Abstract
Chronic kidney disease may be stimulated by many different etiologies, but its progression involves a common, yet complex, series of events that lead to the replacement of normal tissue with scar. These events include altered physiology within the kidney leading to abnormal hemodynamics, chronic hypoxia, inflammation, cellular dysfunction and activation of fibrogenic biochemical pathways. The end result is the replacement of normal structures with extracellular matrix. Treatments are presently focused on delaying or preventing such progression, and are largely nonspecific. In pediatrics, such therapy is further complicated by both pathophysiological issues that render children a unique population.
Progression of glomerular and tubular disease is clinically defined by a persistent decline in glomerular filtration rate (GFR) that results in chronic kidney disease (CKD) and may lead to end-stage kidney disease (ESKD). Clinical assessment of the velocity of GFR deterioration can be a challenging task in children, since not only is GFR a function of age, gender, and method of its measurement [1], but also the decline may take several years, and thus be difficult to assess. This has practical implications for patient care and research. Several methods/formulas for GFR determination and surrogate markers of kidney disease progression have been developed and validated to alleviate this problem [2]. Morphologically, it has been long noted that impairment of GFR correlates better with the extent of tubulointerstitial injury rather than glomerular injury [3]. However, the reciprocal interaction of primary glomerular and tubular injury makes difficult, and perhaps renders moot, the question of the primacy of the glomerular vs the tubulointerstitial lesion.
Diseases Causing Progression in Children
Progression of glomerular and tubular disease depends on several host factors (i.e. age, sex, race, prenatal course, hypertension, genotype, environmental exposure) and the nature of the underlying kidney disease. The most common causes of CKD in children involve congenital renal and urologic anomalies. Other diseases commonly underlying CKD in children include focal segmental glomerulosclerosis (FSGS), hemolytic uremic syndrome (HUS), immune complex diseases, and hereditary nephropathies, such as Alport's disease [4]. The incidence of CKD in children has been stable in the last decade, in contrast to the adult population where the incidence is sharply increasing. It should be noted that the increase in adults may be attributed to the rising prevalence of diabetes and hypertension. Although the resulting CKD occurs primarily in adulthood, it is worth remarking that the underlying causes of diabetes and hypertension likely have their origins in childhood [6].
PATHOPHYSIOLOGY OF CKD
Various animal models of glomerulosclerosis show that nephron loss starts with glomerular extracapillary lesions, and that podocyte injury or dysfunction plays a central role (REFWiggins, KI 2007 review) Kriz and colleagues have proposed that the initial events leading to extracapillary lesions include the formation of adhesions between glomerular capillary loops and Bowman's capsule as a consequence of denudation of the GBM [7], and subsequent activation of mesangial and endothelial cells. The result is decreased renal glomerular filtration surface area and the accumulation of extracellular matrix (ECM) in a segmental pattern (sclerosis) that suggests mesangial involvement [11]. This misdirected filtration hypothesis is in contrast to another hypothesis, one based upon the concept of podocyte depletion, where an absolute number of podocytes is proposed to be present in a particular glomerulus. When these are injured or detached, the remaining podocytes migrate and undergo hypertrophy in order to cover the external aspect of all capillary loops. When the area requiring such “coverage” exceeds the capacity of the remaining podocytes, the resulting hypertrophy and dysfunction causes glomerulosclerosis. It should be recognized that these two hypotheses are not mutually exclusive. For example, a capillary loop denuded because of podocyte failure is more likely to adhere to Bowman's capsule.
Several other morphologic features of CKD are characteristic and affect the entire nephron: loss of normal renal cells, primarily through apoptosis, and infiltration by monocytes and/or macrophages into the tubulointerstitium, with subsequent fibrosis [11]. Review of experimental and clinical studies suggests common mechanisms that contribute to this pathologic process. These include abnormal glomerular hemodynamics, hypoxia, genetic factors, effects of proteinuria, hypertension, and the abnormal production of cytokines and growth factors. Each of these processes represents an abnormal adaptation to the primary injury, resulting in molecular and cellular disarrangement and, ultimately, renal dysmorphology.
In particular, excessive nephron load has been implicated in progressive disease. Increased glomerular hemodynamic stress result from increased glomerular circulation, hydrostatic pressure or filtered load, either from altered autoregulation or in response to the loss of other nephrons. Increased filtered load of proteins taxes the reabsorptive capacity of the renal tubules, particularly the proximal tubules [12]. Reabsorption of biologically active proteins may activate cells in the tubulointerstitium or recruit inflammatory cells from elsewhere. The result is changes in cell phenotype and function that will be discussed later in this review.
Renal scarring results from increased synthesis and decreased breakdown of extracellular matrix (ECM). The abnormal ECM contains an excess of normal components such as fibronectin, laminin, proteoglycans, and type IV collagen [14], as well as matrices not usually found such as type I collagen in the glomerulus. These molecular changes in the ECM composition alter the ways the cells interact with the ECM, which in turn affects gene regulation and expression in response to specific growth factors.
MECHANISMS OF PROGRESSION
Physiological factors in progression
It is known that hypertension is associated with progression, or at least accelerated progression [15]. Increased hydrostatic pressure at the level of both the single nephron and the whole kidney has been implicated. However, Yoshida and colleagues reported an elegant series of studies in which hyperfiltration, rather than hypertension, appeared to be the significant factor [16]. Hypertension also may be a significant cause of tubulointerstitial fibrosis, as indicated by studies in hypertensive rats where one kidney was protected from elevated perfusion pressure [17]. Opinion is divided regarding whether the critical factor is high pressure or whether the hypertension reflects excess renin activity. A large number of studies in humans and in animal models have indicated that renin antagonists, angiotensin-receptor blockade (ARB) or angiotensin converting enzyme (ACE) inhibition slows the progression of renal failure [18]. In some, but not all, studies comparing renin-angiotensin system (RAS) antagonists with calcium channel blockers, it was found that the therapies were equally effective. It is likely that the underlying cause of hypertension or of kidney disease was an important determinant of outcome. RAS antagonists also may have additional beneficial effects on proteinuria or on the excess production of additional mediators that promote real disease progression [19].
New data suggest that specific cellular interactions with the surrounding extracellular matrix (ECM) are important in progression, since integrin-deficient mice may manifest progressive renal disease [20]. The role of integrins in fibrogenic signaling [21,22] suggests that cell-matrix interactions may be one way in which the physiological effects of local hypertension have an impact on ECM accumulation. Few relevant studies comparing calcium channel blockers with ACE/ARB have been performed solely in children.
