Abstract
Background
Left ventricular (LV) contraction displaces the aortic annulus and produces a force that stretches the ascending aorta. We hypothesized that aortic stiffening increases this previously ignored component of LV load and may contribute to hypertrophy. Conversely, aortic stretch-related work represents stored energy that may facilitate early diastolic filling.
Methods and Results
We performed magnetic resonance imaging of the aorta and LV in 347 participants (72 to 91 years old, 189 women) in the Age, Gene/Environment Susceptibility-Reykjavik Study to examine relations of aortic stretch with LV structure and function. Aortic stiffness was evaluated as the product of Young’s modulus and wall thickness. Force was computed from Young’s modulus and longitudinal aortic strain; work was the integrated product of force and annulus displacement during systole. LV mass and dynamic volume were measured using the area-length method. Filling was assessed from time-resolved LV volume curves. In multivariable models that adjusted for age, sex, height, weight, end-diastolic LV volume, augmentation index, end-systolic pressure, and cardiovascular disease risk factors, higher aortic stiffness was associated with increased LV mass (B=3.0±0.8% per SD, P<0.001; sex interaction, P=0.8). Greater stretch-related aortic work was associated with enhanced early filling in men (B=4.0±0.8 mL/SD, P<0.001), but not in women (B=−0.4±0.7 mL/SD, P=0.6).
Conclusions
Higher aortic stiffness was associated with higher LV mass, independently of pressure. Higher stretch-related work was associated with greater early diastolic filling in men only. Impaired diastolic recovery of energy stored by systolic proximal aortic stretch may contribute to increased susceptibility to diastolic dysfunction in women.
Keywords: aorta, left ventricle, pressure, aortic stiffness, epidemiology
Left ventricular (LV) hypertrophy is a risk factor for cardiovascular disease, including heart failure.1 LV structure and function are affected by standard cardiovascular disease risk factors including blood pressure. Evaluation of the interaction between the proximal aorta, which is a major determinant of the pulsatile component of blood pressure, and the LV may facilitate elucidation of the pathophysiology of hypertension and cardiovascular disease, and may provide insight into higher susceptibility to diastolic dysfunction in older women.2;3
LV systolic long-axis shortening causes aortic annulus displacement towards the apex of the heart.4–6 Previously, we showed that in light of modest relative movement of the aorta at the level of the brachiocephalic artery,6;7 axial displacement of the aortic annulus results in longitudinal stretch of the proximal aorta.8 Aortic stretch represents both a previously unrecognized load on the LV and a source of stored elastic energy that may facilitate LV recoil and early diastolic filling. In order to evaluate relations between longitudinal aortic stretch and the LV, we assessed mechanical stiffness of the proximal aorta as the product of Young’s modulus and aortic wall thickness. We also calculated aortic work as the integral of the product of aortic annulus displacement and the force that produced the observed longitudinal aortic stretch.
In this paper we investigate the following hypotheses: 1) aortic stretch imposes a previously unidentified load on the LV that increases with aortic stiffness and may contribute to LV hypertrophy independently of pressure and 2) aortic work performed during stretch of the elastic elements in the proximal ascending aorta represents stored energy that may enhance early diastolic LV filling as the aorta recoils.
Methods
Participants
Participant selection criteria and design of the Age, Gene/Environment Susceptibility – Reykjavik Study (AGES-Reykjavik) have been presented in detail.9 During a second AGES-Reykjavik exam conducted from 2008 to 2011, a subset of participants was recruited to participate in a comprehensive magnetic resonance imaging (MRI) study of aortic structure and function.10 Participants with known MRI contraindications (292 of 3316 participants, 8.8%) or who had previously refused to participate in MRI imaging studies because of claustrophobia or other reasons (279, 8.4%) were excluded prior to recruitment of our initial sample of 633 participants. The study was approved by the National Bioethics Committee in Iceland and the National Institute on Aging Intramural Institutional Review Board. All participants gave their informed written consent.
Tonometry Data Acquisition
Participants were studied supine after 10 minutes of rest. Auscultatory blood pressure was obtained with a semiautomated computer-controlled device (NIHem, Cardiovascular Engineering, Inc, Norwood, MA). Arterial tonometry and simultaneous electrocardiography (ECG) were obtained from brachial and carotid arteries with a custom transducer (Cardiovascular Engineering, Inc, Norwood, MA).
MRI Acquisition
MRI was performed in supine participants using an 8-channel torso coil in a 1.5 Tesla MRI scanner (Signa Excite, General Electric Medical Systems, Waukesha, WI). Two orthogonal multiphase FIESTA localizers of the proximal aorta were taken: a cardiac 3-chamber scan was obtained using the coronal plane localizer, and an oblique coronal scan was obtained using the cardiac 3-chamber localizer. Using the cardiac 3-chamber and oblique coronal scans as localizers, cross-sectional scans of the proximal ascending aorta were obtained approximately 10 mm distal to the sinotubular junction after a 10 ms trigger delay. Two long-axis multiphase FIESTA scans were taken of the heart; a 4-chamber scan was prescribed from the sagittal and cardiac 3-chamber localizers and a 2-chamber scan was prescribed from the cardiac 3-chamber localizer and 4-chamber scan. Detailed imaging parameters are provided in the data supplement. All acquisitions were obtained during one breath-hold, and multi-phase scans were obtained using ECG triggering.
