Abstract
High affinity class-switched antibodies and memory B cells are products of the germinal center. The CD4+ T cell help required for the development and maintenance of the germinal center is delivered by follicular helper T cells (TFH), a CD4+ helper T cell subset characterized by expression of Bcl-6 and secretion of IL-21. The cellular interactions that mediate differentiation of TFH and GC B cells remain an important area of investigation. We previously showed that MHC class II-dependent DC antigen presentation is sufficient for the differentiation of a TFH intermediate (termed pre-TFH), characterized by Bcl-6 expression but lacking IL-21 secretion. Here we examine the contributions of MHCII antigen presentation by B cells to TFH differentiation and GC responses in several contexts. B cells alone do not efficiently prime naïve CD4+ T cells or induce TFH following protein immunization; however, during LCMV infection B cells induce TFH differentiation despite the lack of effector CD4+ T cell generation. Still, MHCII-positive DCs and B cells cooperate for optimal TFH and GC B cell differentiation in response to both model antigens and viral infection. This study highlights the roles for B cells in both CD4+ T cell priming and TFH differentiation and demonstrates that different APC subsets work in tandem to mediate the germinal center response.
Introduction
CD4+ T cells play a central role in immune responses, both as effector cells and by providing help to other cells, including B cells. Naïve CD4+ T cells must be activated by antigen presenting cells (APCs) expressing peptide-MHC class II (MHCII) complexes. MHCII-dependent T cell-effector cell interactions are also required for the delivery of CD4+ T cell help. MHCII-positive dendritic cells (DCs) are uniquely positioned to activate naïve CD4+ T cells (1). However, multiple cell types express MHCII, including B cells, macrophages, basophils, mast cells and some endothelial cells (2–4) and could mediate CD4+ T cell effector functions.
Multiple studies have shown that B cell expression of MHCII is necessary for B cells to “receive” CD4+ T cell help to mediate functions such as isotype class switching (5, 6). However, experiments to define the converse ability of MHCII-positive B cells to present antigen to CD4+ T cells and drive T cell differentiation have yielded conflicting results (7). Early studies in mice lacking B cells suggested that B cells are required for optimal CD4+ T cell responses, including both initial priming and effector functions (8–16). Contrasting studies in B cell deficient mice and allogeneic transfer systems in mice and chickens suggested that B cells activate T cells inefficiently and CD4+ T cells priming was independent of B cells (17–20). However, studies to directly test the sufficiency of B cell antigen presentation in CD4+ T cell priming are lacking.
Primed CD4+ T cells differentiate into multiple effector subsets, including follicular helper T cells (TFH) (21, 22). TFH are necessary to initiate and maintain germinal centers (GCs), structures in secondary lymphoid tissues in which activated B cells undergo class switching and somatic hypermutation to generate high affinity plasma cells (PCs) and memory B cells (23). TFH express the transcription factor Bcl6, which controls their differentiation (24–26), the chemokine receptor CXCR5, allowing them to localize to the CXCL13 rich B cell follicles, (27–29), as well as co-stimulatory molecules, including CD40L, ICOS and PD-1 (21, 30, 31)and cytokines, especially IL-21 and IL-4 (32, 33), that contribute to the formation and function of the germinal center. As TFH play a critical role in the GC process, it is important to understand the cells and cues that mediate their differentiation.
TFH differentiation is initiated early in the immune response, prior to CD4+ T cell interactions with B cells (31, 34, 35). Consistent with these observations, we previously showed that TFH differentiation requires DCs (36). However, DC priming is not sufficient to complete TFH differentiation, but instead drives the production of pre-TFH, a partially-differentiated intermediate that expresses CXCR5 and Bcl6 (36). Pre-TFH lack expression of PD-1 and do not produce significant quantities of the cytokine, IL-21. It has been proposed that B cells mediate the differentiation of pre-TFH into IL-21-producing TFH. Several groups have demonstrated that antigen-specific B cells are necessary for TFH maintenance (24, 29, 31, 32, 37). Similarly, B cell expression of costimulatory molecules, including ICOSL, PD-1 ligands, and CD80, are necessary for TFH and GC B cell differentiation and function (31, 38–41). The notion of unique B cell signaling has been challenged by other groups (42, 43), who instead suggest that TFH differentiation simply requires persistent TCR signals. Concretely delineating the requirement for individual MHCII+ APCs to initiate and maintain TFH differentiation and development of the germinal center should resolve these conflicts.
In this study, we describe a novel mouse model in which MHCII, I-Ab, is restricted to B cells. We define the ability of B cells to prime naïve CD4+ T cells in vivo and the contribution of B cells to TFH differentiation in different contexts. MHCII expression restricted to B cells cannot drive CD4+ T cell priming, TFH differentiation, or initiate GC responses in response to nominal peptide and protein immunization. However, in the context of viral infection, B cell MHCII expression is sufficient to induce limited T cell priming and strikingly endows the vast majority of antigen specific CD4+ T cells with a TFH phenotype. However, the generation of functional antigen-specific germinal centers and subsequent plasma and memory B cell output requires both DC and B cell MHCII expression. Therefore, in the setting of viral infection, MHCII+ B cells may be able to drive the TFH program; however, MHCII-dependent antigen presentation by both DCs and B cells is necessary to induce optimal differentiation of TFH and germinal centers.
Materials and Methods
Mice
C57BL/6J, CD19 Cre and OT-II mice were purchased from Jackson Laboratories. Smarta TCR transgenic mice (44), Foxp3 GFP mice (45) and CD11c/Aβb mice (6) were bred in house. MHCII Aβb STOP/STOP mice were developed at Washington University in St. Louis as described and subsequently bred in house (46). B-MHCII mice were breed as CD19Cre/+ MHCII Aβb STOP/-, B/DC-MHCII mice additionally had the CD11c/Aβb transgene. WT control mice were breed as MHCII STOP/+ CD19 Cre/+. MHCII alleles are denoted in the text as follows: WT as +, MHCII Aβb KO (47) as - and MHCII Aβb STOP as STOP. Mice were housed under pathogen free conditions, in accordance with the University of Pennsylvania Animal Care and Use Guidelines and used at 8–18 weeks of age.
Immunizations and infections
CD4+ OT-II T cells, CD4+ Smarta T cells and CD4+ polyclonal cells from C57/BL6J mice were enriched by negatively selecting out CD8+, B220+, MHCII+ and FcγRII+ cells and labeled with CFSE where indicated, as previously described (48). OT-II cells were transferred i.v. 1 day prior to i.p. immunization with 50µg NP14-OVA (4–Hydroxy-3-nitrophenylacetyl coupled to ovalbumin) (Biosearch Technologies) in alum (Sigma) as previously described (32, 36). 1×104 Smarta cells were transferred i.v. 1 day prior to infection with 2 × 105 PFU LCMV Armstrong (experiments shown in figure 4) or 2×104 PFU Armstrong (experiments shown in figure 6) as previously described (49). Virus was grown and titered as described (49). B-MHCII and B/DC-MHCII mice infected with LCMV Armstrong also received ~107 CD4+ polyclonal T cells isolated from C57/BL6 mice 7–14 days before infection to reconstitute the CD4+ T cell compartment.
Figure 4.