Chronic hypoxia is associated with the loss of peritubular capillaries and the development of interstitial fibrosis that further impairs oxygen diffusion and supply to tubular and interstitial cells, resulting in a vicious cycle [23]. Peritubular capillaries undergo spasm and, eventually apoptosis, during the course of CKD [24]. The hypoxia-inducible transcription factor, HIF-1α, is expressed in high quantities in many cases of progressive renal disease [25] and may play a role in the production of cytokines or of signaling molecules that mediate progression. Recent observations regarding the role of asymmetric dimethyl arginine (ADMA) in the cardiovascular complications of CKD [26] also may have implications for the local circulation in the kidney. Alternatively, tubular loss could be the primary event, with subsequent capillary dropout. Chevalier and Forbes cite data indicating that the immediately post-glomerular proximal tubule is particularly sensitive to ischemia and reactive oxygen species, and its irreversible damage could lead to the generation of atubular glomeruli, with consequent loss of function [27].
Genetic factors
Gene polymorphisms in the RAAS system, including ACE, angiotensinogen and the angiotensin type 1 receptor, have been associated with diabetic nephropathy, IgA nephropathy and uropathies [28] [31] [32] [33]. The ACE DD genotype is associated with increased RAS activity, and was shown to be increased in patients with IgA nephropathy who experienced progressive decline in renal function during follow-up compared with those whose renal function remained stable over the same time period [33]. Polymorphisms of TGF-β have been implicated in hypertension and progressive fibrosis. The Arg 25 polymorphism is increased in African Americans [34].
Additional genetic factors for which specific gene mutations or polymorphisms have not been identified are suggested by the observation that certain ethnic backgrounds or familial patterns may contribute to progression. In the Pima Indians, a population with extremely high incidence of type 2 diabetes mellitus [35], some families experience a very high occurrence of ESRD, whereas others do not. Patients of African American descent experience a higher frequency and rate of progression than do Caucasians with similar disease [36], suggesting a predisposition that has been attributed to increased hypertension [37], increased glomerular size [38], greater levels of circulating TGF-β [34], or additional, unknown factors. Low nephron number has been associated with increased incidence of hypertension [39] and kidney disease [40]; these studies suggest the hypothesis that low nephron number contributes to hyperfiltration and/or nephron hypertrophy. However, it is likely that an additional factor is involved, since the presence of a congenitally solitary kidney or loss of a kidney from injury, or due to organ donation is not commonly associated with CKD [41].
Male gender is associated with a more rapid progression of kidney disease in several animal models of renal injury and in human kidney disease, independent of other risk factors such as systemic blood pressure or serum lipid levels [43]. Experimental data suggest that the impact of gender on renal disease progression may be due to genetically determined differences between the sexes in renal structure and function as well as to receptor-mediated effects of sex hormones.
Environmental exposures
One of the best-documented environmental exposures associated with progression of renal disease is exposure to lead. Large-scale epidemiologic studies have noted associations between environmental exposure to lead, even at low levels, and CKD [45]. Although lead exposure amongst children in U.S. has declined in recent years, the situation is different in developing countries [46], and there is well established longitudinal relationship between lead exposure in early childhood and bone lead levels in adulthood [47].
Exposure to high phosphorus intake that results in high calcium–phosphorus product in patients with established CKD leads to vascular and tubulointerstitial calcifications, which in turn stimulate tubulointerstitial inflammation and fibrosis and lead to progression of renal and cardiovascular disease [48].
Cellular determinants of progression
The podocyte in progressive renal disease
The discovery of nephrin as the gene that is mutated in Finnish-type congenital nephrotic syndrome [49] has led to a major shift in our understanding of how the glomerular filter works and of the role of podocyte injury in chronic, progressive kidney disease. Nephrin is a podocyte-specific protein, and subsequently, mutations of other, podocyte-specific proteins also have been associated with glomerulosclerosis (Table 1). Functionally, the development of progression and glomerulosclerosis (GS) in several human and experimental diseases is associated with podocyte loss and podocytopenia [50] [51] [52]. There is significant correlation between the development of extracapillary lesions on renal histology and urinary podocyte number [55]. Causes of podocytopenia include apoptosis, detachment from the GBM, and the inability or lack of podocytes to proliferate [56]. In turn the remaining podocytes may fail to cover completely the glomerular basement membrane (GBM) and thus parietal epithelial cells of Bowman's capsule may gain access to bare areas of the GBM, forming adhesions and leading to segmental glomerulosclerosis [56] [57] [58].
Table 1.
Podocyte proteins for which mutations have been related to FSGS or its variants*
| Protein | Gene | Function |
|---|---|---|
| Podocyte-specific | ||
| α-actinin-4 | ACTN4 | Cytoskeletal assembly |
| CD2-associated protein | CD2AP | Slit diaphragm complex |
| Nephrin | NPHS1 | Cell-cell interaction |
| Podocin | NPHS2 | Cell-cell interaction |
| Transient receptor potential ion channel 6 | TRPC6 | Channel protein |
| Not podocyte-specific | ||
| Lmx1b | Lmx1b | Transcription factor |
| Laminin β2 | LAMB2 | ECM protein |
| Wilms tumor-1 | WT1 | Transcription factor |
Data taken from reference [118].
Apoptotic podocytes are excreted in the urine [55, 62]. Several reports describe the presence of live podocytes in the urine in glomerular diseases [63, 64]. Quantitative determination of podocyte number in human urine was found to be a useful diagnostic tool for differentiating glomerular from nonglomerular diseases, or inflammatory from noninflammatory diseases, and as a marker of disease progression [55, 64-66]. Pagtalunan et al. [50] showed that subjects type II diabetes who had more advanced proteinuria and glomerular matrix accumulation also had fewer glomerular podocytes than those who had diabetes for the same length of time but did not have proteinuria or glomerulosclerosis. In contrast, other glomerular cells did not decrease in number in the same glomeruli [50]. Pima Indians with a lesser podocyte number developed macroalbuminuria faster than those who had a greater podocyte number [65]. Lemeley et al. [52] showed that podocyte loss in IgA nephropathy (IgAN) is associated with increasing disease severity. In that study, the degree of podocytopenia was related to the degree of glomerular sclerosis, impairment of permselectivity, and GFR. In contrast, the authors did not find corresponding correlations between these indices of injury and the number of mesangial and endothelial cells [52]. Podocyte inability to proliferate prevents the restoration of a normal podocyte number [67]. This is in contrast with mesangial and endothelial cells, which readily proliferate in response to many forms of injury [68]. Tubular cell numbers are affected in polycystic kidney disease, where the precisely controlled balance between cellular proliferation and apoptosis is disturbed with increased proliferation in both non-cystic and cystic tubules [70]. Kidneys from patients with ADPKD have high levels of both apoptosis and cellular proliferation [72].
Mesangial cells
As mentioned above, mesangial cells may proliferate, and increased mesangial cell number in glomerulosclerosis is associated with a poor response to therapy [73]. Given the mesangial pattern of ECM expression seen with many forms of progressive glomerular disease, it is likely that the mesangial cell is a significant contributor to the scarring pattern that is observed.