Tonometry Data Analysis
All data was transferred to the core laboratory (Cardiovascular Engineering, Inc., Norwood, MA) for analysis by trained analyzers blinded to participant characteristics. Tonometry waveforms were signal-averaged with the ECG used as a fiducial point. Blood pressures were over-read in the core laboratory. Systolic and diastolic cuff pressures were used to calibrate the peak and trough of the signal-averaged brachial pressure waveform. Diastolic and integrated mean brachial pressures were used to calibrate carotid pressure tracings.11 End-systolic pressure was taken from the carotid waveform at the time of the dicrotic notch. Augmentation index was calculated as previously described.10
MRI Data Analysis
All cases were analyzed using ImageJ version 1.44p (32-bit, National Institutes of Health, Bethesda, MD) with custom macros and plugins. Oblique coronal scans were used to measure aortic annulus displacement. The proximal aortic contour was located by using ImageJ default thresholding, and a centerline was created from the midpoints of the aortic contours.8 Centerlines were obtained for 30 evenly spaced phases of the cardiac cycle, each representing 1/30th of the RR interval. The aortic annulus was manually located on every third phase in the oblique coronal stack and interpolated across the two intervening phases; aortic annulus displacement was measured along the aortic centerline.
Cross-sectional FIESTA scans were used to acquire circumferential area waveforms of the ascending aorta by applying an ImageJ default threshold for auto-detection of the lumen at each phase. Double inversion recovery images were used to measure the diastolic aortic wall cross-sectional area as previously described.10 The 2-chamber and 4-chamber scans were used to trace the diastolic epicardium of the LV and to acquire contours of the endocardium throughout the cardiac cycle using an ImageJ threshold approach.
Calculations
Aortic stiffness was assessed as the product (Eh) of incremental Young’s modulus (E) and proximal ascending aortic wall thickness (h). Eh was used instead of E in order to account for overall mechanical stiffness of the aorta. Incremental Young’s modulus of the ascending aorta was calculated from measured circumferential strain and calculated stress due to pressure in a constant longitudinal state. The following simplified stress equation12 was used:
to measure circumferential stress at the inner wall of a thick-walled cylinder under pressure with the assumption extravascular pressure was zero. Pi represents intravascular pressure. Ri is the inner radius calculated from aortic lumen area, and Ro is the outer radius calculated from the sum of aortic lumen and wall areas. Wall thickness (h) represents the diastolic difference between the inner and outer aorta radius. Radial strain measurements were adjusted for longitudinal aortic strain to calculate circumferential stress in a longitudinally static state.8
Young’s modulus was assumed to be identical in circumferential and longitudinal directions. Longitudinal aortic stress was calculated as the product of Young’s modulus and ascending aortic longitudinal strain. Aortic force was calculated as the product of longitudinal stress and ascending aortic cross-sectional wall area, which was also corrected for longitudinal strain by dividing the wall area by longitudinal aortic strain at each point in time. Aortic work was calculated as the integral of aortic force and aortic annulus displacement during systole.
LV volumes and mass were computed using the area-length method as previously described.10 Early and late filling volumes were calculated from dynamic LV volume waveforms. Early filling volume was defined as the difference between the volume at LV diastasis and end-systolic volume. Late filling volume was defined as the difference between end-diastolic volume and the volume at diastasis.
As previously described and validated, measured aortic lumen and wall areas and ventricular volumes were highly reproducible when analyzed by different observers using different software.10
Statistical Analyses
Anthropometric and risk factor data were tabulated for included and excluded participants and separately by sex for included participants. Aortic and LV characteristics were tabulated by sex. In order to normalize their distributions, skewed variables were transformed by using the square root, natural logarithm, or square as noted. Sex-differences were assessed by using an independent-samples t-test on transformed variables.
We used multivariable linear regression to examine relations between proximal aortic stiffness and annulus displacement and to examine relations between aortic properties and LV structure and function. To evaluate the hypothesis that proximal aortic stiffness imposes a load on the LV that contributes to hypertrophy and a shift to late diastolic (active) LV filling, we examined models with LV mass or late filling as dependent variables and aortic Eh as an exposure. To evaluate the hypothesis that early diastolic (passive) LV filling is facilitated by recovery of work stored as longitudinal strain of elastic elements in the proximal aorta, we examined a model with early diastolic filling as the dependent variable and aortic work as an exposure variable. Model coefficients were expressed per SD difference in the independent variable.
