Abstract
Spatial encoding in the hippocampus is multifactorial, and it is well established that metric information about space is conferred by place cells that fire when an animal finds itself in a specific environmental location. Visuospatial contexts comprise a key element in the formation of place fields. Nevertheless, hippocampus does not only use visual cues to generate spatial representations. In the absence of visual input, both humans and other vertebrates studied in this context, are capable of generating very effective spatial representations. However, little is known about the relationship between nonvisual sensory modalities and the establishment of place fields. Substantial evidence exists that olfactory information can be used to learn spatial contexts. Here, we report that learning about a distinct odor constellation in an environment, where visual and auditory cues are suppressed, results in stable place fields that rotate when the odor constellations are rotated and remap when the odor constellations are shuffled. These data support that the hippocampus can use nonvisuospatial resources, and specifically can use spatial olfactory information, to generate spatial representations. Despite the less precise nature of olfactory stimuli compared with visual stimuli, these can substitute for visual inputs to enable the acquisition of metric information about space.
Keywords: CA1, hippocampus, olfactory, place cells, sensory
Introduction
The hippocampus plays an essential role in the integration of sensory information such that spatial representation and the creation of declarative memory results. It engages in these tasks by means of long-term alterations of synaptic efficacy in the form of synaptic plasticity (Martin and Buno 2005; Kemp and Manahan-Vaughan 2007), network oscillatory activity (Buzsaki and Draguhn 2004; Hasselmo 2005), and place field formation (O'Keefe and Dostrovsky 1971; Knierim et al. 1995). Most of the studies that have explored spatial representations in the hippocampus to date have used visually defined environments. This approach contrasts with the fact that hippocampal neurons are highly multimodal, and receive information from many sensory modalities (Ranck 1973). Although it has been reported that vision and motion cues can be interchanged without affecting the place field representation of an environment (Quirk et al. 1990; Gothard et al. 1996), little attention has been placed on the role of sensory saliency and dominant modalities on information processing on this level.
Visual information processing is clearly a highly relevant source of navigational and cognitive information for rodents. Most manipulations that describe the properties of place fields have been based on visual cues. The association of place fields with contextual information relates to consistent observations that place fields rotated with the rotation of cue cards on a wall (O'keefe and Conway 1978; Muller and Kubie 1987) or move with objects displaced to the periphery of an enclosure (Cressant et al. 1997). But as nocturnal creatures, other modalities such as olfaction and somatosensation are likely to provide essential input for comprehensive representations of an environment in rodents and, in blind humans, spatial navigation is conducted by nonvisual means. Furthermore, place cells established in blind rats are similar to those in sighted rats, suggesting that nonvisual information is sufficient for spatial representation (Save et al. 1998). The brain has the capacity to either use multimodal sensory information to acquire orientational information or can opt to use the most reliable modality to the exclusion of others (Schlack et al. 2005). Thus, it is not unreasonable to assume that in the absence of reliable visual cues, the hippocampus may resort to information from other modalities to generate memory of space.
From previous studies on hippocampal place cells using odor cues, Save et al. (2000) reported that place fields were more stable when the recording box was not cleaned between recording sessions, suggesting that odor information was used by the animals to support the visual cues. However, since the odor cues were left behind by animals during a previous exploration, it is unclear whether they can utilize novel olfactory cues to generate or stabilize place fields in the absence of visual input. It is also not clear if, for example, olfactory cues within an environment can serve as metric input to the hippocampus for the generation of spatial representations. In contrast, odor cues, which were used as global contextual cues, affect spatial representation. For example, global remapping of place fields was observed when the odor of the familiar recording environment was changed from one trial period to another (Anderson and Jeffery 2003). It is also not known yet whether odor cues control the rotation of place fields in the absence of salient visual input. However, it has been reported that the preferred directions of head direction cells shift in the same direction as olfactory cues (Goodridge et al. 1998), suggesting that place fields may also shift in the same way.
In our study, we explored whether spatial olfactory cues can give rise to stable place fields when presented under circumstances where auditory and visual cues are suppressed. We observed reliable place fields that rotated when the cue constellation was rotated and remapped when the cues were reconfigured. This suggests that hippocampal place cells can use nonvisual sensory modalities to generate place fields in the absence of reliable visual information.
Materials and Methods
Subjects
The present study was carried out in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) for care of laboratory animals. All experiments were performed according to the guidelines of the German Animal Protection Law and were approved by the North Rhine-Westphalia State Authority (Bezirksamt, Arnsberg). All efforts were made to reduce the number of animals used.
Male Wistar rats (8–9 weeks old) were housed individually and maintained on a 12-h light/12-h dark cycle. The animals were given sufficient food to maintain 90% of their free-feeding weight and ad libitum access to water. They were handled individually for 10 min per day, 1 week before surgery.
Electrodes and Microdrives
One lightweight microdrive (Axona Ltd, St. Albans, UK) was chronically implanted in each rat (8–9 weeks at the time of surgery). Each microdrive held 4 tetrodes made of 4 twisted bundles of 25 µm formvar-insulated nichrome wire (A-M systems, USA). The tetrodes were strengthened, respectively, with cyanoacrylate glue and inserted into a cannula, which was attached to the microdrive. One full rotation of the mechanical drive enabled a vertical movement of 200 µm, of all tetrodes simultaneously, without rotating the cannula or the electrodes.
Surgery
Each rat was chronically implanted with a microdrive as follows: Animals were anesthetized with an initial dose of sodium pentobarbital (52 mg/kg, i.p.) and placed in a stereotactic unit. Body temperature was monitored throughout the operation, and the anesthetic dose was adjusted to maintain surgical anesthesia. The skull was exposed and cleaned. A hole was drilled (1.2 mm diameter) over the right hippocampus. The tetrodes were placed in the cortex just above the CA1 hippocampal subfield (bregma −3.8 mm AP, 3.0 mm ML, and 1.5 mm DV). So as to protect exposed part of tetrodes between the skull surface and the bottom of the cannula, a sleeve made of 19-gauge tubing was pulled down over the exposed tetrodes to a depth just below the skull surface, the top of which overlapped the cannula. Three holes were drilled in the frontal, parietal, and occipital bone, respectively, into which small jewelers' screws were inserted. The microdrive was then anchored to the jewelers' screws and the skull surface by dental acrylic (Paladur, Heraeus Kulzer GmbH). One of screws also served as the electrical ground. The wound was dusted with antiseptic powder (“Chlorhexidine” Riemser, Germany). The animals were treated before and after surgery with analgesia (Meloxicam, Vetmedica GmbH, Ingelheim, Germany). The animals were allowed at least 7 days to recover from surgery before screenings were conducted. During this period, they were monitored closely for infection or distress and handled regularly.