Endothelial cells
The endothelial cells deliver oxygen and nutrients and are essential to the survival of other cells [74]. Endothelial proliferation and peritubular capillary growth has been observed in progression models that are based on reduction of nephron mass [75]. Conversely, endothelial cells play a role in the repair of capillaries and microaneurysms in the Thy-1 model of glomerulonephritis [76]. While early production of vascular endothelial cell growth factor (VEGF) may play a role in acute disease pathogenesis, under more chronic conditions VEGF may promote healing [77]. Ostendorf et al. [78] showed that inhibition of VEGF leads to impairment of capillary repair and results in progressive renal damage, and Masuda et al. [79] have shown that VEGF administration enhances capillary repair and improves renal function in Thy-1 model of glomerulonephritis. However, increased angiogenesis and VEGF expression leads to increasing vascular permeability and promotes cyst formation in the cysts of ADPKD [80].
Inflammation
Macrophage colony stimulating factor secreted by tubular cells during renal injury induces local macrophage proliferation and infiltration in the kidney [81]. These cells in turn produce more cytokines that amplify cell proliferation and infiltration. In addition macrophage-derived cytokines, including interleukin IL-1, IL-6, and TNF-α, inhibit expression of vascular endothelial growth factor (VEGF), impairing angiogenesis and promoting capillary loss [82]. Macrophage infiltration in the interstitium correlates with the degree of renal dysfunction [84]. This vicious cycle promotes cell apoptosis and fibrosis [86] [81]. However, macrophages also may play a beneficial role in scarring. In studies of bone marrow transplantation in wild-type mice and mice with unilateral ureteral obstruction reconstituted with either wild type macrophages or macrophages devoid of the AT1a receptor, severe interstitial fibrosis was observed in mice with AT1a deficient macrophages, even though they had fewer infiltrating macrophages. This result suggests that the macrophage AT1a receptor plays a protective role in fibrogenesis [87].
Interstitial mast cells have been associated with severity of interstitial fibrosis in patients with various glomerulonephritides [88]. There is also an association of stem cell factor produced by mast cells and myofibroblasts that suggests, these cells may be involved in progression of interstitial fibrosis as well [88]. Dendritic cells have been identified in the tubulointerstitium and could play a role in antigen presentation and immune activation of the kidney [89].
Epithelial-to-mesenchymal transition (EMT) as a factor in progression
A critical issue in the cellular response is the phenotype of the cell. Epithelial cells are unlikely to produce non-basement membrane ECM when epithelioid in nature, but when they dedifferentiate they produce a more primordial mesenchyme. Thus, the mesangial cell usually produces type IV collagen, but when injured may produce more type I collagen. There is significant disagreement regarding the origin of ECM-producing cells in the renal scar. Sources of fibroblasts include EMT of renal tubular cells or podocytes [63] [90], activation of resident interstitial stem cells that differentiate into myofibroblasts [92], fibroblasts that migrate into tissue from adjacent areas [93], or macrophages that are recruited to the lesion and undergo transition to myofibroblasts. The resulting phenotype, which has features of both smooth muscle cells and fibroblasts, have been reported to play a role in the fibrogenic process in both glomeruli and tubules, producing and secreting α2(I) and α2(III) collagens and fibronectin.
Biochemical pathways regulating ECM accumulation
An important determinant of scarring in the kidney is the balance between ECM synthesis and degradation [94]. In addition to the synthesis of ECM, the net abundance of matrix proteins is controlled by two degradative pathways. In one, the matrix metalloproteinase (MMP) pathway, a large number of enzymes with varying specificity for different ECMs facilitate the degradation of matrix [95]. These are opposed by the tissue inhibitors of metalloproteinases (TIMPs) [96]. A second pathway involves the tissue- and urokinase-type plasminogen activators (tPA and uPA, respectively) [97], which are opposed by the plasminogen activator inhibitors, PAI-1 and PAI-2. Many of these molecules have shown increased expression or activation in kidney disease, particularly TIMP-1 [98] and PAI-1 [99] and mice genetically altered to be deficient in PAI-1 do not respond to fibrogenic stimuli with nearly the degree of scarring shown by wild-type mice [100].
Molecular regulation of progression
A number of cytokines have been implicated in progression. Prominent among them is transforming growth factor (TGF)-β, a pleiotropic molecule that stimulates many of the events involved in progression, including EMT, apoptosis, ECM synthesis, generation of reactive oxygen species (ROS) and PAI-1 production [101]. Cytokine mediators that stimulate proliferative changes include basic fibroblast growth factor (FGF-2) [102] and platelet-derived growth factor (PDGF) [103]. Endothelin-1 mediates vasoconstriction and also stimulates the production of aldosterone, recently implicated in fibrogenesis as well [104]. Connective tissue growth factor (CTGF), a downstream mediator of TGF-β that stimulates the synthesis of several ECM proteins, has been associated with progression of several forms of renal injury [105].
In the cells of the kidney, several signal transduction pathways have been identified that appear to play a prominent role in progression. The TGF-β pathway is mediated by the Smad family of signal transduction proteins [106]. Several other pathways have been identified as being engaged in cross-talk with Smads, including ERK MAP kinase [107], p38 MAP kinase [108], phosphatidylinositol-3-kinase (PI3K) [109], protein kinase C (PKCδ) [110], PKCβ and Rho A. Rho A has been implicated in EMT [111] and diabetic nephropathy [112]. PI3K [113] and PKCβ [114] have been implicated in diabetic nephropathy.
Overview of mechanisms of progression
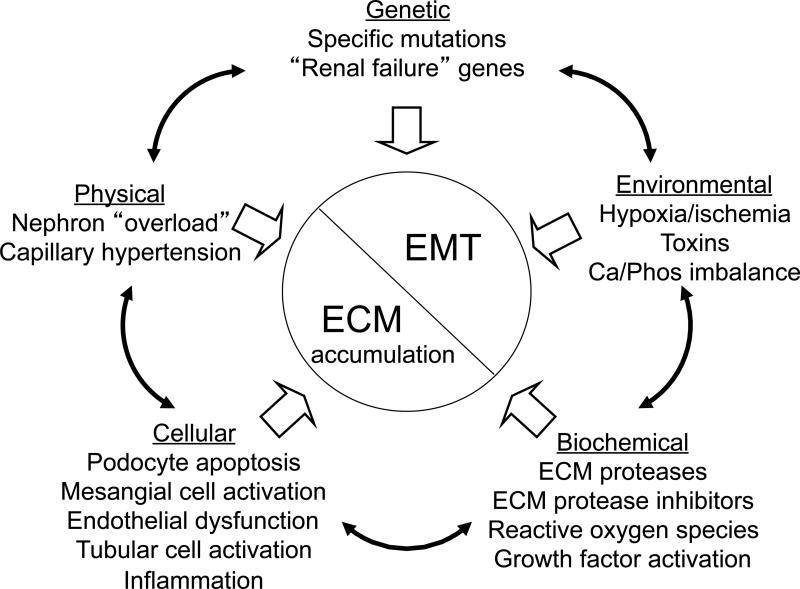
Figure 1 shows an overall schema of mechanisms of progression mentioned in this review. All of the parameters described may interact. For example, genetic factors may influence the development of hypertension, or genes may be activated by environmental factors. Once these factors have been activated, a variety of cellular and biochemical pathways mediate cellular dedifferentiation and ECM accumulation.
