, ICBT = intracavitary brachytherapy, ISBT = interstitial brachytherapy.
Radiotherapy
All three hospitals had facilities for high-dose-rate (HDR) brachytherapy with a 192Iridium source. The treatment strategy was almost the same in the three hospitals. Generally, patients with small, superficial tumors (tumor thickness <5 mm) were treated with local radiotherapy [brachytherapy or external beam radiotherapy (EBRT)] alone. Other tumors were treated with EBRT with brachytherapy, except in two cases with a poor performance status. The initial 20–40 Gy was delivered to the whole pelvis, and then pelvic irradiation with a central shield was performed. If the tumor remained large (tumor thickness >5 mm) at the time of brachytherapy, HDR interstitial brachytherapy (ISBT) was performed instead of HDR intracavitary brachytherapy (ICBT). The EBRT to brachytherapy ratio and the total dose were determined on an individual basis by each physician. EBRT was administered to 42 patients (86%), including 41 with a primary lesion and regional lymph node drainage defined by the primary lesion location.
For primary tumors confined to the proximal two-thirds of the vagina, the clinical target volume (CTV) for EBRT included the areas of the obturator lymph nodes, external and internal iliac lymph nodes, and common iliac nodes. For tumors that had invaded the distal one-third of the vagina, the area of the inguinal lymph nodes was included, in addition to that of the pelvic lymph nodes. The planning target volume (PTV) for EBRT was generated using a 10-mm uniform expansion of the CTV. The prescribed doses of EBRT were at the center of the PTV. The CTV for brachytherapy comprised the whole tumor (at the time of brachytherapy) plus 5 mm in all directions, except for the posterior (rectal) margin. The posterior margin varied from 2 to 5 mm, depending on the distance to the rectal wall. Prophylactic vaginal wall irradiation was not performed. Measurement of the tumor thickness was carried out by palpation and ultrasound.
HDR-ISBT was performed in 28 patients (57%) at a median dose of 30 Gy in 5 fractions (range: 10–50 Gy in 2–10 fractions). HDR-ICBT was performed in 13 patients (27%) at a median dose of 30 Gy in 5 fractions (range: 10–38 Gy in 2–6 fractions). The ICBT dose was defined at a depth of 5 mm from the vaginal surface. In HDR-ISBT, the planning system used was either PLATO or Oncentra (Elekta, Stockholm, Sweden) was used in combination with manual modification to ensure the 100% isodose line encompassed the CTV on every slice after computer optimization using the geometrical optimization algorithm.
Practice patterns of radiotherapy are shown in Table 1. Radiotherapy using EBRT alone, ICBT alone, ISBT alone, EBRT + ICRT and EBRT + ISBT was performed in 16%, 8%, 6%, 19% and 51% of cases, respectively. Collectively, radiotherapy using a single modality and combined modalities was performed in 30% and 70% of cases, respectively.
To compare the combined dose of brachytherapy (ICBT or ISBT) and EBRT with a single modality dose (brachytherapy or EBRT alone), the total dose was calculated as the biologically equivalent dose in 2-Gy fractions (EQD2) using the linear quadratic model. The dose to the primary tumor was the prescription dose of EBRT (excluding the fractions with central shielding) plus the prescription dose of brachytherapy. The value used for assessing effects on the tumor was α/β = 10 Gy. The equation used to calculate the EQD2 was as follows:
where N is the fraction number for EBRT, d is the dose fraction for EBRT, NB is the fraction number for brachytherapy, and dB is the dose fraction for brachytherapy.
We divided the radiation field into three regions: primary lesion, enlarged lymph nodes and prophylactic lymph nodes, and evaluated the correlation between total EQD2 and recurrence rates in the respective regions. Recurrence was defined as a tumor that recurred or persisted in the same region (primary, obturator, external iliac, internal iliac, common iliac or inguinal) after radiotherapy.