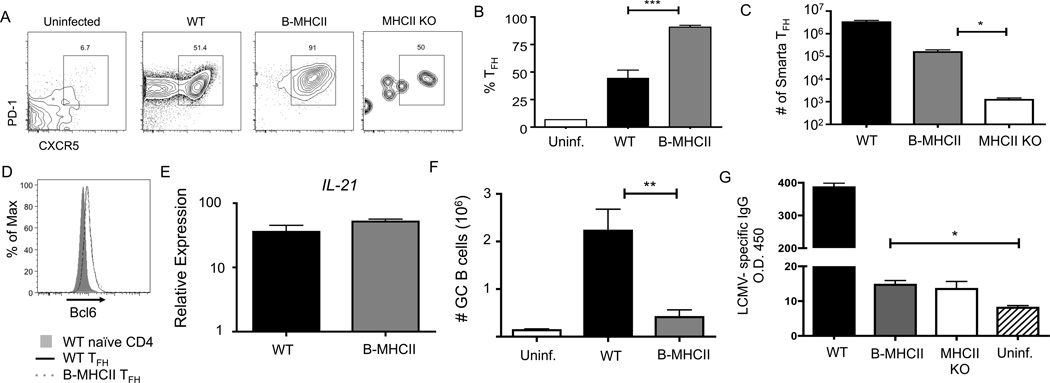
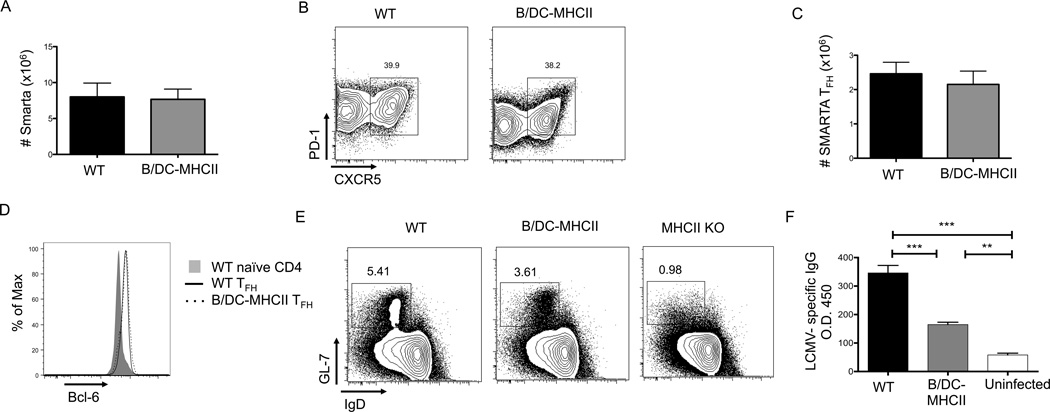
B cell antigen presentation preferentially drives TFH differentiation in response to viral infection. B-MHCII and MHCII KO mice received 1×107 CD4+ T cells from C57/BL6 mice 7–14 days prior to infection to reconstitute the CD4+ T cell compartment. 1×104 SMARTA transgenic CD4+ T cells were transferred to WT and B-MHCII mice and the mice were infected with LCMV Armstrong one day later. Splenocytes were analyzed on day 8 post infection. (A) Representative FACS plots of CD19− TCRβ+ CD4+ SMARTA cells to identify CXCR5+ PD-1+ TFH cells. (B) Percentage of SMARTA cells in WT and B-MHCII mice that are CXCR5+ PD-1+ TFH cells. (C) Total number of splenic SMARTA TFH cells in WT, B-MHCII and MHCII KO mice. (D) Histogram overlay of Bcl6 expression by CXCR5+ PD-1+ SMARTA TFH cells. (E) Relative expression of IL-21 mRNA in sorted CXCR5+ PD-1+ SMARTA cells. (F) Total number of CD19= B220+ IgDlo GL-7+ GC B cells. (G) Measurement of LCMV specific IgG in the serum on d8 p.i., compared to uninfected C57/BL6 mice. * denotes a p value of < 0.05, ** denotes a p value of <0.01 and *** denotes a p value of <0.001 calculated using Student’s t test. Bar graphs show mean ± SEM. Data are representative of 2 independent experiments with 3–6 mice per group.
Figure 6.
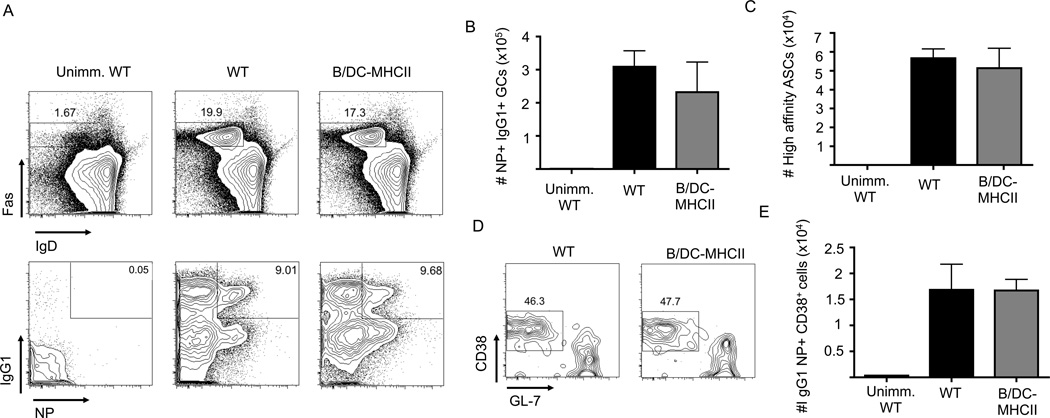
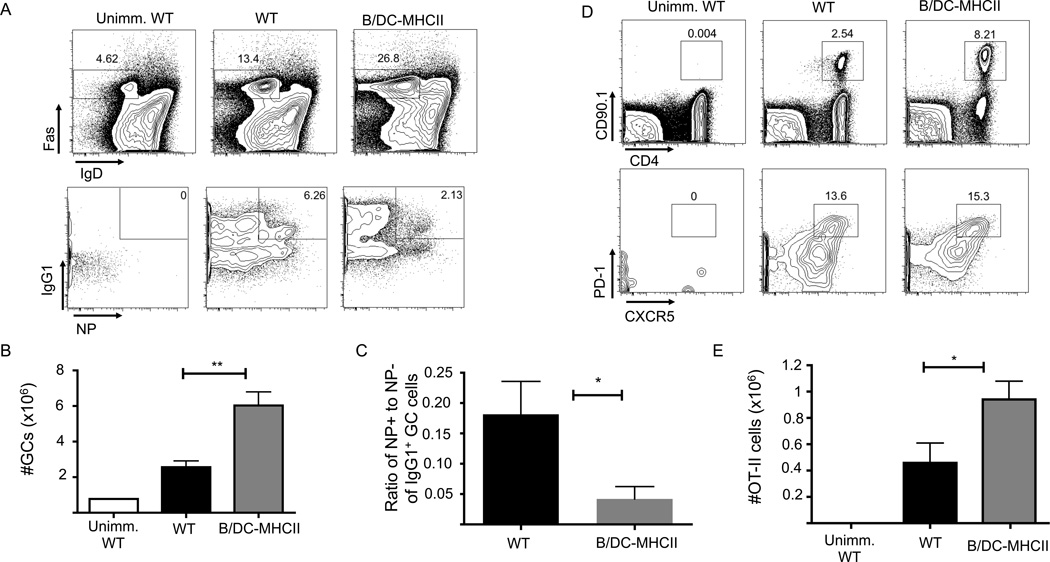
MHCII dependent antigen presentation by DCs and B cells is sufficient for GC B cell responses after protein immunization. 1×105 OT-II cells were transferred to WT and B/DC-MHCII mice and mice were immunized i.p. with NP-OVA in alum. (A) Representative FACS plots of splenic Fas+ GC B cells (gated on CD19+ B220+ cells) (top plots) and IgG1 expression and NP specific cells in the GC population (gated on CD19+ B220+ Fas+ IgDlo cells) (bottom plots) on d7 p.i. Numbers represent the percentage of B cells that are GCs (top) and percent of GC B cells that are NP+ IgG1+ (bottom) (B) Total number of NP specific IgG1+ GC B cells on d7 p.i. quantified from the plots in (A) (C) Total number of high affinity, NP specific IgG1+ ASCs in the spleen on day 14 p.i. as determined by ELISPOT. (D) Representative FACS plots of class-switched NP specific memory B cells on d29 p.i. (gated on CD19+ B220+ dump− IgG1+ IgD−IgM− NP+ cells) (E) Number of IgG1+ NP memory B cells on 29 p.i. quantified from the plots in (D). Bar graphs in (B), (C) and (E) show mean ± SEM. n= 4–5 mice per group, representative of 2–3 independent experiments.
In vitro cultures
Sorted B cells were incubated overnight with 10 µg LPS and one day later were incubated with CFSE labeled purified OT-II cells (see immunizations and infections section above), at a ratio of 1:10, with 10,000 B cells and 100,000 OT-II cells per well, in OVA protein at a concentration of 100µg/mL. CFSE dilution of OT-II cells was analyzed 4 or 5 days later.
Flow cytometry and cell sorting
All antibodies were purchased from Biolegend, eBioscience, BD Pharmingen or Invitrogen. DAPI or Live/Dead AQUA™ (Invitrogen) was used to identify live cells. The FoxP3 fixation and permeabilization kit was used to detect intracellular Bcl6 and Foxp3 staining (eBioscience). Cells were acquired or sorted on an LSR II cytometer or FacsAria II, respectively (BD Biosciences). Data was analyzed using FlowJo software (TreeStar). All FACS plots shown were gated on live, singlet cells.
QPCR
QPCR was conducted as previously described (36). In brief, RNA was extracted using the RNEasy® Mini kit (Qiagen) and cDNA was made using the high capacity cDNA reverse transcription kit (Applied Biosystems). GAPDH was used as the housekeeping gene for TFH cell qPCR, 18s was used as a housekeeping gene for all other qPCRs. qPCRs were performed on an 7500 Real Time PCR system machine (Applied Biosystems). Data were analyzed using the ΔΔct method.
