Abstract
Emerging evidence indicates that type I metabotropic glutamate receptors (mGluRs) in the nucleus accumbens play a critical role in cocaine seeking. The present study sought to determine the role of accumbens core mGluR1, mGluR5 and protein kinase C (PKC) in cocaine priming-induced reinstatement of drug seeking. Here, we show that intra-accumbens core administration of the mGluR1/5 agonist DHPG (250 μM) promoted cocaine seeking in rats. Consistent with these results, administration of an mGluR1 (50.0 μM YM 298198) or mGluR5 (9.0 μM MPEP) antagonist directly into the accumbens core prior to a priming injection of cocaine (10 mg/kg) attenuated the reinstatement of drug seeking. mGluR1/5 stimulation activates a signaling cascade including PKC. Intracore microinjection of PKC inhibitors (10 μM Ro 31–8220 or 30.0 μM chelerythrine) also blunted cocaine seeking. In addition, cocaine priming-induced reinstatement of drug seeking was associated with increased phosphorylation of PKCγ, but not PKCα or PKCβII, in the core. There were no effects of pharmacological inhibition of mGluR1, mGluR5 or PKC in the accumbens core on sucrose seeking. Together, these findings indicate that mGluR1 and mGluR5 activation in the accumbens core promotes cocaine seeking and that these effects are reinforcer specific. Furthermore, stimulation of mGluR1 and mGluR5 in the accumbens core may regulate cocaine seeking, in part, through activation of PKCγ.
Keywords: Addiction, glutamate, psychostimulant, relapse, self-administration, striatum
INTRODUCTION
A growing body of evidence indicates that group I metabotropic glutamate receptors (mGluRs), which include mGluR1 and mGluR5 receptors, play a critical role in the reinstatement of cocaine-seeking behavior, an animal model of relapse. For example, systemic administration of an mGluR1 antagonist (Achat-Mendes, Platt & Spealman 2012) or an mGluR5 antagonist (Lee et al. 2005; Kumaresan et al. 2009) attenuates the ability of a priming injection of cocaine and/or cocaine-associated cues to reinstate cocaine seeking. Group I mGluRs are expressed predominantly on postsynaptic membranes (Rouse et al. 2000) throughout the brain including the nucleus accumbens (Shigemoto et al. 1993; Testa et al. 1995). The nucleus accumbens is a heterogeneous structure that consists of two major subregions, the core and the shell, each of which modulates aspects of cocaine-seeking behavior (Schmidt et al. 2005; Schmidt & Pierce 2010). Recent studies have begun to identify the exact roles of mGluR1 and mGluR5 in the accumbens core and shell in cocaine priming-induced reinstatement of drug seeking. Administration of an mGluR5 antagonist into the accumbens shell attenuated cocaine priming-induced reinstatement of drug seeking (Kumaresan et al. 2009; Schmidt et al. 2013). Interestingly, intrashell administration of an mGluR1 antagonist had no effect on cocaine priming-induced reinstatement of drug seeking (Schmidt et al. 2013). In contrast to studies of mGluR5 in the accumbens shell, studies examining the role of accumbens core mGluR5s in cocaine priming-induced reinstatement of drug seeking have yielded mixed results (Backstrom & Hyytia 2007; Wang et al. 2013). Moreover, no studies, to date, have investigated the role of accumbens core mGluR1s in cocaine priming-induced reinstatement of drug seeking.
Stimulation of group I mGluRs results in activation of protein kinase C (PKC) (Conn & Pin 1997). Previous studies demonstrated a role for PKC in psychostimulant-mediated behaviors. For example, systemic administration of a PKC inhibitor attenuated cocaine-induced conditioned place preference (Cervo et al. 1997). Administration of a PKC inhibitor directly into the nucleus accumbens blocked the expression of cocaine-induced behavioral sensitization (Pierce et al. 1998). Consistent with these findings, repeated experimenter-delivered cocaine infusions increased the phosphorylation of some, but not all, isoforms of PKC in the nucleus accumbens (Steketee, Rowe & Chandler 1998). Recently, our group showed that cocaine priming-induced reinstatement of drug seeking was associated with increased activation of PKCγ, but not PKCα or PKCβII, in the accumbens shell (Schmidt et al. 2013). Collectively, these results strongly suggest that stimulation of mGluR5 in the accumbens shell promotes cocaine seeking through activation of PKCγ. However, the role of accumbens core PKC in cocaine seeking remains unknown.
Here, we initially determined the ability of the mGluR1/5 agonist DHPG microinjected into the accumbens core to promote cocaine seeking. Next, we assessed the effect of intracore administration of an mGluR1 (YM 298198) or mGluR5 (MPEP) antagonist as well as PKC inhibitors (Ro 31–8220 or chelerythrine) on cocaine priming-induced reinstatement of drug seeking. Moreover, we also examined the expression of native and phosphorylated PKC isoforms in the accumbens core during cocaine seeking. Our results indicate that cocaine seeking is mediated by activation of mGluR1, mGluR5 and PKCγ in the accumbens core.
MATERIALS AND METHODS
Animals and housing
Male Sprague Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY). Animals were individually housed with food and water available ad libitum in their home cage. A 12/12 hours light/dark cycle was used with the lights on at 7:00 a.m. All experimental procedures were performed during the light cycle. The experimental protocols were all consistent with the guidelines issued by the National Institutes of Health and were approved by the Perelman School of Medicine’s Institutional Animal Care and Use Committee.
Surgery
Prior to surgery, rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (Sigma-Aldrich, St. Louis, MO, USA). An indwelling catheter (CamCaths, Cambridge, UK) was inserted into the right jugular vein and sutured in place. The catheter was routed to a mesh backmount platform that was implanted subcutaneously dorsal to the shoulder blades. Catheters were flushed daily with 0.3 ml of antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline and sealed with plastic obturators when not in use.
After catheter insertion, some rats were immediately mounted in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA). Guide cannulas (14 mm, 24 gauge) for microinjections were implanted bilaterally 2 mm dorsal to the accumbens core. Guide cannulas were cemented in place by affixing dental acrylic to stainless steel screws secured in the skull. The coordinates for the ventral ends of the guide cannulas, relative to bregma according to the atlas of Paxinos & Watson (1997), were as follows: +1.0 mm A/P, ±2.5 mm M/L and −5.0 mm D/V. An obturator (14 mm, 33 gauge) was inserted into each guide cannula in order to prevent occlusion.
Cocaine self-administration, extinction and reinstatement of cocaine seeking
After surgery, rats were allowed 7 days to recover before behavioral testing commenced. Initially, rats were placed in operant chambers and allowed to lever press for intravenous infusions of cocaine (0.25 mg cocaine/59 μl saline, infused over a 5-second period) on a fixed-ratio 1 (FR1) schedule of reinforcement. Once an animal achieved at least 20 infusions of cocaine in a single daily operant session under the FR1 schedule, the subject was switched to a fixed-ratio 5 (FR5) schedule of reinforcement. For both FR1 and FR5 schedules, the maximum number of injections was limited to 30 per daily self-administration session. A 20-second time-out period followed each cocaine infusion, during which time active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during the operant sessions. Light/tone cues were not incorporated into the present studies.