To account for potential confounding by traditional anthropometrics or cardiovascular disease risk factors, age, height, weight, heart rate, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, diabetes, current smoking, cardiovascular disease, treated hypertension, and statin use were included as standard covariates in all statistical models. Variables with skewed distributions (heart rate, height, fasting glucose, HDL cholesterol, triglycerides) were transformed by using the natural logarithm to normalize distributions. To account for effects of chamber dimension on LV mass and filling volumes, we adjusted for end-diastolic volume. A primary goal of the analyses was to determine whether proximal aortic measures have relations with LV structure and function that are separate from potential effects of stiffness on wave reflection or blood pressure. To account for possible effects of premature wave reflection, augmentation index was included as a covariate. To account for potential effects of LV pressure load, end-systolic pressure was included in models. Relations between independent variables and dependent variables were assessed by examining scatterplots of appropriately transformed variables. Regression models were evaluated for effects of nonlinearity and heteroskedasticity by examining histograms and QQ plots of residuals and plots of residuals versus predicted values.
All dependent and independent continuous variables were assessed for normality and transformed as needed. We screened for nonlinear associations by examining scatterplots of dependent and independent variables. In addition, we examined plots of residuals versus predicted variables to screen for effects of nonlinearity or heteroskedasticity of independent or dependent variables and found a uniform distribution of residuals across the range of predicted values. We examined variance inflation factors for reported exposure variables and found that all were <1.4. Finally, to screen for influential values and ensure that we had not violated assumptions of normality, we examined the distribution of residuals by using histograms and QQ plots, which demonstrated that residuals were normally distributed.
We evaluated effect modification by sex or end-systolic pressure by adding two-way interactions to the model one at a time. Models with a significant sex-interaction were analyzed separately for men and women; otherwise sex was included as a covariate and the interaction term was removed. Interactions between end-systolic pressure and independent variables of interest were similarly evaluated and removed from the model if the interaction term was not significant.
To illustrate relations between aortic and LV measures, we evaluated LV measures in groups defined by sex-specific median values of end-systolic pressure and either aortic Eh (for LV mass and late filling) or aortic work (early filling). Groups were compared by using ANOVA models that adjusted for covariates included in linear regression models. Adjusted mean values were reverse transformed as needed and plotted along with 95% confidence intervals. Results are presented as mean±SD unless stated otherwise. A two-tailed P-value <0.05 was considered significant.
Results
Among the original sample of 633 volunteers, 2 could not lie supine, 4 became fatigued or ill prior to the study, 6 were canceled for logistical reasons, 6 could not fit in the MRI gantry, 6 were claustrophobic, and 7 withdrew from the study leaving 602 participants who had any image data acquired. Of these cases, 81 had unusable hemodynamic information, 66 had unusable oblique coronal images of the aorta, 60 had unusable cross-sectional images of the proximal aorta, 6 had unusable aortic wall images, 25 had unusable 2-chamber or 4-chamber cardiac images, 13 had atrial fibrillation, and 4 had valve replacement or known dilation of the proximal aorta, leaving 347 cases with complete information required for the present analysis. No additional participants were excluded because of missing values for any covariates used in the present analyses. Excluded cases had a higher heart rate, slightly higher diastolic blood pressure, and a higher prevalence of treated hypertension and cardiovascular disease (Supplemental Table). Characteristics of the included study participants are presented by sex in Table 1.
Table 1.
Participant Characteristics
| Variables | Men | Women |
|---|---|---|
| Sample size | 158 | 189 |
| Age range, years | 73 to 88 | 72 to 91 |
| Height, cm | 177 ± 6 | 162 ± 6 |
| Weight, kg | 83 ± 12 | 72 ± 12 |
| Body surface area, m2 | 2.0 ± 0.2 | 1.8 ± 0.1 |
| Body mass index, kg/m2 | 27 ± 4 | 27 ± 4 |
| Heart rate, min−1 | 61 ± 9 | 65 ± 9 |
| Brachial pressures, mm Hg | ||
| Systolic | 138 ± 20 | 144 ± 17 |
| Diastolic | 65 ± 9 | 64 ± 9 |
| Mean | 92 ± 12 | 95 ± 11 |
| Pulse pressure | 74 ± 18 | 80 ± 18 |
| Carotid pressures, mm Hg | ||
| Peak | 137 ± 24 | 146 ± 23 |
| End-systolic | 103 ± 16 | 107 ± 14 |
| Augmentation index | 6 ± 11 | 10 ± 15 |
| Fasting glucose, mmol/L | 5.7 ± 1.0 | 5.5 ± 0.8 |
| Total cholesterol, mmol/L | 4.9 ± 1.0 | 5.7 ± 1.1 |
| HDL cholesterol, mmol/L | 1.4 ± 0.4 | 1.8 ± 0.4 |
| Triglycerides, mmol/L | 1.1 ± 0.5 | 1.2 ± 0.5 |
| Medical history, n (%) | ||
| Diabetes | 18 (11) | 18 (10) |
| Treated hypertension | 101 (64) | 142 (75) |
| Statin use | 71 (45) | 66 (35) |
| Cardiovascular disease | 51 (32) | 21 (11) |
| Current smoker | 15 (10) | 9 (5) |
HDL, high density lipoprotein
Aortic and LV variables are presented in Table 2. Women had greater peak aortic annulus displacement. In a multivariable model, higher Eh was associated with lower annulus displacement (−0.3±0.1 mm/SD, P=0.002) with no evidence of a sex interaction (P=0.3). As expected, men had larger LV volumes and LV mass. Ejection fraction was higher in women, while early filling fraction and aortic Eh were not significantly different between sexes. Aortic force and aortic work were higher in women.
