Abstract
Children transplanted for acute liver failure (ALF) urgently require an optimal graft. Lower post-transplant survival compared to children transplanted for chronic liver disease. Over 10 years, 33 consecutive children transplanted for ALF were followed. Demographics, encephalopathy, intubation, dialysis, laboratory values, graft type (ABO incompatible grafts (ABOI), Large for size grafts(XL)(GRWR>5%),deceased donor segmental liver transplantation(DDSLT), living donor liver transplantation (LDLT) and whole liver transplant (WLT) were evaluated. Complications and survival were determined. ALF accounted for 33/201 (16.4%) of transplants during this period. 12/33 received ABOI, 5 XL grafts, 18 DDSLT, and 3 LDLT. Waiting time pre-transplant was 2.1 days. 1 and 3 year patient survival ALF group was 93% and 93% and graft survivals were 93 and 78.6%. Median follow-up was 1452 days. ABOI one and three year patient and graft survival in the ALF was 92 and 75%. No difference in graft or patient survival was noted in the ALF and chronic liver disease group nor the ABOI and the ABO compatible group. A combination of ABO incompatible donor livers, large for size grafts, DDSLT, LDLT and WLT led to a short wait time and subsequent graft and patient survival not significantly different than that for non-acute liver disease.
Keywords: liver transplantation, pediatric liver transplantation, acute liver failure, fulminant liver failure, ABO incompatible
Introduction
Acute liver failure (ALF) has been defined as the onset of encephalopathy within eight weeks after the appearance of jaundice in the absence of pre-existing liver disease (1). Recently, a pediatric definition for ALF was adopted that includes the presence of biochemical evidence of liver injury, an absence of known chronic liver disease, coagulopathy not corrected by vitamin K administration and an INR > 1.5 if the patient has encephalopathy.
The definition and progression of hepatic encephalopathy (HE) is problematic and atypical in the pediatric population, especially in infants,(2). It is usually a late feature and is not essential for the diagnosis(3). Hepatitis A is the most frequent cause of pediatric ALF in South America and Asia(4,5) while seronegative hepatitis is the most common cause in the Western world. Pediatric mortality without liver transplant has historically been high (3). An accurate model that would predict that all patients urgently requiring a liver transplant would receive one prior to neurological damage, and that all who would survive without one would not, does not exist. Recently, there have been several new prognostic scoring systems including a report from the Denver group, which appears promising but validation of these findings with a larger multi-center trial is necessary.
The diagnosis of fulminant hepatic failure (ALF) accounted for 12.9% of pediatric transplants between 1995 and 2002 in the SPLIT database (6). Post transplant survival at 6 months (74%) and one year for ALF is much lower than for children with chronic liver disease. Previous reports state that patients with encephalopathy grade 4 or higher, infants less than one year of age, children requiring dialysis or mechanical ventilation prior to transplantation had worse results (6,7). Shorter waiting time for children listed for liver transplant for fulminant hepatic failure is more critical than any other group.
We reviewed pediatric liver transplant recipients for ALF at our center over 10.5 years utilizing the following four strategies to increase the donor liver pool;
ABO incompatible grafts (ABOI),
Large for size grafts (XL)(GRWR>5%)
Deceased donor segmental liver transplantation (DDSLT)
Living donor liver transplantation (LDLT).
Materials and Methods
From March 1998 through November 2008, 33 consecutive pediatric patients with a diagnosis of ALF underwent liver transplantation at our hospital. Only children less than 18 years of age with fulminant hepatic failure which was defined as the onset of hepatic encephalopathy within 8 weeks of the first symptoms of liver disease in the absence of pre-existing liver disease were included.
Indications for liver transplantation were progressive hepatic encephalopathy, progressive coagulopathy (INR > than 2), and continued low factor V and VII levels despite supportive management. Encephalopathy was graded as follows:
Grade 1: Slowness of mentation, mildly disturbed sleep–awake cycle.
