Abstract
Objective
To determine whether follicular fluid (FF) cortisol levels affect cumulus cell (CC) lipid content during oocyte meiotic resumption and whether CCs express genes for glucocorticoid action.
Design
Prospective cohort study
Setting
Academic medical center
Patients
Thirty-seven non-obese women underwent ovarian stimulation for IVF
Intervention(s)
At oocyte retrieval, FF was aspirated from the first follicle (>16 mm in size) of each ovary and pooled CC were collected.
Main Outcome Measure(s)
FF cortisol and cortisone analysis was performed by liquid chromatography-tandem mass spectrometry. CCs were stained with lipid fluorescent dye BODIPY FL C16 to determine lipid content by confocal microscopy. Quantitative real-time PCR was used to detect CC gene expression of 11β-hydroxysteroid dehydrogenase (11βHSD) types 1 and 2, glucocorticoid receptor (NR3C1), lipoprotein lipase (LPL) and hormone sensitive lipase (HSL).
Results
Adjusting for maternal age, FF cortisol levels negatively correlated with CC lipid content and positively correlated with numbers of total and mature oocytes. CCs expressed genes for 11βHSD type 1 as the predominant 11βHSD isoform, NR3C1, LPL and HSL.
Conclusion
FF cortisol levels may regulate CC lipolysis during oocyte meiotic resumption and affect oocyte quality during IVF.
Keywords: cortisol, cumulus cell lipid, meiosis, oocyte developmental competence, in vitro fertilization
Introduction
Folliculogenesis is a dynamic process, whereby multiple endocrine and intraovarian paracrine interactions create a changing intrafollicular microenvironment for appropriate oocyte development. Within this microenvironment, cumulus cell-oocyte interactions govern acquisition of oocyte developmental competence, defined as the ability of the oocyte to complete meiosis and undergo fertilization, embryogenesis and term development (1). Crucial for this process is cumulus cell-oocyte signaling, which relies upon free fatty acid (FFA) beta-oxidation as an energy source for meiosis through adenosine triphosphate (ATP) production by the mitochondrial tricarboxylic acid (TCA) cycle and electron transport chain (2, 3, 4, 5, 6, 7). These FFAs likely originate from cumulus cells themselves, which contain abundant lipid as a source of energy for FFA oxidation during oocyte meiotic resumption (8, 9).
During ovarian stimulation for in vitro fertilization (IVF), cumulus cell lipid as an energy source for FFA oxidation may be governed by cortisol, a steroid hormone with lipolytic actions in other target tissues (10, 11). In support of this, cortisol can be converted from cortisone by luteinized granulosa cells that upregulate NADP-dependent type 1, 11β-hydroxysteroid dehydrogenase (11βHSD1) (with bidirectional dehydrogenase-reductase activities) in response to luteinizing hormone (LH)/human chorionic gonadotropin (hCG) compared to NAD-dependent, type 2, 11βHSD (11βHSD2) (with unilateral dehydrogenase activity for cortisone synthesis) (12, 13, 14, 15, 16). As a result, increased cortisol within periovulatory follicles has been positively linked with oocyte maturation (17) and fertilization (17, 18) as well as successful IVF-related pregnancy outcome in some (18, 19, 20), but not all (12, 21, 22), studies, presumably through its anti-inflammatory, anti-apoptotic properties or other functions (17, 19, 21, 22, 23, 24).
Therefore, intrafollicular cortisol during ovarian stimulation for IVF may promote cumulus cell lipid utilization as an energy source for FFA beta-oxidation during oocyte meiotic resumption. The aim of this study investigates whether follicle fluid (FF) cortisol levels in non-obese women undergoing ovarian stimulation for IVF correlate with cumulus cell lipid content. This study also examines whether cumulus cells express mRNA for 11βHSD types 1 and 2 and glucocorticoid receptor (NR3C1), as well as lipoprotein lipase (LPL) and hormone sensitive lipase (HSL) as enzymes controlling cellular lipid uptake and mobilization, respectively.
Materials and Methods
Study Participants
Approval by the UCLA Institutional Review Board was obtained for non-obese women undergoing ovarian stimulation for IVF to enroll in this study by signing informed consent before participation. Study participants were between the ages of 25 and 44 years and had a body mass index (BMI) from 17 to 28.5 kg/m2. Exclusion criteria were galactorrhea, endometriomas, or ovarian cysts greater than 18 mm in diameter as possible modifiers of ovarian responsiveness to gonadotropin therapy (25, 26). Women undergoing IVF who were obese (BMI ≥ 30) were also excluded to eliminate confouding effects of obesity on ovarian cell lipid content or steroidogenesis (8, 27, 28).
Gonadotropin stimulation for IVF and oocyte retrieval
The methods for ovarian stimulation and oocyte retrieval have previously been reported (29). Briefly, women received either a GnRH antagonist (Ganirelix, Merck & Co. Inc., WhiteHouse Station, NJ), luteal phase leuprolide acetate (Lupron, TAP Pharmaceuticals, Deerfield, IL), or microdose leuprolide acetate ovarian stimulation (30, 31, 32), with recombinant human (rh) follicle stimulating hormone (FSH) or urinary gonadotropins starting at a dose of 225–450 IU sc daily for three days and then changed thereafter as clinically indicated. Serial estradiol (E2) levels and transvaginal sonographic measurements of ovarian follicles were performed until at least two follicles reached ≥ 17 mm in diameter and serum E2 levels reached 300 pg/mL per dominant follicle. Human chorionic gonadotropin (hCG, 10,000 IU, intramuscularly), choriogonadotropin alfa (500 ug sc, Ovidrel, EMD Serono, Inc., Rockland, MA) or leuprolide acetate (4 mg sc every 12 hours for 2 doses) was then administered, and transvaginal oocyte retrieval was performed 35.5 hours later.
Follicular fluid preparation
When possible, a single ovarian puncture was used to aspirate all follicles and oocytes within an ovary. At oocyte retrieval, FF uncontaminated by blood was aspirated separately from the first follicle of each ovary, as previously described (27, 29, 31, 33, 34). Each follicle for study was selected by accessibility and size of at least 16 mm in mean diameter of three perpendicular planes to eliminate variability in FF steroid level by follicle size (35). Follicular fluid was transported on ice to the laboratory, centrifuged for 10 min at 1000 rpm at 4°C and stored in 0.5 mL aliquots at −80°C for later hormone determinations.