Table 2.
Aortic and Left Ventricular Characteristics
| Variable | Men | Women | P |
|---|---|---|---|
| Aortic annulus displacement, mm | 6.9 (5.4, 8.1) | 7.8 (6.3, 9.0) | <0.001 |
| Volume, mL | |||
| End-diastolic* | 110 (95, 126) | 90 (79, 103) | <0.001 |
| End-systolic* | 42 (33, 53) | 30 (24, 38) | <0.001 |
| Early filling | 38 (30, 47) | 34 (28, 43) | 0.002 |
| Late filling* | 28 (23, 33) | 23 (19, 29) | <0.001 |
| Ejection fraction, %‡ | 62 (57, 66) | 66 (61, 71) | <0.001 |
| Early filling fraction, % | 58 (49, 66) | 61 (53, 67) | 0.5 |
| Left ventricular mass, g* | 161 (146, 177) | 117 (106, 133) | <0.001 |
| Eh, kdynes/cm* | 3,194 (2,544; 4,252) | 3,058 (2,471; 4,085) | 0.5 |
| Aortic force, kdynes† | 2,123 (1,508; 2,777) | 2,495 (1,860; 3,184) | <0.001 |
| Aortic work, mJ† | 79 (47, 116) | 106 (75, 154) | <0.001 |
Values are presented as median (25th, 75th percentiles) due to skewed distributions for several variables. Values were compared by using an independent-samples t-test on normally-distributed, transformed values with sex as the grouping variable.
log transform;
square root transform;
square transform. Eh, Young’s modulus x aorta wall thickness product.
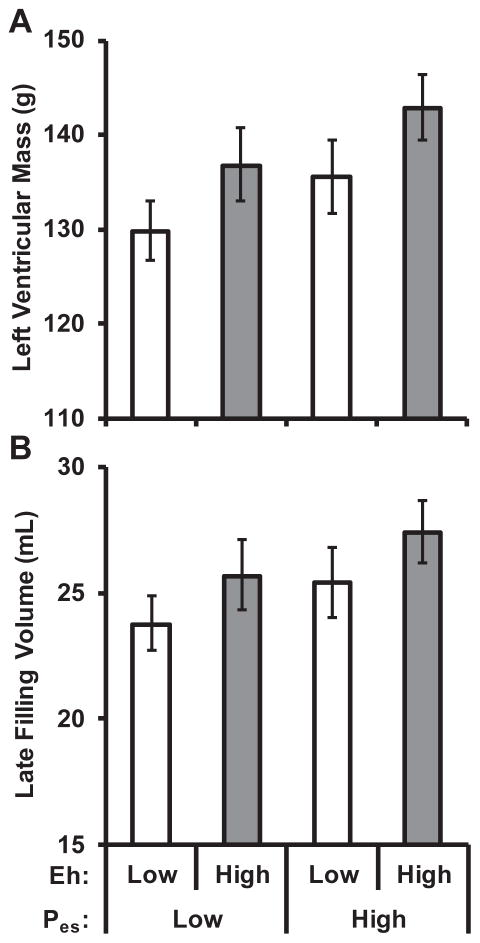
Table 3 presents multivariable linear regression models of LV mass and late filling volume. Greater LV mass was associated with higher Eh and higher end-systolic pressure, while greater late filling volume was only associated with higher Eh. There was no evidence of an interaction between sex and Eh for LV mass (P=0.8) or late filling volume (P=1.0). Comparable relations were observed when LV mass was indexed to body surface area, and when models were adjusted only for sex, age, height, weight, heart rate, and end-diastolic LV volume (data not shown). Mean values for LV mass and late filling volume in groups defined by sex-specific median values of aortic Eh and end-systolic pressure are presented in Figure 1.
Table 3.
Multivariable Model of Left Ventricular Mass and Late Filling Volume
| Independent Variable | LV Mass
|
Late Filling Volume
|
||||
|---|---|---|---|---|---|---|
| B ± SE | P | R2 | B ± SE | P | R2 | |
| Aortic stiffness | 3.0 ± 0.8 | <0.001 | 0.022 | 3.9 ± 1.6 | 0.012 | 0.018 |
| End-systolic pressure | 2.0 ± 0.9 | 0.021 | 0.005 | 2.4 ± 1.6 | 0.1 | 0.004 |
LV mass and late filling volume were log transformed prior to analysis. B values are for the final model with all variables entered. B values were multiplied by 100 to represent % difference in the dependent variable per SD difference in the independent variable. R2 represents the change in model R2 after each variable entered in the order shown. LV mass final model R2=0.699; late filling volume final model R2=0.468). The model adjusted for sex, age, height, weight, heart rate, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, left ventricular end-diastolic volume, augmentation index, diabetes, current smoking, cardiovascular disease, treated hypertension, and statin use. LV, left ventricular.