Following 21 daily cocaine self-administration sessions, drug-taking behavior was extinguished by replacing the cocaine with 0.9% saline such that every five active lever responses resulted in a saline infusion. Daily extinction sessions continued until responding on the active lever was < 15% of the response rate maintained by cocaine self-administration under the FR5 schedule of reinforcement. Typically, it took ~7 days for rats to meet this criterion. The total active lever responses (mean ± SEM) on the last day of extinction for all animals used in the cocaine reinstatement experiments was 12.45 ± 0.80.
Once cocaine self-administration was extinguished, animals entered the reinstatement phase of the experiment. During reinstatement test sessions, satisfaction of the response requirement (i.e. every five active lever responses) resulted in an infusion of saline. Using a between-sessions reinstatement paradigm, each reinstatement test session was followed by extinction sessions until responding was again < 15% of the response rate maintained by cocaine self-administration. Generally, 1–2 days of extinction were necessary to reach extinction criterion between reinstatement test sessions.
Microinjection procedures
The obturators were removed from the guide cannulas and 33 gauge, 16 mm stainless steel microinjectors were inserted. Bilateral infusions were performed simultaneously over 2 minutes in a total volume of 0.5 μl per hemisphere. Following infusion, microinjectors were left in place for an additional 1 minute in order to allow for diffusion of the drug solution away from the tips of the microinjectors. The goal of the experimental design was to have each animal serve as its own control and receive up to four microinjections. However, we were forced to deviate from this experimental design when technical difficulties (i.e. blocked guide cannulas) made it impossible to test all doses of a compound plus vehicle in an entire cohort of subjects. In every case, however, an animal received a minimum treatment of one drug dose and its vehicle. To control for potential rank order effects of drug and vehicle administrations, all treatments were counterbalanced across reinstatement test sessions. However, the loss of some animals from an experiment because of technical difficulties may have comprised the aspects of the counterbalanced design. Therefore, all subjects that failed to receive all of the scheduled microinjections underwent a final reinstatement test session in the absence of any intracranial drug infusion to confirm that the reinstatement response to an acute priming injection of cocaine (10 mg/kg, i.p.) remained robust.
The ability of the mGluR1/5 agonist 3,5-DHPG to reinstate cocaine seeking was assessed. DHPG (25 and 250 μM) was microinjected bilaterally into the core immediately prior to the reinstatement test session. The effect of intracore pretreatment with the mGluR1 antagonist YM 298198, the mGluR5 antagonist MPEP and the PKC inhibitors Ro 31–8220 and chelerythrine on cocaine priming-induced reinstatement of drug seeking was assessed in separate cohorts of rats. YM 298198 (5.0 and 50.0 μM), MPEP (0.9 and 9.0 μM), Ro 31–8220 (1.0 and 10.0 μM), chelerythrine (3.0 and 30.0 μM) and respective vehicles were microinjected into the core 10 minutes prior to a priming injection of cocaine (10 mg/kg, i.p.).
Reinstatement of sucrose seeking
Potential non-specific rate-suppressing effects of intra-core YM 298198, MPEP, Ro 31–8220 and chelerythrine were evaluated by assessing the influence of these compounds on the reinstatement of sucrose-seeking behavior. Separate cohorts of rats were trained initially to self-administer 45 mg sucrose pellets (Research Diets, New Brunswick, NJ, USA) on a FR1 schedule of reinforcement. Once animals achieved stable responding for sucrose (defined as < 20% variation in responding over three consecutive days) on the FR1 schedule of reinforcement, the response requirement was increased to an FR5 schedule of reinforcement. Animals were limited to 30 sucrose pellets within each operant session and were food restricted to ~20 g of laboratory chow (Harlan Teklad, Wilmington, DE, USA) in their home cages for the duration of the experiment. This mild food restriction resulted in a reduction of ~10% of the animals’ free-feeding body weight. Water was continuously available in the home cage.
After 14 days of sucrose-maintained responding on a FR5 schedule of reinforcement, rats underwent an extinction phase where lever pressing no longer resulted in sucrose delivery. Once lever responding decreased to < 15% of the maximum number of responses completed during sucrose self-administration, animals proceeded to reinstatement testing. Doses of YM 298198 (50.0 μM), MPEP (9.0 μM), Ro 31–8220 (10.0 μM) and chelerythrine (30.0 μM) that attenuated cocaine priming-induced reinstatement of drug seeking were microinjected into the core 10 minutes prior to the beginning of the reinstatement session. Using a within-subjects design, each animal served as its own control. The experimenter remotely administered one sucrose pellet every 2 minutes for the first 10 minutes of the reinstatement session. A between-session paradigm was used so that each daily reinstatement test session was followed by an extinction session the following day until responding was again < 15% of the response rate maintained by sucrose. Rats were tested for sucrose seeking in the absence of an intracranial drug infusion at the end of the experiment to ensure that reinstatement of sucrose seeking had not extinguished.
Verification of cannula placements
After completion of all microinjection experiments, rats were given an overdose of pentobarbital (100 mg/kg) and perfused with saline followed by 10% formalin. Brains were removed and coronal sections (100 μm) were taken at the level of the nucleus accumbens with a Vibratome (Buffalo Grove, IL). The sections were mounted on gelatin-coated slides and stained with cresyl violet. An individual blind to behavioral responses determined cannula placements as well as excessive cannula-induced damage (defined as a cannula tract in excess of 500 μm) or drug-induced neurotoxicity (defined as cell death extending beyond 100 μm from the cannula tract) using light microscopy. Animals with cannula placements outside of the core, excessive mechanical damage or neurotoxicity were excluded from subsequent data analysis.
