Abstract
Pancreatic adenocarcinoma (PDAC) is a major unmet medical need and a deeper understanding of molecular drivers is needed to advance therapeutic options for patients. We report here that p21-activated kinase 1 (PAK1) is a central node in PDAC cells downstream of multiple growth factor signalling pathways, including hepatocyte growth factor (HGF) and MET receptor tyrosine kinase. PAK1 inhibition blocks signalling to cytoskeletal effectors and tumour cell motility driven by HGF/MET. MET antagonists, such as onartuzumab and crizotinib, are currently in clinical development. Given that even highly effective therapies have resistance mechanisms, we show that combination with PAK1 inhibition overcomes potential resistance mechanisms mediated either by activation of parallel growth factor pathways or by direct amplification of PAK1. Inhibition of PAK1 attenuated in vivo tumour growth and metastasis in a model of pancreatic adenocarcinoma. In human tissues, PAK1 is highly expressed in a proportion of PDACs (33% IHC score 2 or 3; n = 304) and its expression is significantly associated with MET positivity (p < 0.0001) and linked to a widespread metastatic pattern in patients (p = 0.067). Taken together, our results provide evidence for a functional role of MET/PAK1 signalling in pancreatic adenocarcinoma and support further characterization of therapeutic inhibitors in this indication.
Keywords: PAK1, MET, pancreatic adenocarcinoma, onartuzumab, GDC-0941 (pictilisib)
Introduction
Pancreatic adenocarcinoma (PDAC) accounts for over 90% of reported cases of pancreatic cancer and is the fourth leading cause of cancer-related deaths in the United States [1]. Treatment of pancreatic cancer remains an enormous clinical challenge and 75% of patients die within 12 months of their diagnosis, with a median survival of 6–8 months. Complete surgical resection is still the only potentially curative approach [2]. However, patients with locally advanced or metastatic pancreatic cancer are ineligible for surgical intervention, and even following resection with negative margins and adjuvant chemoradiation, most patients have recurrent disease 1 year after surgery [3,4]. Accordingly, poor prognosis in PDAC is also associated with tumour cell migration and invasion [5], and destruction of adjacent structures, including the duodenum, biliary tree, and neural ganglia in the retroperitoneum, is associated with the reduced quality of life and extreme pain that are characteristic of pancreatic cancer [6]. Although the last decade has seen major improvements in our understanding of the molecular drivers of PDAC [7], molecular events driving these later stages of disease development and dissemination are still not well known.
Hepatocyte growth factor/scatter factor (HGF/SF) was originally identified as a liver mitogen and fibroblast-derived epithelial motility factor, and is the only physiological ligand for the MET receptor tyrosine kinase [8]. Both HGF and MET are elevated in multiple cancers, including PDAC [9], and are associated with increased tumour cell invasion, distant metastases, and poor prognosis [8]. MET has also recently been suggested to be a marker for pancreatic cancer stem cells [10] and its expression is elevated in a model of chronic pancreatitis, a risk factor for PDAC [11]. Based on the accumulating evidence on the importance of HGF/MET signalling in tumourigenesis, various small and large molecule inhibitors have entered preclinical and clinical development [8]. Onartuzumab is a humanized, monovalent monoclonal antibody that binds to the semaphorin domain of MET and thereby blocks ligand binding and subsequent receptor activation [12,13]. Onartuzumab is currently being evaluated in phase II and III clinical studies in combination with erlotinib, bevacizumab, and chemotherapy, and data for both clinical efficacy and therapeutic resistance mechanisms have been disclosed [14,15].
Ligand-induced MET dimerization activates its tyrosine kinase activity and autophosphorylation on multiple catalytic domain and carboxy-terminal residues that serve as docking sites for several adaptor proteins and signalling molecules [8]. The most well-characterized effectors for MET signalling are the RAS–MAPK and PI3K–AKT pathways. However, another key cytoplasmic signalling cascade downstream of HGF/MET is mediated by RAS-related C3 botulinum toxin substrate 1 (RAC1)/cell division control protein 42 (CDC42) and p21-activated kinases (PAKs) to elicit cytoskeletal changes for cell migration and adhesion [16]. Group I PAKs (PAK1–3) are activated by direct interaction with RAC/CDC42 via a conserved p21-binding domain (PBD) and can induce lamellipodium formation [17]. PAK1 also localizes to sites of membrane ruffles and focal adhesions, and co-immunoprecipitates with paxillin, a component of focal adhesions [18]. Moreover, active RAC1 transduces signals via PAK1 to induce disassembly of E-cadherin-based adhesions, a process that may require the interaction of PAK1 with the E-cadherin-associated protein, β-catenin [19,20]. Although a number of PAK substrates have been associated with promoting tumour invasiveness and metastasis [18,21], the manner in which PAK1 orchestrates its effects on the cytoskeleton may be both cell type- and context-dependent.
The PAK family member that is most validated as a therapeutic target in cancer is PAK1 [21–25]. However, the contribution of PAK1 to HGF/MET-mediated tumourigenesis is not yet well understood. Given the potential importance of this signalling axis to pancreatic cancer, we sought to investigate the extent to which this pathway might drive human PDAC. We show that MET and PAK1 are frequently co-expressed in human PDAC tissues and inhibition is efficacious in PDAC cell lines and tumours. Furthermore, although multiple secreted growth factor ligands or direct amplification of PAK1 attenuated the effectiveness of single agent onartuzumab treatment, combined inhibition of MET and PAK1 maximizes inhibition of tumour cell motility and combination therapy increases therapeutic benefit.