ELISPOT and ELISA assays
For NP specific ELISPOTS, splenocytes were incubated on 10 ug/ml of NP5-BSA (high affinity) or NP25-BSA (all affinities) (Biosearch Technologies) coated plates (Millipore) and incubated with biotin-anti-mouse-IgG1or IgM (Southern Biotech) followed by incubation with ExtrAvidin-Alkaline Phosphatase (Sigma) and developed with NBT/BCIP substrate (Sigma). Spots were enumerated on CTL-ImmunoSpot reader (Cellular Technologies). LCMV specific antibodies were detected in serum by ELISA. Lysate from baby hamster kidney (BHK) cells infected with LCMV Armstrong was used to coat ELISA plates. HRP-linked antibodies against mouse IgG were used to detect the LCMV reactive antibodies. Relative OD values were determined at 450 nm, and values at dilutions within a linear range were used to determine final relative absorption.
Results
Restricting MHCII expression to B cells
In order to better study the requirements for various MHCII+ APCs in CD4+ T cell activation, we have recently developed a new mouse strain in which the MHC class II, Aβb, locus is targeted with a “conditional gene repair” cassette (50), permitting expression of MHCII in any cell type to which Cre has been targeted (46). In these mice, designated as MHCII Aβb STOP/STOP, the Aβb gene (which is targeted in traditional MHCII knockout mouse strains (47)) is silenced by insertion of a transcriptional STOP cassette (50) flanked by LoxP sites into Intron 1. This allows MHCII Aβb to be activated under the control of its own promoter and regulatory elements after Cre-mediated recombination and cassette deletion. In the absence of Cre, MHCII Aβb STOP/STOP mice phenotypically resemble MHCII Aβb −/− mice with no I-Ab expression and no conventional CD4+ T cells in the thymus or periphery (46). To generate mice in which MHCII is restricted to B cells, we crossed CD19 Cre/Cre mice (51) on a heterozygous background for MHCII (MHCII+/−, (47)) to MHCII Aβb STOP/STOP mice in order to generate pups that were MHCII Aβb STOP/− CD19Cre/+ (referred to as B-MHCII mice). WT control MHCII STOP/+ CD19 Cre/+ mice had one WT allele of Aβb and therefore expressed MHCII on all APC subsets.
Approximately 97% of B220+ TCRβ− B cells expressed MHCII in the spleen and LNs of WT mice (Supplemental Figure 1A, 46), while approximately 90–95% of B cells expressed MHCII in B-MHCII mice. All subsets of CD19+ B cells examined expressed MHCII in B-MHCII mice and there was no preferential MHCII expression in any one subset (46). All non-B cell APC populations including DCs and macrophages in B-MHCII mice were MHCII negative, whereas they were MHCII positive in WT mice (Supplemental Figure 2). To verify that B cells from B-MHCII mice were indeed transcribing MHCII, we sorted CD19+B220+ B cells from spleens of WT, B-MHCII and MHCII KO mice and performed RT-PCR for the targeted Aβb gene. B cells from B-MHCII mice expressed less MHCII, Aβb, mRNA than did B cells from WT mice (Supplemental Figure 1B), consistent with the heterozygous genotype of the B cells in the B-MHCII mice. To confirm MHCII, Aβb, transcription in B-MHCII mice was restricted to B cells, TCRβ− CD19− splenocytes, a population that contains all non-B cell APC subsets, were sorted from each line of mice. Expression of Aβb in this non-T/B cell population was equivalent in B-MHCII and MHCII Aβb STOP/STOP mice (Supplemental Figure 1C). Targeting the Aβb gene does not disrupt B cell development as we recently showed that the populations of developing B cells are comparable in the BM of MHCII Aβb STOP/STOP, B-MHCII and WT mice (46). Additionally, the follicular and marginal zone B cell compartments in the spleen are also comparable between B-MHCII and WT mice (46).
To verify the functionality of B cells targeted with a “gene-repair cassette”, we examined the ability of B cells from B-MHCII mice to prime naïve CD4+ T cells in vitro. CD19+ B220+ B cells were sorted from the spleens of WT and B-MHCII mice, activated overnight with LPS, pulsed with ovalbumin (OVA) protein and incubated with CFSE labeled ovalbumin-specific TCR transgenic OT-II cells for 4 days. B cells from B-MHCII and WT mice proliferated to a similar extent after activation with LPS (data not shown). OVA-pulsed B cells from WT and B-MHCII mice induced a similar degree of OT-II proliferation (Supplemental Figure 1D). Similar results were obtained using OVA peptide in place of OVA protein (data not shown). These data indicate that activated B cells from B-MHCII mice are functional and have the ability to process and present antigen to activate naïve CD4+ T cells. Additionally, WT and B-MHCII B cells induce comparable CD4+ T cell proliferation in vitro despite expressing different levels of MHCII.
B cells prime naïve CD4 T cells poorly in response to nominal protein antigen in vivo
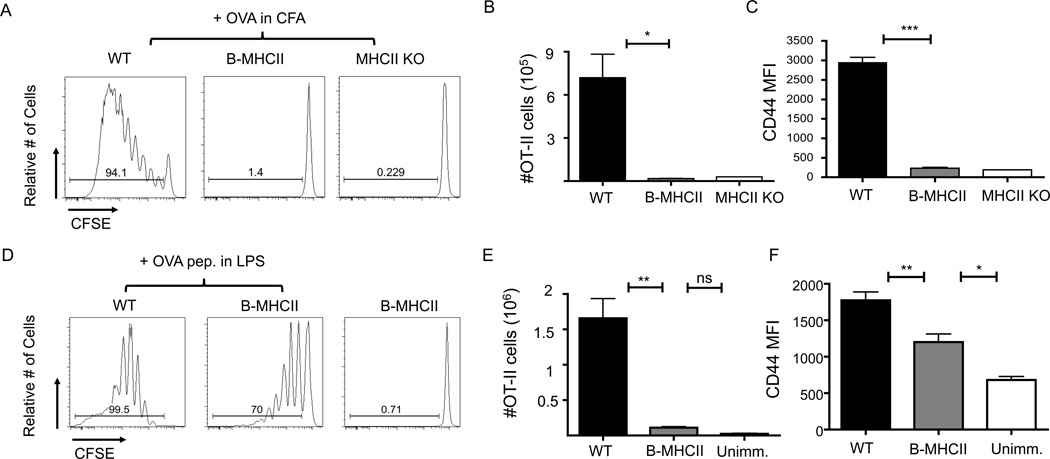
Although B cells are the most numerous MHCII-positive APC in secondary lymphoid tissues, their contribution to the priming of naïve CD4+ T cells in vivo remains unclear. The B-MHCII mice provide the ideal system to examine this question. Thymic cortical epithelium is MHCII-negative in B-MHCII, which therefore lack a mature peripheral CD4+ T cell compartment (46). Despite the lack of conventional CD4+ T cells, B-MHCII mice have an intact CD8+ T cell compartment and normal lymphoid architecture, with segregation of T and B cells, as well as normal T cell zone and B cell follicle structure (data not shown), consistent with published data on mice lacking CD4+ T cells (36, 52). Given the lack of conventional CD4+ T cells in this system, we examined the response of adoptively transferred antigen-specific TCR transgenic CD4+ T cells. CFSE labeled OT-II cells were transferred into MHCII-deficient, B-MHCII, or WT recipients 1 day prior to s.c. immunization with OVA protein emulsified in CFA. Four days after immunization, OT-II cells in the draining LNs of WT mice had undergone extensive proliferation and expansion; whereas there was neither proliferation nor expansion of OT-II cells in the draining LNs of either B-MHCII mice or MHCII KO mice (Figure 1A, B). Consistent with these data, OT-II cells in B-MHCII mice had significantly less CD44 expression than those found in WT mice, verifying defective activation (Figure 1C).
Figure 1.
MHCII+ B cells prime naïve CD4+ T cells very inefficiently. 2×106 CFSE labeled OT-II cells were transferred to WT, B-MHCII and MHCII KO mice. Mice were immunized s.c. with 200 µg OVA emulsified in CFA (A–C) or i.v. with 100µg OVA 323–339 peptide and 75µg LPS (D–F). (A) Proliferation of CD19− TCRβ+ OT-II cells in dLNs 4 days after s.c. immunization with OVA/CFA. (B) Total number of and (C) mean fluorescence intensity of CD44 on OT-II cells in dLNs of WT, BMHCII and MHCII KO mice 4 days after OVA/CFA immunization. (D) Proliferation of CD19− TCRβ+ OT-II cells in the spleen 4 days after OVA peptide immunization. (E) Total number of and (F) mean fluorescence intensity of CD44 on splenic OT-II cells. Bar graphs in (B), (C), (E) and (F) show mean ± SEM. n= 3–5 mice per group, representative of 2–3 independent experiments. * indicates a p value of <0.05, ** indicates a p value of <0.01 and *** indicates a p value of <0.001, calculated using Student’s t test (B,C) or one way ANOVA with Tukey’s analysis (E,F).
