Abstract
Purpose
To develop mitigators for combined irradiation to the lung and skin.
Methods
Rats were treated with X-rays as follows: (1) 12.5 or 13 Gy whole thorax irradiation (WTI) (2) 30 Gy soft X-rays to 10% area of the skin only (3) 12.5 or 13 Gy WTI+30 Gy skin irradiation after 3 hours (4) 12.5 Gy WTI+skin irradiation and treated with captopril (160 mg/m2/day) started after 7 days. Our end points were survival (primary) based on IACUC euthanization criteria and secondary measurements of breathing intervals and skin injury. Lung collagen at 210 days was measured in rats surviving 13 Gy WTI.
Results
After 12.5 Gy WTI with or without skin irradiation, one rat (12.5 Gy WTI) was euthanized. Survival was less than 10% in rats receiving 13 Gy WTI, but was enhanced when combined with skin irradiation (p<0.0001). Collagen content increased at 210 days after 13 Gy WTI vs 13 Gy WTI+30 Gy skin irradiation (p<0.05). Captopril improved radiation-dermatitis after 12.5 Gy WTI+30 Gy skin irradiation (p=0.008).
Conclusions
Radiation to the skin given 3 hours after WTI mitigated morbidity during pneumonitis in rats. Captopril enhanced the rate of healing of radiation-dermatitis after combined irradiations to the thorax and skin.
Keywords: Combined radiation injuries, radiation to skin and lungs, mitigation, radiological terrorism, nuclear accident
INTRODUCTION
For this publication, we honor Dr. Mike Robbins who was a pioneer in testing ACE inhibitors to protect normal tissues from radiation toxicities (Robbins and Hopewell 1986).
Our long term goal is to mitigate radiation injury to the lungs. Radiation induces two phases of injury, pneumonitis, a potentially lethal syndrome that develops after 42–70 days and pulmonary fibrosis that occurs from 6 months to years later in rats. Mitigation is described as the use of a countermeasure to reduce injury after exposure to radiation but before onset of symptoms (Stone et al. 2003). In the past decade we have worked to repurpose angiotensin converting enzyme (ACE) inhibitors (commonly prescribed for hypertension, renal and cardiac diseases) as mitigators of pneumonitis and pulmonary fibrosis induced by ionizing radiation. We have used WAG/RijCmcr rats given total body irradiation (TBI) (Gao et al. 2012) or whole thorax irradiation (WTI) in preclinical models (Gao et al. 2013a, Kma et al. 2012, Szabo et al. 2010, Zhang et al. 2008). The ACE inhibitors captopril and enalapril mitigated pneumonitis and fibrosis after WTI (Kma et al. 2012, Medhora et al. 2012).
In addition to potential utility for patients receiving radiotherapy for malignancies, countermeasures are highly desirable for use in the event of an unexpected radiological terrorism attack or nuclear accident. In the latter case, exposures from such events would injure the lungs as well as other organs, especially the skin (DiCarlo et al. 2008). Our aim in the current study was to develop a combined injury model of irradiation to the thorax coupled with irradiation to 10% of non-overlapping skin of rats in order to test mitigation by the ACE inhibitor captopril.
Because combined injuries are reported to increase mortality (DiCarlo et al. 2008), we anticipated that non-overlapping irradiation of two organs of the same rat might induce more severe damage to each organ than if the same dose was delivered to each organ alone. For example a lower dose of radiation induced the same mortality if it was combined with a non-lethal punch wound to the skin (Kiang et al. 2010, Kiang and Ledney 2013). Similarly radiation with burn injuries enhanced hematopoietic impairment and worsened wound healing (Ran et al. 2004; 2007).
However some studies describe improved survival of mice up to one month after total body irradiation (Ledney et al. 1981, Ledney et al. 1985a, Ledney et al. 1985b) with wounding of the skin in a narrow window of time before or after irradiation. Though the skin and bone marrow were involved in these examples, we know of no reports of the effect of radiation to the skin on indices of injury secondary to irradiation to the thorax.
In pilot studies we used a sublethal dose of 12 Gy WTI combined with 30 Gy soft X-rays to 10% of the skin. Results (not shown here) indicated no exacerbation of injuries to the lungs or skin by combined irradiations, while captopril delayed pneumonitis after combined irradiations. We progressed to a higher dose of 12.5 Gy WTI to injure the lungs in this study. We combined WTI with 30 Gy to 10% of the skin. The two fields of radiation did not overlap. Skin irradiation was restricted to the whole thickness of the skin only, i.e. it did not permeate the underlying tissue. Once again rats which received radiation to both lungs and skin did not demonstrate more severe injury to either organ. We therefore combined a higher dose of WTI alone (13 Gy) that is lethal to >80% of the rats, with the same dose of irradiation to the skin. In this schedule of exposure we observed increased survival in rats with irradiation of both organs as compared to those receiving13 Gy WTI alone through the phase of injury corresponding to pneumonitis. These unexpected results make our model of combined injury to the lung and skin the first to demonstrate mitigation of radiation pneumonitis by injury to the skin. Captopril delayed lung injury and improved radiation-dermatitis in this model.
MATERIAL AND METHODS
Animal Care
The study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical College of Wisconsin. Female rats (WAG/RijCmcr) were housed in a moderate security barrier. Based upon directives from the IACUC of the Medical College of Wisconsin, rats were considered morbid and were euthanized if they met specified veterinarian’s criteria. These included at least 3 of the following: (1) animals are emaciated with prominent skeletal structures; (2) inactivity on 2 consecutive days, as no movement unless actively stimulated; (3) lack of grooming that becomes worse after 24 h; (4) breathing rates of less than 60 or greater than 250 breaths per minute; and (5) hunched posture on 2 consecutive days.
Injury Models
Whole thorax irradiation (WTI or lung irradiation)
Unanesthetized 9- to 10-week-old rats weighing approximately 140 g were immobilized in a plastic jig and irradiated with a 320-kVp orthovoltage X-ray system, with a half-value layer (HVL) of 1.4 mm Cu. Rats were treated with a single dose of 12.5 Gy or 13 Gy to the whole thorax at a dose rate of 1.43 Gy/min. The radiation dose was delivered by two equally weighted lateral beams to improve uniformity. The whole lung, heart and a small amount of liver were in the field.
Skin irradiation (3 hours after WTI)
Twenty-four hours prior to the irradiation, hair from the dorsal surface of the rats was removed under light anesthesia (isoflurane inhalation) using electric clippers. On the day of the irradiation, unanesthetized rats were immobilized in a specially constructed acrylic jig and irradiated with a 10 kVp X-ray beam in the dorsoventral direction. The skin was irradiated 3 hours after WTI for combined irradiations. Rats were treated with a single dose of 30 Gy defined at the dermal layer at a dose rate of 0.68 Gy/min. The jig was open at the top, with four evenly spaced fishing lines (0.35-mm-diameter monofilament) to restrain the animals. The irradiated area of skin corresponded to approximately 10% (30 cm2) of the total body surface and was located on the animal’s back (Jourdan et al. 2011).