Hormone Assays
Follicular fluid levels of cortisol and cortisone were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS: Quest Diagnostics Nichols Institute, San Juan Capistrano, CA), as previously described (29). In each patient, the mean FF cortisol and cortisone concentrations were derived from respective values of the first follicle aspirated for study from each ovary (N=2 follicles per individual). Within each patient, the cortisol and cortisone concentrations in the follicle of one ovary were comparable with those in the follicle of the contralateral ovary (within-subject correlations: 0.66, P<0.001, cortisol; 0.60, P<0.001, cortisone). The inter-follicle coefficients of variation (CV) for cortisol and cortisone were 16% and 15%, respectively. The lower limits of quantification (LOQ) for cortisol and cortisone were 0.026 and 0.15 μg/dL, respectively.
Preparation of cumulus cells
Cumulus cells from all cumulus-oocyte complexes of a single individual were pooled together for lipid determination. Pooled cumulus cells were isolated by mechanical stripping after oocyte retrieval when cumulus-oocyte complexes were prepared for ICSI. Cells were transferred to culture dishes (36, 37) and were washed several times in 5 mL of MOPS (4-morpholinepropanesulfonic acid) buffered medium (G-MOPS™, VitroLife, Englewood, CO), containing 10% serum substitute supplement (Irvine Scientific, Santa Ana, CA). They then were resuspended in 100 μL of recombinant human hyaluronidase (40–120 U/ml) (ICSI Cumulase®, Malov, Denmark), and pipetted up and down for 1 minute before being placed in the MOPS buffered medium. Pooled cumulus cells were initially centrifuged at 1600 rpm for 5 minutes at 24°C in the IVF laboratory. Cell samples were then transported on ice to the research laboratory, where they were resuspended in phosphate buffered saline (PBS) and centrifuged for 5 minutes at 800 rpm at 20 °C within 1–2 hours.
Isolated cumulus cells were immediately fixed with 4% paraformaldehyde in cell suspension for 20 minutes. Endogenous cumulus cell lipid content was determined using the fluorescence probe BODIPY® FL C16 (0.8μg/mL) (1 hour in the dark at room temperature) (Invitrogen, Grand Island, NY). Cell staining included a 5-minute incubation with DAPI (0.5μg/mL) (Invitrogen, Grand Island, NY) to identify the nuclei. Cells were resuspended in 30 uL of PBS and 3 uL of 10% polyvinylpyrrolidone solution with human serum albumin (Irvine Scientific, Santa Ana, CA) before being placed on glass slides for imaging.
Confocal microscopy and analysis
Images were captured using the Leica TCS-SP2-AOBS confocal microscope with x63 oil objective, using identical magnification and gain settings, as previously described (8, 29). The 488-line argon laser was used at 667 V to capture the BODIPY® FL C16 lipid stain; the diode 405 nm laser was used at 436 V to capture DAPI nuclear stain. Image acquisition was performed using Leica Confocal Software (LCS) version 2.61 Build 1537. Fluorescent images of cumulus cell lipid content were quantified by one of two observers using ImageJ (http://rsbweb.nig.gov/ij/) to determine mean fluorescence (fluorescence/unit area) of 20 cells per patient. The reproducibility of quantitative cellular lipid measurements made by the two different observers measuring the same lipid quantity was large (0.96 [95% CI 0.86–0.99]), indicating a very high degree of consistency between the two observers.
Images taken as single channel images were converted to overlay images and all images were saved in Tag Image File Format (TIFF). Single channel images of BODIPY® FL C16 and DAPI were used to create an overlay image, with single channel BODIPY® FL C16 used for lipid quantification in ImageJ. Background staining was accounted for by measuring five negatively stained regions per cell, which were subtracted from the total mean fluorescence.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total cellular RNA was isolated from cumulus cells using an RNeasy kit (Qiagen, Hilden, Germany) and the manufacturer’s protocol. First strand cDNA was synthesized using first strand RT2 kit (Qiagen, Hilden, Germany); messenger ribonucleic acid (mRNA) was quantified by qRT-PCR using RT2 qPCR Master Mix according to manufacturer’s protocol (Qiagen, Hilden, Germany). qRT-PCR was performed on an ABI 7300 (A&B Applied Biosystems, Foster City, CA) using standard temperature cycling conditions. Human primers for NR3C1 (chromosome 4: exons 7-8, length 68 bp), 11βHSD, type 1 (chromosome 1: exons 3-4, length 67 bp) and type 2 (chromosome 16: exons 1-2, length 50 bp), HSL (chromosome 19: exons 1-2, length 67 bp) and LPL (chromosome 8: 6-7) (Life Technologies Corp, Chicago, IL) were used to detect mRNAs. To verify primer sets for 11βHSD types 1 and 2 as well as NR3C1, cDNA obtained from mRNAs of human adipose stem cells (ASCs) and the 295R adrenocortical cell line (ATCC, Mannaras, VA) were used as positive controls (38, 39, 40, 41, 42). To verify primer sets for HSL and LPL, cDNA obtained from mRNA of human adipose tissue was used as a positive control (43). Human primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH: chromosome 12: exons: 6-7, length: 93 bp) and ribosomal protein L15 (RPL: chromosome 3: exons 2-3, length: 87 bp) were used to detect GAPDH and RPL mRNA as internal control housekeeping genes (Life Technologies Corp, Chicago, IL). As a negative control, first strand DNA synthesis was performed without mRNA followed by qRT-PCR. Each measurement was performed in triplicate with at least three independent experiments conducted for each sample.
The relative expression of target genes was measured using the comparative critical threshold (Ct) method. The results were expressed as mRNA expression relative to GADPH using the formula 2−delta Ct (delta Ct is the difference between the mean Ct values of targeted genes and the mean Ct value GAPDH).
Statistical Analysis
Pearson correlation coefficients were calculated to examine the associations between mean FF cortisol and cortisone levels as well as the mean FF cortisol/cortisone ratios with pooled cumulus cell lipid content, the total number of oocytes (mature and immature) retrieved, patient demographics and IVF cycle characteristics. For significant correlations, least squares regression lines were calculated and then plotted (44, 45). Patient age and numbers of oocytes retrieved were incorporated into the regression models as necessary to determine if significant associations held after controlling for these variables (46).
Statistical analyses were performed in SPSS 21 (IBM Corp., Armonk, NY). A P value of ≤0.05 was considered statistically significant. Patient/IVF cycle characteristics, mean FF cortisol and cortisone levels as well as mRNA expression of NR3C1, 11βHSD type 1/2, HSL and LPL relative to GAPDH are expressed as mean ± standard error of the mean (SEM).