Single-Unit Recordings
Rats were screened once or twice daily for unit activity in a screening box that was visually distinct from and in a different room to the test arena. Neural activities were passed through AC-coupled, unity-gain operational amplifiers, which were mounted on a headstage (Axona, UK) connected close to the rat's head through a socket that fitted onto the microdrive plug. The headstage was linked to a preamplifier via lightweight hearing-aid wires. The buffered signal from the headstage was amplified 6000–30 000 times in the preamplifier and then digitized (48 kHz) and bandpass filtered (0.6–7 kHz) in the dacqUSB system unit (Axona, UK). Each tetrode could be recorded differentially being referenced by one electrode of another tetrode. One of the recording channels was dedicated to EEG recording. The position of the rat was monitored by a video camera mounted directly above the platform and converted into x–y coordinates at a sampling rate of 50 Hz by a tracking system which detected a small infrared LED light mounted on the headstage near the rat's head.
Data Analysis
Data analysis was performed using the Tint analysis software (Axona, UK). The waveforms were displayed as clusters by plotting the peak-to-peak amplitude of each spike on one electrode against that of each of the other 3. The clusters were isolated by hand. On the basis of spike shape, firing rate, and firing location, complex-spike cells with 1 or 2 firing fields were separated. At least 50 spikes were isolated for each cluster. After the cluster cutting, firing rate maps for each cell were visualized using Tint, which divided the camera view arena into 64 × 64 square bins with a side length of 2.5 cm. The firing rate for a given cell in each bin indicated the spike number divided by dwell time in that bin. The firing rate maps were presented in color with a lowest firing rate (i.e., 0 Hz) in blue and the highest in red. A place field was defined as the contiguous group of pixels possessing a firing rate higher than half of the peak firing rate and covering <60% of the size of the recording arena. If a place cell was identified with one or more place fields, recordings were repeated 2 to 3 times on the same day and at least once more on the second day to verify its stability. If no qualified cell activity was identified, the tetrodes were advanced 25–50 µm and rats were returned to their home cages for at least 2 h. The maximum movement of tetrodes per day was 150 µm.
For each place cell, the firing rate map for each session was examined to determine: 1) place field size; 2) average firing rate; 3) peak firing rate; 4) mean infield firing rate; 5) mean outfield firing rate; 6) spatial information content; 7) sparsity. The size of the place field was calculated as the percentage of the recording arena by the place field. The average firing rate was determined by dividing the number of spikes that occurred over the entire session by the duration of the session. The peak firing rate was the highest firing rate of all pixels within the place field of the cell. The mean infield and outfield firing rates were defined as the mean values for the firing rates of all pixels within (infield) and outside (outfield) the place field. The spatial information content, measured in bits/spike, is a measure of how much information about the spatial location of the animal is contained within the activity of the cell. It was calculated using the methods described by Skaggs et al. (1993). Sparsity is a measure of how compact the firing field is relative to the recording apparatus and was calculated according to the methods described by Jung et al. (1994). The more confined the firing field of the place cell, the lower the sparsity measure.
The similarity between 2 neighboring firing rate maps was analyzed using a correlation procedure as follows: Each map was decomposed into a 32 × 32-element matrix. Each pixel in one matrix was correlated, by a Pearson's correlation, with its equivalent pixel in the second map. Pixels with a zero firing rate in both metrics were discarded. The outcome, that is, correlation coefficient (ranging from −1 to 1), represents the similarity of 2 rate maps.
It was suggested in some previous studies that place fields need to be normalized to a standard size in order to eliminate cross-correlation bias. Because correlation of larger place fields would produce better stability scores as there are more bins available (Hussaini et al. 2011). This process was not performed in our study for 2 reasons. First, place fields in the “no odor” condition were formed in a much more diffused way with larger place fields, indicating instability. If the place fields were normalized, this phenomenon would be ignored. Second, spatial correlation in the “no odor” condition was still significantly lower than the “4 odor” condition, although it had been already enhanced by larger place fields.
To estimate place field rotation numerically, spatial correlations were made between pairs of firing rate arrays. Firing rate maps in S6 were rotated in steps of 1° until up to 360° counterclockwise. The rotated maps were then compared with the corresponding rate maps in S7. The highest correlation coefficient was compared with the distribution of that from S4 to S6 (mean ± SD: 0.65 ± 0.25). If it fell into the confidence interval, the rotation corresponding to this correlation represented the rotation of the place fields. This was done to exclude the possibility that remapping might occur instead of rotation. This also applied to the case that the place field's shape changed dramatically after odor rotation. A normality test (Kolmogorov-Smirnov) was applied to each data set before any other comparison so as to ascertain whether data matched the pattern expected if the data were drawn from a population with a normal distribution. T-tests were applied to compare data sets from different groups of cells. Paired t-tests were applied to comparisons between 2 specific trials within the same group of cells. In some cases, place cell activities were not successfully recorded in one of the trials as a result of global remapping (i.e. data were lost for paired t-tests if cells became silent or new place cells emerged). T-tests were applied in this case, as well as when comparisons were made between data sets pooled from multiple trials (repeated trials recorded in the same condition). Statistical significance was defined as P ≤ 0.05.
Behavioral Apparatus
All screening for units took place in an open field square box (floor dimensions: 80 × 80 cm, walls: 70 cm). When well-isolated place cells with stable fields were confirmed, experiments were performed in a novel circular arena (80 cm in diameter, 70 cm high) in darkness. The floor of the arena contained 4 clusters of small pinholes located symmetrically in each quadrant, very close (5 cm) to the nearest border. Underneath each cluster of pinholes, a container was attached onto the floor of the arena. When 4 odors were placed in the containers, they diffused through the pinholes into the arena, creating an “odor constellation” with one distinct odor in each quadrant. The odor intensity was kept very low to ensure very low diffusion throughout the chamber. Rats foraging in the arena were not able to see the odor containers. The walls and floor were made of smooth plastic surfaces and lacked constant somatosensory cues. A white noise generator was placed just underneath the arena that masked any of the localized auditory cues that the rats could use for orientation. Noise was measured from 4 locations close to the pinhole clusters for 30 s at a sampling rate of 3 Hz. Data in each location were compared with those in other locations by analysis of variance (ANOVA). No significant difference was observed between any of the 4 locations (Supplementary Fig. 1). A very dim red light (6 W) was turned on while the rats were being connected to the recording system and turned off before they were randomly placed into the recording arena. Wistar rats are unpigmented and cannot see in red light. Curtains were not used since the room was in complete darkness during recording. The monitors from the PC and the recording system were turned off during recordings to avoid contributing visual cues. All indicators that emitted weak light were covered by opaque tape. The recording room had no window. The gaps between doors of the room and the floor were covered so that no light could pass through them. The recordings were initiated by a remote control.