Figure 1.
Factors underlying or influencing progression of kidney disease.
APPROACHES TO TREATMENT
Specific treatment
Progressive kidney disease is multifactorial in nature. Its specific treatment depends upon the underlying etiology. Thus, type 1 diabetic nephropathy is best treated by regulation of glucose metabolism, whereas type 2 diabetic nephropathy may best be treated by a combination of treatments directed at glucose control, vascular reactivity and, where appropriate, weight reduction. CKD secondary to inflammatory disease is best directed at the most appropriate treatment for the underlying cause. In FSGS, therapy largely involves calcineurin antagonists such as cyclosporine or tacrolimus [115] [116]. Additional therapies include glucocorticoids, cytotoxic agents or mycophenolate mofetil [118]. The calcineurin antagonists have proven most effective of these choices, although none of these treatments is fully effective. In part, this may be because FSGS itself is a multifactorial disease. In those cases where the primary cause is a genetic deficiency of a podocyte protein, even these “specific” treatments do not address the primary problem in disease pathogenesis.
Nonspecific treatment
Treatment of proteinuria
In most studies, proteinuria is associated with progression of chronic kidney disease. Although there remains some question regarding whether proteinuria is a marker of disease [119, 120] or a cause, it is clear that the association is a strong one, and that cases in which treatments significantly reduce proteinuria are more likely to show delay or prevention of progression in children and adults [121, 122]. Therefore, ACE inhibition and/or angiotensin receptor blockade is now recommended as adjunct therapy [123] in most cases of CKD.
Treatment of hypertension
Hypertension is associated with accelerated progression to ESKD in children [15]. For this reason, tight BP control is an essential adjunct treatment. Most recent guidelines by the Joint National Committee in the US define 120/80 mmHg as the upper limit of the ‘optimal’ blood pressure range, particularly when proteinuria is present [123]. These blood pressure targets are equivalent to the 50th to 75th distribution percentile in the general young adult population. In adults blood pressure > 130/80 should be actively lowered by therapeutic intervention in CKD patients [124]. Although it is as yet unknown whether these blood pressure targets hold true for pediatric population and whether glomerular damage in children correlates with absolute or age-specific relative blood pressure, K/DOQI guidelines on blood pressure control in CKD children adopted the recommendations of the task force that target blood pressure should be <90th percentile for normal values adjusted for age, gender, and height percentile [125]. In addition to the absolute blood pressure level normal diurnal blood pressure pattern may play a significant role in renal failure progression, as “nondipping” is an independent cardiovascular risk factor associated with more rapid progression of renal failure in adult CKD patients [126]. ACE inhibition and/or angiotensin receptor blockade is an effective and safe antihypertensive and antiproteinuric approach in children with CKD-associated hypertension [127] [128].
Treatment of calcium-phosphate metabolism
Hyperphosphatemia, hyperparathyroidism, lack of active vitamin D, and excess of the phosphaturic hormone FGF 23 lead to disturbances in calcium-phosphate metabolism and play a role in CKD progression [129]. Dietary phosphate restriction in adult patients with CKD is associated with stabilization of kidney function [130]. This however, has not been demonstrated in large scale pediatric studies [131]. Calcium-free phosphate binders may prove beneficial beyond phosphate lowering due to their pleiotropic effects, i.e. lipid-lowering and anti-inflammatory properties [132]. High and prolonged level of PTH exposure is toxic to many organs, including the heart, bones, skeletal muscle, nerves, and reproductive system [133]. Early control of PTH production by parathyroid glands with phosphate restriction and administration of vitamin D is crucial because sustained hyperactivity of parathyroid glands leads to nodular hyperplasia that largely irreversible and resistant to vitamin D and calcium regulation [134]. Treatment with nonhypercalcemic doses of active vitamin D and its analogues attenuates renal failure progression in non-inflammatory and inflammatory models of CKD. Active vitamin D has negative endocrine regulation of the RAS, anti-inflammatory, antifibrotic and antiproteinuric properties [135] [136] [137]. Recently FGF23 has been shown to be an independent predictor of progression of renal disease in adult patients with nondiabetic CKD [138].
Treatment of anemia
Treating anemia early in renal failure patients significantly slows the decline of renal function and delays the need for renal replacement therapy [139]. In animal models of kidney injury the mechanism of renoprotection of recombinant human erythropoietin (EPO) appears to be mainly mediated by a reduction of apoptotic cell death [140] and maintenance of the podocyte actin cytoskeleton and nephrin expression [141]. No conclusive studies have indicated a benefit of EPO in slowing pediatric CKD progression.
Other adjunct treatments
Lipid-lowering therapy reduces cardiovascular morbidity and mortality in adults with CKD although this effect has not been shown in patients with ESRD [142]. Statins may have renoprotective properties not only by their lipid-lowering but also by lipid-independent pleiotropic effects. Statins reduce oxidative stress and improve endothelial function [143]. There is evidence for synergistic effects of statins and RAS inhibitors on retardation of renal disease progression [144]. However, the renoprotective effects of statins, although significant, are quantitatively small [145]. There are no studies demonstrating the usefulness of statins in children with CKD.
Antioxidants such as probucol [146] or Vitamin E [147] have been suggested as treatments for some forms of progressive kidney disease, including that associated with IgA nephropathy. The efficacy of these treatments remains unproven. No conclusive studies have indicated a benefit of plasmapheresis.
Potential new approaches to therapy
New treatments that have been suggested include those directed at some of the signal transduction mechanisms mentioned earlier in this review. These include PI3K antagonists [148]; PKCβ antagonists [149]; and fasudil, a Rho A inhibitor [150]. Other experimental anti-fibrotic agents include sulodexide [151], and PPAR antagonists [152]. Smad7, an inhibitory Smad, may also have therapeutic potential [153].
Pediatric-specific considerations
Although The Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines were initiated in 1997 by the National Kidney Foundation, the large majority of the pediatric guidelines are still opinion based because of the lack of evidence-based data [131] [124]. Outcome measures used in adults i.e. mortality, cardiovascular complications, have only limited validity in pediatric studies. Some K/DOQI guideline goals may not be applicable i.e. cholesterol, and many drugs have not been tested for efficacy and safety in children [154].
Another unique consideration of the pediatric patient with CKD progression includes emphasis on the importance of growth and development. Growth failure has long been recognized in children with chronic renal failure [155]. Abnormalities in GH and IGF-I signal transduction and the interaction of these pathways with ghrelin, myostatin, and the suppressor of cytokine signaling (SOCS) family are responsible for many important complications seen in chronic kidney disease (CKD), such as growth retardation and cachectic wasting, as well as disease progression [156]. Growth retardation in CKD is associated with increased morbidity and mortality [157]. Treatment with recombinant human growth hormone in CKD is safe, efficacious and widely accepted [158]. Newer treatment modalities targeting the GH resistance with recombinant human IGF-1 (rhIGF-1), recombinant human IGFBP3 (rhIGFBP3) and IGFBP displacers are under investigation and may prove to be more effective in treating growth failure in CKD [160] [161].