Utilizing a protein immunization system limits antigen delivery to the small number of B cells with a B cell receptor specific for the immunizing antigen (53), and non-BCR mediated antigen uptake mechanisms such as pinocytosis (54) which are quite inefficient. To examine T cell priming in a scenario in which all B cells could present peptide-MHCII complexes regardless of BCR specificity, mice were immunized IV with OVA 323–339 peptide and LPS. OT-II cells in WT mice exhibited extensive proliferation, with most of the cells found in the 4th division or greater (Figure 1D). In contrast, OT-II cell proliferation induced by B cells alone in B-MHCII mice was sub-optimal as the majority of cells had divided only once or twice (Figure 1D). OT-II cells primed by B cells did have increased CD44 expression in comparison to mice that were not immunized; although, they expressed much less CD44 than OT-II cells primed in WT mice (Figure 1F) and produced significantly interferon gamma and IL-2 (Supplemental Figure 3). However, there was no increase in the number of OT-II cells in either the spleen or peripheral LNs (Figure 1E) of immunized B-MHCII mice compared to unimmunized mice. Thus, B cells are capable of inducing minimal CD4+ T cell priming in vivo when directly targeted with processed antigen, but B cell antigen presentation alone does not induce the activation and expansion observed when other MHCII+ APC populations are also functional.
B cell restricted antigen presentation is not sufficient to elicit TFH and GC formation following peptide or protein immunization
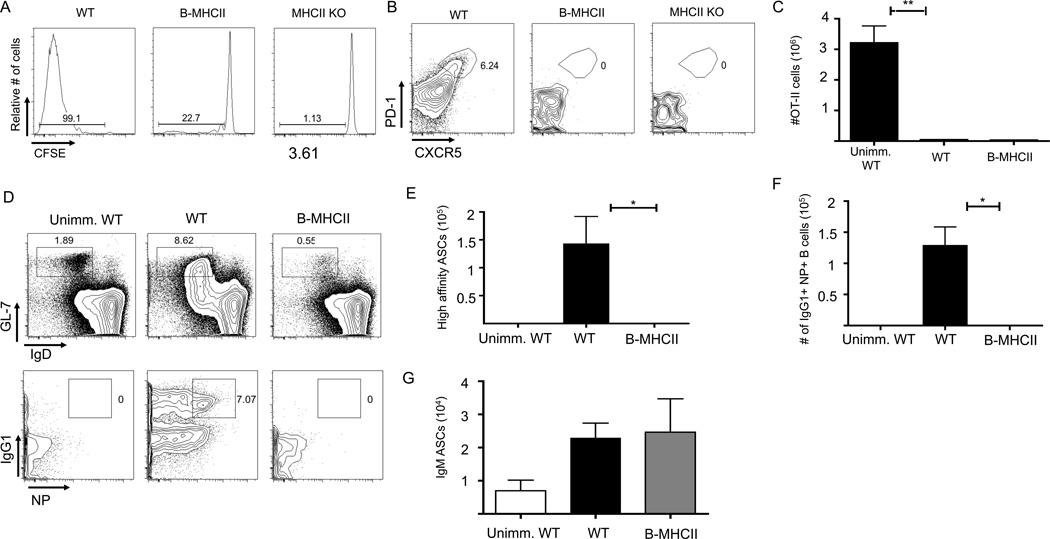
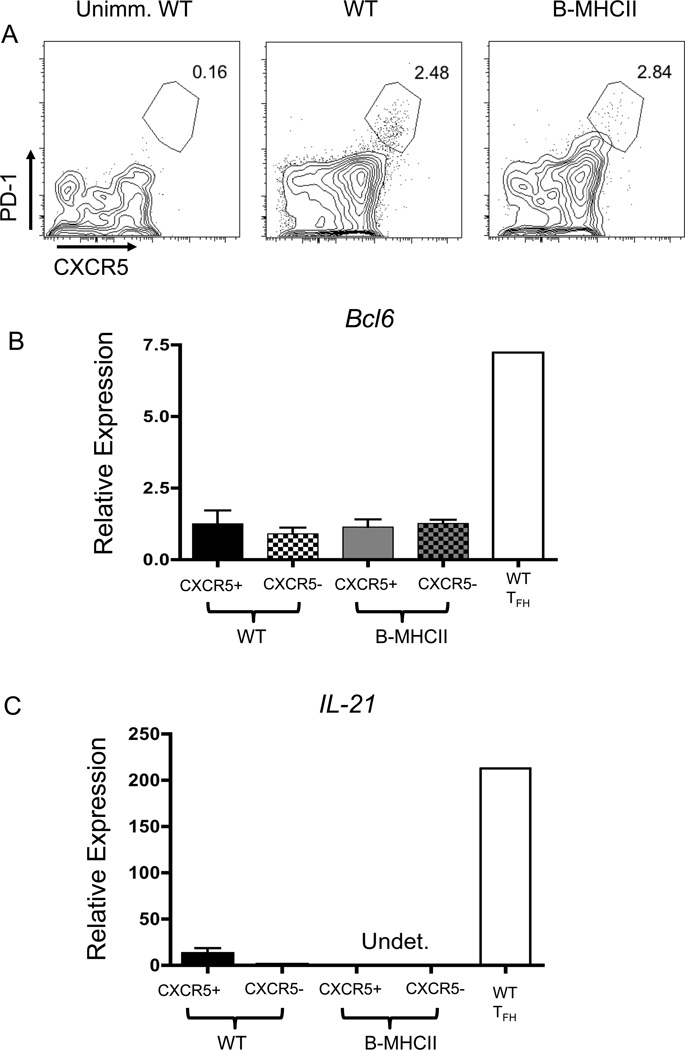
We considered the possibility that B cells could interact with T cells to induce TFH and germinal center differentiation, despite their inability to generate significant CD4+ T cell expansion. To address this, we examined the response of OT-II cells and Ag-specific B cells after immunization with haptenated NP-OVA in alum, which elicits strong germinal center and antibody responses. Differentiation of OT-II TFH and GC B cells were examined 7 and 14 days after immunization. Similar to i.v. immunizations, OT-II cells in B-MHCII mice underwent minimal proliferation and no expansion after NP-OVA immunization (Figure 2A, C). Additionally, up-regulation of CXCR5 was impaired and there was no differentiation of CXCR5+ PD-1+ TFH differentiation on either day 7 (Figure 2B) or d14 post immunization (data not shown). In the absence of TFH, neither antigen-specific germinal centers (Figure 2D, 2F) nor high affinity plasma cells (PCs) in the spleen (Figure 2E) or BM (data not shown) were formed. Importantly, WT and B-MHCII mice had comparable numbers of IgM PC responses in the spleen on d7 (data not shown) and d14 post immunization (Figure 2G), in agreement with previous data suggesting that the early IgM PC response is T cell independent (52). Together, these data show that B cell restricted antigen presentation induces neither TFH nor GC responses after protein immunization.
Figure 2.
MHCII dependent antigen presentation by B cells alone does not elicit TFH and GC responses in response to protein immunization. 1×106 OT-II cells were transferred to WT and B MHCII and mice were immunized i.p. with 50 µg NP-OVA in alum. (A) Representative FACS plots of CFSE diltution of OT-II cells in the spleen on d7 p.i. Gated on CD19− TCRβ+ CD90.1+ OT-II cells (B) Representative FACS plots of OT-II cells to identify CXCR5+PD-1hi TFH on d7 p.i. Numbers indicated percentage of OT-II cells that are CXCR5+ PD-1hi (C) Total number of OT-II TFH in WT and B-MHCII mice on d7 p.i. (D) Representative FACS plots of GL-7+ germinal center B cells (top) and IgG1 expression and NP specific cells of the GC (bottom) on day 7 p.i. Gated on CD19+B220+ cells (top) and further on GL-7+ IgD−. Numbers represent the percentage of B cells that are GCs (top) and percent of GC B cells that are NP+ IgG1+ (bottom) (E) Total number of splenic NP specific, IgG1+ GC B cells on d7 p.i. (F) Total number of high affinity, NP specific IgG1+ ASCs per spleen and (G) Total number of IgM+ ASCs per spleen on day 14 p.i. as determined by ELISPOT. Bar graphs in (C), (E), (F) and (G) show mean ± SEM. n=5–6 mice, data are pooled from two independent experiments. * denotes a p value of <0.05 and ** denotes a p value of <0.01, calculated using Student’s t test.
B cell priming after i.p. NP-OVA immunization induced much less OT-II cell proliferation than did i.v. immunization with OVA peptide in LPS. We therefore examined TFH differentiation in B-MHCII mice after peptide immunization as the increased T cell priming and proliferation might be more conducive to TFH differentiation. While i.v. peptide immunization did induce some CXCR5 expression on OT-II in both WT and B-MHCII mice, there was only a small population of CXCR5+ PD-1+ TFH-like cells present in either strain (Figure 3A). However, there was no induction of either Bcl6 or IL-21 expression in CXCR5+ OT-II cells from either WT or B-MHCII mice after immunization, indicating that i.v. peptide immunization does not induce TFH responses (Figure 3B, C).