Results
Thirty-seven nonobese IVF patients (ages 37.5 ± 0.8 yrs; BMI, 22.2 ± 0.4 kg/m2 [mean ± SEM]) were recruited with the primary diagnoses of endometriosis (N = 4), advanced maternal age (N = 9), unexplained infertility (N = 6), exclusive male factor infertility (N = 8), hypogonadotropic hypogonadism (N = 3), recurrent pregnancy loss (N = 2), tubal factor (N = 1), fertility preservation (N = 1), and oocyte donation (N = 3). Characteristics of IVF cycles included: cycle day 3 serum FSH (7.2 ± 0.5 mIU/mL) and E2 (36.9 ± 2.5 pg/mL) levels; total amounts of rhFSH (3084 ± 161 IU) and hCG (192 ± 21.5 IU) coadministered; maximal serum E2 levels (3029 ± 1563 pg/mL); total gonadotropins administered (41.4 ± 2.1 amps); total basal antral follicle count (14.3 ± 1.3); days of stimulation (8.8 ± 0.2); total numbers of oocytes retrieved (14.9 ± 1.4); number of mature (metaphase II) oocytes (11.5 ± 1.2); percent IVF cycles with intracytoplasmic sperm injection (ICSI) (70.3%); overall fertilization rate (74 ± 3%); and number of embryos transferred (1.6 ± 0.2).
Nine participants underwent day 3 transfer, 20 participants underwent day 5 transfer, and one participant underwent day 6 transfer. Two subjects failed to produce viable embryos. Fourteen participants achieved clinical pregnancy, as defined by a fetal heartbeat, while 18 failed to conceive, resulting in a 43.8% clinical pregnancy rate per fresh IVF cycle. The remaining participants underwent oocyte vitrification (n=5).
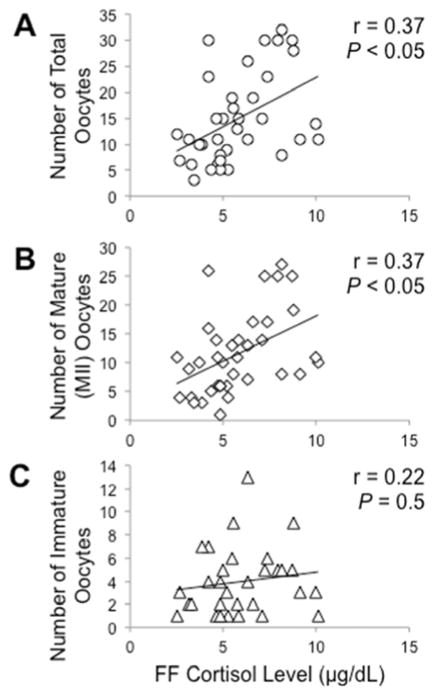
The mean intrafollicular cortisol concentration was 5.8 ± 0.3 μg/dL. Follicle fluid cortisol levels negatively correlated with cumulus cell lipid content (r = −.38, P < 0.025). On the other hand, FF cortisol levels positively correlated with numbers of total and metaphase II oocytes (r = 0.45, P < 0.01; both total and mature oocytes), but not immature oocytes (P = 0.2), retrieved. Intrafollicular cortisol concentrations inversely correlated with patient age (r = −0.33, P < 0.05) so that all FF cortisol data were adjusted for patient age as a covariable. Adjusting for patient age, FF cortisol levels remained negatively correlated with cumulus cell lipid content (r = −.46, P < 0.01) (Figure 1) and positively correlated with numbers of total and metaphase II oocytes retrieved (r = 0.37, P < 0.05; both total and mature oocytes) (Figure 2). Adjusting for both patient age and numbers of oocytes retrieved, FF cortisol levels continued to remain negatively correlated with cumulus cell lipid content (r=0.40, P<0.025).
Figure 1.
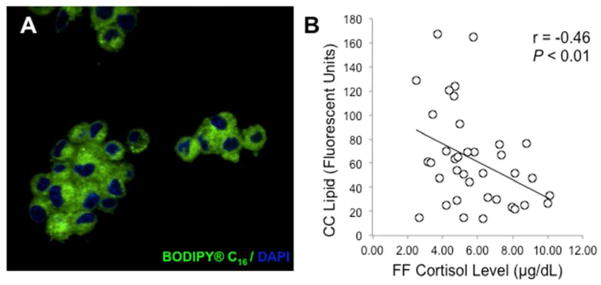
(A) Lipid content of human cumulus cells. Cumulus cells were isolated from cumulus-oocyte-complexes, pooled and fixed in 4% paraformaldehyde at time of oocyte retrieval. Fixed cumulus cells were then stained with BODIPY FL C16 (green) for lipid detection and DAPI (blue) for nuclei. Images were captured with a confocal microscope, using a x63 oil objective, and quantified with ImageJ software (National Institutes of Health) (see Materials and Methods). (B) Regression of FF cortisol with cumulus cell lipid content. FF cortisol levels were determined by liquid chromatography-tandem mass spectrometry. Cumulus cell lipid content of at least 20 cells per patient was determined by immunofluorescent, confocal microscopy and quantified by ImageJ. Adjusted for maternal age, FF cortisol levels negatively correlated with cumulus cell lipid content (r = −.46, P < 0.01).
Figure 2.
Regression of FF cortisol levels with numbers of (A) total oocytes, (B) mature (MII) oocytes and (C) immature oocytes retrieved. Adjusting for maternal age, FF cortisol levels were positively correlated with numbers of total and metaphase II oocytes (r = 0.37, P < 0.05, both oocyte types). There was no correlation between FF cortisol levels and numbers of immature oocytes (r = 0.22, P = 0.5)
Age-adjusted FF cortisol levels were inversely correlated with total amounts of rhFSH (r = −.35, P < 0.05) administered but were unrelated to peak serum E2 levels, total amounts of hCG coadministered with rhFSH (P = 0.5) or BMI (P = 0.5), within the BMI range studied.
Intrafollicular cortisone concentrations averaged 1.4 ± 0.1 μg/dL and were negatively correlated with cumulus cell lipid content (r = −.35, P < 0.05), but not patient age (P=0.2). Unlike cortisol, FF cortisone levels were unrelated to numbers of total, metaphase II and immature oocytes (P = 0.2; total and metaphase II oocytes; P = 0.4; immature oocytes) retrieved; BMI (P = 0.5); or amounts of gonadotropins administered (P = 0.2; rhFSH; P = 0.8; hCG).