Regression
Finally, all of the treatments described here are aimed at preventing the progression of disease. Recent studies by several investigators have suggested that it may be possible to reverse the renal lesion by promoting the formation of new nephrons or capillary structures [122] [162]. Possible approaches include pro-angiogenic therapy, regulating the dedifferentiation and redifferentiation of cells to promote the formation of new structures [163], or the use of stem cell treatments [164]. While the challenges inherent to these approaches are daunting, the potential rewards of success mandate continued studies toward implementation.
REFERENCES
- 1.Goldstein SL, Devarajan P. Progression from acute kidney injury to chronic kidney disease: a pediatric perspective. Adv Chronic Kidney Dis. 2008;15:278–283. doi: 10.1053/j.ackd.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–1848. doi: 10.1007/s00467-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 3.Schainuck LI, Striker GE, Cutler RE, et al. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970;1:631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 4.Seikaly MG, Ho PL, Emmett L, et al. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatr Nephrol. 2003;18:796–804. doi: 10.1007/s00467-003-1158-5. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Hales CN, Fall CH, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Forsen T, Eriksson JG, et al. Growth and living conditions in childhood and hypertension in adult life: a longitudinal study. J Hypertens. 2002;20:1951–1956. doi: 10.1097/00004872-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 8.Radford MG, Jr., Donadio JV, Jr., Bergstralh EJ, et al. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 9.Katafuchi R, Oh Y, Hori K, et al. An important role of glomerular segmental lesions on progression of IgA nephropathy: a multivariate analysis. Clin Nephrol. 1994;41:191–198. [PubMed] [Google Scholar]
- 10.D'Amico G, Minetti L, Ponticelli C, et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med. 1986;59:363–378. [PubMed] [Google Scholar]
- 11.Churg J, Habib R, White RH. Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet. 1970;760:1299–1302. doi: 10.1016/s0140-6736(70)91905-7. [DOI] [PubMed] [Google Scholar]
- 12.Gudehithlu KP, Pegoraro AA, Dunea G, et al. Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int. 2004;65:2113–2122. doi: 10.1111/j.1523-1755.2004.00633.x. [DOI] [PubMed] [Google Scholar]
- 13.Eppel GA, Osicka TM, Pratt LM, et al. The return of glomerular-filtered albumin to the rat renal vein. Kidney Int. 1999;55:1861–1870. doi: 10.1046/j.1523-1755.1999.00424.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee LK, Meyer TW, Pollock AS, et al. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995;96:953–964. doi: 10.1172/JCI118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsnefes M, Ho PL, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol. 2003;14:2618–2622. doi: 10.1097/01.asn.0000089565.04535.4b. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida Y, Fogo A, Ichikawa I. Glomerular hemodynamic changes vs. hypertrophy in experimental glomerular sclerosis. Kidney Int. 1989;35:654–660. doi: 10.1038/ki.1989.35. [DOI] [PubMed] [Google Scholar]
- 17.Mori T, Polichnowski A, Glocka P, et al. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008;19:1472–1482. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korbet SM. Angiotensin antagonists and steroids in the treatment of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:219–228. doi: 10.1053/snep.2003.50020. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Ge Y, Si J, et al. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 2008;74:1128–1138. doi: 10.1038/ki.2008.380. [DOI] [PubMed] [Google Scholar]
- 20.Zent R, Yan X, Su Y, et al. Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 2006;70:460–470. doi: 10.1038/sj.ki.5000359. [DOI] [PubMed] [Google Scholar]
- 21.Hayashida T, Wu MH, Pierce A, et al. MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci. 2007;120:4230–4240. doi: 10.1242/jcs.03492. [DOI] [PubMed] [Google Scholar]
- 22.Lakhe-Reddy S, Khan S, Konieczkowski M, et al. Beta8 integrin binds Rho GDP dissociation inhibitor-1 and activates Rac1 to inhibit mesangial cell myofibroblast differentiation. J Biol Chem. 2006;281:19688–19699. doi: 10.1074/jbc.M601110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nangaku M. Hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. Nephron Exp Nephrol. 2004;98:e8–12. doi: 10.1159/000079927. [DOI] [PubMed] [Google Scholar]
- 24.Goligorsky MS. Frontiers in nephrology: viewing the kidney through the heart--endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2007;18:2833–2835. doi: 10.1681/ASN.2007050598. [DOI] [PubMed] [Google Scholar]
- 25.Higgins DF, Kimura K, Iwano M, et al. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47:42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008;19:197–206. doi: 10.1681/ASN.2007080862. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida H, Kon V, Ichikawa I. Polymorphisms of the renin-angiotensin system genes in progressive renal diseases. Kidney Int. 1996;50:732–744. doi: 10.1038/ki.1996.371. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida H, Kuriyama S, Atsumi Y, et al. Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int. 1996;50:657–664. doi: 10.1038/ki.1996.362. [DOI] [PubMed] [Google Scholar]
- 30.Marre M, Jeunemaitre X, Gallois Y, et al. Contribution of genetic polymorphism in the renin-angiotensin system to the development of renal complications in insulin-dependent diabetes: Genetique de la Nephropathie Diabetique (GENEDIAB) study group. J Clin Invest. 1997;99:1585–1595. doi: 10.1172/JCI119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brock JW, 3rd, Hunley TE, Adams MC, et al. Role of the renin-angiotensin system in disorders of the urinary tract. J Urol. 1998;160:1812–1819. [PubMed] [Google Scholar]
- 32.Boonstra A, de Zeeuw D, de Jong PE, et al. Role of genetic variability in the renin-angiotensin system in diabetic and nondiabetic renal disease. Semin Nephrol. 2001;21:580–592. doi: 10.1053/snep.2001.26804. [DOI] [PubMed] [Google Scholar]
- 33.Hunley TE, Julian BA, Phillips JA, 3rd, et al. Angiotensin converting enzyme gene polymorphism: potential silencer motif and impact on progression in IgA nephropathy. Kidney Int. 1996;49:571–577. doi: 10.1038/ki.1996.81. [DOI] [PubMed] [Google Scholar]
- 34.August P, Leventhal B, Suthanthiran M. Hypertension-induced organ damage in African Americans: transforming growth factor-beta(1) excess as a mechanism for increased prevalence. Curr Hypertens Rep. 2000;2:184–191. doi: 10.1007/s11906-000-0080-5. [DOI] [PubMed] [Google Scholar]
- 35.Pavkov ME, Knowler WC, Hanson RL, et al. Predictive power of sequential measures of albuminuria for progression to ESRD or death in Pima Indians with type 2 diabetes. Am J Kidney Dis. 2008;51:759–766. doi: 10.1053/j.ajkd.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duru OK, Li S, Jurkovitz C, et al. Race and sex differences in hypertension control in CKD: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2008;51:192–198. doi: 10.1053/j.ajkd.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao MV, Qiu Y, Wang C, et al. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999-2004. Am J Kidney Dis. 2008;51:S30–37. doi: 10.1053/j.ajkd.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Marcantoni C, Ma LJ, Federspiel C, et al. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 2002;62:172–180. doi: 10.1046/j.1523-1755.2002.00420.x. [DOI] [PubMed] [Google Scholar]