One group of rats was not irradiated (unirradiated controls or 0 Gy group) but maintained under identical conditions. Groups of irradiated rats and their age-matched controls were followed for at least 84 days after irradiation. Some rats were sacrificed at 42 days for invasive assays to monitor radiation-pneumonitis.
Administration of Drug
A group of randomly selected rats that received 12.5 Gy WTI and 30 Gy skin irradiation were treated with captopril (Sigma Chemicals, St. Louis, MO) added to the drinking water (300 mg/L) as described previously (Kma et al. 2012). This concentration delivers ~160 mg/m2 of drug per day. Drug was started at 7 days after irradiation.
Groups, sizes (n) and time points
No irradiation (0 Gy); n=4
12.5 Gy WTI; n=5
30 Gy skin; n=6
12.5 Gy WTI+ 30 Gy skin; n=7
12.5 Gy WTI + 30 Gy skin + captopril (160 mg/m2/day started from 7 days and continued); n=6
13 Gy WTI; n=16
13 Gy WTI+ 30 Gy skin; n=16
Groups (i)–(v) were followed to 84 days after irradiation. In addition, a separate set of rats from each of these groups (n=5/group) were sacrificed at 42 days after irradiation for invasive analysis of lung structure. Groups (vi) and (vii) were followed to 210 days to estimate radiation-induced fibrosis.
Breathing Rate assay to determine breathing intervals
Breathing rates were measured every week from 28 to 84 days in groups (i)–(v) as described previously (Gao et al. 2012, Zhang et al. 2008). Rats were restrained in a Plexiglas jig and placed in a transparent, airtight box connected to a differential pressure transducer. The mean breathing rate in each rat was calculated from a minimum of four steady regions of recording lasting at least 15 seconds each. The breathing interval (minutes/breath) were calculated as the inverse of the breathing rates.
Sircol Collagen Assay
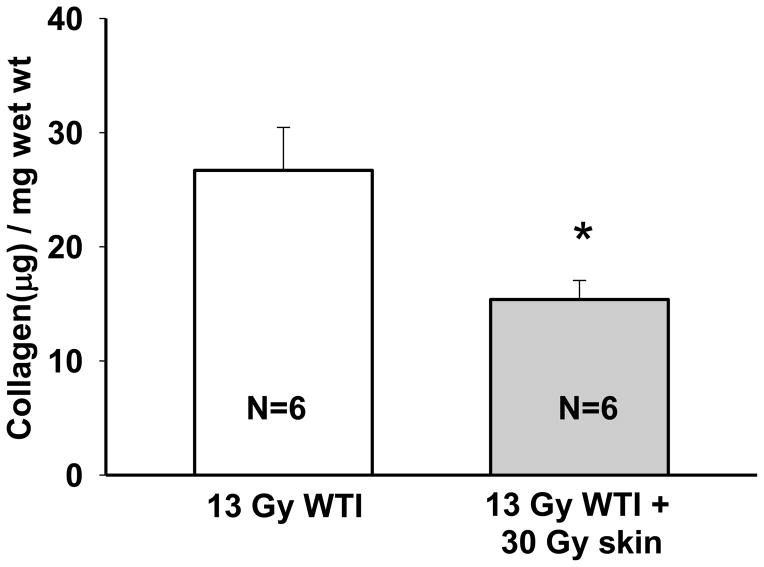
The Sircol collagen assay (Biocolor Ltd., Carrickfergus, Northern Ireland) was used to measure newly synthesized collagen as a marker of fibrosis. The assay was performed in rat lungs at 210 days after 13 Gy as described previously (Gao et al. 2012, Gao et al. 2013b, Kma et al. 2012). Collagen content in test samples were normalized by the lung wet weight and expressed as “collagen (μg)/wet weight (mg)”. Lung samples from 4 survivors after 13 Gy WTI only chosen at random from a separate experiment were added to increase the sample size to n=6 as seen in Figure 8. All Sircol assays (n=6 for 13 Gy WTI and n=6 for 13 Gy+30 Gy skin) were performed at the same time along with known collagen standards.
FIGURE 8.
Irradiation to the skin decreases newly synthesized collagen in the lungs. Acid and pepsin soluble collagen in the lung was measured using Sircol, at 210 days after irradiation in 2 groups of rats: (i) pooled 13 Gy whole thorax irradiation (WTI) from two separate experiments (open bar) (ii) 13 Gy WTI+30 Gy to the skin (grey bar). Number of rats in each group are indicated in the bar. * indicates p<0.05 between lung collagen in the 2 groups of rats. Collagen has been previously reported to be increased at 210 days after 13 Gy as compared to unirradiated age-matched control rats (Kma et al. 2012). Unirradiated rats using the same model and collagen assay at 210 days had 14.6±0.2 μg collagen/mg wet weight of lung (n=5) (Kma et al. 2012).
Monitoring of radiation induced skin injury
The skin injury was assessed every week from one week to twelve weeks after irradiation according to a previously published method (Jourdan et al. 2011). The radiation-induced wounds were traced on clear autoclaved plastic, and the images were scanned and used to calculate surface area. Wound size was compared to the original irradiation size. Wound area was determined as described previously (Jourdan et al. 2011).
Lung wet weight and right ventricular hypertrophy
Whole lungs were removed at 42 days after irradiation from 5 rats sacrificed after treatments described in groups (i)–(v). Lungs were trimmed of tissue such as adventitia, esophagus, thymus, then excess fluid gently wiped and weighed. The hearts were dissected and the ventricles and septa were weighed separately to calculate right ventricular hypertrophy (RVH) according to previously published method (Ghosh et al. 2009a). The left lungs were saved for histological evaluation of mast cells.
Mast cell count/histology
The left lung from rats in groups (i)–(v) was removed after the lungs were weighed at 42 days (see previous paragraph), fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburg, PA) and embedded in paraffin. Whole-mount sections were cut (4 μm), processed and stained with anti-tryptase antibody (Imgenex, San Diego, CA) for mast cell counts as described previously (Gao et al. 2013a). Number of cells stained positively with anti-tryptase were counted by two people blinded to the treatment groups (3 high power fields per sample). Results were expressed as number of mast cells/field.
Statistical methods
The morbidity/mortality of rats following treatments was evaluated by a Kaplan- Meier survival plot and expressed as percent morbidity. The significance was analyzed by the Peto-Peto Wilcoxon test. Results for mast cell count, lung wet weight and right ventricular hypertrophies were compared by one-way analysis of variance (ANOVA). All pair-wise multiple comparisons were done by the Holm-Sidak test to determine significance. Results for sircol collagen assay were compared by t-test. Graph values are expressed as mean ± SEM (standard error of the mean).