Statistical analysis
Overall survival (OS), disease-free survival (DFS) and loco-regional control (LRC) rates were calculated from the start of initial treatment. In evaluating LRC, common iliac LN recurrence was defined as regional recurrence. Rates were estimated using the Kaplan–Meier method, and differences between factors were examined by log-rank test. A Cox proportional hazard model was used for multivariate analysis. P < 0.05 or a 95% confidence interval (CI) of the hazard ratio >1.0 was considered to indicate a significant difference. All statistical analysis was performed using Stat Mate IV (ATMS Co., Ltd, Tokyo, Japan).
RESULTS
Outcome analysis
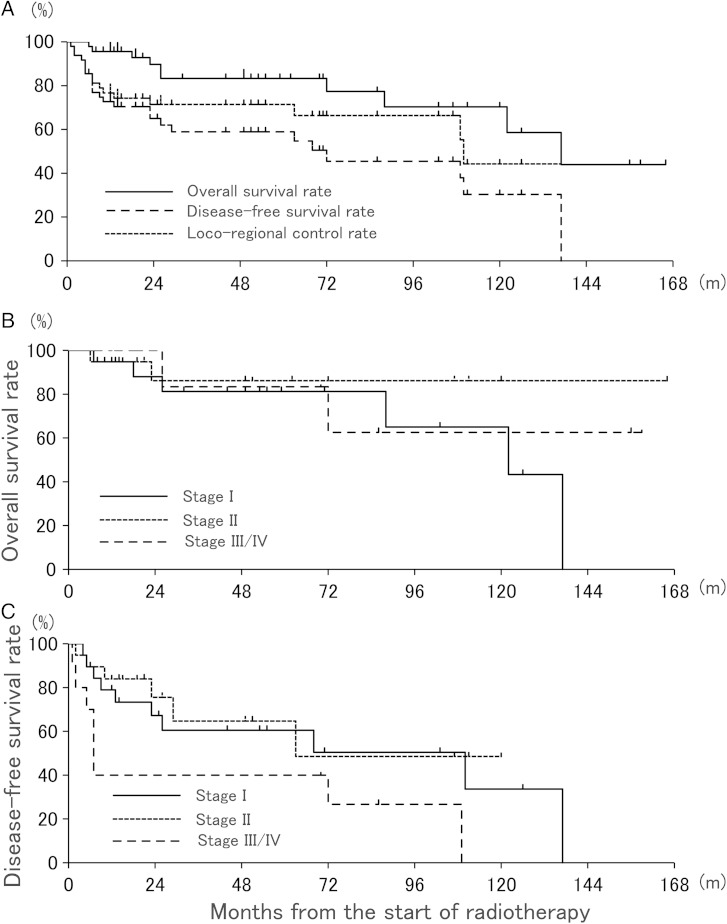
At the time of analysis, the median follow-up time of the 49 patients was 33 months (range: 1–169 months). The 3-year OS, DFS and LRC rates were 83%, 59% and 71%, respectively (Fig. 1A). According to FIGO stage, the 3-year OS for Stages I, II and III–IV patients was 81%, 86% and 83%, respectively (Fig. 1B), and the corresponding 3-year DFS was 60%, 65% and 40%, respectively (Fig. 1C). Relationships among outcomes, tumor types, and treatment factors are summarized in Table 2. The histological type (P = 0.037) and FIGO stage (P = 0.026) were significantly associated with DFS; and histological type (P = 0.028), FIGO stage (P = 0.019), and clinical N stage (P = 0.023) were significantly associated with LRC. In patients treated with brachytherapy, LRC did not differ significantly between patients treated with ISBT and ICBT. Multivariate analysis was performed with histological type (SCC vs others), FIGO stage (I/II vs III/IV) and clinical N stage (N0 vs N1), which were judged to be potential risk factors in univariate analysis. In multivariate analysis, the histological type (HR = 3.82, 95% CI = 1.04–13.08, P = 0.044) was a significant risk factor for LRC. OS showed no significant differences between different tumor types and treatment factors.
Fig. 1.
(A) Overall survival, disease-free survival, and loco-regional control rates after definitive radiotherapy for vaginal cancer. (B, C) Overall survival and disease-free survival rates according to FIGO stage.
Table 2.