Experimental Protocol
The experimental structure is shown in Figure 1. All recording sessions were conducted in total darkness. White noise was applied before the animals were transported into the room and remained on during the whole experimental process. In order to motivate the exploration of animals during recording, the animals' weight was sustained at 90% of pretesting body weight and small chocolate pieces were randomly scattered into the arena during recordings. Animals were first allowed to explore the arena for 2 min before the protocol started. This was done to minimize the factor that place fields were less stable during the first minutes. The protocol for the test group consisted of 10 sessions, each for 5 min, with an intersession interval of 10 min. During the interval, rats were removed from the arena and the floor was cleaned.
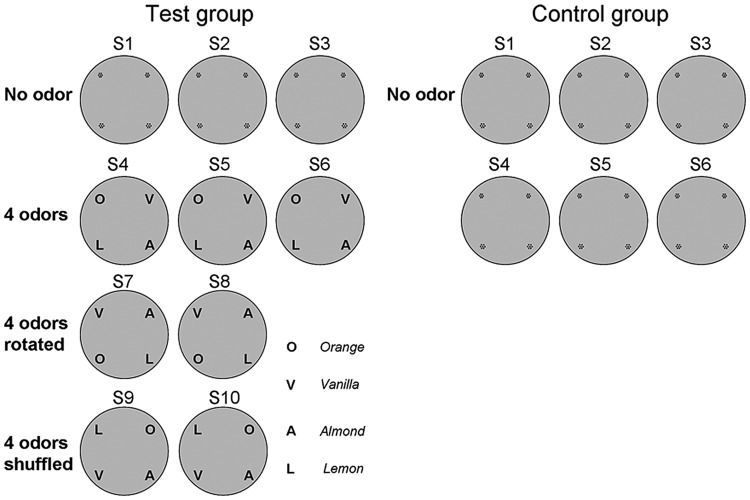
Figure 1.
Overview of the experimental protocol for place field recordings. All recordings were made during 1 experimental day in a circular arena that contained small pinholes on the floor clustered in each of the 4 quadrants of the arena, as indicated in S1–S3. The protocol for the test group consisted of 10 sessions and was divided into 4 conditions. During S1–S3 where no specific odors were present, exploration was in complete darkness and white noise excluded auditory cues. In the second condition (S4–S6), 4 odors (O: orange, V: vanilla, A: almond or L: lemon) were placed underneath each of the 4 pinhole clusters respectively. During recordings, odors diffused into the arena through pinholes. The arena was ventilated between trials. In the third condition (S7–S8), odors were rotated 90° counterclockwise to test whether place fields also rotate accordingly. In the last condition (S9–S10), the 4 odors were shuffled so that none of the odors remained at the same location compared with S7–S8. The protocol for the control group consisted of 6 sessions without introduction of any odor cue.
In each session, rats were randomly placed into the arena and allowed to explore in darkness. From session S1–S3, no odors were placed into the arena. These sessions were to test to what extent place fields were formed in a novel environment without visual information. From S4 to S6, 4 distinct odors (i.e., orange, vanilla, almond, and lemon) were placed into 4 quadrants. Here, we tested how locally placed novel odor cues affect place fields. In S7 and S8, odor cues were rotated 90° counterclockwise in space. The question here was to ask whether these odor cues were used as a spatial landmark and whether place fields followed the rotation of odor cues. In S9 and S10, odor cues were shuffled in space so that none of the odors were kept at the same location and the relative spatial relationship between any 2 odors was altered compared with the S4–S8 conditions. This was done to clarify if place fields encode a fixed spatial constellation of odors, or whether they simply follow one particular odor. When the odors were shuffled, the “odor constellation” was broken and remapping was assumed thereafter. Odor diffusion was carefully controlled during the entire experiment so as to ensure they were space-specific. There was a 2-min interval between odor placement and rat exploration, during which rats were connected to the recording system and were remained on a holding platform. During cleaning between sessions, pinholes were tightly sealed by cellophane to prevent excessive odor leakage, the air within the arena was dispersed and the room was ventilated. In order to assess if repeated sessions in the protocol affected place field responses, the responses of 2 control rats were scrutinized. For the control group, 6 recording sessions, in which no visual, olfactory, and auditory cues were present, were conducted with an intersession interval of 10 min, the same conditions as the ones described for S1–S3 in the test group (Fig. 1). Each rat went through the protocol 3 times. No “temporal” effect was detected. In the test group (n = 6), each animal went through the protocol once.
Histological Analysis
The location of the recording and stimulation electrodes was verified by postmortem histological visualization. The tissue was fixed, coronal slices were obtained and Nissl-stained according to established procedures (Manahan-Vaughan et al. 1998) (Supplementary Fig. 2). Animals with misplaced electrodes were not included in the data analysis.