Based on prior experience and examination of residuals, breathing rate was analyzed on an inverse scale, which corresponds to analyzing inter-breath interval (Medhora et al. 2012). The main analysis tool used to compare the breathing intervals or body weights was mixed-effects modeling with a random effect accounting for within-animal correlation, and treatment group and measurement time as crossed fixed effects. For significance testing only the pre-planned pair-wise comparisons of the treatment groups to WTI only for each time-point and the comparison of each time-point to baseline within each treatment groups were considered. Analyses were performed using R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria) with the lme4 1.0–6 and multcomp 1.3–1 packages.
For wound healing, the family-wise type I error rate was controlled at 5% for each outcome using the single-step adjustment method based on the multivariate normal distribution. The dynamics of wound growth and healing were modeled using a bi-exponential model Area=A [exp(-rhT) – exp(-rgT)], where T is the time since irradiation in days, rg and rh are the rates of growth and healing, respectively, and A is a scaling constant. The model parameters were allowed to vary with treatment group; the within-animal repeated measures were incorporated including animal-specific random effects for the model parameters. The maximum wound area and the time of the largest wound size were expressed as functions of the parameters. The inference concentrated on comparing the ‘WTI+skin’ group to the other groups. The resulting nonlinear mixed effects model was fitted using the NLMIXED procedure in SAS 9.2 (SAS Institute, Cary NC).
RESULTS
Survival through pneumonitis after 12.5 Gy to the lungs combined with 30 Gy to the skin
We used 5 groups of rats (groups (i) – (v)) described for group sizes in Material and Methods. As shown in the Kaplan Meier plot in Figure 1, only one rat from the WTI group (after 12.5 Gy) met the IACUC criteria for euthanization, also described in Material and Methods. This rat was morbid at a time corresponding to pneumonitis (42–70 days after WTI) (Medhora et al. 2012, Szabo et al. 2010). All rats with combined irradiations survived to termination of the studies.
FIGURE 1.
Kaplan-Meier plot of morbidity after 12.5 Gy whole thorax irradiation (WTI) with and without 30 Gy irradiation to the skin. Captopril was given in drinking water from day 7 after irradiation, at a dose of 300 mg/L (~160 mg/m2/day) to rats receiving combined irradiation to the thorax and skin. Numbers in parentheses indicate the number of rats at risk at 56 days after irradiation. There is no difference between groups. Plots are shifted slightly to avoid overlap.
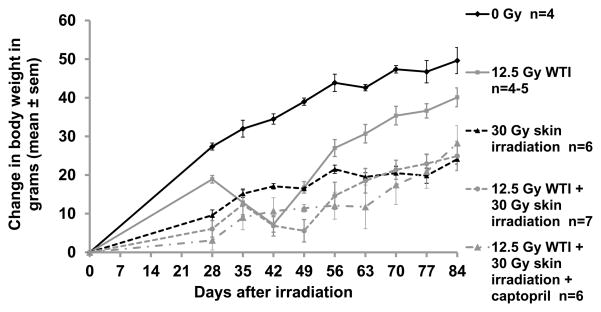
Changes in body weights in these rats are graphically represented in Figure 2. Body weights were different from baseline (day 28) after 12.5 Gy WTI at 35, 42 and 49 days (p<0.05 for each time point). Rats irradiated to the skin only did not lose body weight during the period corresponding to pneumonitis (42–70 days) but had lower body weights than unirradiated controls. (Figure 2). The nadir for weight loss was delayed in the combined irradiations group as compared to 12.5 Gy WTI alone.
FIGURE 2.
Changes in body weight versus time (X-axis) after 12.5 Gy WTI±30 Gy to the skin. For clarity in presentation, significant differences are denoted in this figure legend and the results section, though not in the graph. There was a significant decrease in body weight at 35, 42 and 49 days after 12.5 Gy as compared to the weights of the same rats at 28 days after irradiation (p<0.05 for each time point). The changes in body weights between the 12.5 Gy WTI only and combined irradiation group were not different until 56 days, after which they remained higher until the end of the experiment (p<0.05). The body weights after combined irradiation with captopril were less than those for the rats irradiated to the thorax only from 56 – 77 days (p<0.05). Rats irradiated to the skin only had lower body weights than unirradiated controls (p<0.05). Plots represent mean values+SEM.
Lung injury after combined WTI and skin irradiations
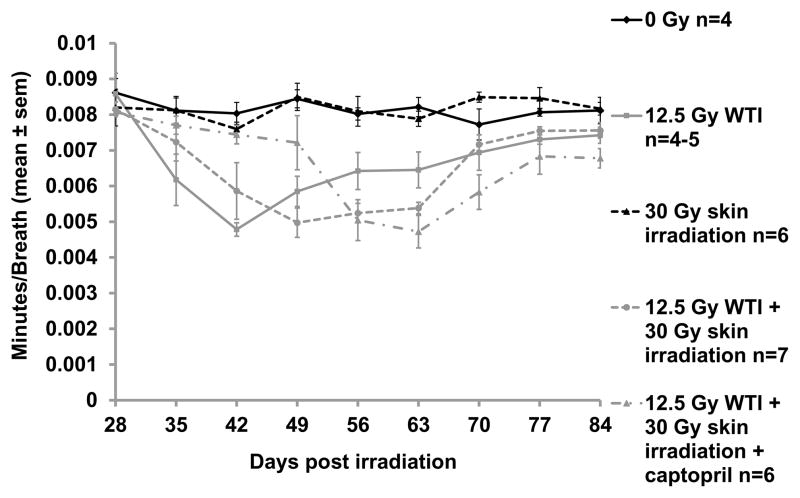
Injury to the lung was assayed by non-invasive measurements of breathing intervals (the inverse of breathing rates) in rats from groups (i) – (v) (see Materials and Methods), as well as by histological examination of the lungs in these groups at 42 days after irradiation. Results were analyzed by mixed-effects modeling. After 12.5 Gy WTI alone, the nadir of the breathing interval was at 42 days (Figure 3, Table I). The first decrease in the breathing interval in the WTI alone group was at 35 days after irradiation (p=0.0031, Table I). In the combined WTI + skin irradiation group, the breathing intervals decreased at 42 days with a nadir at 49 days. We did not observe a difference in the in breathing intervals between rats irradiated to the thorax alone from those in the combined groups at 42 or 49 days (Figure 3, Table I). The fall in breathing intervals were further delayed to 56 days in this combined injury group (12.5 Gy WTI+30 Gy skin) that was treated with captopril (Figure 3, Table I). The nadir for captopril-treated combined injury group was at 63 days (Figure 3). These results indicate a delay in pneumonitis in rats receiving combined injury and a further delay if they were given captopril, as compared to rats treated with WTI alone. Breathing intervals in all groups of rats returned close to baseline by or after 70 days (Table I).