Univariate analysis of prognostic factors for OS, PFS and LRC in patients with carcinoma of the vagina treated with definitive radiotherapy.
| Characteristics | n | 3-year OS | P | 3-year DFS | P | 3-year LRC | P |
|---|---|---|---|---|---|---|---|
| % | % | % | |||||
| Age, years | 0.114 | 0.207 | 0.846 | ||||
| <70 | 25 | 90 | 66 | 70 | |||
| ≥70 | 24 | 73 | 47 | 73 | |||
| Histological type | 0.591 | 0.037 | 0.028 | ||||
| SCC | 42 | 88 | 66 | 77 | |||
| Others | 7 | 50 | 19 | 38 | |||
| FIGO stage | 0.687 | 0.026 | 0.019 | ||||
| I/II | 39 | 83 | 63 | 77 | |||
| III/IV | 10 | 83 | 40 | 48 | |||
| Clinical N stage | 0.395 | 0.100 | 0.023 | ||||
| N0 | 34 | 84 | 65 | 80 | |||
| N1 | 15 | 82 | 47 | 53 | |||
| size, cm | 0.477 | 0.582 | 0.117 | ||||
| <4 | 31 | 82 | 59 | 77 | |||
| ≥4 | 18 | 85 | 58 | 62 | |||
| Involvement of lower 1/3 | 0.976 | 0.831 | 0.280 | ||||
| NO | 30 | 85 | 59 | 77 | |||
| YES | 19 | 81 | 58 | 62 | |||
| Chemotherapy | 0.268 | 0.657 | 0.764 | ||||
| NO | 43 | 85 | 58 | 72 | |||
| Yes | 6 | 67 | 63 | 63 | |||
| Brachytherapy | 0.555 | 0.258 | 0.075 | ||||
| YES | 41 | 84 | 61 | 76 | |||
| NO | 8 | 67 | 50 | 50 |
OS = overall survival rate, DFS = disease-free survival rate, LRC = loco-regional control rate, SCC = squamous cell carcinoma, FIGO = Federation of Gynecology and Obstetrics.
Correlation between total EQD2 and recurrence rate
Correlations between total EQD2 doses to primary lesions, enlarged lymph nodes and prophylactic lymph nodes with tumor recurrence rates for lesions of different FIGO stages are shown in Table 3. In primary lesions, recurrence clearly increased for a primary tumor with a diagnosis of Stage III or higher, despite use of a relatively high dose (median EQD2 dose: 79 Gy). For enlarged lymph nodes, 11 cases (73%) with good control of the tumor received a total dose of >50 Gy (median EQD2 dose: 60 Gy), whereas all four cases with recurrence received a total dose of ≤50 Gy. In FIGO Stage I cases, three of eight patients (38%) who did not undergo prophylactic lymph node irradiation had lymph node recurrence, compared with two of 12 patients (17%) who received prophylactic pelvic irradiation (median EQD2 dose: 50 Gy), but the difference was not significant (P = 0.29). The rate of lymph node recurrence remained high (40%), even with prophylactic irradiation, in all Stage III or IV patients (median EQD2 dose: 50 Gy).
Table 3.
Correlation between total EQD2 dose and tumor control according to FIGO stage
| FIGO | n | Mean EQD2 | Median EQD2 | Range | Percent recurrence (number) |
|||
|---|---|---|---|---|---|---|---|---|
| (Gyα/β10) | (Gyα/β10) | |||||||
| Primary | Overall | 65 | 70 | 38–96 | 18 | (9/49) | ||
| I | 20 | 57 | 57 | 38–80 | 5 | (1/20) | ||
| II | 19 | 70 | 70 | 53–96 | 16 | (3/19) | ||
| III–IV | 10 | 73 | 79 | 44–90 | 50 | (5/10) | ||
| Gross node | Overall | 15 | 56 | 60 | 44–66 | 27 | (4/15a) | |
| Prophylactic | Overall | 39 | 50 | 0–60 | 22 | (11/49) | ||
| Ib | 8 | 0 | 0 | 0 | 38 | (3/8) | ||
| I | 12 | 46 | 50 | 30–50 | 17 | (2/12) | ||
| II | 19 | 47 | 50 | 30–60 | 11 | (2/19) | ||
| III–IV | 10 | 49 | 50 | 40–60 | 40 | (4/10) | ||
aAll four cases with recurrence received a total dose of 50 Gy or less. bProphylactic lymph node irradiation was not performed. EQD2 = equivalent dose in 2-Gy fractions, FIGO = Federation of Gynecology and Obstetrics.