Results
Exploration by Means of Spatial Odor Cues Enables Stable Place Field Formation
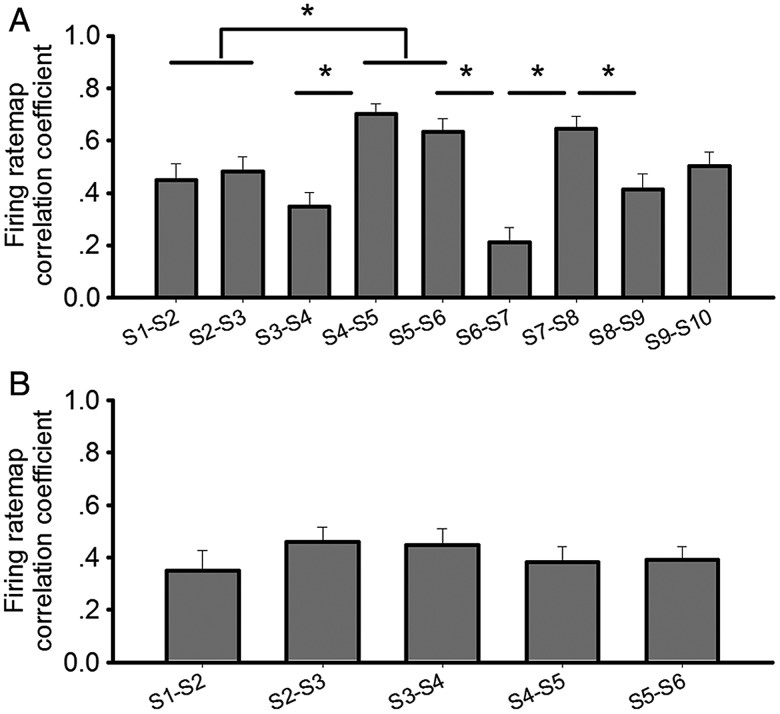
Animals were placed in the test arena in the absence of salient visual, auditory, or olfactory cues (S1–S3) following the protocol shown in Figure 1. Three cells were selected as representative in the test group (Fig. 2) and in the control group (Fig. 3), respectively. Thirty-one well-isolated cells from 6 rats were assessed (Supplementary Fig. 3). Here, 21 cells exhibited place fields in the S1–S3 condition. Twenty-seven cells showed place fields from S4 to S8. Thirty cells showed place fields during S9 and S10. Ten cells which were initially silent during S1–S3, started to form place fields when odors were introduced into the arena (S4–S6). One cell that showed initially stable place fields from S1 to S3, switched off upon odor introduction. This cell was later verified in a familiar screening box and re-formed its place fields there, which suggests that it was not lost during experiments. In the test group from S1 to S3, a relatively low spatial correlation between S1 and S2 (mean ± SEM: 0.35 ± 0.06) and between S2 and S3 (mean ± SEM: 0.37 ± 0.06) indicated low stability of place fields in a context without salient landmarks or orientation cues (Fig. 4a). In contrast, when odor cues were introduced (S4–S6), that were arranged in a fixed constellation, the place fields became by comparison significantly more stable, with higher spatial correlation between S4–S5 (mean ± SEM: 0.69 ± 0.04) and between S5–S6 (mean ± SEM: 0.65 ± 0.05) (Fig. 4a). Data pooled from S1–S2 and S2–S3 was compared with data pooled from S4–S5 and S5–S6. A significant difference was verified by t-test (P < 0.005, F = 99). In the control group, correlation coefficients were not significantly changed from S1 to S6 and remained in a low level similar to that in S1–S3 in the test group. (t-test, P > 0.1, degrees of freedom (F) = 131). Thus, in the absence of reliable sensory cues, place fields were less stable compared with conditions where odors were introduced.
Figure 2.
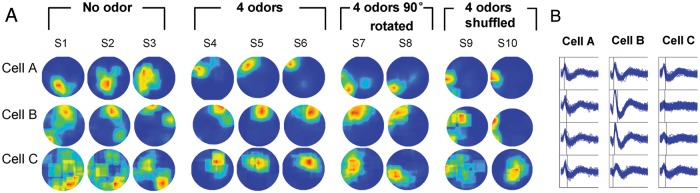
Example of firing rate maps of 3 cells across the test protocol. (a) Firing rate maps of 3 cells during the test protocol are shown. Recordings were conducted in a circular arena in dark. S1–S3 were recorded in a context with no specific odor cues. From S4 to S6, 4 odor cues were placed into 4 locations, respectively, each in a different quadrant. In S7 and S8, 4 odors were rotated 90° counterclockwise in space. In S9 and S10, 4 odors were shuffled in space. (b) Spike waveforms of illustrated cells were recorded by respective tetrode and were shown in a time window of 2 ms.
Figure 3.
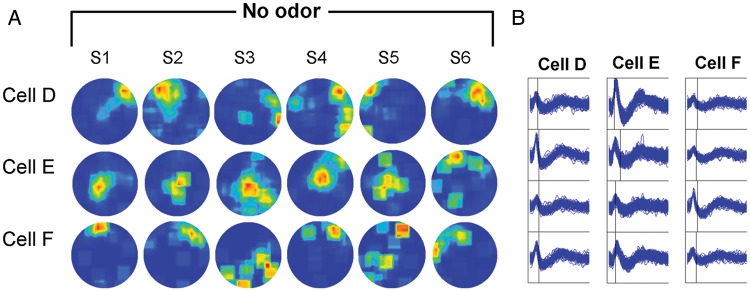
Example of firing rate maps of 3 cells across the control protocol. (a) Firing rate maps of 3 cells during the control protocol are shown. Recordings were conducted in a circular arena. Animals were allowed to explore the arena in dark with no specific odor cues. (b) Spike waveforms of illustrated cells were recorded by respective tetrode and were shown in a time window of 2 m s.
Figure 4.
Correlations of firing patterns in each neighboring session. (a) Each correlation is shown in bars indicating the mean value (± SEM). Each group of correlations in neighboring sessions was compared with a previous/following correlation group by t-test or paired t-test. *P < 0.005. (b) In the control group, each correlation is shown in bars indicating the mean value (± SEM). Each group of correlations in neighboring sessions was compared with a previous/following correlation group by paired t-test. No significant difference was observed.
Remapping upon Odor Introduction
After an initial period where no visual, auditory or odor cues were available (S1–S3), 4 odors were introduced to the area in a constellation that was kept constant for each rat. Eleven cells (cell 2, 4, 5, 6, 11, 12, 15, 21, 25, 30, 31) switched their place fields on or off (Supplementary Fig. 3). Furthermore, statistical analysis indicated that the spatial similarity of place fields between S3 and S4 was significantly reduced (t-test: P < 0.005, F = 48), with lower correlation (mean ± SEM: 0.25 ± 0.05) compared with that between S4 and S5 (mean ± SEM: 0.69 ± 0.04) or between S5 and S6 (mean ± SEM: 0.65 ± 0.05) (Fig. 4a). This reduction was mainly due to 11 cells (cell 1, 3, 7, 8, 9, 13, 14, 19, 20, 23, 24), which changed their firing patterns dramatically with distinct place fields from S3 to S4 (Supplementary Fig. 3). It suggests that remapping might have occurred in these cells. These data suggest that the introduction of the odor constellation was perceived as a context change that gave rise to place field remapping.