FIGURE 3.
Decrease in breathing interval during pneumonitis after 12.5 Gy whole thorax irradiation (WTI). For statistical comparisons, see Table I; differences are not identified in this graph for purposes of clarity in presentation. Note the decrease in breathing interval during pneumonitis at days 35, 42, 49 and 63 after 12.5 Gy WTI alone (p<0.05 at each of these time points, nadir at 42 days). Unirradiated rats and those given 30 Gy to the skin alone did not show changes in breathing intervals. Rats in the combined groups (12 Gy WTI+30 Gy skin) with or without captopril also exhibited decrease in breathing intervals that recovered by 84 days, with a nadir at 49 days.
Table I.
Comparison of breathing intervals over time. The table shows weekly p-values between breathing intervals from comparisons at 35 – 84 days vs 28 days (baseline for each group). Breathing intervals were not different between groups at 28 days (Figure 3). However, the breathing intervals decreased in all groups receiving 12.5 Gy WTI with or without combined irradiation to the skin (30 Gy). Breathing intervals remained unchanged over 84 days in unirradiated rats or those receiving 30 Gy to the skin only (Figure 3). Decreases from baseline values were delayed in rats receiving combined irradiation to the lung and skin relative to those receiving radiation to the lung along. Addition of captopril to the treatment plan further delayed radiation-evoked decreases in the breathing intervals.
| Comparisons in time (days) vs baseline @ 28 days | P-value | ||
|---|---|---|---|
| 12.5 Gy lung | 12.5 Gy lung+skin | 12.5 Gy lung+skin+cap300 | |
| 35 vs 28 | 0.0031* | 0.9362 | 1 |
| 42 vs 28 | <0.001* | <0.001* | 1 |
| 49 vs 28 | <0.001* | <0.001* | 0.9898 |
| 56 vs 28 | 0.0153* | <0.001* | <0.001* |
| 63 vs 28 | 0.0178* | <0.001* | <0.001* |
| 70 vs 28 | 0.343 | 0.8712 | 0.0021* |
| 77 vs 28 | 0.827 | 0.9999 | 0.6203 |
| 84 vs 28 | 0.9291 | 0.9999 | 0.5302 |
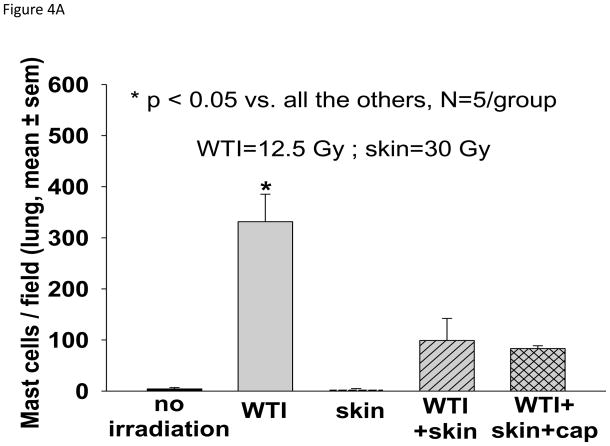
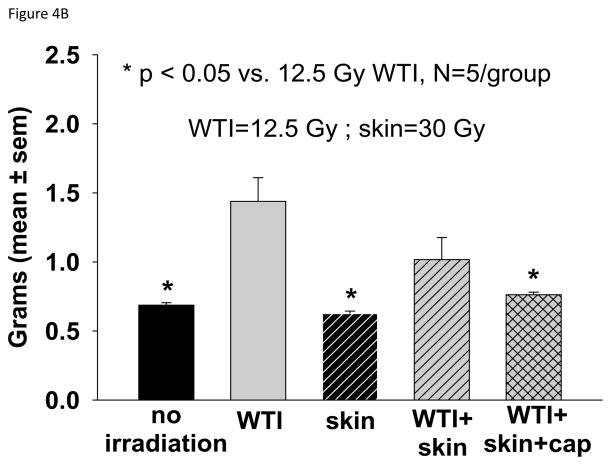
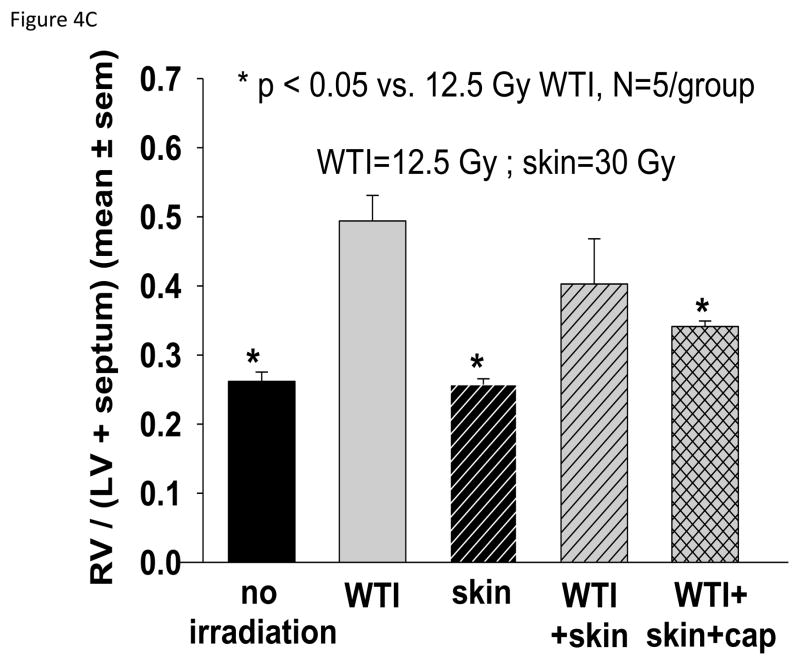
Histological analysis of lung sections at 42 days showed an impressive increase in tryptase-positive mast cells in the 12.5 Gy WTI only group as compared to all the other groups (Figure 4A). Lung wet weight (Figure 4B) and RVH (Figure 4C) 42 days after irradiation were increased in the 12.5 Gy group as compared to matched rats in the unirradiated, skin irradiation only and combined irradiations with captopril groups.
FIGURE 4.
Histology and structure of the lung and heart at 42 days after 12.5 Gy irradiation (for details see Materials and Methods). Lungs and hearts were harvested from 5 groups (treatments i–v, see Materials and Methods). The lungs were weighed, fixed, sectioned and stained with anti-tryptase antibody. The hearts were dissected and the right ventricle as well as the left ventricle and septum were weighed separately. 4A. Representations of the mean±SEM of the number of mast cells/field in 5 experimental groups as labeled. N= 5 rats/group. Note the large increase in mast cells from lung sections of rats irradiated to the thorax only. B. Mean±SEM of wet weight of the lungs in each group. The lung weight doubled at 42 days after 12.5 Gy WTI. C. Depicts the ratio of the right ventricle:left ventricle+septum. Note the right ventricular hypertrophy in rats that received WTI, was mitigated by captopril.