Grade 2: Drowsiness, confusion, inappropriate behavior, disorientation, apathy, mood swings, anxiety, restlessness.
Grade 3: Markedly confused, combative and hyperreflexic, reaction only to vocal stimuli.
Grade 4: Unconscious, without reaction to vocal stimuli, response only to noxious physical stimuli.
All patients underwent computed tomographic head evaluation. Invasive intracranial pressure monitoring was performed shortly after intubation in patients with either clinical or radiological evidence of increased intracranial pressure. Data was obtained by utilizing our computerized transplant tracking registry.
Immunosuppression was based on induction with a single dose of daclizumab, tacrolimus, mycophenolate mofetil, 5 day methylprednisolone taper followed by daily prednisone maintenance. Intravenous methylprednisolone 20 mg/kg (up to a one gram maximum) was given at the time of the biliary anastomosis. Daclizumab was given 1 mg/kg intravenously 12 to 24 hours post-transplantation. Mycophenolate mofetil was started orally at 30 mg/kg/day divided BID on postoperative day one. Oral tacrolimus began on postoperative day 7. In the absence of rejection, steroid weans were initiated to discontinuation between 3 to 12 months after transplantation. If acute rejection occurred during the prednisone wean, acute treatment was given with pulsed steroids, reinstitution of the oral prednisone, and increasing the calcineurin inhibitor trough to a therapeutic level. Patients were followed and monitored for the development of complications, and patient and graft survival were also determined.
The obtained data was analyzed by with quantitative data being expressed as means ± standard deviations and qualitative data expressed with frequency and rate. Statistical analysis was performed using Student’s t test (2-tailed) for all continuous data. Chi-square and Fisher’s exact tests were used, when appropriate, to compare nominal data. Survival was calculated using Kaplan-Meier analysis and significance determined by the log rank method. A P value of 0.05 or less was considered significant.
Results
Etiology of liver disease in the ALF group (n=33) consisted of drug toxicity in one patient, hepatitis B in two patients and cryptogenic in the remainder. Average follow-up was 1452 ± 1284 days. Median pre-transplant age and weight was 3.84 years (10 days to 17.58 years) and 15 kilograms (1.7 to 86 kg). 13/33 (39%) of recipients were less than one year of age at time of transplant with average weight of 6.2 kg (1.9 – 12.2 kg). Four patients in this group were transplanted for diagnosis of neonatal hemachromatosis at an average age of 19 days old (11–25 days) and weight 2.4 kg (1.7–3.3 kg). 24/33 (73%) recipients were less than 6 years old at the time of transplant. Recipients consisted of 19 males and 14 females. Twelve of thirty-three (36%) patients received a whole sized graft, 18/33 (54%) received a DDSLT, and 3/33 (9%) received a living donor (LDLT). 12/33 (36%) of patients received an ABO incompatible graft (ABOI) and 5 patients received an extra large for size graft (XL-GRWR>5%). Mean time on the waiting list was 2.1 ± 1.5 days (range - 0–33 days).
The number and grade of hepatic encephalopathy at time of transplantation consisted of 16 patients with grade 2, 4 patients with grade three and 13 patients with grade 4, respectively. Fourteen patients (42%) were ventilator dependent at the time of surgery. The mean values for pre-transplant INR, total bilirubin level and serum creatinine were 2.9 ± 2.5 seconds, 10.7 ± 6.5 mg/dl, and 0.4 ± 0.2 mg/dl, respectively.
Six patients were referred to the liver transplant team, placed on the waiting list and then removed for the following reasons:
Two patients – A seven day old with neonatal hepatitis and a 12 month old with mushroom poisoning, both of which improved, and did not require transplant.
Three patients were referred late and were taken off less than 24 hours after listing secondary to brain stem herniation.
One patient was removed less than 48 hours after listing secondary to development of acute respiratory distress syndrome.