Figure 3.
Minimal TFH differentiation in response to peptide immunization. 2×106 CFSE labeled OT-II cells were transferred to WT and B-MHCII mice. Mice were immunized i.v. with 100µg OVA 323–339 peptide and 75µg LPS. Splenocytes were examined 7 days after immunization. (A) Representative FACS plots of CD19− TCRβ+ CD4+ OT-II cells to identify CXCR5+ PD-1+ TFH cells. CXCR5+ and CXCR5− OT-II cells were sorted from WT and B-MHCII mice after immunization and examined for (B) Bcl6 mRNA and (C) IL-21 mRNA. Bar graphs in (B) and (C) show mean ± SEM. n=3–4 mice per group, representative of 3 independent experiments.
B cell restricted antigen presentation induces TFH differentiation following viral infection
Immunization with model antigens in adjuvant is a useful tool for understanding the biology of an immune response, but does not always mimic the processes that occur in the context of infection. To examine B cell restricted antigen presentation during acute viral infection, we reconstituted the CD4+ T cell comparment of B-MHCII mice with 107 polyclonal CD4+ T cells and transfered 1×104 LCMV GP61–80 specific SmartaTCR Tg T cells to WT and B-MHCII mice one day prior to infection with LCMV Armstrong. On day 8 post infection, there was much less expansion of Smarta T cells in infected B-MHCII mice than in WT littermates with approximately 100x fewer cells (Figure 4C). However, Smarta cells did not expand in infected MHCII KO mice; thus, the expansion observed in B-MHCII mice was antigen-specific. Strikingly, upwards of 90% of the Smarta cells in B-MHCII spleens exhibited a TFH phenotype (Figure 4A), (Figure 4C). TFH cells primed in WT and B-MHCII mice had equivalent levels of Bcl-6 mRNA (data not shown) and protein (Figure 4D), and also expressed equivalent levels of IL-21 mRNA (Figure 4E), suggesting that the CXCR5+ cells primed only by B cells were indeed TFH cells. Overall, these data demonstrate that in the setting of acute viral infection, B cells can induce partial TFH differentaion, and skew T cells almost exclusively toward the TFH lineage.
As TFH play a critical role in the GC B cell reponse, we next asked if LCMV-specific GC responses were present in LCMV-infected B-MHCII mice. As there are no reagents to asses LCMV specific B cells by FACS, we quantified the number of GL-7+ IgDlo B cells in spleens of WT and B/DC-MHCII mice by FACS and measured serum IgG antibodies by ELISA on day 8 post infection.After infection, WT mice generated significant numbers of IgDlo GL-7+ GC B cells; however, B-MHCII mice had almost no GC B cells, close to the background level observed in uninfected mice (Figure 4F). Consistent with these data, B-MHCII mice generated only minimal LCMV specific IgG+ antibody titers, though greater then the levels in uninfected mice (Figure 4G). Thus, the small number of TFH cells generated in B-MHCII mice after LCMV infection were insufficient for GC formation.
The combination of DC and B cell antigen presentation is sufficient for TFH differentiation and GC development after protein immunization
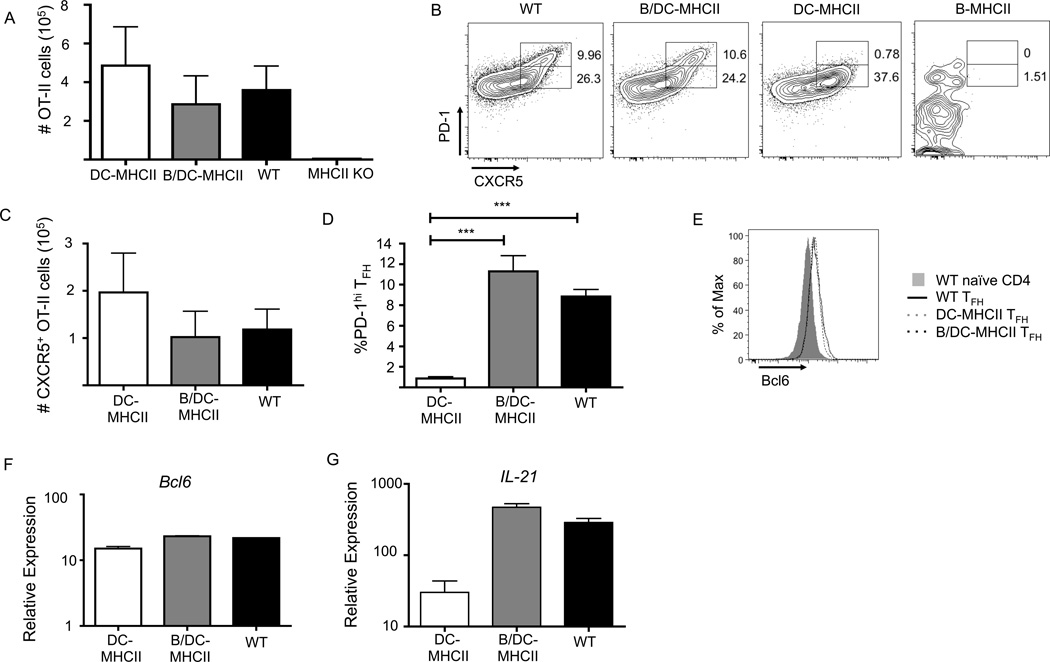
Previous work has shown that generation of a partially differentiated TFH cell (pre-TFH) (36) is initiated by MHCII-positive DCs prior to cognate T-B interactions (31, 34–36). We and others have proposed that B cell antigen presentation completes the TFH program (21, 36). However, the ability of MHCII-positive B cells to complete TFH differentiation has not been directly examined. We therefore crossed B-MHCII mice to mice in which only CD11chi lymphoid resident DCs are MHCII+ [(DC-MHCII, referred to as CD11c/Aβb (6, 36)], to generate mice in which MHCII is expressed by conventional DCs and B cells together (B/DC-MHCII mice). To examine TFH differentiation in the presence of DC and B cell MHCII expression, we again analyzed transferred OT-II cells in mice immunized i.p. with NP-OVA in alum. OT-II cells expanded similarly in DC-MHCII, B/DC-MHCII, and WT mice (Figure 5A) and generated similar numbers of CXCR5+ OT-II cells with equivalent expression of Bcl6 mRNA and protein (Figure 5B, C, E, F). Consistent with our prior work, antigen-specific CD4+ T cells primed by DCs alone lack the PD-1hi TFH population found in WT mice (Figure 5B, D); however, PD-1hi TFH are restored in B/DC-MHCII mice (Figure 5B, D). While CXCR5+ OT-II cells primed only by DCs exhibit approximately a 10 fold reduction in IL-21 mRNA levels when compared to WT-TFH, TFH primed by both DCs and B cells exhibit similar levels of IL-21 transcript as WT-TFH (Figure 5G). Together these data demonstrate that MHCII-positive DCs and B cells cooperate for TFH differentiation after immunization, as neither population alone is sufficient for TFH differentiation but the combination is.
Figure 5.
MHCII antigen presentation by DCs and B cells cooperates for TFH differentiation. 1×105 OT-II cells were transferred to WT, B-MHCII, DC-MHCII and B/DC-MHCII mice. Mice were immunized with NP-OVA in alum i.p. and analyzed on day 7 p.i. (A) Total number of OT-II cells (CD19− TCRβ+CD90.1+) in the spleen on day 7 p.i. (B) Representative FACS plots of OT-II cells for expression of CXCR5 and PD-1 to identify TFH. Numbers represent the percent of OT-II cells that are CXCR5+ PD-1hi and PD-1int (C) Total number of CD62L− CXCR5+ OT-II cells in the spleen on day 7 p.i. (D) Quantification of PD-1hi OT-II TFH from the plots shown in (B) (E) Histogram overlay of Bcl6 expression by CD6L−CXCR5+ OT-II cells. Relative expression of (F) Bcl6 and (G) IL-21 mRNA in sorted CXCR5+ OT-II cells relative to naïve CD4+ T cells. Bar graphs in (A), (C), (D), (F) and (G) show mean ± SEM. n=3–5 mice per group, representative of 3–4 independent experiments. *** denotes a p value of <0.001 calculated using a one way ANOVA with Tukey’s analysis.