Practice patterns and recurrence rate
Practice patterns (single modality vs combined therapy) were analyzed according to tumor or patient characteristics (Table 4). Patients with FIGO Stage I/II or clinical N1 stage had a higher recurrence rate in treatment with a single modality compared with that with combined modalities. However, all three patients with clinical N1 stage who had recurrence had received EBRT alone as a single modality. Additionally, these patients received ≤50 Gy to the enlarged lymph node and subsequently had recurrence in the same lesion. Age, histological type, tumor size and length of vaginal invasion did not influence the recurrence rate in either single or combined modalities.
Table 4.
Practice pattern and recurrence rate according to tumor and patient characteristics
| Combined modalities recurrence rate |
Single modality recurrence rate |
||||
|---|---|---|---|---|---|
| % | Number | % | Number | P | |
| Age, years | |||||
| <69 | 29 | (6/21) | 75 | (3/4) | 0.076 |
| ≥70 | 23 | (3/13) | 36 | (4/11) | 0.476 |
| Histological type | |||||
| SCC | 21 | (6/30) | 42 | (5/12) | 0.149 |
| Others | 75 | (3/-4) | 67 | (2/3) | 0.809 |
| FIGO stage | |||||
| I/II | 15 | (4/26) | 46 | (6/13) | 0.038 |
| III/IV | 50 | (4/8) | 100 | (2/2) | 0.197 |
| Clinical N stage | |||||
| N0 | 18 | (4/22) | 42 | (5/12) | 0.137 |
| N1 | 33 | (4/12) | 100 | (3/3) | 0.038 |
| Size, cm | |||||
| <4 | 17 | (4/23) | 43 | (3/8) | 0.241 |
| ≥4 | 45 | (5/11) | 57 | (4/7) | 0.629 |
| Involvement of lower 1/3 | |||||
| No | 19 | (4/21) | 44 | (4/9) | 0.149 |
| Yes | 38 | (5/13) | 50 | (3/6) | 0.636 |
SCC = squamous cell carcinonma, FIGO = International Federation of Gynecology and Obstetrics.
Toxicities
Treatment-related late toxicity was evaluated using the Common Terminology Criteria for Adverse Events ver. 4.0. Six patients (12%) had Grade 3 late toxicities, including rectovaginal fistula (n = 5) and perforation of the sigmoid colon (n = 1). All of these patients were treated with ISBT, and two had a history of radiotherapy for pelvic lesions. Patients with previous pelvic irradiation had higher rates of Grade 3 complications compared with those without previous pelvic irradiation [2/5 (40%) vs 4/44 (9%), P = 0.04]. There were no vaginal complications of Grade 3 or higher and no Grade 4–5 late toxicities.
DISCUSSION
The outcomes in the current study are similar to or better than those in previous studies and showed 3-year and 5-year OS rates of 39–63% and 21–57%, respectively, for patients treated with HDR brachytherapy with or without EBRT [, , 5], and rates of 0–15.8% for serious late complications [–6 ]. However, the main reason for the better outcome may be the shorter median follow-up period of 33 months in the current study.
The varying outcomes for the three hospitals and the lack of a pre-specified protocol were significant limitations in the analysis and interpretation of outcomes. To overcome these limitations and to compare the combined total dose in several different modalities and the extent of the radiation field, we calculated the total dose as an EQD2 dose using the linear–quadratic model. We also divided the radiation field into three regions (primary lesion, enlarged lymph nodes, and prophylactic lymph nodes) and then evaluated the respective recurrence rates. Additionally, practice patterns (single modality vs combined therapy) were analyzed according to tumor or patient characteristics.