Figure 1. Relations of left ventricular mass and late filling volume with aortic stiffness and end-systolic pressure.
Left ventricular mass (A) and late filling volume (B) summarized according to groups defined by sex-specific median aortic stiffness (Eh) and end-systolic pressure (Pes). Aortic stiffness was evaluated as the product of Young’s modulus and aortic wall thickness and had a positive relation with both mass (P=0.001) and late filling volume (P=0.010). Aortic pressure was also positively related with mass (P=0.008) and late filling volume (P=0.033). Results were consistent when left ventricular mass was indexed to body surface area. There were 106 participants with low stiffness and low pressure, 68 participants had low stiffness and high pressure, 69 participants had high stiffness and low pressure, and 104 participants had high stiffness and high pressure. Left ventricular mass and late filling volume were log-transformed prior to analysis and reverse transformed for presentation. Values represent the geometric mean and 95% confidence intervals. Values were adjusted for sex, age, height, weight, heart rate, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, left-ventricular end-diastolic volume, augmentation index, diabetes, current smoking, cardiovascular disease, treated hypertension, and statin use.
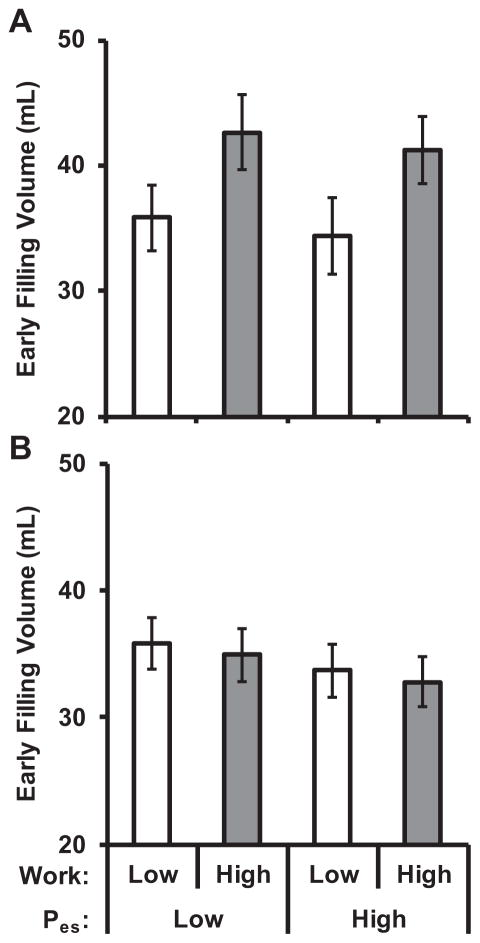
A multivariable linear regression model of early filling volume demonstrated an interaction between sex and aortic work (P<0.001); models were therefore estimated separately for men and women (Table 4). Higher stored aortic work was associated with higher early filling volume in men but not in women. Results were comparable when models were adjusted only for age, height, weight, heart rate, and end-diastolic LV volume (data not shown). Figure 2 presents early filling volumes separately by sex for groups based on sex-specific median values of aortic work and end-systolic pressure.
Table 4.
Multivariable Model of Early Filling Volume
| Independent Variable | Men
|
Women
|
||||
|---|---|---|---|---|---|---|
| B ± SE | P | R2 | B ± SE | P | R2 | |
| Aortic work | 4.0 ± 0.8 | <0.001 | 0.066 | −0.4 ± 0.7 | 0.6 | 0.002 |
| End-systolic pressure | −1.0 ± 0.8 | 0.2 | 0.005 | −1.1 ± 0.7 | 0.1 | 0.006 |
B values are for the final model with all variables entered and represent mL difference in early filling volume per SD difference in the independent variable. R2 represents the change in model R2 after each variable entered in the order shown. For men, final model R2=0.592; for women, final model R2=0.628. The model adjusted for age, height, weight, heart rate, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, left ventricular end-diastolic volume, augmentation index, diabetes, current smoking, cardiovascular disease, treated hypertension, and statin use.
Figure 2. Relations of early filling volume with aortic work and end-systolic pressure.