- 39.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 40.Hoy WE, Bertram JF, Denton RD, et al. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez E, Gutierrez E, Morales E, et al. Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int. 2005;68:263–270. doi: 10.1111/j.1523-1755.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- 42.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25:515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 43.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 44.Lombet JR, Adler SG, Anderson PS, et al. Sex vulnerability in the subtotal nephrectomy model of glomerulosclerosis in the rat. J Lab Clin Med. 1989;114:66–74. [PubMed] [Google Scholar]
- 45.Muntner P, He J, Vupputuri S, et al. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63:1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 46.Pirkle JL, Brody DJ, Gunter EW, et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). Jama. 1994;272:284–291. [PubMed] [Google Scholar]
- 47.Kim R, Hu H, Rotnitzky A, et al. Longitudinal relationship between dentin lead levels in childhood and bone lead levels in young adulthood. Arch Environ Health. 1996;51:375–382. doi: 10.1080/00039896.1996.9934425. [DOI] [PubMed] [Google Scholar]
- 48.Coburn JW. An update on vitamin D as related to nephrology practice: 2003. Kidney Int Suppl. 2003:S125–130. doi: 10.1046/j.1523-1755.64.s87.19.x. [DOI] [PubMed] [Google Scholar]
- 49.Wartiovaara J, Ofverstedt LG, Khoshnoodi J, et al. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest. 2004;114:1475–1483. doi: 10.1172/JCI22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim YH, Goyal M, Kurnit D, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 52.Lemley KV, Lafayette RA, Safai M, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- 53.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 54.Matsusaka T, Xin J, Niwa S, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 55.Hara M, Yanagihara T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron. 2001;89:342–347. doi: 10.1159/000046097. [DOI] [PubMed] [Google Scholar]
- 56.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 57.Kihara I, Yatoita E, Kawasaki K, et al. Limitations of podocyte adaptation for glomerular injury in puromycin aminonucleoside nephrosis. Pathol Int. 1995;45:625–634. doi: 10.1111/j.1440-1827.1995.tb03514.x. [DOI] [PubMed] [Google Scholar]
- 58.Kriz W. Progressive renal failure--inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant. 1996;11:1738–1742. [PubMed] [Google Scholar]
- 59.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 60.Hara M, Yamamoto T, Yanagihara T, et al. Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron. 1995;69:397–403. doi: 10.1159/000188509. [DOI] [PubMed] [Google Scholar]
- 61.Hara M, Yanagihara T, Takada T, et al. Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis. Am J Nephrol. 1998;18:35–41. doi: 10.1159/000013302. [DOI] [PubMed] [Google Scholar]
- 62.Vogelmann SU, Nelson WJ, Myers BD, et al. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng YY, Huang TP, Yang WC, et al. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998;54:864–876. doi: 10.1046/j.1523-1755.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- 64.Yu D, Petermann A, Kunter U, et al. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733–1741. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- 65.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 66.Hara M, Yanagihara T, Kihara I. Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schonlein-Henoch purpura nephritis. Clin J Am Soc Nephrol. 2007;2:231–238. doi: 10.2215/CJN.01470506. [DOI] [PubMed] [Google Scholar]
- 67.Wiggins JE, Goyal M, Sanden SK, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 68.Pabst R, Sterzel RB. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983;24:626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- 69.Rasch R, Norgaard JO. Renal enlargement: comparative autoradiographic studies of 3H-thymidine uptake in diabetic and uninephrectomized rats. Diabetologia. 1983;25:280–287. doi: 10.1007/BF00279944. [DOI] [PubMed] [Google Scholar]
- 70.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 71.Tao Y, Kim J, Schrier RW, et al. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 72.Lanoix J, D'Agati V, Szabolcs M, et al. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene. 1996;13:1153–1160. [PubMed] [Google Scholar]
- 73.Ito C, Yamamoto H, Furukawa Y, et al. Role of cyclins in cAMP inhibition of glomerular mesangial cell proliferation. Clin Sci (Lond) 2004;107:81–87. doi: 10.1042/CS20030335. [DOI] [PubMed] [Google Scholar]
- 74.Kang DH, Kanellis J, Hugo C, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 75.Pillebout E, Burtin M, Yuan HT, et al. Proliferation and remodeling of the peritubular microcirculation after nephron reduction: association with the progression of renal lesions. Am J Pathol. 2001;159:547–560. doi: 10.1016/S0002-9440(10)61726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iruela-Arispe L, Gordon K, Hugo C, et al. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol. 1995;147:1715–1727. [PMC free article] [PubMed] [Google Scholar]
- 77.Kang DH, Johnson RJ. Vascular endothelial growth factor: a new player in the pathogenesis of renal fibrosis. Curr Opin Nephrol Hypertens. 2003;12:43–49. doi: 10.1097/00041552-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 78.Ostendorf T, Kunter U, Eitner F, et al. VEGF(165) mediates glomerular endothelial repair. J Clin Invest. 1999;104:913–923. doi: 10.1172/JCI6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masuda Y, Shimizu A, Mori T, et al. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bello-Reuss E, Holubec K, Rajaraman S. Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int. 2001;60:37–45. doi: 10.1046/j.1523-1755.2001.00768.x. [DOI] [PubMed] [Google Scholar]
- 81.Isbel NM, Hill PA, Foti R, et al. Tubules are the major site of M-CSF production in experimental kidney disease: correlation with local macrophage proliferation. Kidney Int. 2001;60:614–625. doi: 10.1046/j.1523-1755.2001.060002614.x. [DOI] [PubMed] [Google Scholar]
- 82.Kang DH, Joly AH, Oh SW, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 83.Hooke DH, Gee DC, Atkins RC. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987;31:964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- 84.Lan HY, Nikolic-Paterson DJ, Mu W, et al. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int. 1995;48:753–760. doi: 10.1038/ki.1995.347. [DOI] [PubMed] [Google Scholar]
- 85.Yang N, Wu LL, Nikolic-Paterson DJ, et al. Local macrophage and myofibroblast proliferation in progressive renal injury in the rat remnant kidney. Nephrol Dial Transplant. 1998;13:1967–1974. doi: 10.1093/ndt/13.8.1967. [DOI] [PubMed] [Google Scholar]