During the 10 year study period, prospective tracking showed no hepatic artery thrombosis, portal vein thrombosis or hepatic vein outflow obstruction within the ALF group. 5 of 33 (15%) patients developed biliary complications consisting of one cut edge leak, one anastomotic leak and three biliary strictures. All were successfully treated by placement of a percutaneous transhepatic stent.
Two patients (6%) died within the first year after transplant. One of which, a 16 day old, 1.9 kg female transplanted with an ABO incompatible DDSLT graft for neonatal hemachromatosis, developed fulminant cytomegalovirus sepsis on day 64 and died with a functioning graft. The second patient, a 2.8 kg 27 day old male with an ABO compatible DDSLT graft, expired on day 161 post-transplant dead on arrival after being brought to our emergency room with presumed septicemia.
One patient was re-transplanted at 1031 days after her primary transplant for chronic rejection due to noncompliance and died at 1262 days from sepsis. Another patient was re-transplanted 675 days secondary to parental noncompliance. He was re-transplanted successfully, adopted by foster parents and is alive and well.
One patient died 753 days after liver transplant from chronic medication and follow-up non-adherence. One patient died 1901 days after her liver transplant. She suffered neurological sequelae at the time of transplant and died from a refractory seizure episode with a functioning graft. One patient died 2826 days post-transplant from non-adherence 3 months after moving to Mexico.
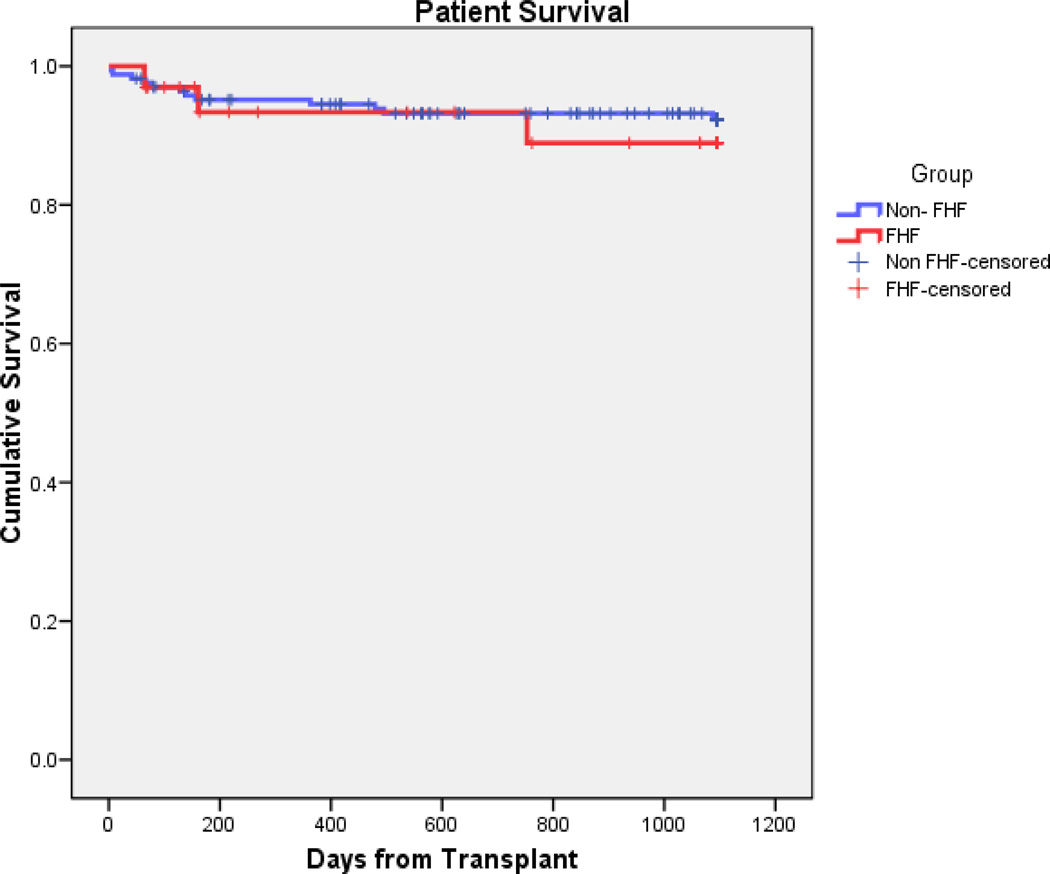
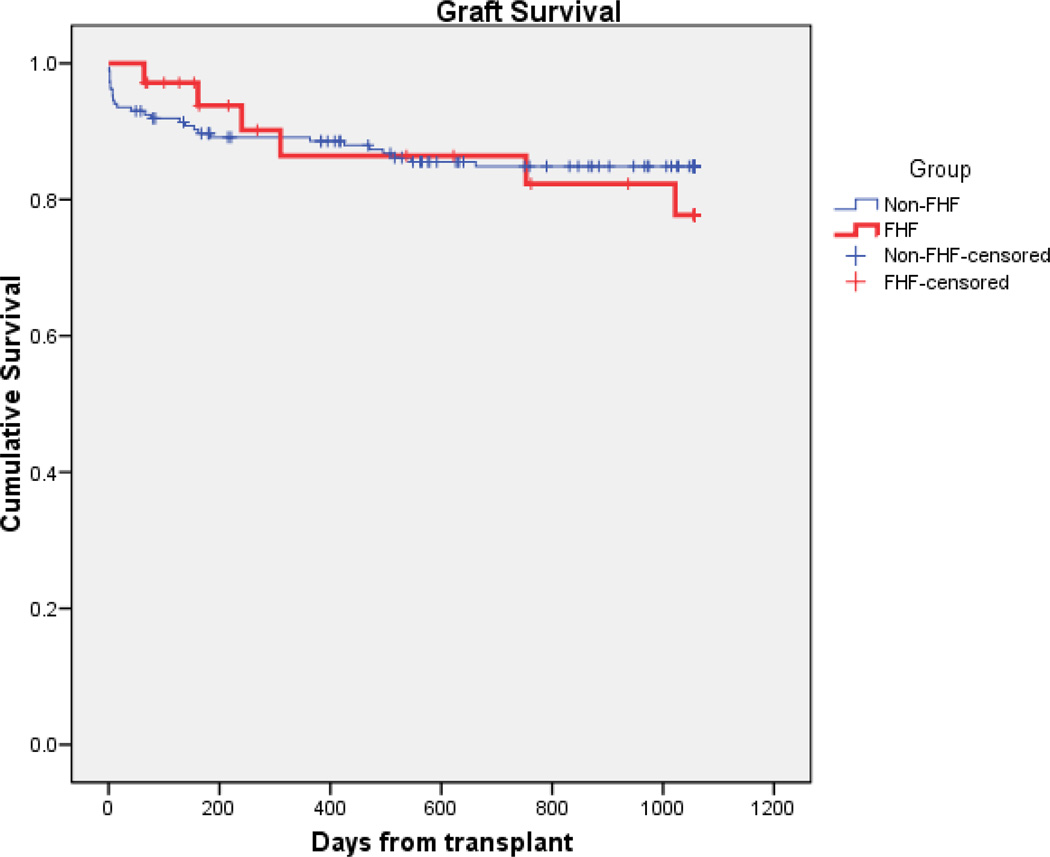
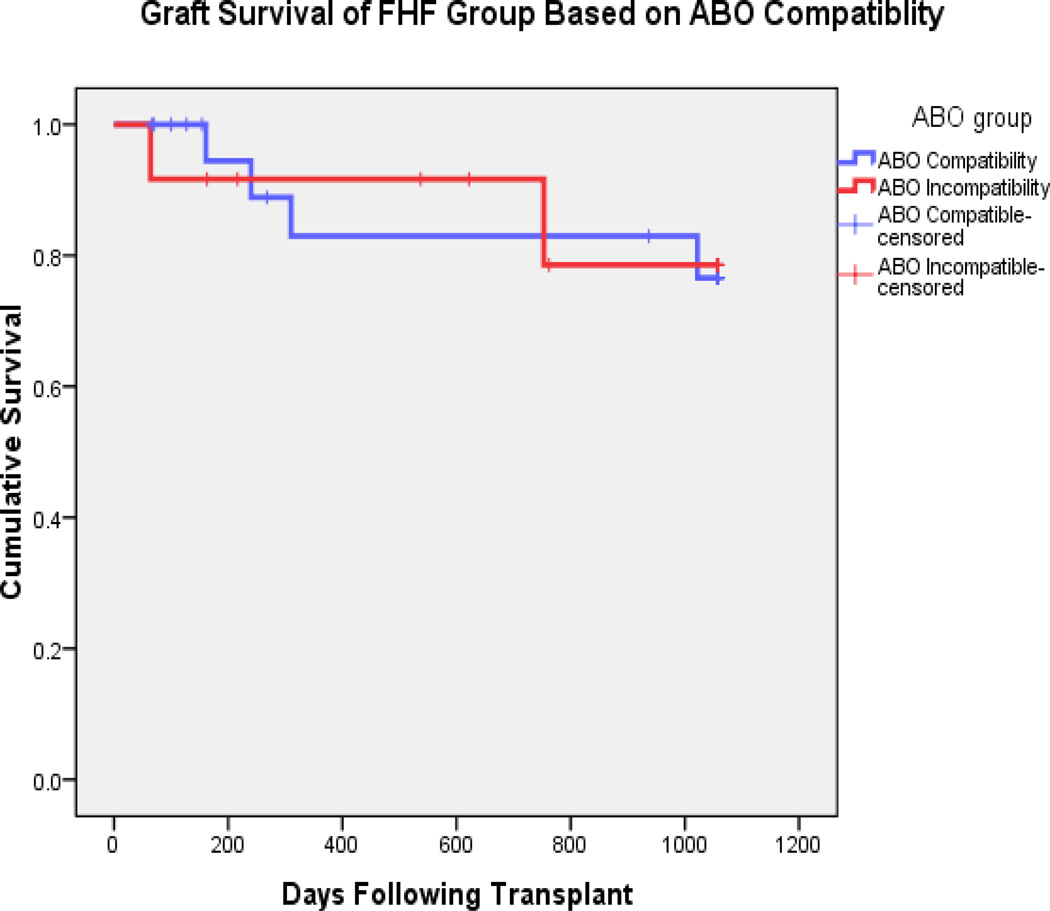
Actuarial patient survival were similar between the fulminant failure group and patients with chronic hepatic disease (n = 168) at both one (93.4% vs. 94.5%) and three years (88.9% vs. 88.9%; p = 0.595) as shown in Figure #1. Graft survival was also similar for ALF patients compared to chronic patients at both 1 (86.4% vs. 88.6%) and 3 years (77.7% vs. 84.1%; p = 0.634; Figure #2). In the 21 fulminant transplant patients who were ABO compatible, actuarial patient survival at 1 and 3 years were 93.9% and 93.9%, and graft survival of 93.9% and 80%. This is compared to the 12 ABO incompatible (ABOI) patients with actuarial patient and graft survival of 91.6% and 78.6 % for both graft and patient survival. (fig. #3 and #4).
Figure #1.
Kaplan-Meier patient survival curves comparing the Non-ALF group to the ALF group (p = 0.595 by log rank method)
Figure #2.
Kaplan-Meier survival curves comparing the Non-ALF group graft survival to the ALF group (p = 0.634 by log rank method)
Fig. #3.
Survival of the ALF group comparing ABO incompatible group to the ABO compatible group (p = 0.283 by log rank method)
Fig. #4.