Drugs
Cocaine was obtained from the National Institute on Drug Abuse (Rockville, MD, USA) and dissolved in bacteriostatic 0.9% saline. YM 298198 hydrochloride (6-Amino-N-cyclohexyl-N, 3-dimethylth-iazolo[3,2-a]benzimidazole-2-carboxamide hydrochloride), MPEP hydrochloride (2-Methyl-6-(phenylethynyl)pyridine), Ro 31–8220 mesylate (3-[3-[2, 5-Dihydro-4-(1-methyl-1H-indol-3-yl)-2, 5-dioxo-1H-pyrrol-3-yl]-1H-indol-1-yl]propyl carbamimidothioic acid ester mesylate), chelerythrine chloride (1,2-Dimethoxy-12-methyl[1,3]benzodioxolo [5,6]phenanthridinium chloride) and 3,5-DHPG (3,5-dihydroxyphenylglycine) were purchased from Tocris (Minneapolis, MN, USA). YM 298198, chelerythrine and 3,5-DHPG were dissolved in sterile 0.9% saline. MPEP and Ro 31–8220 were dissolved in 100% DMSO to make stock solutions and then diluted in sterile 0.9% saline to required final working concentrations, resulting in final vehicle concentrations of 1% DMSO. The dose ranges for each of the aforementioned pharmacological compounds were based on the following rat intracranial microinjection experiments: YM 298198 (Titley, Heskin-Sweezie & Broussard 2010; Timmer & Steketee 2012), MPEP (Backstrom & Hyytia 2007; Kumaresan et al. 2009), Ro 31–8220 mesylate (Loweth et al. 2009), chelerythrine (Narita et al. 2004; Li et al. 2011), 3,5-DHPG (Swanson et al. 2001; Schwendt, Sigmon & McGinty 2012). While unlikely, it is possible that a solution of 1% DMSO may enhance the diffusion of drug in brain tissue in our study.
Cocaine self-administration and yoked saline controls for Western blotting experiments
Rats underwent catheterization as described above and were allowed to recover for 7 days before cocaine self-administration commenced. Rats were randomly assigned to one of four groups (self-administration/challenge injection): cocaine/cocaine, cocaine/saline, yoked saline/cocaine or yoked saline/saline. Within each individual experiment, rats were randomly assigned to experimental and control groups. Each rat trained to respond for contingent cocaine infusions was paired with a yoked subject that received infusions of saline. Lever pressing for the saline-yoked rats had no scheduled consequences, but these animals received the same number and temporal pattern of infusions as self-administered by the paired cocaine-experimental rat.
Initially, cocaine-experimental rats were placed in the operant chambers and allowed to lever press for intravenous cocaine infusions (0.25 mg cocaine/59 μl saline) on a FR1 schedule of reinforcement. Once a cocaine-experimental rat achieved stable responding on the FR1 schedule, they were switched to a FR5 schedule of reinforcement. For responding on both FR1 and FR5 schedules, the maximum number of cocaine infusions was limited to 30 per daily self-administration session and a 20-second time-out period followed each cocaine infusion. Daily 2-hour operant sessions (5–6 days/week) were conducted for a total of 21 days. Cocaine self-administration was then extinguished. After rats met their extinction criteria, one-half of the cocaine self-administration rats and one-half of the yoked saline controls received an acute injection of cocaine (10 mg/kg, i.p.), whereas the remaining animals received an injection of saline. All rats were then placed in the operant chambers under extinction conditions. Thirty minutes after the cocaine or saline injection, rats were removed from the operant chambers and immediately decapitated. Brains were then removed and the accumbens core was dissected on ice. Brain tissue samples were stored at −80°C until further analysis.
Western blotting
Accumbens core tissue was homogenized with a Polytron (Brinkman Instruments, Westbury, NY) in 10 volumes of homogenization buffer (pH 7.4) consisting of 20 mM Tris, 10 mM EGTA, 2 mM EDTA, 0.25 M sucrose, 1 mM phenylmethylsulfonyl fluoride and a 1:100 dilution of a protease inhibitor cocktail (Sigma-Aldrich) and a serine/threonine phosphatase inhibitor cocktail (Sigma-Aldrich). Following homogenization, samples were centrifuged at 10 000 g at 4°C for 10 minutes. Protein content was determined with a Bio-Rad protein assay kit (Hercules, CA). For Western analysis, 20 μg of protein was separated on 10% Bis-Tris gels (Invitrogen, Carlsbad, CA, USA) using SDS-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes, which were then preblocked with phosphate-buffered saline containing 0.1%Tween 20 and 5% bovine serum albumin for 1 hour before overnight incubation with primary antibodies. Membranes were incubated overnight at 4°C with the following primary antibodies that have been used previously to examine expression of native and phosphorylated PKC isoforms in whole cell tissue extracts from rat brain: anti-PKCα (Abcam Cambridge, MA, 1:1000) (Zhang et al. 2011), anti-PKCβII (Santa Cruz Biotechnology, Santa Cruz, CA, 1:500 dilution) (Olive et al. 2005), anti-PKCγ (Abcam, 1:1000) (Zhang et al. 2011), anti-phospho-PKCα/PKCβII Thr638/641 (Cell Signaling Technology, Beverly, MA, 1:1000 dilution) (Olive et al. 2005) and anti-phospho-PKCγ Thr674 (Abcam, 1:500) (Wilkie et al. 2007). Membranes were concurrently incubated with mouse monoclonal anti-GAPDH (Cell Signaling Technology, 1:3000) as a loading control. Primary antibody incubation was followed by three washes in Tris-buffered saline containing 0.2% Tween 20. Membranes were then incubated for 1 hour at RT with secondary antibodies (IRDye 800 goat anti-mouse and IRDye 680 goat anti-rabbit, 1:5000) in Odyssey blocking buffer + 0.05% Tween 20 (LI-COR Biosciences, Lincoln, NE, USA). Antibody/protein complexes were visualized using the Odyssey IR imaging system (LI-COR Biosciences). Band intensities were quantified using the Odyssey software. For data analysis, native and phosphorylated PKC bands were normalized to GAPDH and divided by the mean of the control group. The ratio of phosphorylated to native protein was then calculated.
Statistics
For the cocaine reinstatement experiments utilizing YM 298198, MPEP, Ro 31–8220, chelerythrine chloride or DHPG in the accumbens core, the total mean lever responses were analyzed with two-way mixed factors analyses of variance (ANOVAs). Total mean active and inactive lever responses for all sucrose reinstatement tests sessions were analyzed with unpaired t-tests. Two-way mixed factors ANOVAs were used to analyze PKC isoform expression data. Pairwise comparisons were made with Bonferroni post hoc test following two-way ANOVAs (P < 0.05).