- 86.Savill J. Regulation of glomerular cell number by apoptosis. Kidney Int. 1999;56:1216–1222. doi: 10.1046/j.1523-1755.1999.00707.x. [DOI] [PubMed] [Google Scholar]
- 87.Nishida M, Fujinaka H, Matsusaka T, et al. Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 2002;110:1859–1868. doi: 10.1172/JCI200215045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El-Koraie AF, Baddour NM, Adam AG, et al. Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int. 2001;60:167–172. doi: 10.1046/j.1523-1755.2001.00783.x. [DOI] [PubMed] [Google Scholar]
- 89.Li L, Huang L, Sung SS, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008 doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan JM, Huang XR, Ng YY, et al. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1- dependent mechanism in vitro. Am J Kidney Dis. 2001;37:820–831. doi: 10.1016/s0272-6386(01)80132-3. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki T, Kimura M, Asano M, et al. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am J Pathol. 2001;158:75–85. doi: 10.1016/S0002-9440(10)63946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61:387–395. doi: 10.1046/j.1523-1755.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 93.van Goor H, van der Horst ML, Fidler V, et al. Glomerular macrophage modulation affects mesangial expansion in the rat after renal ablation. Lab Invest. 1992;66:564–571. [PubMed] [Google Scholar]
- 94.Schnaper HW. Balance between matrix synthesis and degradation: a determinant of glomerulosclerosis. Pediatr Nephrol. 1995;9:104–111. doi: 10.1007/BF00858986. [DOI] [PubMed] [Google Scholar]
- 95.Lovett DH, Johnson RJ, Marti HP, et al. Structural characterization of the mesangial cell type IV collagenase and enhanced expression in a model of immune complex-mediated glomerulonephritis. Am J Pathol. 1992;141:85–98. [PMC free article] [PubMed] [Google Scholar]
- 96.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 97.Zhang G, Eddy AA. Urokinase and its receptors in chronic kidney disease. Front Biosci. 2008;13:5462–5478. doi: 10.2741/3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- 99.Fogo AB. Renal fibrosis: not just PAI-1 in the sky. J Clin Invest. 2003;112:326–328. doi: 10.1172/JCI19375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma J, Weisberg A, Griffin JP, et al. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney Int. 2006;69:1064–1072. doi: 10.1038/sj.ki.5000201. [DOI] [PubMed] [Google Scholar]
- 101.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 102.Strutz F, Zeisberg M, Ziyadeh FN, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 103.Taneda S, Hudkins KL, Topouzis S, et al. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol. 2003;14:2544–2555. doi: 10.1097/01.asn.0000089828.73014.c8. [DOI] [PubMed] [Google Scholar]
- 104.Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66:1–9. doi: 10.1111/j.1523-1755.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 105.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 106.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 107.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. Faseb J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 108.Wang L, Kwak JH, Kim SI, et al. Transforming growth factor-beta1 stimulates vascular endothelial growth factor 164 via mitogen-activated protein kinase kinase 3-p38alpha and p38delta mitogen-activated protein kinase-dependent pathway in murine mesangial cells. J Biol Chem. 2004;279:33213–33219. doi: 10.1074/jbc.M403758200. [DOI] [PubMed] [Google Scholar]
- 109.Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem. 2004;279:2632–2639. doi: 10.1074/jbc.M310412200. [DOI] [PubMed] [Google Scholar]
- 110.Runyan CE, Schnaper HW, Poncelet AC. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am J Physiol Renal Physiol. 2003;285:F413–422. doi: 10.1152/ajprenal.00082.2003. [DOI] [PubMed] [Google Scholar]
- 111.Masszi A, Di Ciano C, Sirokmany G, et al. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284:F911–924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 112.Kolavennu V, Zeng L, Peng H, et al. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57:714–723. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 113.Price PM. Diabetes: caught in the Akt? Kidney Int. 2007;71:839–841. doi: 10.1038/sj.ki.5002200. [DOI] [PubMed] [Google Scholar]
- 114.Toyoda M, Suzuki D, Honma M, et al. High expression of PKC-MAPK pathway mRNAs correlates with glomerular lesions in human diabetic nephropathy. Kidney Int. 2004;66:1107–1114. doi: 10.1111/j.1523-1755.2004.00798.x. [DOI] [PubMed] [Google Scholar]
- 115.Cattran DC. Cyclosporine in the treatment of idiopathic focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:234–241. doi: 10.1053/snep.2003.50022. [DOI] [PubMed] [Google Scholar]
- 116.Bhimma R, Adhikari M, Asharam K, et al. Management of steroid-resistant focal segmental glomerulosclerosis in children using tacrolimus. Am J Nephrol. 2006;26:544–551. doi: 10.1159/000097864. [DOI] [PubMed] [Google Scholar]
- 117.Cattran DC, Wang MM, Appel G, et al. Mycophenolate mofetil in the treatment of focal segmental glomerulosclerosis. Clin Nephrol. 2004;62:405–411. doi: 10.5414/cnp62405. [DOI] [PubMed] [Google Scholar]
- 118.Schnaper HW, Robson AM, Kopp JB. Nephrotic syndrome: minimal change nephropathy, focal segmental glomerulosclerosis and collapsing glomerulopathy. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. 8th Edition Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 1585–1673. [Google Scholar]
- 119.Ardissino G, Testa S, Dacco V, et al. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol. 2004;19:172–177. doi: 10.1007/s00467-003-1268-0. [DOI] [PubMed] [Google Scholar]
- 120.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 121.Hogg RJ, Portman RJ, Milliner D, et al. Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics. 2000;105:1242–1249. doi: 10.1542/peds.105.6.1242. [DOI] [PubMed] [Google Scholar]
- 122.Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. doi: 10.1016/S0140-6736(00)04728-0. [DOI] [PubMed] [Google Scholar]
- 123.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 124.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- 125.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 126.Timio M, Venanzi S, Lolli S, et al. “Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol. 1995;43:382–387. [PubMed] [Google Scholar]
- 127.Wuhl E, Mehls O, Schaefer F. Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney Int. 2004;66:768–776. doi: 10.1111/j.1523-1755.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 128.Hilgers KF, Dotsch J, Rascher W, et al. Treatment strategies in patients with chronic renal disease: ACE inhibitors, angiotensin receptor antagonists, or both? Pediatr Nephrol. 2004;19:956–961. doi: 10.1007/s00467-004-1554-5. [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez M, Felsenfeld AJ. PTH, FGF-23 and early CKD. Nephrol Dial Transplant. 2008;23:3391–3393. doi: 10.1093/ndt/gfn438. [DOI] [PubMed] [Google Scholar]