The FF cortisol to cortisone ratio positively correlated with numbers of total and mature oocytes (r = 0.45: P < 0.01, total oocytes; r = 0.46: P < 0.005, mature oocytes), but not immature oocytes (P = 0.3), retrieved. Adjusting for patient age, the FF cortisol to cortisone ratio remained positively correlated with numbers of total and mature oocytes (r = 0.38: P < 0.05, total oocytes; r = 0.40: P < 0.025, mature oocytes), but not immature oocytes (P = 0.5), retrieved. Adjusting for both patient age and numbers of oocytes retrieved, the FF cortisol to cortisone ratio was not correlated with cumulus cell lipid content (P=0.5) due to significant inverse relationships of both FF cortisol and cortisone levels with cumulus cell lipid content (r=0.40: P<0.025, cortisol; r=0.37: P<0.05, cortisone).
Gene Expression Analysis
Cumulus cells expressed mRNA for the target genes, 11βHSD type 1 and 2, NR3C1, LPL and HSL. Messenger RNA expression of 11βHSD1 and 11βHSD2 (relative to GADPH) in cumulus cells were 1.65 ± 0.48 (delta Ct = 0.058 ± 0.51) and 0.009 ± 0.006 (delta Ct = 8.74 ± 0.72), respectively, resulting in a 183-fold increase in 11βHSD1 versus 11βHSD2 mRNA expression (p<0.001) (Figure 3A). ASCs and H295R adrenocortical cells (positive controls) showed expression of 11βHSD1 mRNA (relative to GADPH) with values of 1.24 ± 0.04 (delta Ct = −0.31 ± 0.05) and 0.001 ± 0.00004 (delta Ct = 10.26 ± 0.07), respectively. ASCs and H295R adrenocortical cells (positive controls) also showed expression of 11βHSD2 mRNA (relative to GADPH) with values of 0.002 ± 0.00003 (delta Ct = 9.04 ± 0.02) and 0.001 ± 0.00006 (delta Ct = 10.33 ± 0.11).
Figure 3.
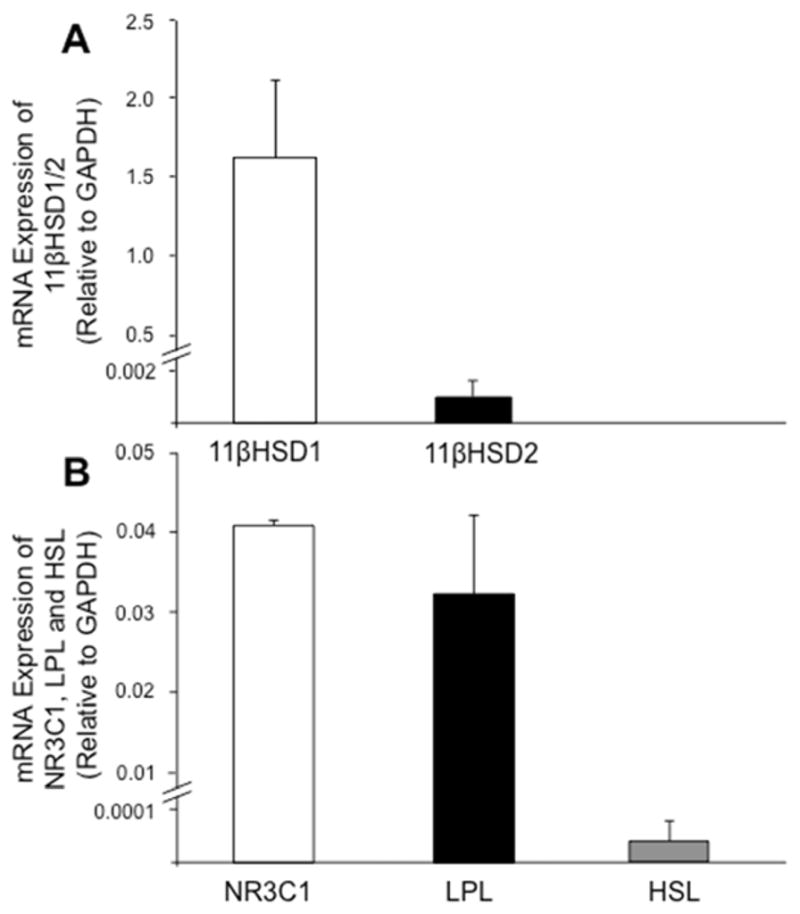
mRNA expression of target genes in human cumulus cells. RNA from pooled cumulus cells was isolated at time of oocyte retrieval (see Materials and Methods). mRNA levels of (A) 11βHSD types 1 and 2; and (B) NR3C1, LPL and HSL were determined by qRT-PCR and calculated using the formula 2ΔCt. Cumulus cells preferentially expressed mRNA for 11βHSD1 over 11βHSD2, as well as LPL over HSL. H295R adrenocortical cells, adipose stem cells and human adipose tissue were used as positive controls for all target genes, respectively. Error bars represent 1 SEM.
Cumulus cell mRNA expression of NR3C1 (relative to GADPH) was 0.04 ± 0.001 (delta Ct = 5.43 ± 0.62) (Figure 3B). Human adipose tissue and H295R adrenocortical cell mRNAs (positive controls) showed NR3C1 expression (relative to GADPH) with values of 0.003 ± 0.0004 (delta Ct = 8.74 ± 0.23) and 0.04 ± 0.001 (delta Ct = 4.60 ± 0.02), respectively.
Cumulus cell mRNA expression of LPL and HSL (relative to GADPH) were 0.033 ± 0.01 (delta Ct = 5.44 ± 0.39) and 0.00006 ± 0.0001 (delta Ct =1.24 ± 0.04), respectively, resulting in a 577-fold increase in LPL versus HSL mRNA expression (Figure 3B). Human adipose tissue mRNA (positive control) showed expression of LPL (relative to GADPH) with values of 0.024 ± 0.0065 (delta Ct = 5.39 ± 0.42) and HSL (relative to GADPH) with values of 0.0013 ± 0.00017 (delta Ct = 9.54 ± 0.18), respectively.
Similar results were obtained for the mRNA expression of 11βHSD type 1 and 2, NR3C1, HSL and LPL relative to the housekeeping gene RPL (data not shown). Messenger RNA expression of 11βHSD type 1 and 2, NR3C1 as well as HSL and LPL was not detected in the absence of mRNA during first strand DNA synthesis (data not shown).