Place Fields Appeared to be More Spatially Selective After Odor Introduction
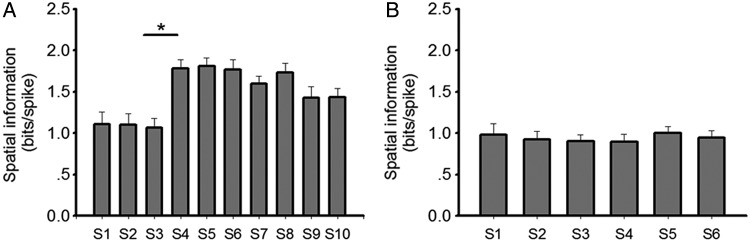
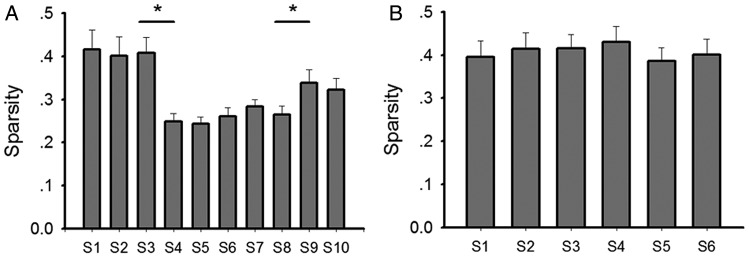
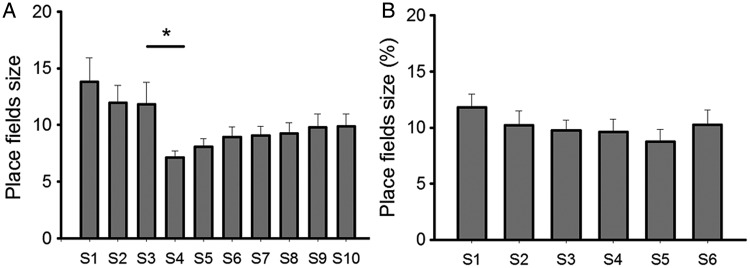
Spatial information and sparsity describe to what extent information, carried by a single spike, can predict the location of an animal, and how sparse neuronal spikes of a given cell are distributed in the recording apparatus. We assessed these parameters here and found that in the context with no salient cues (S1–S3 in the test group), diffuse firing patterns were evident in place cells as indicated by low spatial information (mean ± SEM: 1.11 ± 0.15 for S1; 1.10 ± 0.13 for S2; 1.07 ± 0.12 for S3; Fig. 5) and high sparsity (mean ± SEM: 0.42 ± 0.04 for S1; 0.41 ± 0.04 for S2; 0.40 ± 0.03 for S3; Fig. 6). In contrast, when odor cues were introduced (S4–S6), place fields became significantly more spatially selective, with higher spatial information (mean ± SEM: 1.78 ± 0.10 for S4; 1.81 ± 0.10 for S5; 1.77 ± 0.12 for S6; Fig. 5; t-test: P < 0.001, F = 151) and lower sparsity occurring (mean ± SEM: 0.24 ± 0.02 for S4; 0.23 ± 0.02 for S5; 0.25 ± 0.02 for S6; Fig. 6; t-test: P < 0.001, F = 151). Furthermore, place fields were more compact after odor introduction. The size of place fields, which occupied 14.0 ± 2.23% (mean ± SEM) of the arena in S3 decreased significantly in S4 which occupied only 8.3 ± 0.66% (mean ± SEM) of the arena (Fig. 7; t-test: P < 0.05, F = 48). This suggests that the place fields became spatially more selective after introduction of the spatial constellation of odors. In the control group, corresponding place field characteristics, including spatial information sparsity and place field size, remained stable across 6 recording sessions. It suggests that an improvement of spatial selectivity was not induced by the repeated exposure to an environment, but rather was enabled by the exposure to spatial odor configurations.
Figure 5.
Spatial information content of place cells in each session. Spatial information content of place cells in each session was calculated and plotted in a bar chart for the test group (a) and for the control group (b). Data in each session were compared with those in a previous/following session by t-test or paired t-test (bar charts: mean ± SEM, *P < 0.001).
Figure 6.
Sparsity of place cells in each session. Sparsity of place cells in each session was calculated and plotted in a bar chart for the test group (a) and for the control group (b). Data in each session were compared with those in a previous/following session by t-test or paired t-test (bar charts: mean ± SEM, *P < 0.05).
Figure 7.
Sizes of place fields in each session. Sizes of place fields were calculated with regard to the size of the arena in each session and were plotted in a bar chart in the test group (a) and in the control group (b). Data in each session were compared with those in a previous/following session by t-test or paired t-test (bar charts: mean ± SEM, *P < 0.001).
Place Fields Followed the Rotation of Odor Cues
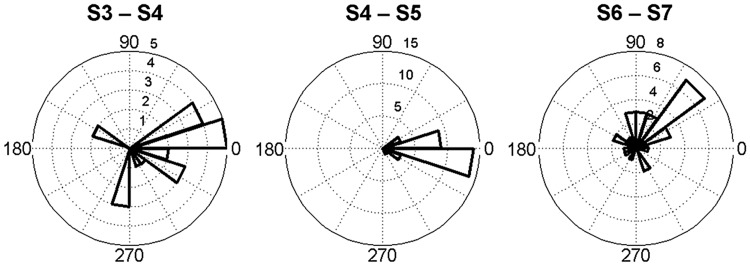
A low spatial correlation of firing rate maps was observed between S6 with 4 odor cues, and S7 with 4 odor cues rotated (mean ± SEM: 0.25 ± 0.05) (Fig. 4a). Due to the fact that the only change to the environment from S6 to S7 comprised the rotation of odor cues, we interpret this as meaning that the place fields followed the rotation of odor cues. For each cell, firing rate maps in S6 were rotated in steps of 1° until up to 360°. The rotated maps were later correlated to those in S7. The highest correlation coefficient was then compared with the distribution of data derived from S4 to S6 (mean ± SD: 0.65 ± 0.25). If it fell into the confidence interval, the rotation angle corresponding to the highest correlation coefficient represented the rotation of the place fields. Here, it was found that the rotation of the place fields from S6 to S7 was 83.3° ± 8.7° (mean ± SEM), while the odors were rotated by 90°. CA1 place cells can be split when there is conflicting information from salient proximal and distal visual cues (Knierim 2002). Our results support that this may also be the case for olfactory cues. A small proportion of the cells (cells 8, 10, 13, 21, 29) rotated <45°. Four cells (cells 12, 18, 22, 24) counter-rotated and 2 cells (cells 15, 24) remapped, which were excluded from this analysis. This amounted to roughly one third of the cells studied. Rotation angles of all place fields (from S6 to S7) were plotted in a polar histogram, which illustrates that the majority of place fields followed the rotation of odor cues (Fig. 8). Place fields were consistent in their orientation when odor cues were not rotated, that is, from S4 to S5 (Fig. 8). It strengthens the possibility that place fields can follow spatially configured odor cues. The rotation angles from S3 to S4 were also plotted in spite of their low correlations. This was conducted to test whether there was any tendency for cells to fire in the same overall location before and after introduction of odors. However, no specific pattern was observed. Taken together, the data indicate the place fields typically followed the rotation of odor cues.