RESULTS
Intra-accumbens core administration of the mGluR1/5 agonist DHPG promotes cocaine seeking
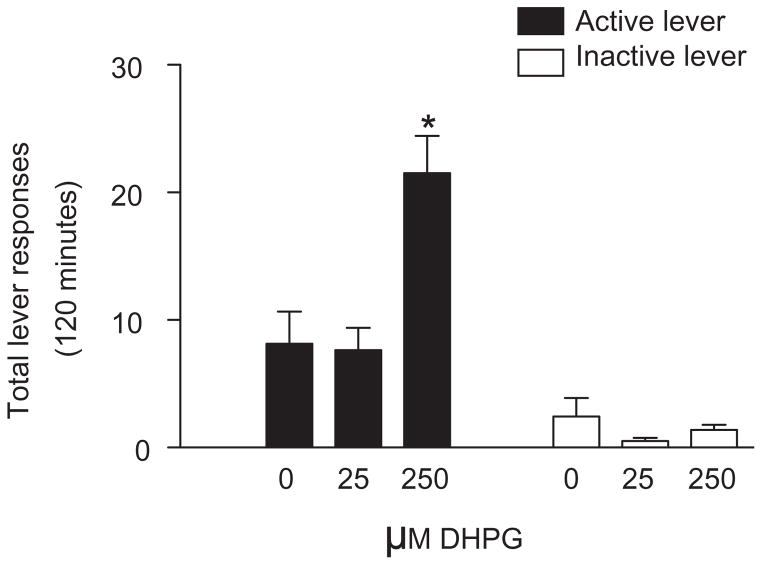
Total lever responses (mean ± SEM) following intra-accumbens core administration of DHPG are shown in Fig. 1. Total lever responses were analyzed with a two-way ANOVA, which revealed significant main effects of treatment [F(2,40) = 9.59, P < 0.0001] and lever [F(2,40) = 53.69, P < 0.0001] as well as a significant interaction between these factors [F(2,40) = 9.52, P < 0.0001]. Subsequent pairwise analyses (Bonferroni, P < 0.05) showed that the total active lever responses were significantly different between vehicle (n = 7) and 250 μM DHPG (n = 8) treatments. These findings indicate that activation of mGluR1 and/or mGluR5 in the accumbens is sufficient to reinstate cocaine seeking.
Figure 1.
Microinjection of the mGluR1/5 agonist DHPG into the accumbens core reinstated cocaine seeking. Total number of responses (mean ± SEM) on the active and inactive levers during the reinstatement test session following intra-accumbens core administration of vehicle (n = 7), 25.0 μM DHPG (n = 8) or 250 μM DHPG (n = 8). There was a significant increase in active lever responding in animals treated with 250 μM DHPG when compared with animals treated with vehicle (Bonferroni, *P < 0.05). No significant differences in responding on the inactive lever were found between treatments
Pharmacological inhibition of mGluR1s and mGluR5s in the accumbens core attenuated the reinstatement of cocaine seeking
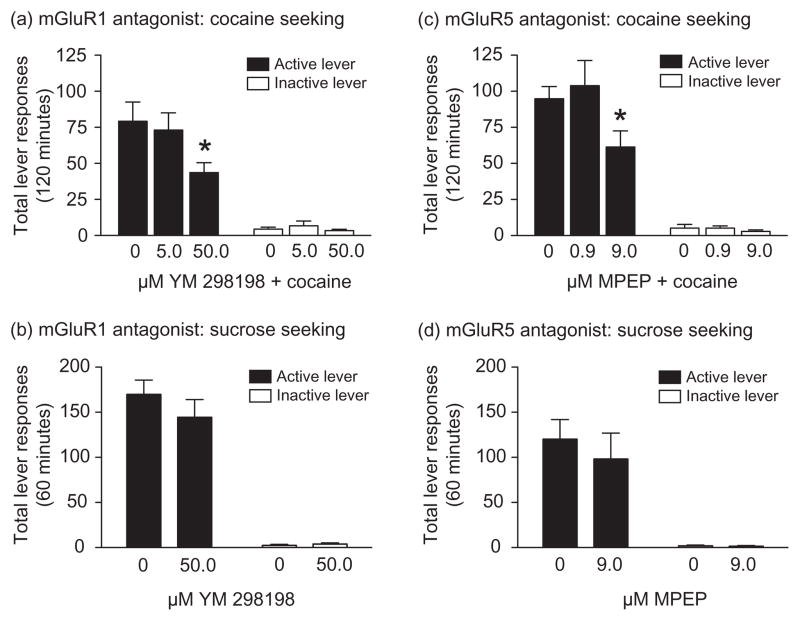
Total active and inactive lever responses (mean ± SEM) following a systemic priming injection of cocaine in animals pretreated with microinfusions of the mGluR1 antagonist YM 298198 (vehicle, 5.0 or 50.0 μM, n = 13/treatment) into the accumbens core are shown in Fig. 2a. These data were analyzed with a two-way ANOVA, which revealed significant main effects of treatment [F(2,72) = 4.35, P < 0.05] and lever [F(1,72) = 111.2, P < 0.0001] as well as a significant treatment × lever interaction [F(2,72) = 3.28, P < 0.05]. Post hoc analyses showed that the total active responses were significantly different between the vehicle and 50.0 μM YM 298198 treatments (Bonferroni, P < 0.05). The effects of YM 298198 pretreatment (n = 9) in the accumbens core on sucrose reinstatement are shown in Fig. 2b. There was no effect of drug treatment on active [t(16) = 0.98, P = 0.34] or inactive [t(16) = 0.83, P = 0.42] lever responding. Total lever responses (mean ± SEM) in rats pretreated with the mGluR5 antagonist MPEP are shown in Fig. 2c. These data were analyzed with a two-way ANOVA. The results of this analysis indicated significant main effects of treatment [F(2,56) = 5.561, P < 0.01] and lever [F(1,56) = 64.71, P < 0.0001] as well as a significant treatment × lever interaction [F(2,56) = 4.42, P < 0.05]. Subsequent pairwise analyses (Bonferroni, P < 0.05) showed that the total active lever responses were significantly different between vehicle and 9.0 μM MPEP treatments (n = 15/treatment). The effects of MPEP pretreatment (n = 8) in the accumbens core on sucrose reinstatement are shown in Fig. 2d. There was no effect of drug treatment on active [t(14) = 0.62, P = 0.55] or inactive [t(14) = 0.35, P = 0.73] lever responding. Taken together, these results suggest that both mGluR1 and mGluR5 in the accumbens core play a critical role the reinstatement of cocaine seeking.
Figure 2.