As TFH function to drive and sustain the GC B cell response, we hypothesized that the combination of DC and B cell antigen would also suffice for differentiation of GC B cells. Indeed, seven days after immunization, both WT and B/DC-MHCII spleens contained equivalent numbers of Fas+ IgDlo NP-binding, IgG1+ GC B cells (Figure 6A, B). Germinal centers function to generate high affinity class-switched plasma cells and memory B cells. Fourteen days after NP-OVA immunization there were similar numbers of high affinity IgG1+ NP+ ASCs in the spleen (Figure 6C), as well as in the BM (data not shown) of WT and B/DC-MHCII mice. Similarly, on day 14 post-immunization (data not shown), as well as day 29 post immunization (Figure 6D, E) B/DC-MHCII spleens contained NP-binding IgG1+ memory B cells in similar numbers to WT mice. In combination with our published data, these data suggest that MHCII expression by both DCs and B cells are both necessary and sufficient for germinal center B cell differentiation after protein immunization.
DC and B cell antigen presentation during viral infection
As B cell priming alone was insufficient to induce optimal TFH or antibody responses following acute LCMV infection, we hypothesized that the addition of DC antigen presentation was necessary. We, therefore, compared WT and B/DC-MHCII mice acutely infected with 2 × 104 PFU LCMV Armstrong. Smarta cells had expanded equivalently in WT and B/DC-MHCII mice on day 8 post infection (Figure 7A) and similar numbers of Smarta cells had differentiated into CXCR5+ PD-1hi Bcl6+TFH cells in B/DC-MHCII and WT mice (Figure 7B, C, D), indicating that DC and B cell MHCII expression is sufficient for TFH differentiation in the setting of viral infection.
Figure 7.
MHCII antigen presentation by DCs and B cells cooperates for TFH and GC differentiation during LCMV infection. B/DC-MHCII mice received 1×107 CD4+ T cells from C57/BL6 mice 7 days prior to infection to reconstitute the CD4+ T cell compartment. 1×104 SMARTA transgenic CD4+ T cells were transferred to WT and B/DC-MHCII mice and the mice were infected with LCMV Armstrong one day later. Splenocytes were analyzed on day 8 post infection. (A) Total number of SMARTA cells per spleen in WT and B/DC-MHCII mice on d8 post infection (gated on CD19−TCRβ+ CD45.1+ cells). Numbers represent the percent of Smarta cells that are CXCR5+ PD-1+ (B) Representative FACS plots of CD19−TCRβ+ CD45.1+ Smarta cells for PD-1 and CXCR5 expression. (C) Total number of PD-1hi CXCR5+ Smarta TFH per spleen in WT and B/DC-MHCII mice on day 8 p.i., quantified from the plots in (A) (D) Histogram overlay of Bcl6 expression of PD-1+ CXCR5+ Smarta TFH from the plots shown in (A) WT are shown with the black line and B/DC-MHCII by the dashed line. (E) Representative FACS plots of GC B cells on day 8 post infection (gated on CD19+ B220+ F4/80− GR-1− TCRβ− cells). Numbers represent the percent of B cells that are GCs. (F) Measurement of LCMV specific IgG in the serum of WT and B/DC-MHCII mice on d8 p.i., compared to uninfected C57/BL6 mice ** denotes a p value of < 0.01 and *** denotes a p value of <0.001 calculated using a one way ANOVA with Tukey’s analysis. Bar graphs in (C) and (F) show mean ± SEM. Data are representative of 2 independent experiments with 4–5 mice per group.
As DC and B cell MHCII expression was sufficient for antigen specific GC B cell responses to immunzation, we asked if this was also true following viral infection. While GC B cells did develop in B/DC-MHCII mice, the GC population was significantly smaller than in WT mice (Figure 7E). In agreement, B/DC-MHCII mice generated lower titers of IgG+ LCMV-specific antibodies than did WT mice, although the levels were significantly greater than those of uninfected mice (Figure 7F). We suspect the decreased GC responses in B/DC-MHCII mice represent the limitations of reconstituting the T cell compartment with transferred CD4+ T cells and the requirement for viral-specific CD4+ T cells of multiple different specificities with diverse Ag-specific B cells in the GC response. Nonetheless, MHCII-positive DCs and B cells do generate both TFH and GCs following viral infection, in contrast to MHCII+ B cells alone.
B/DC-MHCII mice have increased GCs in the absence of peripheral Tregs
Follicular regulatory T cells (TFR) express Foxp3 and Bcl6 and localize to the GC to limit the humoral response mediated by Bcl6-positive TFH cells (55–57). TFR numbers increase during later stages of the GC response, suggesting that TFRs regulate the GC as the immune response progresses (55). TFR cells limit the size of the GC response, as well as, maintaining the production of antigen-specific antibodies (55, 57). OT-II cells do not become TFR cells after immunization (Figure 9F, (55, 57)) and TFR may differentiate from thymically-derived Foxp3+ nTregs. As we previously noted, B/DC-MHCII mice lack thymic selection of CD4+ T cells and, therefore, also lack functional Tregs (data not shown) and provide a model to study the GC response in the absence of TFR.
Figure 9.
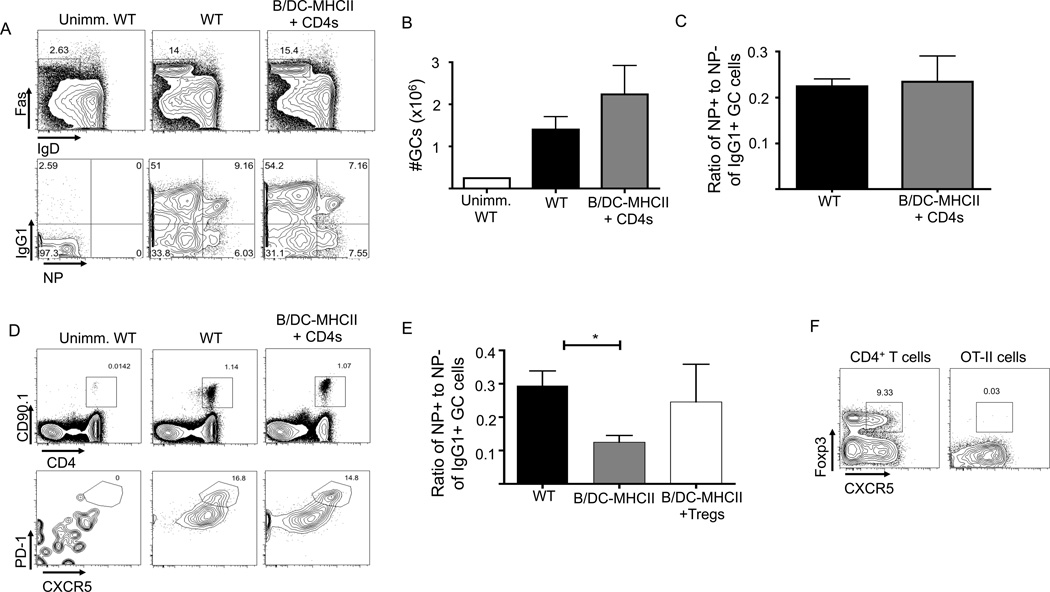
Germinal Centers and OT-II cell responses in the presence of either polyclonal CD4+ T cells or Tregs. (A–D) 1×107 CD4+ T cells from WT mice were transferred to B/DC-MHCII mice. One week later, 1×105 OT-II cells were transferred to WT and B/DC-MHCII mice and mice were immunized with NP-OVA /alum. Mice were analyzed on day 14 p.i. (A) Representative FACS plots of CD19+B220+ splenic GC B cells (top) and NP specific cells in the GC (bottom). Numbers represent the percentage of B cells that are GCs (top) and percent of GC B cells that are NP+ IgG1+ (bottom). (B) Total number of NP specific IgG1+ GCs on d14 p.i. quantified from the plots in (A) (C) Ratio of NP+ to NP− cells of CD19+ B220+ IgDlo Fas+ IgG1+ GC B cells (D) Representative FACS plots of OT-II cells (top) and OT-II TFH (bottom) on d14 p.i. Numbers represent the percent of CD4+ T cells that are OT-II (top) and the percent of OT-II cells that are CXCR5+ PD-1hi (bottom). (E) 5×105 sorted GFP+ Foxp3+ Tregs from FoxP3 GFP mice and 105 OT-II cells were transferred to WT and B/DC-MHCII mice and mice were immunized with NP-OVA in alum. Spleens were analyzed on day 14 p.i. Ratio of the percentage of NP+ to NP− cells of CD19+ B220+ IgDlo Fas+ IgG1+ GC B cells. (F) Analysis of Foxp3 and CXCR5 of endogenous CD4+ T cells (gated on TCRβ+ CD19− splenocytes) and OT-II cells (gated on TCRβ+ CD19− CD4+ CD090.1+) from the spleens of C57/BL6 mice immunized with NP-OVA in alum on day 8 p.i. Numbers represent the percentage of FoxP3+ CXCR5+ cells. * denotes p value of <0.05 using a one way ANOVA with Tukey’s analysis. Bar graphs in (B), (C) and (E) show mean ± SEM. n=3–6 mice per group, representative of two experiments. Data in E are pooled from two independent experiments.