Figure 8.
Rotation of place field. Rotation angles (20° bins) of all place fields were plotted corresponding to 3 conditions: odor introduction (S3–S4), odor maintenance (S4–S5), and odor rotation (S6–S7). Remapping induced by odor introduction resulted in dramatic angular shifts of most place fields with low correlation values. Place fields remained consistent in orientation when odor cues were stable. When odor cues were rotated 90° counterclockwise, the majority of place fields rotated in the same direction.
Remapping When Odors Were Shuffled in Space
In sessions S9 and S10, the odors were shuffled such that the position of the odors and their spatial correlation shared no similarity with the previous sessions (S7–S8). The spatial correlation of place fields between S8 and S9 was significantly reduced (t-test: P < 0.005, F = 56; Fig. 4a), with a lower correlation occurring (mean ± SEM: 0.35 ± 0.06), compared with that between S7 and S8 (mean ± SEM: 0.65 ± 0.05). This suggests that when odor cues are shuffled in space an alteration of firing patterns of place cells occurs. Specifically, 2 cells (cell 11 and 25) became silent from S7 to S8 indicating rate remapping, 15 cells (cell 4, 5, 7, 9, 12, 14, 18, 19, 20, 23, 24, 26, 27, 30, 31) changed their firing location conspicuously which was considered as remapping, and 14 cells remained stable (Supplementary Fig. 3). This response was distinct to that seen in animals that explored the arena in the dark in the absence of spatially configured odor cues (Supplementary Fig. 4). In order to verify whether some cells followed specific odors after shuffling the odor cues, cells with place fields close to one odor quadrant in S8 were examined as to whether their place fields in S9 fell into the same odor quadrant. Cell 6 turned out to be silent after odor introduction, and was excluded. Seven of 30 cells (cells 4, 7, 12, 14, 16, 20, 23) were observed to follow the odor cues from S8 to S9, which is close to 25% of random possibility (Supplementary Fig. 2). Moreover, among the 10 cells (cells 2, 4, 5, 11, 12, 15, 21, 25, 30, 31) that formed place fields after odor introduction, which were highly suspected to be odor-specific cells, only 2 of them followed a specific odor cue after odor shuffling (Supplementary Fig. 3) (These 2 cells were excluded from analysis). Thus, the current data does not support the possibility that the cells we identified as generating place fields were in fact odor-specific cells. Rather, the data indicate that remapping occurred when the odors were reconfigured in space. Interestingly, the sparsity of place fields from S8 to S9 increased significantly (t-test: P < 0.05, F = 56; Fig. 6a) provoking the interesting possibility that the cues were perceived as less reliable.
Firing Frequencies and Behavior Status Remain Stable Across the Protocol
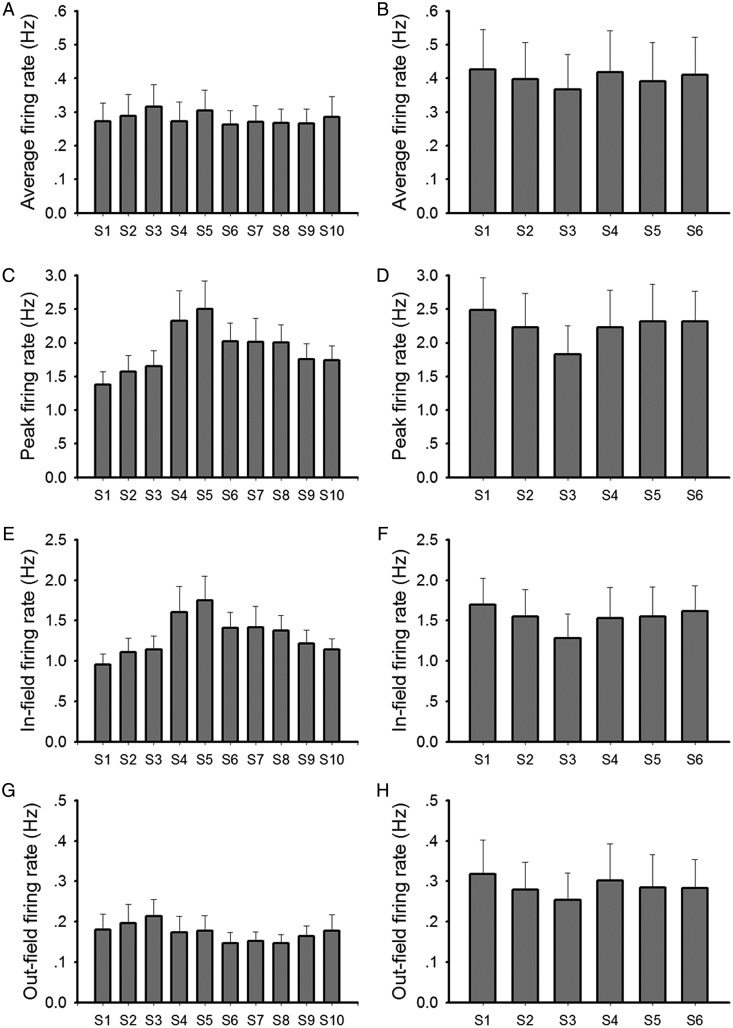
The average firing rate was calculated by dividing the number of spikes recorded over the entire session by the duration of the session. The peak firing rate was defined as the highest firing rate of all pixels in the rate map of a place field. Mean infield and outfield firing rates were defined as the average firing rates of all pixels within (infield) and outside (outfield) the place field. None of these measures was significantly altered across the protocol (Fig. 9). The average velocity of animals was not significantly altered and the behavioral status was not notable changed (Supplementary Fig. 5). This excludes the likelihood that changes in these parameters influenced the outcome of the place field patterns in the various conditions.