Discussion
Glucocorticoid metabolism within the human ovarian follicle is a balance between NAD-dependent, 11βHSD2 dehydrogenase activity (with high cortisol binding affinity) and NADP-dependent, 11βHSD1 dehydrogenase-reductase activities (with low cortisol binding affinity) (12, 14, 16, 47). Normally, granulosa cells within the growing antral follicle convert cortisol to inactive cortisone via the NAD-dependent 11βHSD2 enzyme. Conversely, luteinized granulosa cells exposed to LH/hCG gain the capacity to regenerate active cortisol via NADP(H)-dependent 11βHSD1 reductase activity (14, 15, 16). Consequently, responsiveness of granulosa cell 11βHSD to gonadotropins favors metabolism of cortisol to cortisone in immature follicles, while the converse is true in periovulatory follicles (16, 22), with increased cortisol production by luteinized granulosa cells positively associated with oocyte maturation (17) and fertilization (17, 18) as well as successful pregnancy outcome in some (18, 19, 20), but not all (12, 21, 22), IVF studies.
In support of this concept, intrafollicular cortisol levels in our IVF patients adjusted for age positively correlated with the numbers of total and metaphase II oocytes retrieved. Within these follicles, the amount of cortisol was approximately 4-fold greater than that of cortisone, while cortisone levels did not correlate with either the numbers or maturity of oocytes retrieved. As a result, the FF cortisol to cortisone ratios, adjusting for patient age, also positively predicted the numbers of total and metaphase II oocytes retrieved. Our finding of a positive correlation of FF cortisol levels with numbers of total and metaphase II oocytes retrieved suggests that both variables share as a common denominator the ovarian response to gonadotropin stimulation, consistent with FF cortisol levels being negatively correlated with total amounts of rhFSH administered. The amount of cortisol in follicles, however, did not predict the rate of oocyte fertilization, as in other studies (17, 18), likely due to the partial or complete use of ICSI in 70.3% of our IVF cycles.
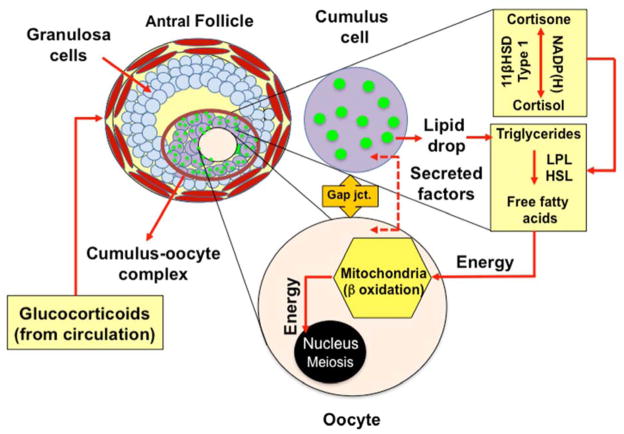
Nevertheless, FF cortisol levels were independent of peak serum E2 concentrations, consistent with the previous finding that circulating cortisol and E2 levels are unrelated to each other during ovarian stimulation for IVF (48). Yet, FF cortisol levels remained negatively correlated with cumulus cell lipid content, adjusting for patient age and numbers of oocytes retrieved, implying a role for cortisol in ovarian cellular lipid metabolism as shown in other lipid-containing cells (10, 11). In animal models, fatty acid metabolism is a major source of energy for oocyte meiotic resumption and fertilization as well as early embryogenesis (2, 3, 4, 5, 6, 7). In maturing murine cumulus cell-oocyte complexes, for example, entry of FFAs into the mitochondria is catalyzed by carnitine palmitoyl transferase-I (CPTI) as the rate-limiting step for beta-oxidation, after which FFAs are converted into acetyl CoA molecules that enter the TCA cycle and electron transport chain to produce ATP for oocyte meiosis (2, 3, 4, 5). We have previously reported that cumulus cells of IVF patients contain abundant lipid stores (8), which we now show to be inversely correlated with FF cortisol levels, adjusting for patient age and numbers of oocytes retrieved. We further demonstrate that these lipid-laden cumulus cells also express the genes for glucocorticoid receptor, LPL and HSL, suggesting that cortisol-induced lipolysis of cumulus cell lipid during IVF may promote FFA beta-oxidation as an energy source during oocyte meiotic resumption (Figure 4).
Figure 4.
Hypothesis of work. Proper oocyte meiotic resumption requires energy in the form of FFA, likely derived from cumulus cells through cumulus-oocyte signaling via gap junctions and secreted factors. Breakdown of triglycerides into readily available FFA requires enzymatic action of LPL and HSL, which can be activated by the glucocorticoid cortisol in other target tissues. Cortisol and cortisone enter the follicle from the circulation and are interconverted by the enzymes 11BHSD types 1 and 2 present in the cumulus cells. In response to the LH surge/hCG administration, 11BHSD type 1 is the predominant isoform, giving rise to cortisol elevation within the follicle. Thus, intrafollicular cortisol may interact with cumulus cell LPL and HSL enzymes to break down triglyceride into FFA, providing a source of energy via mitochondrial beta-oxidation during oocyte meiotic resumption.
One caveat, however, is that human granulosa cells have the capacity to convert cholesterol to bile acids rather than steroids (49). Synthesized from cholesterol via pathways initiated by CYP7A1 or CYP27A1, bile acids can act through bile acid-activated receptors in various tissues to regulate lipid and glucose homeostasis in conjunction with the glucocorticoid receptor (50, 51). Whether similar events affect the relationship between FF cortisol and cumulus cell lipid is unknown, although redundant energy pathways within the periovulatory follicle during meiosis likely account for our finding of only a moderate inverse relationship between FF cortisol and cumulus cell lipid content (52, 53).
To our knowledge, this study is the first to show a weak inverse correlation of FF cortisol level with patient age, perhaps of questionable relevance since circulating cortisol levels do not decline with age (54). Nevertheless, a larger study controlling for patient age, infertility-related disease, ovarian stimulation protocol and embryo transfer technique is needed to resolve the long-standing controversy regarding cortisol action on oocyte quality and pregnancy outcome (12, 18, 19, 20, 21, 22), perhaps mediated through cortisol-induced lipolysis of cumulus cell lipid as energy substrate for the developing oocyte (55, 56).
Our finding that pooled cumulus cells predominantly express mRNA for 11βHSD1 versus 11βHSD2, as reported in luteinized granulosa cells (14, 15, 16), does not consider heterogeneity of 11βHSD gene expression among individual follicles that differ in cortisol production and/or oocyte quality. This is an important study limitation because 11βHSD1 dehydrogenase-reductase activity controlling cortisol-cortisone interconversion requires NADP(H) production by hexose-6-phosphate dehydrogenase (57, 58, 59, 60) in response to glucose (61, 62) and other factors, which may have caused subtle differences in cortisol levels between follicles of the same patient.