Skin injury after combined irradiations to the lung and skin
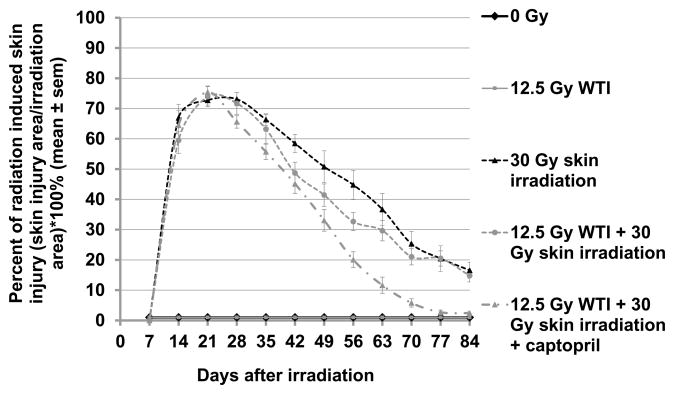
Skin injury was measured as the percent irradiated area that had a visible skin lesion (Figures 5 and 6). In the combined WTI (12.5 Gy) + skin (30 Gy) irradiation group, average maximum wound size was 1755 ± 57 mm2 achieved at 19.8 ± 0.6 days post irradiation, with growth rate rg=0.44 ± 0.04 and healing rate rh=0.28 ± 0.02 (see Materials and Methods). Both the skin only and captopril groups had the same maximal wound size and wound growth rate as the combined group (all p>0.15), however the peak size was 2.4 ± 0.8 days later in the skin only group (p=0.006), and 2.9 ± 0.7 days earlier in the captopril group (p=0.0001). The captopril treated animals had 1.23 ± 0.09 fold higher healing rate than the combined group (p=0.008), while the skin only group had a healing rate similar to the combined group (0.90 ± 0.09 fold lower, p=0.30).
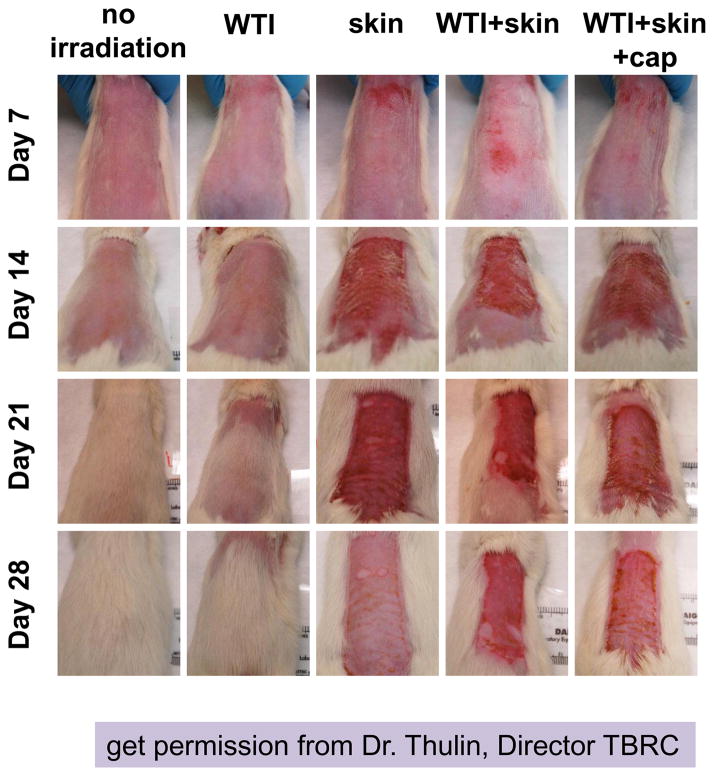
Figure 5.
Skin injury in 5 groups (i–v, see Materials and Methods). The skin was shaved and irradiated alone (30 Gy) or at 3 hours after whole thorax irradiation (WTI, 12.5 Gy). Representative images from each group taken weekly after irradiation are shown. Columns show rats from the same group while rows show progression of the wound with time. Maximal injury was observed around days 14–28 days. Rats that did not receive skin irradiation showed complete recovery at day 28, though the hair grew back slower in rats after 12.5 Gy WTI.
FIGURE 6.
Mitigation of injury to the skin after irradiation in 5 groups of rats (i–v, see Materials and Methods). Injury (Y-axis) was measured as % of total irradiated area that was injured (mean ± SEM). There was no injury in rats receiving no irradiation to the skin. Of rats given 30 Gy to the skin, the least injury was observed after combined thorax and skin irradiation followed by treatment with captopril at 7 days after irradiation and continued. Also see text for sample numbers and statistical analyses.