Figure 9.
Calculations of firing rate in each session. Average firing rate, peak firing rate, infield firing rate, and outfield firing rate of place cells in each session were calculated and plotted in bar charts for the test group (a,c,e,g) and for the control group (b,d,f,h). Data in each session were compared with those in a previous/following session by t-test or paired t-test. No significant difference was observed (bar charts: mean ± SEM).
Discussion
This study demonstrates that in the absence of reliable spatial cues from the visual sensory modality, rodents will use spatial configurations of odors to generate reliable and stable place fields. This observation suggests that the hippocampus can use nonvisuospatial resources, and specifically can use spatial olfactory information, to generate spatial representations. Despite the less precise nature of olfactory stimuli compared with visual stimuli, this form of sensory information can substitute for visual inputs to enable the acquisition of metric information about space.
The influence of olfaction on spatial representation has been scrutinized in other circumstances by others that revealed that place fields are more stable when the recording box remains uncleaned between recording sessions in both light and dark conditions (Save et al. 2000). In addition, a global olfactory effect was described by Anderson and Jeffery (2003), who observed global remapping of place fields in a visuospatial environment when the scent of the recording box was entirely changed. Activity of hippocampal pyramidal cells is also modulated by nonspatial but task-dependent olfactory cues. Muzzio et al. (2009) demonstrated that spatially shifting reward-associated olfactory cues reduce the stability of former established place fields in visual environments and increase the stability of reward-associated odor representations. In addition, hippocampal pyramidal cells exhibit conjunctive properties, responding optimally to a particular combination of position and odor (Manns and Eichenbaum 2009).
In this study, rats were trained to search randomly scattered food pellets in a recording box during cell screening. Experiments were conducted in total darkness. In previous studies, place fields were shown to be unaffected by darkness when animals were previously allowed to explore the environment in the light. However, they changed dramatically when the animals were put directly into the same but already darkened chamber (Quirk et al. 1990). The latter phenomenon was also observed in this study, in which rats were always placed into the recording arena in the dark. Correlations between sessions with no odor cues (S1–S3 in the test group, and all sessions in the control group) were relatively lower than sessions with odor cues. When novel odor cues were included, spatial correlations between neighboring sessions were significantly enhanced, which clearly suggested that local olfactory information was successfully adopted in the form of a spatial representation.
This study demonstrates that the hippocampus can utilize novel spatial olfactory information to enable stable place field formation in the absence of salient visual cues. This suggests that, in line with findings with regard to other brain structures (Schlack et al. 2005), the hippocampus can opt to use the most salient sensory information in stabilizing spatial representations. There are 2 possible ways to explain this phenomenon. First, place fields drift in the dark (Quirk et al. 1990), due to cumulative directional errors coming from the head direction cell and path integration systems (Knierim et al. 1995; McNaughton et al. 2006). This kind of drifting can be fixed by including visual information (Knierim, et al. 1995). Thus, it is likely that olfactory information is adopted in the same way that visual information is processed. Second, in anatomical terms, olfactory information projects to the hippocampus from the periphery without being preprocessed by the thalamus, where the head direction system is also partially located. It is very likely the effect of olfactory information on hippocampus is therefore different from that of visual information.
Our observation that place fields become more compact upon presentation of odor cues extends interesting links to observations with regard to learning-facilitated plasticity (Kemp and Manahan-Vaughan 2007, 2012). Learning-facilitated plasticity describes the ability of selected hippocampal synapses to respond with persistent synaptic plasticity when afferent stimulation of the synaptic population is coupled with a spatial learning event (Kemp and Manahan-Vaughan 2007). Typically, under conditions where spatial context is learned, long-term depression is facilitated (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2008; Goh and Manahan-Vaughan 2013). Interestingly, LTD is also facilitated in response to exploration of novel odor cues in space (André and Manahan-Vaughan 2013). This facilitation of LTD is tightly associated with learning about the spatial odor configuration (André and Manahan-Vaughan 2013). The odor configuration protocol used in that study was matched to the protocol we used in the present study, and even with only one exposure to the odor configuration, evidence of significant learning was obtained (André and Manahan-Vaughan 2013), indicating that odor configuration learning was a component of the place field stabilization we observed here.
LTD comprises a reduction of synaptic efficacy. Our observations that place fields became more stable and compact upon the inclusion of spatially arranged odor cues, may reflect a function of LTD in suppressing “background noise” such that the signal-to-noise ratio favors place field formation and strong signal contrast. The place field thus appears (with an optimized signal-to-noise ratio) upon the background of low synaptic activity in selected neuronal populations. Alternatively, LTD and place fields cooperate, where LTD serves to help place fields to become more compact and more place-specific, by “pruning” the size of place cells' firing fields. Thus, whereas the development of LTD and place fields in the spatial olfactory environment may comprise distinct and autonomous processes, it is also plausible that under these circumstances LTD may contribute to informational processing by place cells such that place field stability and spatial selectivity are increased.
In the current study, we observed that when odor cues were locally introduced into the arena, place fields became orientationally more stable and some place fields followed the rotation of odor cues. This kind of behavior of place fields with regard to odor rotation is similar to reports in which place fields rotate with visual cue cards (Jeffery and O'Keefe 1999). It may suggest that olfactory information could be utilized not only as contextual cues, as described by others (Anderson and Jeffery 2003). Interestingly, activity of head direction cells could also be controlled by olfactory cue rotations, as shown when blindfolded rats navigated in a cylinder (Goodridge et al. 1998).