Administration of the mGluR1 antagonist YM 298198 or the mGluR5 antagonist MPEP into the accumbens core attenuated cocaine, but not sucrose, seeking. (a) Rats were administered vehicle, 5.0 or 50.0 μM YM 298198 into the accumbens core 10 minutes prior to a priming injection of cocaine (10 mg/kg, i.p.) during the reinstatement phase (n = 13/treatment). *P < 0.05 for active lever responding between vehicle and 50.0 μM YM 298198 (Bonferroni). (b) No differences in total active or inactive lever responses (mean ± SEM) during sucrose reinstatement test sessions following intra-accumbens core administration of vehicle or 50.0 μM YM 298198 (n = 9) were observed (unpaired t-tests, P > 0.05). (c) Total active and inactive lever responses (mean ± SEM) during the reinstatement test session following a 10 mg/kg priming injection of cocaine in rats pretreated with intra-accumbens core vehicle, 0.9 or 9.0 μM MPEP (n = 15/treatment). *P < 0.05 for active lever responding between vehicle and 9.0 μM MPEP (Bonferroni). (d) No significant differences in responding on the active or inactive levers (mean ± SEM) were found between treatments following intra-accumbens core administration of vehicle or 9.0 μM MPEP (n = 8) during sucrose reinstatement test sessions (unpaired t-tests, P > 0.05)
Microinjection of the PKC inhibitors Ro 31–8220 or chelerythrine into the accumbens core attenuated the reinstatement of cocaine seeking
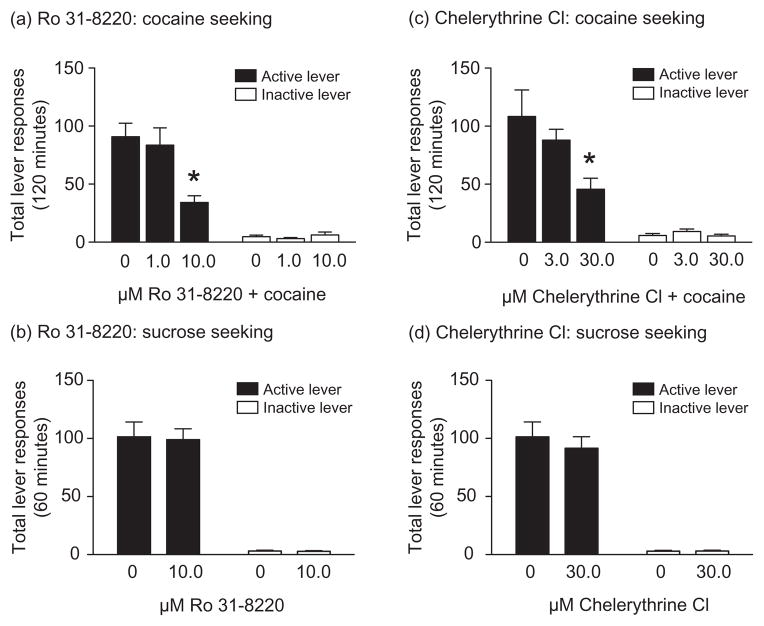
Total lever responses (mean ± SEM) following intra-accumbens core administration of the PKC inhibitors Ro 31–8220 and chelerythrine prior to a systemic priming injection of cocaine are shown in Fig. 3. Total active and inactive lever responses in animals pretreated with microinfusions of Ro 31–8220 (vehicle, n = 12; 1.0 μM Ro 31–8220, n = 11; and 10.0 μM Ro 31–8220, n = 12) directly into the core are plotted in Fig. 3a. These data were analyzed with a two-way ANOVA, which revealed significant main effects of treatment [F(2,64) = 7.31, P < 0.01] and lever [F(1,64) = 100.2, P < 0.0001] as well as a significant treatment × lever interaction [F(2,64) = 8.48, P < 0.001]. Subsequent pairwise analyses showed significant differences in responding on the active lever between animals pretreated with vehicle and those treated with 10.0 μM Ro 31–8220 (Bonferroni, P < 0.05). The effects of Ro 31–8220 pretreatment in the core on sucrose reinstatement are shown in Fig. 3b. No significant effects of treatment on active [t(16) = 0.70, P = 0.51] or inactive [t(16) = 0.19, P = 0.85] lever responding were observed. Total active and inactive lever responses in a separate cohort of animals pretreated with intracore infusions of chelerythrine (vehicle, 3.0 and 30 μM chelerythrine, n = 12/treatment) prior to a priming injection of cocaine are shown in Fig. 3c. Total lever responses were analyzed with a two-way ANOVA, which revealed significant main effects of treatment [F(2,66) = 3.93, P < 0.05] and lever [F(1,66) = 61.80, P < 0.0001] as well as a significant treatment × lever interaction [F(2,66) = 3.70, P < 0.01]. Subsequent pairwise analyses showed that the total active lever responses were significantly different between vehicle and 30 μM chelerythrine treatments (Bonferroni, P < 0.05). Total lever responding for animals pretreated with chelerythrine in the core prior to sucrose reinstatement tests are shown in Fig. 3d. No significant effects of treatment on active [t(18) = 1.21, P = 0.24] or inactive [t(18) = 0.18, P = 0.86] lever responding were observed. Taken together, these data suggest that accumbens core PKC plays a critical role in cocaine reinstatement and that attenuation of cocaine seeking following administration of Ro 31–8220 and chelerythrine into the core is not due to drug-induced motor impairments. Microinjection sites targeting the core for all behavioral pharmacology experiments are shown in Fig. 4.
Figure 3.
Microinjection of the PKC inhibitors Ro 31–8220 and chelerythrine chloride into the accumbens core dose-dependently attenuated cocaine, but not sucrose, seeking. (a) Total number of responses (mean ± SEM) on the active and inactive levers during the reinstatement test session following a priming injection of cocaine (10 mg/kg, i.p.) in rats pretreated with vehicle (n = 12), 1.0 (n = 11) or 10.0 (n = 12) μM Ro 31–8220 into the accumbens core. *P < 0.05 between vehicle and 10.0 μM Ro 31–8220 with regard to active lever responses (Bonferroni). (b) No differences in total active or inactive lever responses (mean ± SEM) during sucrose reinstatement test sessions following intra-accumbens core administration of vehicle or 10.0 μM Ro 31–8220 (n = 9) were observed (unpaired t-tests, P > 0.05). (c) Rats were administered vehicle, 3.0 or 30.0 μM chelerythrine chloride (n = 12/treatment) into the accumbens core before a priming injection of cocaine (10 mg/kg, i.p.) during the reinstatement phase. Depicted are the total (mean ± SEM) active and inactive lever responses during the reinstatement test sessions. The asterisk represents a significant difference in active lever responding between rats treated with vehicle and rats treated with 30.0 μM chelerythrine chloride (Bonferroni, P < 0.05). (d) No significant differences in responding on the active or inactive levers (mean ± SEM) were found between treatments following intra-accumbens core administration of vehicle or 30.0 μM chelerythrine chloride (n = 10) during sucrose reinstatement test sessions (unpaired t-tests, P > 0.05)
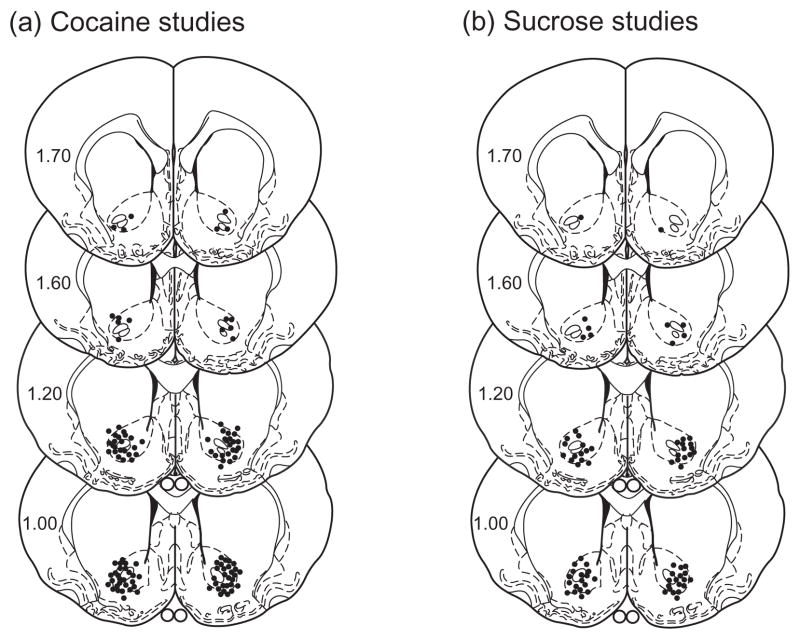
Figure 4.