Patients with FIGO Stage I/II had a higher recurrence rate in a single modality. Additionally, among Stage I patients, 40% received radiotherapy for the primary lesion alone without prophylactic lymph node coverage. In cases without prophylactic lymph node irradiation, the recurrence rate in the prophylactic lesion tended to be higher, compared with cases with pelvic node irradiation (38% vs 17%) (P = 0.29). In a study of 21 FIGO Stage I patients treated with local radiation only (without regional node coverage), Frank et al. [10] found that three of nine patients (33%) treated with brachytherapy alone developed recurrent disease in the pelvis, whereas patients who had received EBRT with or without brachytherapy did not have pelvic recurrence. Collectively, these findings indicate that the optimal radiation practice is EBRT with brachytherapy (interstitial or intracavitary) regardless of FIGO stage. Patients with clinical N1 stage had a higher recurrence rate after treatment with a single modality, and all three patients with clinical N1 stage who had recurrence had received EBRT as a single modality. These patients received ≤50 Gy to the enlarged lymph node and had recurrence in the same lesion. These data indicate that recurrence in these patients was due mainly to a suboptimal EBRT dose to enlarged lymph nodes, and not to the practice pattern.
Improvement of treatment outcome in cases of FIGO Stage III or IV vaginal cancer remains a significant challenge. In previous studies, 5-year OS rates for patients with Stage III disease have ranged from 4% to 58% [, , 15], with LRC rates of 57% to 69% [,9,10]. The outcome for Stage IV disease is even worse, with survival rates of 0% to 35% [, ,15].
In this study, the local recurrence rate was very high (50%) with a median EQD2 of 79 Gy, and the rate of prophylactic lymph node recurrence was also high (40%) with a median EQD2 of 50 Gy for Stage III–IV tumors. The total dose for the primary lesion or prophylactic lymph node may be considered as the upper limit for normal tissue. However, 3D image-based HDR brachytherapy has recently been used in cervical cancer. Therefore, these results using EQD2 of the prescribed dose require verification in studies using image-based brachytherapy.
For achievement of higher LRC, concurrent chemoradiation (CCRT) therapy has been attempted for locally advanced disease. In a study of 14 patients with vaginal cancer [including 11 (71%) with Stage II or III disease] who received CCRT with a 5-FU-based regimen, only one patient had local recurrence and died of the disease [16]. In a review of 12 patients with vaginal cancer in Stages II to IV who were treated with concurrent weekly cisplatin at a dose of 40 mg/m2 for 5 weeks, Samant et al. found 5-year OS and PFS rates of 66% and 75%, respectively [17]. These findings led to the conclusion that CCRT is feasible and effective for management of primary vaginal cancer and should be considered as an option for patients being treated with curative intent [17].
Despite these promising outcomes in patients with vaginal cancer treated with CCRT, a randomized trial comparing radiation alone with radiation plus chemotherapy has not been performed in vaginal cancer. Additionally, many retrospective studies of CCRT for primary vaginal cancer are limited by the small number of patients or inclusion of other cancers, such as cervical and vulvar cancers. Therefore, further studies are needed to clarify the potential therapeutic benefits of CCRT. However, the design and execution of prospective randomized trials is challenging because of the rarity of this disease. In this study, the treatment and outcomes for vaginal cancer were acceptable, but EBRT with brachytherapy (interstitial or intracavitary) was needed regardless of FIGO stage. Thus, improvement of treatment outcome in cases of FIGO Stage III or IV remains a significant challenge.
FUNDING
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 25861097. Funding to pay the Open Access publication charges for this article was provided by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 25861097.