Early diastolic filling volume summarized according to groups defined by median values of aortic work and end-systolic pressure (Pes) in men (A) and women (B). In men, greater early diastolic filling was associated with higher aortic work (P<0.001), but was not related to end-systolic pressure (P=0.4). In women, early filling volume was unrelated to aortic work (P=0.5) and end-systolic pressure (P=0.1). There were 45 men with low aortic work and low pressure, 34 with low aortic work and high pressure, 35 with high aortic work and low pressure, and 44 with high aortic work and high pressure. There were 55 women with low aortic work and low pressure, 40 with low aortic work and high pressure, 40 with high aortic work and low pressure, and 54 with high aortic work and high pressure. Values represent the mean and 95% confidence intervals. Values were adjusted for age, height, weight, heart rate, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, left ventricular end-diastolic volume, augmentation index, diabetes, current smoking, cardiovascular disease, treated hypertension, and statin use.
Discussion
This study examined relations between aortic stiffness, longitudinal aortic stretch, and LV structure and function. Higher aortic stiffness, as assessed by the product of Young’s modulus and wall thickness in the proximal aorta (Eh), was associated with higher LV mass and higher late filling volume in women and men in models that adjusted for end-systolic pressure, augmentation index, and standard cardiovascular disease risk factors, suggesting that coupling of the LV to a stiff proximal aorta may impose a pressure-independent load on the LV that has not been considered previously. Greater aortic work was positively related to early filling volume in men, suggesting that aortic stretch during systole stores elastic energy that may be recovered as enhanced early diastolic filling. In women, however, aortic work was not associated with early filling volume, which suggests that in older women, energy stored by systolic stretch of the ascending aorta is not recovered as enhanced early diastolic filling. Failure to recover stored aortic work during early diastole may enhance the susceptibility of older women to develop heart failure with preserved LV function.
Several prior studies have examined relations between aortic stiffness and LV mass. Some studies reported a positive relation between aortic stiffness and LV mass that persisted after adjusting for pressure,13–15 while others attributed the relation to effects of aortic stiffness on blood pressure.16 In our study, end-systolic pressure and aortic stiffness assessed as Eh had additive positive relations with LV mass in men and women. The association between LV mass and Eh was consistent when carotid peak-systolic or pulse pressure, brachial peak-systolic or pulse pressure, or mean arterial pressure were used in place of end-systolic pressure (data not shown). Additionally, we adjusted for augmentation index in our models. Thus, the residual relation between aortic stiffness and LV mass was separate from potential effects of aortic stiffness on blood pressure, pressure amplification, and wave reflection. Studies that found relations between LV mass and aortic stiffness that were independent of blood pressure attributed the relation to possible concurrent exposure to risk factors such as obesity or treated hypertension, or possible volume overload.13–15 In our study, the pressure-independent relation between aortic stiffness and LV mass persisted after adjustment for various risk factors, including prevalent cardiovascular disease and treated hypertension.
Early LV filling is the result of a pressure gradient from the left atrium to the LV. This pressure gradient is enhanced by early diastolic suction due to rapid expansion of LV volume. Factors that are thought to enhance early diastolic filling include elastic recoil of the LV wall after systolic myocardial compression and twist,17–19 erectile effect of coronary perfusion,20 and recoil of the left atrium after being stretched during systole.21 In light of negligible movement of the apex when the LV contracts,22;23 long axis shortening pulls the aortic annulus towards the apex, producing longitudinal stretch in the proximal aorta. The resulting stretch-related work represents stored elastic energy in the walls of the aorta. Ideally, when the LV stops contracting, aortic stretch-related work is recovered as elastic recoil, which pulls upward on the base of the heart, increasing LV volume and enhancing early diastolic filling.
We observed a strong positive relation between stretch-related aortic work and early LV filling in men that was independent of end-systolic pressure. However, we did not observe any relation between aortic work and early filling in women. Prior studies have noted that women have greater global longitudinal LV strain than men,24 which is consistent with our observation of greater aortic annulus displacement in women (Table 2). Although older women tend to have preserved systolic function, they are more susceptible to impaired diastolic function.2;3 Our results suggest that work stored as longitudinal aortic stretch from systolic LV contraction represents an important contributor to early diastolic LV filling and that sex difference in diastolic recovery of that work may contribute to diastolic dysfunction and increased risk for heart failure with preserved ejection fraction in older women.2;3
Limitations
Our study has limitations that need to be acknowledged. All participants were over 70 years of age and from white European descent. Thus, additional studies should be performed in other age groups and ethnicities in order to establish the generalizability of our results. In particular, our results may not be generalizable to adults younger than 50 years of age, when a prominent, nonlinear transition in age relations of key hemodynamic variables is known to occur.25 For logistical reasons, tonometry was performed immediately before MRI acquisition. In future studies, intermittent blood pressure measurement during the course of the MRI acquisition would be a useful addition in order to confirm our assumption of a stable blood pressure during aortic imaging. To minimize the confounding effects of time delay between measurements, participants were placed in the body coil on a detachable MRI gurney for blood pressure and tonometry measurements and then immediately transferred into the MRI machine with no further change in posture.
Several types of acquisitions were needed for this study, rendering the final sample size sensitive to acquisition issues. For example, oblique coronal images were initially acquired as localizers without the intention of quantitative analysis and therefore were not repeated at the time of acquisition if there were minor deficiencies that did not interfere with image localization; in some cases these deficiencies did, however, obviate quantitative analysis.