Cannula placements from all of the animals included in the behavioral pharmacology experiments. Coronal sections depicting microinjection sites, as indicated by closed circles, targeting the medial nucleus accumbens core in the cocaine (a) and sucrose (b) experiments. Numbers on the left side of the coronal sections denote distance from bregma in the anteroposterior direction
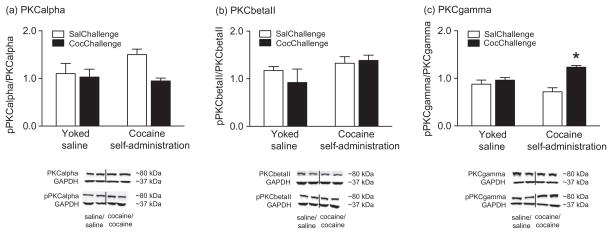
The reinstatement of cocaine seeking was associated with increased phosphorylated PKCγ in the nucleus accumbens core
In these experiments, rats with a previous history of cocaine self-administration or yoked saline controls were administered 10 mg/kg cocaine or saline (i.p.) and were allowed to self-administer saline under extinction conditions for 30 minutes at which point they were killed and their brains removed for the Western blot analyses. As expected, animals with a history of cocaine self-administration showed robust reinstatement of cocaine seeking, whereas the yoked-saline controls did not [total active lever responding (mean ± SEM) during the reinstatement test session for the four treatment groups was as follows: saline/saline, 1.82 ± 0.65; saline/cocaine, 0.33 ± 0.25; cocaine/saline, 8.64 ± 2.66; cocaine/cocaine, 56.33 ± 4.85]. The fluorescent densitometry results from the Western blots are shown in Fig. 5. Unpaired t-tests indicated that there was no significant difference in expression of GAPDH between the saline and cocaine groups in the accumbens core (data not shown). Ratios of phosphorylated to native PKCα (Fig. 5a), PKCβII (Fig. 5b) and PKCγ (Fig. 5c) were calculated and analyzed with two-way mixed factors ANOVAs, with factors of self-administration (saline or cocaine) and reinstatement challenge (saline or cocaine). No significant effects of self-administration or reinstatement challenge on pPKCα/PKCα (Fig. 5a) or pPKCβII/PKCβII (Fig. 5b) expression were observed between treatments. Analysis of pPKCγ/PKCγ expression data (Fig. 5c) revealed a significant main effect of reinstatement [F(1,20) = 19.0, P < 0.001] as well as a significant self-administration × reinstatement interaction [F(1,20) = 9.5, P < 0.01]. Subsequent pairwise analyses showed that there was a significant difference between the cocaine/cocaine group (n = 7) and the saline/saline (n = 7), saline/cocaine (n = 6) and cocaine/saline (n = 4) groups (Bonferroni, P < 0.05).
Figure 5.
The reinstatement of cocaine seeking was associated with increased expression of phosphorylated PKCγ in the nucleus accumbens core. Representative Western blots for PKC isoforms and GAPDH (loading control) in the accumbens core from cocaine self-administration/cocaine challenge injection and yoked saline/cocaine challenge injection treatments are shown (yoked saline/saline challenge injection and cocaine self-administration/saline challenge injection blots not shown). Florescence values from all Western blots were normalized to GAPDH and then plotted as the ratio of phosphorylated to native PKC isoform expression. The results of these analyses are plotted in (a), (b) and (c). There were no significant differences among treatments in terms of pPKCα/PKCα expression (a) or pPKCβII/PKCβII expression (b) in the accumbens core. There was a significant increase in pPKCγ/PKCγ expression (c) in the accumbens core of the cocaine/cocaine group compared with the saline/saline, saline/cocaine and cocaine/saline treatment groups (Bonferroni, *P < 0.05). There were four to seven rats per treatment
DISCUSSION
The present results indicate that administration of an mGluR1/5 agonist into the nucleus accumbens core is sufficient to reinstate cocaine seeking. Consistent with these results, administration of an mGluR1 or mGluR5 antagonist directly into the accumbens core attenuated cocaine priming-induced reinstatement of drug seeking. Moreover, pharmacological inhibition of PKC in the accumbens core attenuated cocaine seeking. Intra-accumbens core administration of mGluR1, mGluR5 or PKC antagonists did not influence sucrose seeking indicating that these effects were reinforcer specific and not due to general motor-suppressant effects of drug treatment. Western blot analyses revealed that the reinstatement of cocaine-seeking behavior is associated with increased activation of PKCγ in the core. Collectively, these data suggest that stimulation of both mGluR1 and mGluR5 in the accumbens core promotes cocaine seeking, in part, through activation of PKCγ.
The present findings contribute to and expand upon previous studies demonstrating a role for accumbens group I mGluR signaling in cocaine seeking. While type I mGluRs are expressed in the ventral striatum, mGluR1 is expressed at much lower levels than mGluR5 in the nucleus accumbens (Testa et al. 1994). Administration of a selective mGluR1 antagonist directly into the accumbens core, but not shell, attenuated context-induced reinstatement of cocaine seeking (Xie et al. 2012). Similarly, administration of a selective mGluR1 antagonist directly into the accumbens core (present findings), but not shell (Schmidt et al. 2013), reduced the ability of a priming injection of cocaine to reinstate drug-seeking behavior. These results suggest that mGluR1 signaling in the accumbens core plays a critical role in cocaine reinstatement regardless of the environmental stimuli used to precipitate drug seeking (i.e. context- versus drug priming-induced reinstatement). In contrast to the accumbens subregion-specific role for mGluR1 signaling in the reinstatement of cocaine seeking, activation of mGluR5s in both the accumbens core and shell subregions is important for cocaine seeking. Thus, administration of an mGluR5 antagonist into the accumbens shell attenuated cocaine priming-induced reinstatement of drug seeking (Kumaresan et al. 2009; Schmidt et al. 2013). Furthermore, pharmacological inhibition of mGluR5s in the accumbens core attenuated cocaine seeking precipitated by a priming injection of cocaine (present findings and Wang et al. 2013) or re-exposure to cues previously associated with cocaine taking (Knackstedt, Trantham-Davidson & Schwendt 2013; Wang et al. 2013). Consistent with these results, administration of the mGluR1/5 agonist DHPG into the core (present findings) or shell (Schmidt et al. 2013) reinstated cocaine seeking. Taken together, these results indicate that cocaine seeking, regardless of the environmental stimuli used to precipitate reinstatement, is dependent upon activation of mGluR5, but not mGluR1, in the accumbens shell and both mGluR1 and mGluR5 signaling in the accumbens core.