Survival is improved by irradiation to the skin
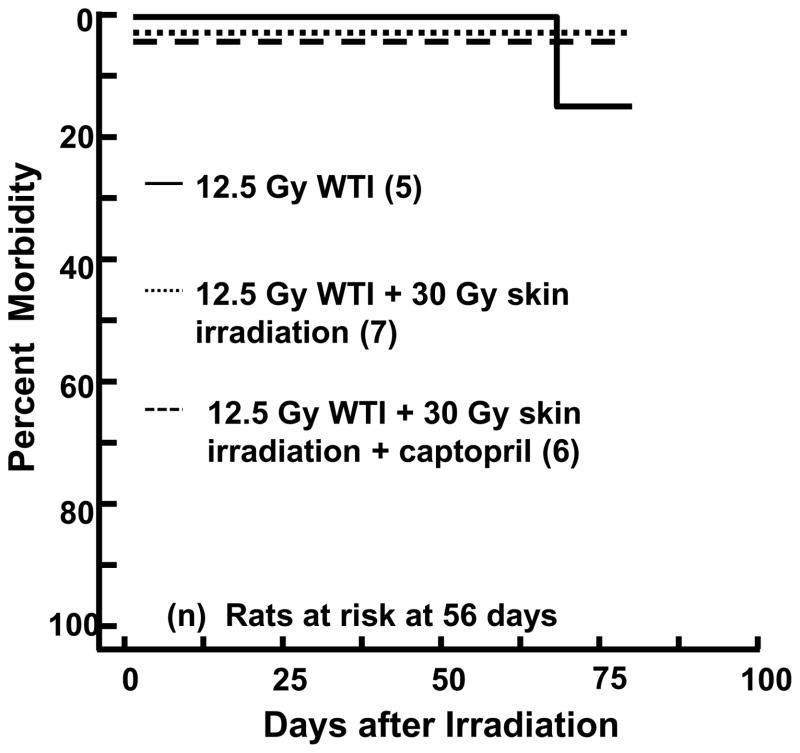
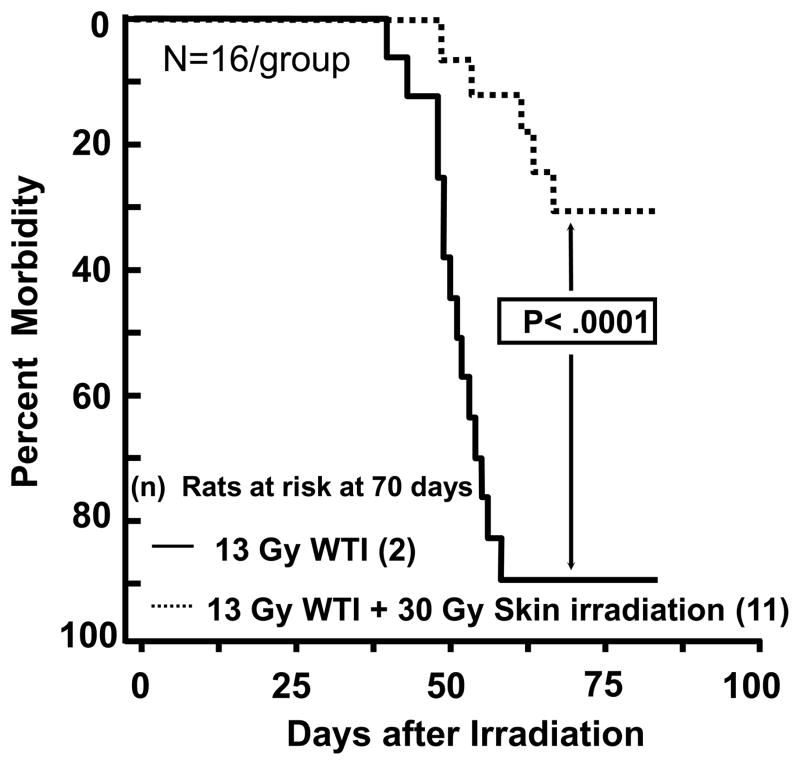
Morbidity, as defined by the IACUC criteria, was not enhanced as hypothesized in rats receiving irradiation with 12.5 Gy WTI+30 Gy skin (Figure 1). Combined injury did not worsen survival nor exacerbate injury to either organ, in fact it delayed the fall in breathing intervals (Figure 3, Table I) and improved wound closure in the skin at 42 days (Figure 6). In order to test if combined irradiation to both organs mitigated injury to the lung, we escalated the WTI dose to 13 Gy (Ghosh et al. 2009b, Kma et al. 2012, Szabo et al. 2010). Survival was <10% in this model (Figure 7). Survival through pneumonitis was considerably improved by irradiating the skin after WTI (p=0.0001, n=16 rats/group; Peto-Peto Wilcoxon test).
FIGURE 7.
Kaplan-Meier plot of morbidity after 13 Gy whole thorax irradiation (WTI) with and without 30 Gy irradiation to the skin. Numbers in the parentheses indicate the number of rats at risk at 70 days after irradiation. Radiation to the skin at 3 hours after WTI protects rats from morbidity due to radiation pneumonitis.
Irradiation to the skin mitigates radiation fibrosis
There is a ≥50% increase in synthesis of collagen in WAG/RijCmcr rat lungs after 210 days following 13 Gy WTI (Kma et al. 2012). Lung collagen is a marker of radiation fibrosis, and indicates onset of this injury. We examined if combined injury to the lung and skin attenuated newly synthesized collagen in rat lungs using a biochemical Sircol assay. There was a decrease in lung collagen after 210 days in rats given combined WTI (13 Gy) and skin irradiations (30 Gy) as compared to those receiving 13 Gy WTI only (from 26.7±3.8 μg/mg wet weight to 15.4±1.7 μg/mg wet weight, p<0.05, n=6 rats/group) (Figure 8). The results demonstrate mitigation of radiation fibrosis by irradiation of the skin.
DISCUSSION
We examined the combined effect of localized irradiation to the thorax and skin in rats. The whole thorax was irradiated first followed three hours later by whole thickness skin irradiation to 10% of total skin surface area. Damaging doses of irradiation to the whole volume of lung (12.5 Gy) and a partial volume of the skin (30 Gy to 10% surface area) did not exacerbate injury to either organ by non-invasive monitoring for up to 84 days after irradiation. Lung injury was estimated by measuring decreases in breathing intervals while skin injury was measured by the area of skin damaged. Body weights were altered by both types of radiation. Invasive evaluations of lung weight and histological infiltration by mast cells confirmed the results obtained by the breathing interval measurements. Analysis of the results from both organs showed that after combined irradiation to the lung and the skin, decreases in breathing intervals were delayed. Similar results (not shown) were obtained in a pilot study with the same 5 treatment groups, but using 12 Gy WTI instead of 12.5 Gy WTI. However, no rats were morbid after 12 Gy WTI while one animal was euthanized due to morbidity after 12.5 Gy WTI only.
We also assessed the ability of the drug captopril to mitigate these injuries. Rats receiving captopril after combined irradiation to the thorax and skin showed nadirs in breathing intervals later than those treated similarly but not given any drug, a pattern similar to that observed in rats treated with ACE inhibitors receiving WTI alone. Captopril-treated rats also had a faster healing rate for the skin lesion than the combined group without the drug. The most significant effect of combined injury was on our primary end point – survival (as per the IACUC euthanasia criteria). Surprisingly, survival was not decreased by combining sub-lethal doses of irradiation, 12 Gy to the thorax with 30 Gy to the skin 3 hours later. In fact when we increased the dose of irradiation to the lung to a lethal dose (13 Gy to the thorax, (Kma et al. 2012), survival increased by combining it with irradiation to the skin.
Total body irradiation (5 Gy) in C57BL/6 mice combined with 15% scald burn to the skin, increased mortality (Palmer et al. 2011) and exaggerated early pulmonary inflammation in the combined injury group (Palmer et al. 2013). The lung is reported to often be one of the first organs to fail after burn injury alone even in the absence of smoke inhalation (Dancey et al. 1999, Turnage et al. 2002). We did not test the potential of irradiation to the skin to injure the lung acutely, but we did not observe any decrease in breathing intervals, mast cells infiltration or lung weight during pneumonitis in the skin irradiation only group. Kiang et al 2010 (Kiang et al. 2010) also observed increase in mortality in mice treated with total body irradiation followed by skin wounding within 1 hour (Kiang et al. 2010). While the lung was not evaluated in their study, injury to the gastrointestinal tract was measured. Skin wounding exacerbated the acute radiation effects on gastrointestinal injury after 8.95–10.0 Gy TBI, and increased lethality after 10–20 days. Wound closure times were delayed in mice with combined injuries. It is not clear if the mice succumbed to gastrointestinal or hematological toxicity following total body irradiation in these studies. Messerschmidt et al (1989) reported increased susceptibility to shock in combined radiation injuries and delayed formation of callus in bone fractures. Additional injuries worsened the development and prognosis of radiation-induced disease in a number of such reports (Alpen and Sheline 1954, Brooks et al. 1952, Brooks et al. 1956, Langendorff et al. 1964, Messerschmidt 1989, Stromberg et al. 1968). Often the mortality was partially reversed by antimicrobials indicating infection played a role in the outcomes probably due to hematopoietic depression caused by irradiation (Stromberg et al. 1968).