- 130.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 131.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 132.Takei T, Otsubo S, Uchida K, et al. Effects of sevelamer on the progression of vascular calcification in patients on chronic haemodialysis. Nephron Clin Pract. 2008;108:c278–283. doi: 10.1159/000127361. [DOI] [PubMed] [Google Scholar]
- 133.Bro S, Olgaard K. Effects of excess PTH on nonclassical target organs. Am J Kidney Dis. 1997;30:606–620. doi: 10.1016/s0272-6386(97)90484-4. [DOI] [PubMed] [Google Scholar]
- 134.Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl. 1999;73:S14–19. doi: 10.1046/j.1523-1755.1999.07304.x. [DOI] [PubMed] [Google Scholar]
- 135.Lemire JM. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 136.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Agarwal R, Acharya M, Tian J, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 138.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 139.Gouva C, Nikolopoulos P, Ioannidis JP, et al. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–760. doi: 10.1111/j.1523-1755.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 140.Sharples EJ, Patel N, Brown P, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- 141.Eto N, Wada T, Inagi R, et al. Podocyte protection by darbepoetin: preservation of the cytoskeleton and nephrin expression. Kidney Int. 2007;72:455–463. doi: 10.1038/sj.ki.5002311. [DOI] [PubMed] [Google Scholar]
- 142.Holdaas H, Wanner C, Abletshauser C, et al. The effect of fluvastatin on cardiac outcomes in patients with moderate to severe renal insufficiency: a pooled analysis of double-blind, randomized trials. Int J Cardiol. 2007;117:64–74. doi: 10.1016/j.ijcard.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 143.Epstein M, Campese VM. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. Am J Kidney Dis. 2005;45:2–14. doi: 10.1053/j.ajkd.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 144.Zoja C, Corna D, Rottoli D, et al. Effect of combining ACE inhibitor and statin in severe experimental nephropathy. Kidney Int. 2002;61:1635–1645. doi: 10.1046/j.1523-1755.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 145.Sandhu S, Wiebe N, Fried LF, et al. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 146.Neale TJ, Ojha PP, Exner M, et al. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest. 1994;94:1577–1584. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chan JC, Mahan JD, Trachtman H, et al. Vitamin E therapy in IgA nephropathy: a double-blind, placebo-controlled study. Pediatr Nephrol. 2003;18:1015–1019. doi: 10.1007/s00467-003-1205-2. [DOI] [PubMed] [Google Scholar]
- 148.Winbanks CE, Grimwood L, Gasser A, et al. Role of the phosphatidylinositol 3-kinase and mTOR pathways in the regulation of renal fibroblast function and differentiation. Int J Biochem Cell Biol. 2007;39:206–219. doi: 10.1016/j.biocel.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 149.Bakris GL. Protein kinase C-beta inhibition: a promise not yet fulfilled. Clin J Am Soc Nephrol. 2007;2:619–620. doi: 10.2215/CJN.01940507. [DOI] [PubMed] [Google Scholar]
- 150.Kanda T, Wakino S, Hayashi K, et al. Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int. 2003;64:2009–2019. doi: 10.1046/j.1523-1755.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 151.Dedov I, Shestakova M, Vorontzov A, et al. A randomized, controlled study of sulodexide therapy for the treatment of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:2295–2300. doi: 10.1093/ndt/12.11.2295. [DOI] [PubMed] [Google Scholar]
- 152.Li Y, Wen X, Spataro BC, et al. hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-gamma agonists. J Am Soc Nephrol. 2006;17:54–65. doi: 10.1681/ASN.2005030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lan HY. Smad7 as a therapeutic agent for chronic kidney diseases. Front Biosci. 2008;13:4984–4992. doi: 10.2741/3057. [DOI] [PubMed] [Google Scholar]
- 154.Andreoli SP, Brewer ED, Watkins S, et al. American Society of Pediatric Nephrology position paper on linking reimbursement to quality of care. J Am Soc Nephrol. 2005;16:2263–2269. doi: 10.1681/ASN.2005020186. [DOI] [PubMed] [Google Scholar]
- 155.Abitbol CL, Warady BA, Massie MD, et al. Linear growth and anthropometric and nutritional measurements in children with mild to moderate renal insufficiency: a report of the Growth Failure in Children with Renal Diseases Study. J Pediatr. 1990;116:S46–54. doi: 10.1016/s0022-3476(05)82925-7. [DOI] [PubMed] [Google Scholar]
- 156.Mak RH, Cheung WW, Roberts CT., Jr. The growth hormone-insulin-like growth factor-I axis in chronic kidney disease. Growth Horm IGF Res. 2008;18:17–25. doi: 10.1016/j.ghir.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Furth SL, Hwang W, Yang C, et al. Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol. 2002;17:450–455. doi: 10.1007/s00467-002-0838-x. [DOI] [PubMed] [Google Scholar]
- 158.Fine RN, Ho M, Tejani A, et al. Adverse events with rhGH treatment of patients with chronic renal insufficiency and end-stage renal disease. J Pediatr. 2003;142:539–545. doi: 10.1067/mpd.2003.189. [DOI] [PubMed] [Google Scholar]
- 159.Lowman HB, Chen YM, Skelton NJ, et al. Molecular mimics of insulin-like growth factor 1 (IGF-1) for inhibiting IGF-1: IGF-binding protein interactions. Biochemistry. 1998;37:8870–8878. doi: 10.1021/bi980426e. [DOI] [PubMed] [Google Scholar]
- 160.Ranke MB, Savage MO, Chatelain PG, et al. Long-term treatment of growth hormone insensitivity syndrome with IGF-I. Results of the European Multicentre Study. The Working Group on Growth Hormone Insensitivity Syndromes. Horm Res. 1999;51:128–134. doi: 10.1159/000023345. [DOI] [PubMed] [Google Scholar]
- 161.Rosenbloom AL. Recombinant human insulin-like growth factor I (rhIGF-I) and rhIGF-I/rhIGF-binding-protein-3: new growth treatment options? J Pediatr. 2007;150:7–11. doi: 10.1016/j.jpeds.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 162.Fogo AB. New capillary growth: a contributor to regression of sclerosis? Curr Opin Nephrol Hypertens. 2005;14:201–203. doi: 10.1097/01.mnh.0000165883.08675.ab. [DOI] [PubMed] [Google Scholar]
- 163.Kang DH, Hughes J, Mazzali M, et al. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 164.Chen J, Park HC, Addabbo F, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–889. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaplan JM, Kim SH, North KN, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 166.Palmen T, Lehtonen S, Ora A, et al. Interaction of endogenous nephrin and CD2-associated protein in mouse epithelial M-1 cell line. J Am Soc Nephrol. 2002;13:1766–1772. doi: 10.1097/01.asn.0000019842.50870.41. [DOI] [PubMed] [Google Scholar]
- 167.Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 168.Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 169.Miner JH, Morello R, Andrews KL, et al. Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest. 2002;109:1065–1072. doi: 10.1172/JCI13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zenker M, Tralau T, Lennert T, et al. Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet A. 2004;130A:138–145. doi: 10.1002/ajmg.a.30310. [DOI] [PubMed] [Google Scholar]
- 171.Wagner KD, Wagner N, Schedl A. The complex life of WT1. J Cell Sci. 2003;116:1653–1658. doi: 10.1242/jcs.00405. [DOI] [PubMed] [Google Scholar]