At day 7 p.i. with NP-OVA, B/DC-MHCII mice have a comparable GC response to WT mice (Figure 6A), suggesting that Tregs and TFRs do not impact the early stages of the GC response. However, on day 14 p.i., B/DC-MHCII mice had at least twice the number of splenic Fas+ GC B cells as did WT mice (Figure 8A, B). In parallel, B/DC-MHCII mice also had increased numbers of OT-II T cells; the numbers of OT-II TFH cells were also increased in B/DC-MHCII mice but this reflected the overall increase in OT-II cells rather than a selective increase in TFH (Figure 8D). Although B/DC-MHCII and WT spleens contained a similar number of antigen specific NP+ GC B cells, B/DC-MHCII mice also had a large number of NP− IgG1+ GC B cells (Figure 8A). Thus, the ratio of NP-binding to NP-negative cells within the IgG1+ GC population was significantly reduced in B/DC-MHCII mice, (Figure 8C), indicating an outgrowth of NP-nonbinding clones in the absence of endogenous CD4+ T cells and Tregs.
Figure 8.
Increased OT-II and Germinal Center responses in the absence of endogenous CD4+ T cells. 1×105 OT-II cells were transferred to WT and B/DC-MHCII mice and mice were immunized with NP-OVA in alum. (A) Representative FACS plots of splenic GC B cells (gated on CD19+B220+ splenocytes, top) IgG1 expression and NP specific cells of the GC population (gated on CD19+ B220+ Fas+ IgDlo cells, bottom plots) on day 14 p.i. Numbers represent the percentage of B cells that are GCs (top) and percent of GC B cells that are NP+ IgG1+ (bottom). (B) Total number of NP specific IgG1+ GCs on day 14 p.i. as quantified from the plots in (A) (C) Ratio of the percentage of NP+ to NP− cells of CD19+ B220+ IgDlo Fas+ IgG1+ GC B cells (D) Representative FACS plots of CD19− TCRβ+ OT-II cells (top) and CXCR5+ PD-1hi OT-II TFH (bottom) on day 14 p.i. Numbers represent the percent of CD4+ T cells that are OT-II (top) and the percent of OT-II cells that are CXCR5+ PD-1hi (bottom). (E) Total number of splenic CD19− TCRβ+ OT-II cells on day 14 p.i. as quantified from the plots in (D). Bar graphs in (B), (C) and (E) show mean ± SEM. n= 5–6 mice per group, representative of two independent experiments. ** denotes a p value of <0.01 and * denotes a p value of <0.05 calculated with Student’s t test.
We reconstituted B/DC-MHCII mice with 1×107 polyclonal WT CD4+ cells, (containing approximately 10–15% FoxP3+ Tregs (58)) which resulted in normalization of the numbers of both OT-II T cells and GC B cells (Figure 9A, B, D). The ratio of NP+ to NP- GC B cells also returned to WT levels (Figure 9C). We hypothesized that the presence of TFRs in the polyclonal CD4+ T cells transferred into B/DC-MHCII mice was responsible for the normalization of the GC response. To directly determine if Foxp3+ T cells could mediate this process, we transferred 5×105 Foxp3+ GFP+ Tregs from WT Foxp3-GFP reporter mice (45) (a number equivalent to approximately 5×106 bulk CD4+ T cells) in addition to 1×105 OT-II cells and immunized the mice with NP-OVA. On day 14 post-immunization, GC numbers in B/DC-MCHII mice were reduced to the levels of WT in those mice that also received Foxp3+ Tregs (data not shown), though this difference was more variable than B/DC-MHCII mice that received polyclonal CD4+ T cells. However, the transfer of Foxp3+ Tregs increased the ratio of NP+ to NP− IgG1+ GC B cells to approximately that of WT mice (Figure 9E). These data confirm and support a critical role for TFR cells in the control of the GC response.
Overall, these data support previous observations describing a role for regulatory T cells in the control of the GC response. They also agree with a previous observation that TFR cannot differentiate from activated OT-II cells but differentiate from previously generated Tregs (Figure 8F, (55)). These results also demonstrate that the MHCII dependent interaction of TFR with DCs and/or B cells is sufficient for TFR to exert their function in the germinal center and that MHCII expression by other cell types is not required.
Discussion
In this study we investigated the role for B cell antigen presentation in naïve CD4+ T cell priming, TFH differentiation and development of the GC. We found that MHCII antigen presentation restricted to B cells mediates very inefficient CD4+ T cell priming in response to either nominal protein or peptide antigens, without the induction of either TFH or a GC response. However, in response to acute viral infection, B cell antigen presentation skews the antigen specific T cell response toward the TFH subset. Nevertheless, MHCII expression restricted to DCs and B cell mediates optimal TFH differentiation and expansion, as well as GC formation with affinity maturation and isotype switching of antigen specific antibodies in response to immunization and viral infection. These studies highlight the requirement for cooperation amongst multiple cells during the initiation of a humoral immune response.
The ability of B cells to activate naïve CD4+ T cells has been previously examined with conflicting results. It has been shown that B cells are poor CD4+ activators (19) and may tolerize CD4+ T cells (53, 59). However, others have demonstrated that LPS-activated B cells can activate CD4+ T cells in vitro (60), in agreement with our in vitro data. Teleologically, the inability of B cells to efficiently prime T cells is somewhat perplexing as they are the most numerous professional APC in secondary lymphoid tissues. The inability of B cells to prime naïve CD4+ T cells after immunization may reflect the absence of an appropriate combination of co-stimulatory molecules and inflammatory cytokines expressed by DCs or may simply be a problem of anatomy as T and B cells are found in different locations in secondary lymphoid tissues. In response to acute viral infection, inflammation and the disruption of lymphoid architecture may enhance the activation of naïve, antigen specific B cells and permit them to interact with antigen specific T cells (61). Thus, the reasons for the inability of B cells to effectively prime naïve CD4+ T cells are not clear but may be a combination of location and signal quality.
Our data demonstrate that antigen presentation by DCs and B cells together is sufficient for optimal TFH differentiation in multiple settings, though the role of B cell antigen presentation in the process may be different following immunization and in response to infection. Recent studies have demonstrated that the differentiation of TFH precursors requires DCs and is initiated prior to interactions with B cells (31, 34, 35, 62). In agreement with these latter studies, we also identified a pre-TFH in mice with MHCII antigen presentation restricted to DCs (36). Multiple recent investigations have examined the requirement for B cells in the differentiation of TFH. Earlier studies had demonstrated that mice lacking B cells or the ability to maintain T-B conjugates lack TFH (24, 63). Additionally, examination of gene-deficient mice also suggested that B cell expression of the costimulatory molecules, ICOS and PD-L2, were necessary for TFH differentiation (30, 31).
These data suggest that DCs and B cells may provide qualitatively distinct signals to T cells that contribute to TFH differentiation. For example, IL-6, presumably produced by DCs, has an in vitro role in the induction of Bcl6 and may contribute to the differentiation of TFH after protein immunization (64–66). However, more work has been done to identify costimulatory molecules expressed by B cells that may affect TFH differentiation. B cells can provide many signals to TFH and one specific ligand/receptor pair may not be responsible. ICOS/ICOS ligand signals have been implicated in GC formation and IL-21 production (31, 38–40), and other receptor/ligand pairs, including PD-1 and its ligands as well CD80, are important in TFH and GC B cell differentiation (21, 22, 30, 67). Although it has been suggested that TFH differentiation does not require unique B cell signals but rather sustained antigen presentation (42, 43), most studies support the alternative model that cognate, antigen-specific B cells maintain TFH that differentiate early after DC interactions. The striking observation described here that B cell restricted antigen presentation exclusively primes TFH cells, at the expense of CXCR5 negative effector T cells after viral infection suggests that B cells may express and provide unique signals to T cells to induce the TFH program. B cells alone exclusively generated TFH cells after infection but at greatly reduced numbers. The addition of DC antigen presentation is sufficient to induce optimal antigen specific T cell expansion after infection, as well as restore the normal proportion of TFH and effector T cells. Therefore, DCs drive CD4+ effector T cell differentiation and T cell expansion following infection; whereas, B cell antigen presentation is the force behind TFH differentiation.