Equally important, measuring total cortisol levels in follicles of IVF patients likely underestimates bioactivity of free cortisol in the vicinity of the cumulus-oocyte complex (63). Free cortisol in the periovulatory follicle (53 nM) is almost 10 times higher in amount than in serum (6 nM) due to high production of progesterone and 17-hydroxyprogesterone, which compete with cortisol for the steroid-binding site on cortisol-binding globulin (CBG) (21). Consequently, the percentages of free and CBG-bound cortisol in serum are 2.1% and 94%, respectively, while those of free and CBG-bound cortisol in FF are 22% and 47.9%, respectively (21). The remaining cortisol in FF binds to albumin with lower affinity than progesterone, 17-OH progesterone and estradiol, all of which exist in excess of cortisol (21, 29). Our study did not examine the impact of cortisol bioactivity on the cumulus-oocyte complex that results from displacement of cortisol from CBG and albumen by the large amounts of sex steroids in the follicle.
In conclusion, intrafollicular cortisol levels during ovarian stimulation for IVF negatively correlate with cumulus cell lipid content and positively correlate with oocyte maturation. Given cumulus cell gene expression of 11βHSD1 as the predominant 11βHSD isoform, glucocorticoid receptor and lipid metabolic enzymes, cumulus cell lipolysis induced by cortisol may facilitate FFA beta-oxidation as an energy source during final acquisition of oocyte developmental competence (Figure 4).
Acknowledgments
This study was funded by the UCLA Department of Obstetrics and Gynecology, and in part by a grant from the David Geffen School of Medicine, Stein/Oppenheimer Endowment Award. Confocal laser scanning microscopy was performed at the CNSI Advanced Light Microscopy/Spectroscopy Shared Resource Facility at UCLA, supported with funding from NIH-NCRR shared resources (CJX1-44385-WS-29646) and NSF Major Research Instrumentation grant (CHE-0722519). Statistical analyses were funded by NIH/NCATS/UCLA CTSI Grant UL1TR000124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63(1):39–48. doi: 10.1097/OGX.0b013e31815e85fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev. 2009;76(9):844–53. doi: 10.1002/mrd.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83(6):909–18. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 4.Dunning KR, Robker RL. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim Reprod Sci. 2012;134(1–2):69–75. doi: 10.1016/j.anireprosci.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Dunning KR, Anastasi MR, Zhang VJ, Russell DL, Robker RL. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE. 2014;9(2):e87327. doi: 10.1371/journal.pone.0087327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valsangkar D, Downs SM. A requirement for fatty acid oxidation in the hormone-induced meiotic maturation of mouse oocytes. Biol Reprod. 2013;89(2):1–9. doi: 10.1095/biolreprod.113.109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol Reprod. 2013;88(5):1–11. doi: 10.1095/biolreprod.113.108548. [DOI] [PubMed] [Google Scholar]
- 8.Singh P, Amin M, Keller E, Simerman A, Aguilera P, Briton-Jones C, et al. A novel approach to quantifying ovarian cell lipid content and lipid accumulation in vitro by confocal microscopy in lean women undergoing ovarian stimulation for in vitro fertilization (IVF) J Assist Reprod Genet. 2013;30(5):733–40. doi: 10.1007/s10815-013-9976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carman GM. Thematic minireview series on the lipid droplet, a dynamic organelle of biomedical and commercial importance. J Biol Chem. 2012;287(4):2272. doi: 10.1074/jbc.R111.323931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djurhuus CB, Gravholt CH, Nielsen S, Mengel A, Christiansen JS, Schmitz OE, et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab. 2002;283(1):E172–7. doi: 10.1152/ajpendo.00544.2001. [DOI] [PubMed] [Google Scholar]
- 12.Thomas FJ, Thomas MJ, Tetsuka M, Mason JI, Hillier SG. Corticosteroid metabolism in human granulosa-lutein cells. Clin Endocrinol (Oxf) 1998;48(4):509–13. doi: 10.1046/j.1365-2265.1998.00457.x. [DOI] [PubMed] [Google Scholar]
- 13.Tetsuka M, Thomas FJ, Thomas MJ, Anderson RA, Mason JI, Hillier SG. Differential expression of messenger ribonucleic acids encoding 11beta-hydroxysteroid dehydrogenase types 1 and 2 in human granulosa cells. J Clin Endocrinol Metab. 1997;82(6):2006–9. [PubMed] [Google Scholar]
- 14.Thurston LM, Norgate DP, Jonas KC, Gregory L, Wood PJ, Cooke BA, et al. Ovarian modulators of type 1 11beta-hydroxysteroid dehydrogenase (11betaHSD) activity and intra-follicular cortisol:cortisone ratios correlate with the clinical outcome of IVF. Hum Reprod. 2003;18(8):1603–12. doi: 10.1093/humrep/deg322. [DOI] [PubMed] [Google Scholar]
- 15.Michael AE, Evagelatou M, Norgate DP, Clarke RJ, Antoniw JW, Stedman BA, et al. Isoforms of 11beta-hydroxysteroid dehydrogenase in human granulosa-lutein cells. Mol Cell Endocrinol. 1997;132(1–2):43–52. doi: 10.1016/s0303-7207(97)00118-4. [DOI] [PubMed] [Google Scholar]
- 16.Yong PY, Thong KJ, Andrew R, Walker BR, Hillier SG. Development-related increase in cortisol biosynthesis by human granulosa cells. J Clin Endocrinol Metab. 2000;85(12):4728–33. doi: 10.1210/jcem.85.12.7005. [DOI] [PubMed] [Google Scholar]
- 17.Fateh M, Ben-Rafael Z, Benadiva CA, Mastroianni L, Jr, Flickinger GL. Cortisol levels in human follicular fluid. Fertil Steril. 1989;51(3):538–41. doi: 10.1016/s0015-0282(16)60572-1. [DOI] [PubMed] [Google Scholar]
- 18.Michael AE, Gregory L, Piercy EC, Walker SM, Shaw RW, Cooke BA. Ovarian 11 beta-hydroxysteroid dehydrogenase activity is inversely related to the outcome of in vitro fertilization-embryo transfer treatment cycles. Fertil Steril. 1995;64(3):590–8. [PubMed] [Google Scholar]