Group I mGluRs in the accumbens core appear to have distinct roles in cocaine seeking depending upon the self-administration/reinstatement model studied. mGluR1 and mGluR5 signaling in the core play an essential role in cocaine priming-induced reinstatement of drug seeking in rats whose short-access self-administration behavior has been extinguished in ~7 days (present findings and Wang et al. 2013). In contrast, mGluR1, and not mGluR5, in the accumbens core may play a role in cocaine seeking during protracted withdrawal from extended-access cocaine self-administration (McCutcheon et al. 2011; Loweth, Tseng & Wolf 2013). Collectively, these findings suggest that with short-access/extinction paradigms both mGluR1 and mGluR5 predominate during cocaine seeking while mGluR1 plays a critical role in cocaine seeking with extended-access paradigms.
Stimulation of type I mGluRs leads to activation of PKC (Conn & Pin 1997). Activation of PKC has been shown to regulate psychostimulant-induced behavioral plasticity (Lee & Messing 2008; Olive & Newton 2010) and psychostimulant-induced increases in extracellular dopamine levels in the nucleus accumbens (Loweth et al. 2009). While currently available pharmacological inhibitors of PKC are non-specific in that they do not differentiate between PKC isoforms, there is evidence that accumbens PKC regulates cocaine-mediated behaviors (Cervo et al. 1997; Pierce et al. 1998). With regard to cocaine seeking, administration of a PKC inhibitor into either the accumbens core (present findings) or shell (Schmidt et al. 2013) significantly attenuated the ability of a priming injection of cocaine to reinstate drug-seeking behavior. Our results suggest that stimulation of mGluR5s in the accumbens shell and mGluR1/5 in the accumbens core promotes cocaine seeking, in part, by activating PKC.
There are 10 PKC isoforms that are broadly divided into three subfamilies (i.e. conventional, novel and atypical PKCs) depending upon their molecular structure, calcium dependence and lipid activators (Mellor & Parker 1998). While the role of PKC isoforms in psychostimulant-induced behavioral plasticity is relatively unknown, emerging evidence indicates that repeated exposure to psychostimulants alters expression of conventional PKC isoforms (i.e. PKCα, PKCβI, PKCβII and PKCγ) in the brain (Olive & Newton 2010). Previous studies demonstrated that repeated cocaine administration increased the phosphorylation of some, but not all, isoforms of PKC and that these effects are brain region-specific (Steketee et al. 1998; Chen et al. 2007). For example, repeated experimenter-delivered cocaine is associated with increased PKCβI expression only in the medial prefrontal cortex and no change in the expression of any PKC isoforms in the accumbens (Steketee et al. 1998). Furthermore, PKCγ mRNA expression is increased in the nucleus accumbens following 5 days of withdrawal from self-administered cocaine (Thomas & Everitt 2001). Our group recently demonstrated that cocaine priming-induced reinstatement of drug seeking is associated with selective activation of PKCγ, but not PKCα or PKCβII, in the accumbens shell (Schmidt et al. 2013). We have expanded these findings to include similar studies of conventional PKC isoforms in the accumbens core. Consistent with our previous study, we found that activation of PKCγ, but not PKCα or PKCβII, in the accumbens core was associated with cocaine priming-induced reinstatement of drug seeking. Administration of the mGluR1/5 agonist DHPG into the core (present findings) or shell (Schmidt et al. 2013) promotes cocaine seeking through activation of PKC (Schmidt et al. 2013). Previous studies have shown that DHPG administration selectively increased phosphorylation of PKCγ, but not PKCα or PKCβI/II isoforms, which is consistent with the present findings demonstrating that cocaine reinstatement is associated with increased activation of PKCγ in the accumbens core (Sanchez-Perez & Felipo 2005; Takagi et al. 2010). While not statistically significant, there was a trend toward decreased activation of accumbens core PKCα following an acute priming injection of cocaine in cocaine-experienced rats compared with all other treatments. The functional significance of this decrease is not clear but could involve cell-specific changes in neuronal excitability and/or synaptic glutamate receptor expression (Sanchez-Perez & Felipo 2005; Deng et al. 2009; Ahn & Choe 2010).
The downstream targets that mediate the effects of PKC on cocaine seeking are not known but may include AMPA receptors (Famous et al. 2008). PKC phosphorylates GluA1 at Ser831, which facilitates GluA1 insertion into the plasma membrane (Lin et al. 2009). In contrast, PKC-induced phosphorylation of GluA2 at Ser880 results in rapid internalization of GluA2-containing AMPA receptors (Chung et al. 2000; Perez et al. 2001; Collingridge, Isaac & Wang 2004) (but see, Gardner et al. 2005; Liu & Cull-Candy 2005). An emerging literature indicates that AMPA receptor trafficking plays a prominent role in cocaine seeking (Schmidt & Pierce 2010; Pierce & Wolf 2013). Cocaine priming-induced reinstatement of drug seeking is associated with increased surface expression of GluA1-containing AMPA receptors in the accumbens shell (Anderson et al. 2008) and increased phosphorylation of accumbens GluA2 at Ser880 (Famous et al. 2008; Wiggins et al. 2011). PKCγ, the same PKC isoform identified in the present study to play a role in cocaine seeking, phosphorylates GluA2 at Ser880 and promotes internalization of GluA2 subunits (Patten & Ali 2009). Therefore, it is possible that stimulation of group I mGluR signaling in the accumbens core promotes cocaine seeking, in part, by promoting the transport of GluA1-containing AMPA receptors to synapses and/or internalization of GluA2-containing AMPA receptors. This hypothesis is supported by a recent study demonstrating that DHPG-induced phosphorylation of GluA2 in the striatum is blocked by a PKC inhibitor (Ahn & Choe 2010). Future studies are required to determine whether cocaine seeking is associated with mGluR1/5-mediated trafficking of GluA1 and/or GluA2 AMPA receptor subunits in the accumbens.
In conclusion, the present results indicate that blocking both mGluR1 and mGluR5 transmission in the accumbens core attenuates the reinstatement of cocaine seeking. Furthermore, stimulation of accumbens core group I mGluRs reinstates cocaine seeking and this effect is likely mediated by increased activation of PKC. During the reinstatement of cocaine seeking, increased phosphorylation of PKCγ was observed in the core, which may promote insertion of GluA1-containing AMPA receptors and/or removal of GluA2-containing AMPA receptors from synapses (Pierce & Wolf 2013). Thus, enhanced mGluR1/5-PKC signaling in the accumbens core may produce a dynamic, rapid exchange between a GluA1-containing population of AMPA receptors and a GluA2-containing population of AMPA receptors, which combine to promote cocaine priming-induced reinstatement of drug seeking.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health: K01 DA030445 (H.D.S.), DA022339 (R.C.P.), DA033641 (R.C.P.) and K02 DA18678 (R.C.P.). The authors would like to thank Rachel Schassburger and Kelsey Ige for their technical assistance.