Graft survival of the ALF group based on ABO compatibility (p = 0.963 by log rank method)
Six (50%) of the 12 ABOI allograft children had 14 rejection episodes, whereas 7 of 21(33.3%) of the ABO compatible allograft children had 22 rejection episodes (p = 0.300). There was no difference in the mean time to the first rejection episode in the ABOI group when compared to the compatible group of (968.6 ± 1167.3 days 1 vs. 067.6 ± 1318.5; p = 0.300).
There was an age difference with patients receiving a partial liver graft (DDSLT & LR) compared to children receiving whole organs. Children with partial grafts were younger (2.4 ± 2.3 yrs. vs. 10.1 ± 6.6 years, p = 0.002). There was no difference in race or gender (p = 0.566). Graft survival was similar between ALF patients receiving whole organs compared to those receiving partial liver grafts (1 yr. – 100% vs. 81.9%, 3 yr 83.3% vs. 75.6%; p = 0.761). Likewise, patient survival (1 yr - 100% vs. 90.5%, 3 yr 83.3% vs. 90.5%; p = 0.0.951) was also similar between children receiving whole organs when compared to children receiving partial liver grafts (DDSLT & LR). Following transplant for AHF, only one patient experienced adverse neurological sequelae with none of the patients having to receive treatment with polyclonal antibodies for cytopenia. In surviving children, the most recent follow-up indicated normal graft function.
Discussion
ALF accounts for almost 13% of pediatric transplants (6). The time from diagnoses to transplant is crucial for children with ALF. While the post- transplant survival overall yields patient survival in the range of 90% for children with chronic liver disease, survival rates for ALF in the largest multi-center series is significantly less as it approximates 74% at 6 months (5). Technical variants including DDSLT and LDLT have been utilized to expand the donor pool by overcoming size mismatches from adult donors (7,8,9,10). Split/reduced do not have decreased survival compared to whole grafts after transplant for ALF (6,7). In the more recent series, whole pediatric liver transplants have better immediate postoperative survivals, but by one year patient and graft survival are similar for pediatric recipients with chronic liver disease (11). Auxiliary liver transplant may have great promise with one major preliminary report with 6 children over a ten year period (12).
We also utilized large for sized hepatic grafts (GRWR>5%) in combination with polytetraflouroethylene (PTFE) abdominal mesh to further expand the donor pool. While small for size grafts have been reported as a cause of graft failure, large for size grafts may have less of an negative impact. Perceived variables which may result in decreased survival are vascular complications or large for size mismatch with poor tissue oxygenation possibly related to surgical modifications in the closure of the abdominal wall (13,14,15). We utilized PTFE for enlargement of the abdominal cavity to avoid compression in large for size grafts. We used this method in 5 of our fulminant failure patients.
While others have categorized the use of ABOI donors as marginal with a high retransplant rate, the risk of using ABO-I grafts in our center has been historically small (16). Therefore, we were not hesitant to use this form of donor pool expansion in acute situations such as ALF. With the use of IL-2 blockers for induction we were able to successfully cross blood groups without medications outside of our standard protocol as previously reported (17).
The two patients who died in the first year after transplant were both small (1.7 & 2.8 kg) and young (16 days and 27 days). Both died with functioning grafts on day 64 and 161 post transplant.
The limitation of the study is directly related to the small sample size secondary to a single center study. The above strategies may contribute to a reduced time on the waiting list in other liver programs and thus achieve survival rates that approximate those of children with non-acute disease.