Besides the data described in the current paper, there are other reports of reduced mortality by combining radiation with skin injuries, usually wounding (Langendorff et al. 1964, Ledney et al. 1981, Ledney et al. 1985a, Ledney et al. 1985b, Stromberg et al. 1968). In most studies the timing of the wound with respect to radiation played an important role in the outcome (Ledney et al. 1981, Ledney et al. 1985a, Stromberg et al. 1968). Skin wounding of mice shortly before TBI (up to 3 days depending on the dose of radiation) or after TBI (up to 2 days depending on the dose of radiation), enhanced survival (Ledney et al. 1985a). A dose reduction factor of 1.2 was achieved by skin wounding 24 hours before total body irradiation of mice (Ledney et al. 1981). The mechanism was suggested to be due to enhanced hematopoietic recovery by combined injury as measured by endogenous spleen cell colony formation. Stromberg et al 1967 surmized that thermal burns and rotating drum injuries increased mortality after total body irradiation, while wounds or other specific stresses prior to total body irradiation tended to decrease mortality (Stromberg et al. 1968).
Another insult that attenuated acute radiation injury in rodents is bacterial endotoxin or lipopolysaccharide (LPS). It has been known for decades that there is increased survival of rodents 28 days after total body irradiation and other insults if the animals were treated with LPS (Smith et al. 1955, Smith et al. 1957). Interestingly, LPS is most beneficial when delivered 24 hours before a lethal dose of irradiation in mice, protecting up to 76% of animals. Injection immediately after irradiation was less protective (Smith et al. 1957). Less impressive effects (though still beneficial) were observed in rats given LPS before and immediately after irradiation (Smith et al. 1957). Hamsters on the other hand demonstrated an excellent response to LPS when injected after rather than before irradiation (Smith et al. 1957). From these results the authors concluded that the effects of LPS were not dependent on interactions with primary radiation reaction products or that irradiation destroyed the ability of the rodent to respond to LPS. In a follow up paper (Smith et al. 1958) the authors reported that recovery of granulocytes, platelets and hemoglobin were improved in mice injected with LPS 24 hours before lethal total body irradiation. LPS given to mice one hour after whole thorax irradiation revealed mitigating effects on lung injury (Zaidi et al. 2012). It is not clear if endogenous endotoxin associated with infection from wounding or irradiated skin could mitigate the radiation toxicities we and other observed, though no infection was evident on close inspection and monitoring of our rats by a dermatologist (ZL) on a weekly basis through the study.
We examined complete and differential blood cell counts as well as levels of circulating inflammatory cytokines in the blood of all groups of rats in the 12 or 12.5 Gy studies at 1 and 6 weeks after irradiation (results not shown). These values were not different between groups to account for the survival advantage or mitigation of radiation injuries. We cannot rule out that other time points or factors or mechanisms would be informative to explain the beneficial effects of combined irradiation in our model.
In summary, our studies are the first to report mitigation of radiation injuries to the lungs, including survival during the pneumonitis phase and reduced fibrosis, by combined but localized irradiation to the skin. We also demonstrate additional mitigation of the skin injury in the same model by the ACE inhibitor captopril (Figure 6). ACE inhibitors can be added to the list of mitigators of combined injuries, an ongoing area of research focus that has been reviewed in detail (Zou et al. 2008). It will be interesting to use our combined WTI and skin irradiation model to identify the mechanism for mitigation of lung injury. Discovery of a natural, circulating factor or stimulatory signal that causes the immune system to mitigate radiation damage to the lung will not only be useful in radiological-accident or terrorism scenarios, but will also benefit radiotherapy given to patients with lung and breast cancers. Isolating such a factor may overcome the need to provide a second insult at a specific time with respect to irradiation. This is particularly important since we do not have data to inform us if humans will benefit from a second injury before or after irradiation.
Acknowledgments
We thank Marylou Mäder for irradiating the rats and for the excellent care of these animals. This work was funded by NIH/NIAID agreements U19-AI091036 (Rochester) & 67734 (MCW) and RC1 AI81294 & 01S1 (BARDA).
Footnotes
Conflict of interest: None
Declarations of Interest:
This work honors Dr. Michael Robbins who was an external advisor for the Rochester U-19 award which reviewed and funded the pilot project that initiated our study. Dr. Robbins also contributed to reviewing the progress of this work.