- 19.Keay SD, Harlow CR, Wood PJ, Jenkins JM, Cahill DJ. Higher cortisol:cortisone ratios in the preovulatory follicle of completely unstimulated IVF cycles indicate oocytes with increased pregnancy potential. Hum Reprod. 2002;17(9):2410–4. doi: 10.1093/humrep/17.9.2410. [DOI] [PubMed] [Google Scholar]
- 20.Lewicka S, von Hagens C, Hettinger U, Grunwald K, Vecsei P, Runnebaum B, et al. Cortisol and cortisone in human follicular fluid and serum and the outcome of IVF treatment. Hum Reprod. 2003;18(8):1613–7. doi: 10.1093/humrep/deg352. [DOI] [PubMed] [Google Scholar]
- 21.Andersen CY, Hornnes P. Intrafollicular concentrations of free cortisol close to follicular rupture. Hum Reprod. 1994;9(10):1944–9. doi: 10.1093/oxfordjournals.humrep.a138364. [DOI] [PubMed] [Google Scholar]
- 22.Andersen CY, Morineau G, Fukuda M, Westergaard LG, Ingerslev HJ, Fiet J, et al. Assessment of the follicular cortisol:cortisone ratio. Hum Reprod. 1999;14(6):1563–8. doi: 10.1093/humrep/14.6.1563. [DOI] [PubMed] [Google Scholar]
- 23.Komiyama J, Nishimura R, Lee HY, Sakumoto R, Tetsuka M, Acosta TJ, et al. Cortisol is a suppressor of apoptosis in bovine corpus luteum. Biol Reprod. 2008;78(5):888–95. doi: 10.1095/biolreprod.107.065656. [DOI] [PubMed] [Google Scholar]
- 24.Boumela I, Assou S, Aouacheria A, Haouzi D, Dechaud H, De Vos J, et al. Involvement of BCL2 family members in the regulation of human oocyte and early embryo survival and death: gene expression and beyond. Reproduction. 2011;141(5):549–61. doi: 10.1530/REP-10-0504. [DOI] [PubMed] [Google Scholar]
- 25.Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Impact of endometriosis on in vitro fertilization and embryo transfer cycles in young women: a stage-dependent interference. Acta Obstet Gynecol Scand. 2011;90(11):1232–8. doi: 10.1111/j.1600-0412.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 26.Qublan HS, Amarin Z, Tahat YA, Smadi AZ, Kilani M. Ovarian cyst formation following GnRH agonist administration in IVF cycles: incidence and impact. Hum Reprod. 2006;21(3):640–4. doi: 10.1093/humrep/dei371. [DOI] [PubMed] [Google Scholar]
- 27.Dumesic DA, Lesnick TG, Abbott DH. Increased adiposity enhances intrafollicular estradiol levels in normoandrogenic ovulatory women receiving gonadotropin-releasing hormone analog/recombinant human follicle-stimulating hormone therapy for in vitro fertilization. J Clin Endocrinol Metab. 2007;92(4):1438–41. doi: 10.1210/jc.2006-2161. [DOI] [PubMed] [Google Scholar]
- 28.Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88(2):142–8. doi: 10.1016/j.jri.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Amin M, Simerman A, Cho M, Singh P, Briton-Jones C, Hill D, et al. 21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis. The J Clin Endocrinol Metab. 2014;99(4):1299–306. doi: 10.1210/jc.2013-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamonki MI, Oktay K. Oocyte and ovarian tissue cryopreservation: indications, techniques, and applications. Semin Reprod Med. 2005;23(3):266–76. doi: 10.1055/s-2005-872455. [DOI] [PubMed] [Google Scholar]
- 31.Dumesic DA, Lesnick TG, Stassart JP, Ball GD, Wong A, Abbott DH. Intrafollicular antimullerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer. Fertil Steril. 2009;92(1):217–21. doi: 10.1016/j.fertnstert.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shastri SM, Barbieri E, Kligman I, Schoyer KD, Davis OK, Rosenwaks Z. Stimulation of the young poor responder: comparison of the luteal estradiol/gonadotropin-releasing hormone antagonist priming protocol versus oral contraceptive microdose leuprolide. Fertil Steril. 2011;95(2):592–5. doi: 10.1016/j.fertnstert.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Phy JL, Conover CA, Abbott DH, Zschunke MA, Walker DL, Session DR, et al. Insulin and messenger ribonucleic acid expression of insulin receptor isoforms in ovarian follicles from nonhirsute ovulatory women and polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89(7):3561–6. doi: 10.1210/jc.2003-031888. [DOI] [PubMed] [Google Scholar]
- 34.Foong SC, Abbott DH, Lesnick TG, Session DR, Walker DL, Dumesic DA. Diminished intrafollicular estradiol levels in in vitro fertilization cycles from women with reduced ovarian response to recombinant human follicle-stimulating hormone. Fertil Steril. 2005;83(5):1377–83. doi: 10.1016/j.fertnstert.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 35.Desforges-Bullet V, Gallo C, Lefebvre C, Pigny P, Dewailly D, Catteau-Jonard S. Increased anti-Mullerian hormone and decreased FSH levels in follicular fluid obtained in women with polycystic ovaries at the time of follicle puncture for in vitro fertilization. Fertil Steril. 2010;94(1):198–204. doi: 10.1016/j.fertnstert.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Zhang SS, Carrillo AJ, Darling DS. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol Hum Reprod. 1997;3(7):555–62. doi: 10.1093/molehr/3.7.555. [DOI] [PubMed] [Google Scholar]
- 37.Minasi MG, Fabozzi G, Casciani V, Ferrero S, Litwicka K, Greco E. Efficiency of slush nitrogen vitrification of human oocytes vitrified with or without cumulus cells in relation to survival rate and meiotic spindle competence. Fertil Steril. 2012;97(5):1220–5. doi: 10.1016/j.fertnstert.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Bujalska IJ, Walker EA, Hewison M, Stewart PM. A switch in dehydrogenase to reductase activity of 11 beta-hydroxysteroid dehydrogenase type 1 upon differentiation of human omental adipose stromal cells. J Clin Endocrinol Metab. 2002;87(3):1205–10. doi: 10.1210/jcem.87.3.8301. [DOI] [PubMed] [Google Scholar]