The antigen presentation requirements for GC B cell differentiation largely parallel those required for optimal TFH differentiation. Following protein immunization, the combination of DC and B cell MHCII expression is necessary and sufficient for the differentiation of functional GCs. However, despite the fact that B-MHCII mice were able to induce antigen specific TFH cells after viral infection, they were unable to form GCs and LCMV specific IgG antibody. This may be due to the fact that overall numbers of SMARTA TFH were greatly reduced in B-MHCII mice compared to WT mice. The addition of DC antigen presentation was able to induce some GCs and antigen specific IgG after LCMV infection, but this response was still less than that observed in WT mice. It is possible that other MHCII cells are required for GC formation during viral infection. However, we presume that this reflects an incomplete CD4+ T cell compartment in B/DC-MHCII mice. Given the demonstrated requirement for cognate B-T interactions in the GC, the bulk CD4+ T cells that we transferred probably contain insufficient numbers of CD4+ T cells specific for many LCMV epitopes. Thus, GC B cells and class-switched antibodies are produced, but at reduced frequencies. These data do highlight the limitations of the protein immunization system and show that the minimal MHCII requirements for GC differentiation and functional antibody responses may be context-dependent.
Finally, previous studies have demonstrated increased germinal center and T cell responses in the absence of follicular regulatory T cells (TFRs) (55–57), and our data also suggest a role for these cells. In the absence of endogenous CD4+ T cells, including nTregs, we observed increased antigen specific T cells in the absence of Tregs, including an increase in TFH cells, associated with increased GCs. Both the overabundance of GCs and T cells can be rectified by reconstituting a polyclonal CD4+ T cell population (which includes FoxP3+ Tregs) or by adding back only Foxp3+ Tregs. As an overabundance of TFH cells is linked to autoantibody production (38), TFR cells may play a critical role in the prevention of autoimmunity. The system we have developed will allow for further study of the role of TFR cells in other contexts, as well as dissecting the role(s) of DC and B cell antigen presentation in other settings.
The results described here highlight the controlled and cooperative nature of CD4+ T cell activation, TFH differentiation and germinal B cell formation after protein immunization and LCMV infection. It remains to be seen however, if these same requirements are also in place in the context of other infections, autoimmunity or acute inflammation. One might imagine that in the setting of inflammation and disruption of the lymphoid tissue architecture, such as toxoplasma gondii (68), B cells may contribute to the activation of naïve CD4+ T cells. Additionally, the stringent requirements for TFH activation may be altered in infection or autoimmunity and perhaps a signal from either a DC or a B cell is sufficient for TFH differentiation. The multiple steps required in TFH differentiation may serve as a checkpoint in the prevention of autoimmunity by ensuring the antigen specificity of responding TFH and ensuring that they make IL-21 only when it is appropriate.
Supplementary Material
Acknowledgments
This work was supported by a Target Identification in Lupus award from the Alliance for Lupus research award (to T.M.L.), a Veteran’s Affairs Merit Award (1I01BX000435-01 to T.M.L.), grants from the NINDS (5K08NS062138) and the Dana Foundation (to G.F.W.) and NIH grants AI083005, AI095776, AI083022, AI095608, AI082630, AI045008 and HHSN266200500030C (to E.J.W.). L.L.K. was supported by NIH training grant T32 AR 0077442. A.L.J. is supported by a fellowship from the Brody Family Medical Trust.
Abbreviations used in this work
- DC
dendritic cell
- GC
germinal center
- MHCII
MHC class II
- NP-OVA
(4-hydroxy-3-nitrophenyl) acetyl coupled to OVA
- PD-1
programmed cell death 1
- TFH
follicular helper T cell
- WT
wild type
- p.i.
post immunization
- LCMV
lymphocytic choriomeningitis virus.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, Caton AJ, Koretzky GA. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. The Journal of Immunology. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehling HJ, Viville S, van Ewijk W, Benoist C, Mathis D. Fine-tuning of MHC class II gene expression in defined microenvironments. Trends Genet. 1989;5:342–347. doi: 10.1016/0168-9525(89)90140-6. [DOI] [PubMed] [Google Scholar]
- 5.Williams GS, Oxenius A, Hengartner H, Benoist C, Mathis D. CD4+ T cell responses in mice lacking MHC class II molecules specifically on B cells. Eur J Immunol. 1998;28:3763–3772. doi: 10.1002/(SICI)1521-4141(199811)28:11<3763::AID-IMMU3763>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Lemos MP, Fan L, Lo D, Laufer TM. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol. 2003;171:5077–5084. doi: 10.4049/jimmunol.171.10.5077. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Jensen PE. The role of B lymphocytes as antigen-presenting cells. Archivum immunologiae et therapiae experimentalis. 2008;56:77–83. doi: 10.1007/s00005-008-0014-5. [DOI] [PubMed] [Google Scholar]
- 8.Hayglass KT, Naides SJ, Scott CF, Benacerraf B, Sy MS. T cell development in B cell-deficient mice. IV. The role of B cells as antigen-presenting cells in vivo. J Immunol. 1986;136:823–829. [PubMed] [Google Scholar]
- 9.Janeway CA, Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–1055. [PubMed] [Google Scholar]
- 10.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 11.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. Journal of Experimental Medicine. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, Lund FE. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 14.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 15.Ho WY, Ho WY, Cooke MP, Cooke MP, Goodnow CC, Goodnow CC, Davis MM, Davis MM. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994;179:1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakiuchi T, Chesnut RW, Grey HM. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983;131:109–114. [PubMed] [Google Scholar]
- 17.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips JA, Romball CG, Hobbs MV, Ernst DN, Shultz L, Weigle WO. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassila O, Vainio O, Matzinger P. Can B cells turn on virgin T cells? Nature. 1988;334:253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 20.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 22.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 24.Johnston RJ, Poholek AC, Ditoro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Y-H, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Yan X, Zhong B, Nurieva RI, Wang A, Wang X, Martin-Orozco N, Wang Y, Chang SH, Esplugues E, Flavell RA, Tian Q, Dong C. Bcl6 expression specifies the T follicular helper cell program in vivo. Journal of Experimental Medicine. 2012;209:1841–1852. S1–S24. doi: 10.1084/jem.20120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes NM, Allen C, Lesley R. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. The Journal of Immunology. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 30.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) The Journal of Immunology. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. Journal of Experimental Medicine. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal Center B Cell and T Follicular Helper Cell Development Initiates in the Interfollicular Zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 Protein Expression Shapes Pre-Germinal Center B Cell Dynamics and Follicular Helper T Cell Heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Goenka R, Barnett LG, Silver JS, O'Neill PJ, Hunter CA, Cancro MP, Laufer TM. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. The Journal of Immunology. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistant antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38(3):596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 39.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho I-C, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 41.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular Helper T Cell Differentiation Requires Continuous Antigen Presentation that Is Independent of Unique B Cell Signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. Journal of Experimental Medicine. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Immunity. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 46.Archambault AS, Carrero JA, Barnett LG, McGee NG, Sim J, Wright JO, Raabe T, Chen P, Ding H, Allenspach EJ, Dragatsis I, Laufer TM, Wu GF. Cutting Edge: Conditional MHC Class II Expression Reveals a Limited Role for B Cell Antigen Presentation in Primary and Secondary CD4 T Cell Responses. The Journal of Immunology. 2013;191:545–550. doi: 10.4049/jimmunol.1201598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 48.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dragatsis I, Zeitlin S. A method for the generation of conditional gene repair mutations in mice. Nucleic acids research. 2001;29:E10. doi: 10.1093/nar/29.3.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rickert RC, Rickert RC, Roes J, Roes J, Rajewsky K, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic acids research. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 53.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 55.Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. The Journal of Immunology. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 56.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang Y-H, Lim H, Reynolds JM, Zhou X-H, Fan H-M, Liu Z-M, Neelapu SS, Dong C. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KGC, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 59.Morris SC, Morris SC, Morris SC, Lees A, Lees A, Lees A, Finkelman FD, Finkelman FD, Finkelman FD. In vivo activation of naive T cells by antigen-presenting B cells. J Immunol. 1994;152:3777–3785. [PubMed] [Google Scholar]
- 60.Krieger JI, Grammer SF, Grey HM, Chesnut RW. Antigen presentation by splenic B cells: resting B cells are ineffective, whereas activated B cells are effective accessory cells for T cell responses. J. Immunol. 1985;135:2937–2945. [PubMed] [Google Scholar]
- 61.Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci USA. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct waves of BCL6 expression during T follicular helper cell development. The Journal of Immunology. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- 63.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting Edge: STAT1 Is Required for IL-6-Mediated Bcl6 Induction for Early Follicular Helper Cell Differentiation. J. Immunol. 2013;190:3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang Y-H, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, Anderson SM, Wei L, Sun H, O'Shea JJ, Schwartzberg PL. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35:622–632. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Good-Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. The Journal of Immunology. 2012;188:4217–4225. doi: 10.4049/jimmunol.1102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glatman Zaretsky A, Silver JS, Siwicki M, Durham A, Ware CF, Hunter CA. Infection with Toxoplasma gondii alters lymphotoxin expression associated with changes in splenic architecture. Infect Immun. 2012;80:3602–3610. doi: 10.1128/IAI.00333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.