Footnotes
Conflict of Interest
The authors declare no potential conflict of interest relating to this study.
Authors Contribution
HDS was responsible for the study concept and design, supervised and contributed to the acquisition of data, analyzed the data and drafted the manuscript. RCP was also responsible for the study design and provided critical revisions of the manuscript. BAK and ACA contributed to the acquisition of animal data and editing of the manuscript. All authors reviewed content and approved the final version for publication.
References
- Achat-Mendes C, Platt DM, Spealman RD. Antagonism of metabotropic glutamate 1 receptors attenuates behavioral effects of cocaine and methamphetamine in squirrel monkeys. J Pharmacol Exp Ther. 2012;343:214–224. doi: 10.1124/jpet.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SM, Choe ES. Alterations in GluR2 AMPA receptor phosphorylation at serine 880 following group I metabotropic glutamate receptor stimulation in the rat dorsal striatum. J Neurosci Res. 2010;88:992–999. doi: 10.1002/jnr.22275. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Cervo L, Mukherjee S, Bertaglia A, Samanin R. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res. 1997;775:30–36. doi: 10.1016/s0006-8993(97)00866-4. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lee TH, Wetsel WC, Sun QA, Liu Y, Davidson C, Xiong X, Ellinwood EH, Zhang X. Reversal of cocaine sensitization-induced behavioral sensitization normalizes GAD67 and GABAA receptor alpha2 subunit expression, and PKC zeta activity. Biochem Biophys Res Commun. 2007;356:733–738. doi: 10.1016/j.bbrc.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Deng P, Pang ZP, Lei Z, Xu ZC. Excitatory roles of protein kinase C in striatal cholinergic interneurons. J Neurophysiol. 2009;102:2453–2461. doi: 10.1152/jn.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol. 2013;19:87–101. doi: 10.1111/adb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Messing RO. Protein kinases and addiction. Ann N Y Acad Sci. 2008;1141:22–57. doi: 10.1196/annals.1441.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Li YQ, Xue YX, He YY, Li FQ, Xue LF, Xu CM, Sacktor TC, Shaham Y, Lu L. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Svoboda R, Austin JD, Guillory AM, Vezina P. The PKC inhibitor Ro31–8220 blocks acute amphetamine-induced dopamine overflow in the nucleus accumbens. Neurosci Lett. 2009;455:88–92. doi: 10.1016/j.neulet.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2013;76:287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332 (Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Akai H, Nagumo Y, Sunagawa N, Hasebe K, Nagase H, Kita T, Hara C, Suzuki T. Implications of protein kinase C in the nucleus accumbens in the development of sensitization to methamphetamine in rats. Neuroscience. 2004;127:941–948. doi: 10.1016/j.neuroscience.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Olive MF, Newton PM. Protein kinase C isozymes as regulators of sensitivity to and self-administration of drugs of abuse-studies with genetically modified mice. Behav Pharmacol. 2010;21:493–499. doi: 10.1097/FBP.0b013e32833d8bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Patten SA, Ali DW. PKCgamma-induced trafficking of AMPA receptors in embryonic zebrafish depends on NSF and PICK1. Proc Natl Acad Sci U S A. 2009;106:6796–6801. doi: 10.1073/pnas.0811171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Wolf ME. Psychostimulant-induced neuro-adaptations in nucleus accumbens AMPA receptor transmission. In: Pierce RC, Kenny PJ, editors. Addiction. Cold Spring Harbor; NewYork: Cold Spring Harbor Laboratory Press; 2013. pp. 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Bradley SR, Awad H, Wittmann M, Conn PJ. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson’s disease and related disorders. Pharmacol Ther. 2000;88:427–435. doi: 10.1016/s0163-7258(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez AM, Felipo V. Serines 890 and 896 of the NMDA receptor subunit NR1 are differentially phosphorylated by protein kinase C isoforms. Neurochem Int. 2005;47:84–91. doi: 10.1016/j.neuint.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann NY Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Schassburger RL, Guercio LA, Pierce RC. Stimulation of mGluR5 in the accumbens shell promotes cocaine seeking by activating PKC gamma. J Neurosci. 2013;33:14160–14169. doi: 10.1523/JNEUROSCI.2284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Sigmon SA, McGinty JF. RGS4 overexpression in the rat dorsal striatum modulates mGluR5-and amphetamine-mediated behavior and signaling. Psychopharmacology (Berl) 2012;221:621–635. doi: 10.1007/s00213-011-2606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Rowe LA, Chandler LJ. The effects of acute and repeated cocaine injections on protein kinase C activity and isoform levels in dopaminergic brain regions. Neuropharmacology. 1998;37:339–347. doi: 10.1016/s0028-3908(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N, Besshoh S, Morita H, Terao M, Takeo S, Tanonaka K. Metabotropic glutamate mGlu5 receptor-mediated serine phosphorylation of NMDA receptor subunit NR1 in hippocampal CA1 region after transient global ischemia in rats. Eur J Pharmacol. 2010;644:96–100. doi: 10.1016/j.ejphar.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Landwehrmeyer GB, Penney JB, Jr, Young AB. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol. 1995;354:241–252. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression. J Neurosci. 2001;21:2526–2535. doi: 10.1523/JNEUROSCI.21-07-02526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer KM, Steketee JD. Examination of a role for metabotropic glutamate receptor 5 in the medial prefrontal cortex in cocaine sensitization in rats. Psychopharmacology (Berl) 2012;221:91–100. doi: 10.1007/s00213-011-2548-1. [DOI] [PubMed] [Google Scholar]
- Titley HK, Heskin-Sweezie R, Broussard DM. The bidirectionality of motor learning in the vestibulo-ocular reflex is a function of cerebellar mGluR1 receptors. J Neurophysiol. 2010;104:3657–3666. doi: 10.1152/jn.00664.2010. [DOI] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins A, Smith RJ, Shen HW, Kalivas PW. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31:16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie MB, Besheer J, Kelley SP, Kumar S, O’Buckley TK, Morrow AL, Hodge CW. Acute ethanol administration rapidly increases phosphorylation of conventional protein kinase C in specific mammalian brain regions in vivo. Alcohol Clin Exp Res. 2007;31:1259–1267. doi: 10.1111/j.1530-0277.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17:287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Lin N, Dong Y, Su Q, Luo M. Effect of perinatal thyroid hormone deficiency on expression of rat hippocampal conventional protein kinase C isozymes. Mol Cell Biochem. 2011;353:65–71. doi: 10.1007/s11010-011-0775-8. [DOI] [PubMed] [Google Scholar]