References
- 1.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 2.Gao F, Fish BL, Szabo A, Doctrow SR, Kma L, Molthen RC, Moulder JE, Jacobs ER, Medhora M. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat Res. 2012;178:468–480. doi: 10.1667/RR2953.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao F, Narayanan J, Joneikis C, Fish BL, Szabo A, Moulder JE, Molthen RC, Jacobs ER, Rao RN, Medhora M. Enalapril mitigates focal alveolar lesions, a histological marker of late pulmonary injury by radiation to the lung. Radiat Res. 2013a;179:465–474. doi: 10.1667/RR3127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res. 2012;53:10–17. doi: 10.1269/jrr.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo S, Ghosh SN, Fish BL, Bodiga S, Tomic R, Kumar G, Morrow NV, Moulder JE, Jacobs ER, Medhora M. Cellular inflammatory infiltrate in pneumonitis induced by a single moderate dose of thoracic x radiation in rats. Radiat Res. 2010;173:545–556. doi: 10.1667/RR1753.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: Injury and recovery. Int J Radiat Biol. 2008;84:487–497. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medhora M, Gao F, Fish BL, Jacobs ER, Moulder JE, Szabo A. Dose-modifying factor for captopril for mitigation of radiation injury to normal lung. J Radiat Res. 2012;53:633–640. doi: 10.1093/jrr/rrs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins ME, Hopewell JW. Physiological factors effecting renal radiation tolerance: A guide to the treatment of late effects. Br J Cancer Suppl. 1986;7:265–267. [PMC free article] [PubMed] [Google Scholar]
- 9.DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, Ramakrishnan N. Medical countermeasures for radiation combined injury: Radiation with burn, blast, trauma and/or sepsis. report of an NIAID workshop, march 26–27, 2007. Radiat Res. 2008;169:712–721. doi: 10.1667/RR1295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiang JG, Ledney GD. Skin inqjuries reduce survival and modulate corticosterone, C-reactive protein, complement component 3, IgM, and prostaglandin E 2 after whole-body reactor-produced mixed field (n + gamma-photons) irradiation. Oxid Med Cell Longev. 2013;2013:821541. doi: 10.1155/2013/821541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC, Ledney GD. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 2010;173:319–332. doi: 10.1667/RR1892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ran XZ, Su YP, Zong ZW, Guo CH, Zheng HE, Chen XH, Ai GP, Cheng TM. Effects of serum from rats with combined radiation-burn injury on the growth of hematopoietic progenitor cells. J Trauma. 2007;62:193–198. doi: 10.1097/01.ta.0000215434.24726.72. [DOI] [PubMed] [Google Scholar]
- 13.Ran X, Cheng T, Shi C, Xu H, Qu J, Yan G, Su Y, Wang W, Xu R. The effects of total-body irradiation on the survival and skin wound healing of rats with combined radiation-wound injury. J Trauma. 2004;57:1087–1093. doi: 10.1097/01.ta.0000141885.72033.c7. [DOI] [PubMed] [Google Scholar]
- 14.Ledney GD, Stewart DA, Exum ED, Sheehy PA. Skin wound-enhanced survival and myelocytopoiesis in mice after whole-body irradiation. Acta Radiol Oncol. 1981;20:29–38. doi: 10.3109/02841868109130187. [DOI] [PubMed] [Google Scholar]
- 15.Ledney GD, Exum ED, Jackson WE., 3rd Wound-induced alterations in survival of 60Co irradiated mice: Importance of wound timing. Experientia. 1985a;41:614–616. doi: 10.1007/BF02007684. [DOI] [PubMed] [Google Scholar]
- 16.Ledney GD, Stewart DA, Gruber DF, Gelston HM, Jr, Exum ED, Sheehy PA. Hematopoietic colony-forming cells from mice after wound trauma. J Surg Res. 1985b;38:55–65. doi: 10.1016/0022-4804(85)90010-1. [DOI] [PubMed] [Google Scholar]
- 17.Jourdan MM. Laminin 332 deposition is diminished in irradiated skin in an animal model of combined radiation and wound skin injury. Radiat Res. 2011;176:636–648. doi: 10.1667/rr2422.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Enalapril mitigates radiation-induced pneumonitis and pulmonary fibrosis if started 35 days after whole-thorax irradiation. Radiat Res. 2013b;180:546–552. doi: 10.1667/RR13350.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh SN, Wu Q, Mader M, Fish BL, Moulder JE, Jacobs ER, Medhora M, Molthen RC. Vascular injury after whole thoracic x-ray irradiation in the rat. Int J Radiat Oncol Biol Phys. 2009a;74:192–199. doi: 10.1016/j.ijrobp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009b;75:1528–1536. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer JL, Deburghgraeve CR, Bird MD, Hauer-Jensen M, Kovacs EJ. Development of a combined radiation and burn injury model. J Burn Care Res. 2011;32:317–323. doi: 10.1097/BCR.0b013e31820aafa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer JL, Deburghgraeve CR, Bird MD, Hauer-Jensen M, Chen MM, Yong S, Kovacs EJ. Combined radiation and burn injury results in exaggerated early pulmonary inflammation. Radiat Res. 2013;180:276–283. doi: 10.1667/RR3104.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dancey DR, Hayes J, Gomez M, Schouten D, Fish J, Peters W, Slutsky AS, Stewart TE. ARDS in patients with thermal injury. Intensive Care Med. 1999;25:1231–1236. doi: 10.1007/pl00003763. [DOI] [PubMed] [Google Scholar]
- 24.Turnage RH, Nwariaku F, Murphy J, Schulman C, Wright K, Yin H. Mechanisms of pulmonary microvascular dysfunction during severe burn injury. World J Surg. 2002;26:848–853. doi: 10.1007/s00268-002-4063-3. [DOI] [PubMed] [Google Scholar]
- 25.Messerschmidt O. Combined effects of radiation and trauma. Adv Space Res. 1989;9:197–201. doi: 10.1016/0273-1177(89)90438-9. [DOI] [PubMed] [Google Scholar]
- 26.Stromberg LW, Woodward KT, Mahin DT, Donati RM. Combined surgical and radiation injury. the effect of timing of wounding and whole body gamma irradiation on 30 day mortality and rate of wound contracture in the rodent. Ann Surg. 1968;167:18–22. doi: 10.1097/00000658-196801000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langendorff H, Messerschmidt O, Melching HJ. Studies on combined injuries. I. significance of the time elapsed between total body irradiation and skin lesion in the survival of mice. Strahlentherapie. 1964;125:332–340. [PubMed] [Google Scholar]
- 28.Alpen EL, Sheline GE. The combined effects of thermal burns and whole body X irradiation on survival time and mortality. Ann Surg. 1954;140:113–118. doi: 10.1097/00000658-195407000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks JW, Evans EI, Ham WT, Jr, Reid JD. The influence of external body radiation on mortality from thermal burns. Ann Surg. 1952;136:533–545. doi: 10.1097/00000658-195209000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks JW, Ham WT, Jr, Haynes BW, Jr, Schmidt F, Williams R. A comparison of local and systemic effects following contact and flash burns. Ann Surg. 1956;144:768–777. doi: 10.1097/00000658-195610000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith WW, Alderman IM, Gillespie RE. Increased survival in irradiated animals treated with bacterial endotoxins. Am J Physiol. 1957;191:124–130. doi: 10.1152/ajplegacy.1957.191.1.124. [DOI] [PubMed] [Google Scholar]
- 32.Smith WW, Smith F, Alderman IM. Effect of parenteral injection of particulate matter on resistance of x-irradiated mice to infection. Am J Physiol. 1955;182:400–402. doi: 10.1152/ajplegacy.1955.182.2.400. [DOI] [PubMed] [Google Scholar]
- 33.Smith WW, Alderman IM, Gillespie RE. Hematopoietic recovery induced by bacterial endotoxin in irradiated mice. Am J Physiol. 1958;192:549–556. doi: 10.1152/ajplegacy.1958.192.3.549. [DOI] [PubMed] [Google Scholar]
- 34.Zaidi A, Jelveh S, Mahmood J, Hill RP. Effects of lipopolysaccharide on the response of C57BL/6J mice to whole thorax irradiation. Radiother Oncol. 2012;105:341–349. doi: 10.1016/j.radonc.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou Z, Sun H, Su Y, Cheng T, Luo C. Progress in research on radiation combined injury in china. Radiat Res. 2008;169:722–729. doi: 10.1667/RR1284.1. [DOI] [PubMed] [Google Scholar]