Pressure at the outer wall of the aorta was assumed to be zero, but is known to be slightly negative, which may have caused an underestimation of circumferential stress and Young’s modulus. The proximal aorta was assumed to be isotropic with identical circumferential and longitudinal Young’s moduli.8;26 Additionally, because of elongation of the aortic arch in older cohorts,27 it is possible that systolic movement of the aortic annulus could be caused, in part, by release of a compressive strain rather than imposition of positive stretch in the ascending aorta. Presence of diastolic compression would result in overestimation of aortic force and work because of the presence of a negative force at the onset of systole, when force was assumed to be zero. Compression of the aorta seems unlikely, however, based on positive residual longitudinal strain in excised aortas in older people.28
In order to limit acquisition time, LV volumes and mass were derived from two long-axis views rather than multiple short-axis sections; the latter approach may have provided more precise estimates of volume and mass. Future studies should include additional measures such as LV strain measurements from speckle-tracking echocardiography or dynamic 3-dimensional aortic and LV imaging analysis to extend the results presented in this study. The strength of our study is the large, community-based sample of well characterized participants with central pressure measurements, sophisticated imaging, and detailed risk factor data.
Conclusions
During systole, LV contraction produces a highly variable stretching force in the proximal aorta that is proportional to proximal aortic stiffness and represents a previously ignored, potentially deleterious load on the LV. In contrast, the product of aortic force and stretch represents work stored in the elastic elements of the proximal aorta during systole that is recovered as proportional elastic recoil during diastole. Therefore, while aortic stretch represents a load on the heart, it also provides a mechanism to facilitate early diastolic filling. We found that stiffer aortas were associated with higher LV mass in men and women whereas greater stretch-related energy storage in the proximal aorta during systole was associated with greater early diastolic filling only in men. The observation that aortic stiffness is associated with LV hypertrophy in men and women whereas work stored in the aortic root is associated with greater early diastolic filling only in men may provide insights into the greater susceptibility of older women to develop heart failure with preserved ejection fraction. Further study is required to elucidate mechanisms that may contribute to blunted recovery during diastole of energy stored in the aortic root during systole.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health [contract N01-AG-12100]; the National Institute on Aging Intramural Research Program; Hjartavernd (the Icelandic Heart Association); the Althingi (the Icelandic Parliament); and a grant from the National Institutes of Health, National Heart, Lung and Blood Institute [HL094898].
Footnotes
Disclosures
Dr. Mitchell is the owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck, and Servier and is funded by research grants HL094898, DK082447, HL107385 and HL104184 from the National Institutes of Health. VB, JDG, and AAT are employees of Cardiovascular Engineering, Inc.
References
- 1.Aronow WS, Ahn C. Association of electrocardiographic left ventricular hypertrophy with the incidence of new congestive heart failure. J Am Geriatr Soc. 1998;46:1280–1281. doi: 10.1111/j.1532-5415.1998.tb04546.x. [DOI] [PubMed] [Google Scholar]
- 2.Alagiakrishnan K, Banach M, Jones LG, Datta S, Ahmed A, Aronow WS. Update on diastolic heart failure or heart failure with preserved ejection fraction in the older adults. Ann Med. 2013;45:37–50. doi: 10.3109/07853890.2012.660493. [DOI] [PubMed] [Google Scholar]
- 3.Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJ, Solomon SD. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:535–542. doi: 10.1002/ejhf.67. [DOI] [PubMed] [Google Scholar]
- 4.Beller CJ, Labrosse MR, Thubrikar MJ, Robicsek F. Role of aortic root motion in the pathogenesis of aortic dissection. Circulation. 2004;109:763–769. doi: 10.1161/01.CIR.0000112569.27151.F7. [DOI] [PubMed] [Google Scholar]
- 5.Kozerke S, Scheidegger MB, Pedersen EM, Boesiger P. Heart motion adapted cine phase-contrast flow measurements through the aortic valve. Magn Reson Med. 1999;42:970–978. doi: 10.1002/(sici)1522-2594(199911)42:5<970::aid-mrm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Weber TF, Muller T, Biesdorf A, Worz S, Rengier F, Heye T, Holland-Letz T, Rohr K, Kauczor HU, von Tengg-Kobligk H. True four-dimensional analysis of thoracic aortic displacement and distension using model-based segmentation of computed tomography angiography. Int J Cardiovasc Imaging. 2014;30:185–194. doi: 10.1007/s10554-013-0307-6. [DOI] [PubMed] [Google Scholar]