References
- 1.Trey C, Davidson C. The Management of Fulminant Hepatic Failure. Progress in Liver Disease. 1970;3:282–298. [PubMed] [Google Scholar]
- 2.Liu E, MacKenzie T, Dobyns E, Parikh C, Karrer F, et al. Characterization of acute liver failure and development of a continuous risk of death staging system in children. Journal of Hepatology. 2006;44:134–141. doi: 10.1016/j.jhep.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Dhawan A. Etiology and prognosis of acute liver failure in children. Pediatr Transplantation. 2008;12:167–173. doi: 10.1002/lt.21641. [DOI] [PubMed] [Google Scholar]
- 4.Poddar A, Thapa BR, Prasas A, et al. Natural History and Risk Factors in Fulminant Hepatic Failure. Arch Dis Child. 2002;87:54–56. doi: 10.1136/adc.87.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah U, Habib Z, Kleinman RE. Liver failure attributable to hepatitis A infection in a developing country. Pediatrics. 2000;105:436–438. doi: 10.1542/peds.105.2.436. [DOI] [PubMed] [Google Scholar]
- 6.Baliga P, Alvarez S, Lindblad A, Zeng L, et al. Posttransplant Survival in Pediatric Fulmiant Hepatic Failure: the SPLIT Experience. Liver Transplant. 2004;10(11):1364–1371. doi: 10.1002/lt.20252. [DOI] [PubMed] [Google Scholar]
- 7.Goss J, Shackleton C, Melinda M, Swenson K, Seu P, et al. Liver Transplantation for Fulminant Hepatic Failure in the Pediatric Patient. Archives of Surgery. 1998;133(8):839–844. doi: 10.1001/archsurg.133.8.839. [DOI] [PubMed] [Google Scholar]
- 8.Goss JA, Shackleton CR, McDiarmid SV, Maggard M, Swenson K, Seu P, et al. Long term results of pediatric liver transplantation: an analysis of 569 transplants. ANN Surg. 1998;228:411–420. doi: 10.1097/00000658-199809000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced size liver transplants as split grafts, auxiliary orthotopic grafts and living related segmental liver transplants. Ann Surg. 1990;212:14–22. doi: 10.1097/00000658-199009000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broelsch CE, Whittington PF, Emond JC, Heffron TG, Thistlethwaite JR, Stevens L, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg. 214:428–437. doi: 10.1097/00000658-199110000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker NS, Barshes NR, Aloia TA, Nguyen T, Rojo J, Rodriguez JA, O’Mahony CA, Karpen SJ, Goss JA. Analysis of recent pediatric orthotopic liver transplantation outcomes indicates that allograft type is no longer a predictor of survivals. Liver Transplantation. 2008;14:1125–1132. doi: 10.1002/lt.21491. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Selvaggi G, Levi D, Hernandez E, Takahashi H, Velasco M, Moon J, Nishida J, Thompson P, Ruiz P, Sfakianakis G, Tzakis A. Routine use of auxiliary partial orthotopic liver transplantation for children with fulminant hepatic failure: preliminary report. Transplant Proceedings. 38:3607–3608. doi: 10.1016/j.transproceed.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Kiuchi T, Kasahar M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. doi: 10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka A, Tanaka K, Tokuka A, et al. Graft size matching in living related partial liver transplantation in relation to tissue oxygenation and metabolic capacity. Transplant Int. 1996;9:15. doi: 10.1007/BF00336807. [DOI] [PubMed] [Google Scholar]
- 15.Pinelli D, Spada M, Lucianetti S, et al. Transplantation for acute liver failure in children. Transplantation Proceedings. 2005;37:1146–1148. doi: 10.1016/j.transproceed.2004.12.302. [DOI] [PubMed] [Google Scholar]
- 16.Heffron T, Welch D, Pillen T, et al. Successful ABO-Incompatible Pediatric Liver Transplantation Utilizing Standard Immunosuppression with Selective Postoperative Plasmapheresis. Liver Transplantation. 2006;12:972–978. doi: 10.1002/lt.20760. [DOI] [PubMed] [Google Scholar]
- 17.Heffron TG, Pillen T, Smallwood GA, Welch D, Oakley B, et al. Pediatric liver transplantation with daclizumab induction. Transplantation. 2003;75(12):2040–2043. doi: 10.1097/01.TP.0000065740.69296.DA. [DOI] [PubMed] [Google Scholar]