We observed that some place fields remapped when odor cues were introduced into the arena, which is similar to previous findings (Anderson and Jeffery 2003). What is striking is that some of the place fields remapped when odor positions were shuffled. This suggests that rats, foraging in darkness under white noise conditions, might perceive 4 odor cues as a whole “spatial constellation” (or spatial unit), where each odor was assigned to a part of the space. When the odor cues were shuffled, this constellation was altered and some of the place cells remapped. Another explanation would be that these hippocampal neurons might have encoded sequence of odors during exploration. This possibility is supported by the finding that hippocampal complex-spike cells fire differentially, depending on the sequence in which odors were presented (Ginther et al. 2011). A typical behavior for rats entering a new environment comprises exploration along the borders of the arena. In this study, the rats commonly ran either clockwise or counterclockwise during the first seconds after entering the circular box. If such a temporal pattern of odor sequence is stored and coupled with path integration, spatial representations could also be stabilized and retrieved faster due to pattern completion. For example, if a rat was running counterclockwise, the odor sequence along his pathway would be initially orange-lemon-almond-vanilla-orange. When odor cues were rotated in the next condition, animals would thus not notice any changes in odor sequence during counterclockwise running. Therefore, no remapping would be expected. However, when odor cues were shuffled in the next condition, and the odor sequence during running in the same direction as previously was detected as new pattern, that is, orange-lemon-vanilla-almond-orange, remapping would be expected. It is therefore plausible that some place field remapped due to the mismatch of odor sequence pattern along their pathway. However, the behavior of animals in the recording chamber during a complete session was very random. The hypothetical clockwise/counterclockwise running pattern would thus not apply to every session in the arena.
Evidence exists that certain neurons engage in olfactory recognition memory (Ramus and Eichenbaum 2000). But it is not likely that the cells found in this study subserve the simple representation of this type of memory, because they did not follow any single odor during odor rotation and odor shuffling. The brain structure responsible for this kind of olfactory information encoding comprises the orbitofrontal cortex (Ramus and Eichenbaum 2000). These cells are distinct from item-place cells or odor-place cells that occur in the CA1 and CA3 regions and fire when odors are associated with specific items (Komorowski et al. 2009). Here, we used purely olfactory information as spatial landmarks with no items in the arena to avoid that these kinds of cells would be activated.
Although the place cells in this study were controlled by olfactory information in the dark, this was not the case when animals were exposed to the same spatial environment with lights on. Others have reported that brain structures such as the posterior parietal cortex process sensory information in a multimodal way, but will also resort to choosing the most salient or reliable modality in order to process space (Schlack et al. 2005). This would also appear to be the case for the hippocampus.
In conclusion, our results suggest that olfactory information is processed in a similar way as visual information regarding place field formation in the CA1 region. These data support that olfactory information can help to stabilize place fields when other sensory modalities are not salient enough.
Supplementary Material
Supplementary can be found at: http://www.cercor.oxfordjournals.org/
Funding
Funding to pay the Open Access publication charges for this article was provided by the collaborative research centre (SFB 874) of the German Research foundation (DFG).
Supplementary Material
Notes
We gratefully acknowledge the technical assistance of Jens Klausnitzer and Juliane Boege and the technical contribution of Marion André. We are deeply indebted to K. Jeffery and J. Donnett for technical advice and instruction on procedures and analysis. We thank Nadine Gomell for animal care. This study was supported by a grant (SFB 874/B3) from the German Research Foundation (DFG). Conflict of Interest: None declared.
References
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André MA, Manahan-Vaughan D. Spatial olfactory learning facilitates long-term depression in the hippocampus. Hippocampus. 2013 doi: 10.1002/hipo.22158. Forthcoming. doi:10.1002/hipo.22158. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cressant A, Muller RU, Poucet B. Failure of centrally placed objects to control the firing fields of hippocampal place cells. J Neurosci. 1997;17:2531–2542. doi: 10.1523/JNEUROSCI.17-07-02531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther MR, Walsh DF, Ramus SJ. Hippocampal neurons encode different episodes in an overlapping sequence of odors task. J Neurosci. 2011;31:2706–2711. doi: 10.1523/JNEUROSCI.3413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D. Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb Cortex. 2013;23:1118–1125. doi: 10.1093/cercor/bhs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS. Cue control and head direction cells. Behav Neurosci. 1998;112:749–761. doi: 10.1037//0735-7044.112.4.749. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, McNaughton BL. Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci. 1996;16:8027–8040. doi: 10.1523/JNEUROSCI.16-24-08027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?—linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hussaini SA, Kempadoo KA, Thuault SJ, Siegelbaum SA, Kandel ER. Increased size and stability of CA1 and CA3 place fields in HCN1 knockout mice. Neuron. 2011;72:643–653. doi: 10.1016/j.neuron.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, O'Keefe JM. Learned interaction of visual and idiothetic cues in the control of place field orientation. Exp Brain Res. 1999;127:151–161. doi: 10.1007/s002210050785. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Passive spatial perception facilitates the expression of persistent hippocampal long-term depression. Cereb Cortex. 2012;22:1614–1621. doi: 10.1093/cercor/bhr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci. 2002;22:6254–6264. doi: 10.1523/JNEUROSCI.22-14-06254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Behnisch G, Vieweg S, Reymann KG, Behnisch T. Semi-automated analysis of NMDA-mediated toxicity in digitised colour images from rat hippocampus. J Neurosci Methods. 1998;82:85–95. doi: 10.1016/s0165-0270(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Buno W. Stabilizing effects of extracellular ATP on synaptic efficacy and plasticity in hippocampal pyramidal neurons. Eur J Neurosci. 2005;21:936–944. doi: 10.1111/j.1460-9568.2005.03925.x. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the “cognitive map”. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzio IA, Levita L, Kulkarni J, Monaco J, Kentros C, Stead M, Abbott LF, Kandel ER. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biol. 2009;7:e1000140. doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. The firing of hippocampal place cells in the dark depends on the rat's recent experience. J Neurosci. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus SJ, Eichenbaum H. Neural correlates of olfactory recognition memory in the rat orbitofrontal cortex. J Neurosci. 2000;20:8199–8208. doi: 10.1523/JNEUROSCI.20-21-08199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Save E, Cressant A, Thinus-Blanc C, Poucet B. Spatial firing of hippocampal place cells in blind rats. J Neurosci. 1998;18:1818–1826. doi: 10.1523/JNEUROSCI.18-05-01818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Nerad L, Poucet B. Contribution of multiple sensory information to place field stability in hippocampal place cells. Hippocampus. 2000;10:64–76. doi: 10.1002/(SICI)1098-1063(2000)10:1<64::AID-HIPO7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schlack A, Sterbing-D'Angelo SJ, Hartung K, Hoffmann KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J Neurosci. 2005;25:4616–4625. doi: 10.1523/JNEUROSCI.0455-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs ME, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. Adv Neural Inform Process Syst. 1993;5:1030–1037. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.