- 7.Cinthio M, Ahlgren AR, Bergkvist J, Jansson T, Persson HW, Lindstrom K. Longitudinal movements and resulting shear strain of the arterial wall. Am J Physiol Heart Circ Physiol. 2006;291:H394–H402. doi: 10.1152/ajpheart.00988.2005. [DOI] [PubMed] [Google Scholar]
- 8.Bell V, Mitchell WA, Sigurethsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, de RA, Gudnason V, Harris TB, Mitchell GF. Longitudinal and circumferential strain of the proximal aorta. J Am Heart Assoc. 2014;3:e001536. doi: 10.1161/JAHA.114.001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torjesen AA, Sigurethsson S, Westenberg JJ, Gotal JD, Bell V, Aspelund T, Launer LJ, de RA, Gudnason V, Harris TB, Mitchell GF. Pulse Pressure Relation to Aortic and Left Ventricular Structure in the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Hypertension. 2014;64:756–761. doi: 10.1161/HYPERTENSIONAHA.114.03870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O’Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433–1439. doi: 10.1161/hy1201.098298. [DOI] [PubMed] [Google Scholar]
- 12.Love AEH. A Treatise on the Mathematical Theory of Elasticity. 2. Cambridge: Cambridge University Press Warehouse; 1906. [Google Scholar]
- 13.Boutouyrie P, Laurent S, Girerd X, Benetos A, Lacolley P, Abergel E, Safar M. Common carotid artery stiffness and patterns of left ventricular hypertrophy in hypertensive patients. Hypertension. 1995;25:651–659. doi: 10.1161/01.hyp.25.4.651. [DOI] [PubMed] [Google Scholar]
- 14.Rabkin SW, Chan SH. Correlation of pulse wave velocity with left ventricular mass in patients with hypertension once blood pressure has been normalized. Heart Int. 2012;7:e5. doi: 10.4081/hi.2012.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbina EM, Dolan LM, McCoy CE, Khoury PR, Daniels SR, Kimball TR. Relationship between elevated arterial stiffness and increased left ventricular mass in adolescents and young adults. J Pediatr. 2011;158:715–721. doi: 10.1016/j.jpeds.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. doi: 10.1161/01.hyp.36.4.489. [DOI] [PubMed] [Google Scholar]
- 17.Firstenberg MS, Smedira NG, Greenberg NL, Prior DL, McCarthy PM, Garcia MJ, Thomas JD. Relationship between early diastolic intraventricular pressure gradients, an index of elastic recoil, and improvements in systolic and diastolic function. Circulation. 2001;104:I330–I335. doi: 10.1161/hc37t1.094834. [DOI] [PubMed] [Google Scholar]
- 18.Katz LN. The role played by the ventricular relaxation process in filling the ventricle. American Journal of Physiology. 1930;95:542–533. [Google Scholar]
- 19.Notomi Y, Martin-Miklovic MG, Oryszak SJ, Shiota T, Deserranno D, Popovic ZB, Garcia MJ, Greenberg NL, Thomas JD. Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation. 2006;113:2524–2533. doi: 10.1161/CIRCULATIONAHA.105.596502. [DOI] [PubMed] [Google Scholar]
- 20.Vogel WM, Apstein CS, Briggs LL, Gaasch WH, Ahn J. Acute alterations in left ventricular diastolic chamber stiffness. Role of the “erectile” effect of coronary arterial pressure and flow in normal and damaged hearts. Circ Res. 1982;51:465–478. doi: 10.1161/01.res.51.4.465. [DOI] [PubMed] [Google Scholar]
- 21.Henein MY, Gibson DG. Normal long axis function. Heart. 1999;81:111–113. doi: 10.1136/hrt.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers WJ, Jr, Shapiro EP, Weiss JL, Buchalter MB, Rademakers FE, Weisfeldt ML, Zerhouni EA. Quantification of and correction for left ventricular systolic long-axis shortening by magnetic resonance tissue tagging and slice isolation. Circulation. 1991;84:721–731. doi: 10.1161/01.cir.84.2.721. [DOI] [PubMed] [Google Scholar]
- 23.Rushmer RF, Crystal DK, Wagner C. The functional anatomy of ventricular contraction. Circ Res. 1953;1:162–170. doi: 10.1161/01.res.1.2.162. [DOI] [PubMed] [Google Scholar]
- 24.Kocabay G, Muraru D, Peluso D, Cucchini U, Mihaila S, Padayattil-Jose S, Gentian D, Iliceto S, Vinereanu D, Badano LP. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed) 2014;67:651–658. doi: 10.1016/j.rec.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azadani AN, Chitsaz S, Matthews PB, Jaussaud N, Leung J, Wisneski A, Ge L, Tseng EE. Biomechanical comparison of human pulmonary and aortic roots. Eur J Cardiothorac Surg. 2012;41:1111–1116. doi: 10.1093/ejcts/ezr163. [DOI] [PubMed] [Google Scholar]
- 27.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1:739–748. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Horny L, Adamek T, Gultova E, Zitny R, Vesely J, Chlup H, Konvickova S. Correlations between age, prestrain, diameter and atherosclerosis in the male abdominal aorta. J Mech Behav Biomed Mater. 2011;4:2128–2132. doi: 10.1016/j.jmbbm.2011.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.