REFERENCES
- 1.Hacker NF, Eifel PJ, van der Velden J. Cancer of the vagina. Int J Gynaecol Obstet. 2012;119S2:S97–9. doi: 10.1016/S0020-7292(12)60022-8. [DOI] [PubMed] [Google Scholar]
- 2.Nonaka T, Nakayama Y, Mizoguchi N, et al. Definitive radiation therapy for invasive carcinoma of the vagina: impact of high-dose rate intracavitary brachytherapy. Int J Clin Oncol. 2013;18:314–20. doi: 10.1007/s10147-012-0379-7. [DOI] [PubMed] [Google Scholar]
- 3.Mock U, Kucera H, Fellner C, et al. High-dose-rate (HDR) brachytherapy with or without external beam radiotherapy in the treatment of primary vaginal carcinoma: long-term results and side effects. Int J Radiat Oncol Biol Phys. 2003;56:950–7. doi: 10.1016/s0360-3016(03)00217-7. [DOI] [PubMed] [Google Scholar]
- 4.Nanavati PJ, Fanning J, Hilgers RD, et al. High-dose-rate brachytherapy in primary stage I and II vaginal cancer. Gynecol Oncol. 1993;51:67–71. doi: 10.1006/gyno.1993.1248. [DOI] [PubMed] [Google Scholar]
- 5.Kushner DM, Fleming PA, Kennedy AW, et al. High dose rate 192Ir afterloading brachytherapy for cancer of the vagina. Br J Radiol. 2003;76:719–725. doi: 10.1259/bjr/15634046. [DOI] [PubMed] [Google Scholar]
- 6.Murakami N, Kasamatsu T, Sumi M, et al. Radiation therapy for primary vaginal carcinoma. J Radiat Res. 2013;54:931–7. doi: 10.1093/jrr/rrt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chyle V, Zagars GK, Wheeler JA, et al. Definitive radiotherapy for carcinoma of the vagina: outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 1996;35:891–905. doi: 10.1016/0360-3016(95)02394-1. [DOI] [PubMed] [Google Scholar]
- 8.Jang WI, Wu HG, Ha SW, et al. Definitive radiotherapy for treatment of primary vaginal cancer: effectiveness and prognostic factors. Int J Gynecol Cancer. 2012;22:521–7. doi: 10.1097/IGC.0b013e31823fd621. [DOI] [PubMed] [Google Scholar]
- 9.Tran PT, Su Z, Lee P, et al. Prognostic factors for outcomes and complications for primary squamous cell carcinoma of the vagina treated with radiation. Gynecol Oncol. 2007;105:641–9. doi: 10.1016/j.ygyno.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Frank SJ, Jhingran A, Levenback C, et al. Definitive radiation therapy for squamous cell carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 2005;62:138–47. doi: 10.1016/j.ijrobp.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Shah CA, Goff BA, Lowe K, et al. Factors affecting risk of mortality in women with vaginal cancer. Obstet Gynecol. 2009;113:1038–45. doi: 10.1097/AOG.0b013e31819fe844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creasman WT, Phillips JL, Menck HR. The National Cancer Data Base report on cancer of the vagina. Cancer. 1998;83:1033–40. [PubMed] [Google Scholar]
- 13.Perez CA, Camel HM, Galakatos AE, et al. Definitive irradiation in carcinoma of the vagina: long-term evaluation of results. Int J Radiat Oncol Biol Phys. 1988;15:1283–90. doi: 10.1016/0360-3016(88)90222-2. [DOI] [PubMed] [Google Scholar]
- 14.Stock RG, Chen AS, Seski J. A 30-year experience in the management of primary carcinoma of the vagina: analysis of prognostic factors and treatment modalities. Gynecol Oncol. 1995;56:45–52. doi: 10.1006/gyno.1995.1008. [DOI] [PubMed] [Google Scholar]
- 15.Fine BA, Piver MS, McAuley M, et al. The curative potential of radiation therapy in the treatment of primary vaginal carcinoma. Am J Clin Oncol. 1996;19:39–44. doi: 10.1097/00000421-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Dalrymple JL, Russell AH, Lee SW, et al. Chemoradiation for primary invasive squamous cell carcinoma of the vagina. Int J Gynecol Cancer. 2004;14:110–7. doi: 10.1111/j.1048-891x.2004.014066.x. [DOI] [PubMed] [Google Scholar]
- 17.Samant R, Lau B, EC, et al. Primary vaginal cancer treated with concurrent chemoradiation using Cis-platinum. Int J Radiat Oncol Biol Phys. 2007;69:746–50. doi: 10.1016/j.ijrobp.2007.04.015. [DOI] [PubMed] [Google Scholar]