- 39.Jiang S, Wei H, Song T, Yang Y, Peng J, Jiang S. Transcriptome comparison between porcine subcutaneous and intramuscular stromal vascular cells during adipogenic differentiation. PloS one. 2013;8(10):e77094. doi: 10.1371/journal.pone.0077094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tacon LJ, Soon PS, Gill AJ, Chou AS, Clarkson A, Botling J, et al. The glucocorticoid receptor is overexpressed in malignant adrenocortical tumors. J Clin Endocrinol Metab. 2009;94(11):4591–9. doi: 10.1210/jc.2009-0546. [DOI] [PubMed] [Google Scholar]
- 41.Mazzocchi G, Rossi GP, Neri G, Malendowicz LK, Albertin G, Nussdorfer GG. 11beta-hydroxysteroid dehydrogenase expression and activity in the human adrenal cortex. FASEB J. 1998;12(14):1533–9. doi: 10.1096/fasebj.12.14.1533. [DOI] [PubMed] [Google Scholar]
- 42.Smith SR, Gawronska-Kozak B, Janderova L, Nguyen T, Murrell A, Stephens JM, et al. Agouti expression in human adipose tissue: functional consequences and increased expression in type 2 diabetes. Diabetes. 2003;52(12):2914–22. doi: 10.2337/diabetes.52.12.2914. [DOI] [PubMed] [Google Scholar]
- 43.Richelsen B, Pedersen SB, Kristensen K, Borglum JD, Norrelund H, Christiansen JS, et al. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism. 2000;49(7):906–11. doi: 10.1053/meta.2000.6738. [DOI] [PubMed] [Google Scholar]
- 44.Petrie A, Sabin C. Medical statistics at a glance. Oxford; Malden, MA: Blackwell Science; 2000. [Google Scholar]
- 45.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 46.Hull MG, Fleming CF, Hughes AO, McDermott A. The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril. 1996;65(4):783–90. doi: 10.1016/s0015-0282(16)58214-4. [DOI] [PubMed] [Google Scholar]
- 47.Stewart PM, Whorwood CB. 11 beta-Hydroxysteroid dehydrogenase activity and corticosteroid hormone action. Steroids. 1994;59(2):90–5. doi: 10.1016/0039-128x(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 48.Tica VI, Mares P, Gouzes C, Badea P, Popescu G, Tica I. The variation of serum cortisol during ovarian stimulation for in vitro fertilization. Gynecol Endocrinol. 2008;24(1):12–7. doi: 10.1080/09513590701325509. [DOI] [PubMed] [Google Scholar]
- 49.Smith LP, Nierstenhoefer M, Yoo SW, Penzias AS, Tobiash E, Usheva A. The bile acid synthesis pathway is present and functional in the human ovary. PLoS One. 2009;4 (10):e7333. doi: 10.1371/journal.pone.0007333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30(11):570–80. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Renga B, Mencarelli A, D’Amore C, Cipriani S, Baldelli F, Zampella A, Distrutti E, Fiorucci S. Glucocorticoid receptor mediates the gluconeogenic activity of the farnesoid X receptor in the fasting condition. FASEB J. 2012;26(7):3021–31. doi: 10.1096/fj.11-195701. [DOI] [PubMed] [Google Scholar]
- 52.Gutnisky C, Morado S, Dalvit GC, Thompson JG, Cetica PD. Glycolytic pathway activity: effect on IVM and oxidative metabolism of bovine oocytes. Reprod Fertil Dev. 2013A;25(7):1026–35. doi: 10.1071/RD12193. [DOI] [PubMed] [Google Scholar]
- 53.Gutnisky C, Dalvit GC, Thompson JG, Cetica PD. Pentose phosphate pathway activity: effect on in vitro maturation and oxidative status of bovine oocytes. Reprod Fertil Dev. 2013B;26(7):931–42. doi: 10.1071/RD12397. [DOI] [PubMed] [Google Scholar]
- 54.Campino C, Martinez-Aguayo A, Baudrand R, Carvajal CA, Aglony M, Garcia H, et al. Age-related changes in 11beta-hydroxysteroid dehydrogenase type 2 activity in normotensive subjects. Am J Hypertens. 2013;26(4):481–7. doi: 10.1093/ajh/hps080. [DOI] [PubMed] [Google Scholar]
- 55.Coticchio G, Albertini DF, De Santis L. Oogenesis. London; New York: Springer Verlag; 2013. [Google Scholar]
- 56.Bentov Y, Casper RF. The aging oocyte--can mitochondrial function be improved? Fertil Steril. 2013;99(1):18–22. doi: 10.1016/j.fertnstert.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 57.Tannin GM, Agarwal AK, Monder C, New MI, White PC. The human gene for 11 beta-hydroxysteroid dehydrogenase. Structure, tissue distribution, and chromosomal localization. J Biol Chem. 1991;266(25):16653–8. [PubMed] [Google Scholar]
- 58.Zhang YL, Zhong X, Gjoka Z, Li Y, Stochaj W, Stahl M, et al. H6PDH interacts directly with 11beta-HSD1: implications for determining the directionality of glucocorticoid catalysis. Arch Biochem Biophys. 2009;483(1):45–54. doi: 10.1016/j.abb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Czegle I, Piccirella S, Senesi S, Csala M, Mandl J, Banhegyi G, et al. Cooperativity between 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase is based on a common pyridine nucleotide pool in the lumen of the endoplasmic reticulum. Mol Cell Endocrinol. 2006;248(1–2):24–5. doi: 10.1016/j.mce.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Atanasov AG, Nashev LG, Gelman L, Legeza B, Sack R, Portmann R, et al. Direct protein-protein interaction of 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase in the endoplasmic reticulum lumen. Biochim Biophys Acta. 2008;1783(8):1536–43. doi: 10.1016/j.bbamcr.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson SE, Pallikaros Z, Michael AE, Cooke BA. The effects of different culture media, glucose, pyridine nucleotides and adenosine on the activity of 11beta-hydroxysteroid dehydrogenase in rat Leydig cells. Mol Cell Endocrinol. 1999;158(1–2):37–44. doi: 10.1016/s0303-7207(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 62.Dzyakanchuk AA, Balazs Z, Nashev LG, Amrein KE, Odermatt A. 11beta-Hydroxysteroid dehydrogenase 1 reductase activity is dependent on a high ratio of NADPH/NADP(+) and is stimulated by extracellular glucose. Mol Cell Endocrinol. 2009;301(1–2):137–41. doi: 10.1016/j.mce.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Slaunwhite WR, Lockie GN, Back N, Sandberg AA. Inactivity in vivo of transcortin-bound cortisol. Science. 1962;135(3508):1062–3. doi: 10.1126/science.135.3508.1062. [DOI] [PubMed] [Google Scholar]