Materials and methods
Tissue samples
For pancreatic adenocarcinomas from University Hospital Dresden, tissue microarrays of 143 patients were obtained after surgical resection for pancreatic cancer. Samples were collected from 1993 to 2010, and most of the patients did not undergo adjuvant chemotherapy (39 patients were treated with 5FU or gemcitabine). Informed consent was obtained for each patient, following review by the Human Ethics Committee Ethikkommission an der Technischen Universität Dresden.
Tissues from Johns Hopkins University were obtained from patients with pancreatic adenocarcinoma who enrolled in a phase II study of an allogeneic granulocyte macrophage colony-stimulating factor-secreting whole cell vaccine [26]. Written informed consent was obtained in compliance with the Johns Hopkins Medical Institution Institutional Review Board-approved protocol with approval number #00–01–13–02. An additional cohort of patient samples was obtained from the Gastrointestinal Cancer Rapid Medical Donation Program. Use of all human tissue samples from resection specimens and autopsy participants was approved by Johns Hopkins Institutional Review Board and obtained after informed consent [27,28]. Each patient was classified as having either oligometastatic (10 or fewer metastases) or widespread (>10) metastatic PDAC.
Immunohistochemical analysis
For immunohistochemistry (IHC), formalin-fixed, paraffin-embedded tissue sections were incubated with anti-PAK1 rabbit polyclonal antibody (catalogue number 2602; Cell Signaling Technology, Danvers, MA, USA) [25], anti-total MET rabbit monoclonal antibody (clone SP44; Ventana, Tucson, AZ, USA) [29] or anti-β-catenin mouse monoclonal antibody (clone 14; BD Pharmingen, San Diego, CA, USA) [30,31]. PAK1 IHC was performed as described previously [25]. For p53 IHC, anti-p53 mouse monoclonal antibody (clone DO-1; Calbiochem, EMD Biosciences, Billerica, MA, USA) was used to detect wild-type and mutated p53 expression. The slides were pretreated in a pressure cooker in citrate buffer (pH 6.0) for 25 min at 98°C. For staining, the p53 antibody in a 1 : 1500 dilution was applied for 30 min and detection was performed using the Ultra Vision LP Kit with DAB. In addition, for MET and β-catenin IHC, detection was carried out on the Ventana Benchmark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA), and pretreatment was done with Cell Conditioner 1 using the standard incubation time. For MET IHC, sections were incubated with primary antibody for 16 min at 37°C, followed by incubation with Ventana Ultra View HRP reagent. For β-catenin IHC, sections were incubated with primary antibody for 60min at 37 °C, followed by incubation with Ventana Rabbit OmniMap HRP reagent for 32 min. Ventana DAB and Hematoxylin II reagents were used for chromogenic detection and counterstaining. Isotype-matched antibody and tissue controls were included in all experiments. The intensity of PAK1 and MET expression was scored separately in the cytoplasm and nuclei of neoplastic cells on a semi-quantitative scale of 0–3. The highest intensity score among replicate cores was used as the score for each patient. All cases were scored blind to clinicopathological data.
Cell culture and viability assays
Cells were acquired from the American Type Culture Collection (Manassas, VA, USA). DNA copy number was determined by Illumina HumanOmni SNP Array [32]. Cells were treated with short interfering RNAs (Dharmacon, Chicago, IL, USA), IPA-3 [33], crizotinib (PF02341066) [34], and onartuzumab [13]. Cellular viability was assessed via CellTiter-Glo (Promega, Madison, WI, USA).
Secreted factor screen
Recombinant purified secreted factors were prepared as described previously [35]. Equal volumes of diluted factor (final concentration 50 ng/ml) were arrayed into 96-well plates pre-seeded with AsPC-1 cells transfected with either non-targeting or PAK1-specific siRNA oligonucleotides. Cell migration was determined via Incucyte wound healing assay (Essen Bioscience, Ann Arbor, MI, USA). Secreted factors were prioritized for follow-up experiments based on the extent of ligand-induced tumour cell motility (>2-fold over control) and significant PAK1 dependency (>25% reduction following PAK1 knockdown relative to non-targeting control siRNA).
Mouse models of pancreatic adenocarcinoma
All animals were maintained in accordance with guidelines of the American Association of Laboratory Animal Care (Approval # MO08M142). The mouse liver metastasis model was established using a previously described hemispleen injection technique [36,37]. KPC cells (2 × 106) stably transduced with doxycycline (DOX)-inducible short-hairpin RNA constructs targeting either LacZ control or murine PAK1 were injected into the splenic bed (splenic artery and veins) through one hemispleen, followed by a flush with the HBSS buffer. All mice received drinking water supplemented with 5% sucrose or 5% sucrose plus 1 mg/ml DOX. At necropsy, mice were examined macroscopically and multiple sections of livers and splenic bed injection sites were examined microscopically with H&E staining. Xenograft tumour studies with the KP4 line were carried out as previously described [38].
Results
PAK1 is downstream of multiple growth factors and is essential for the motility of pancreatic adenocarcinoma cells
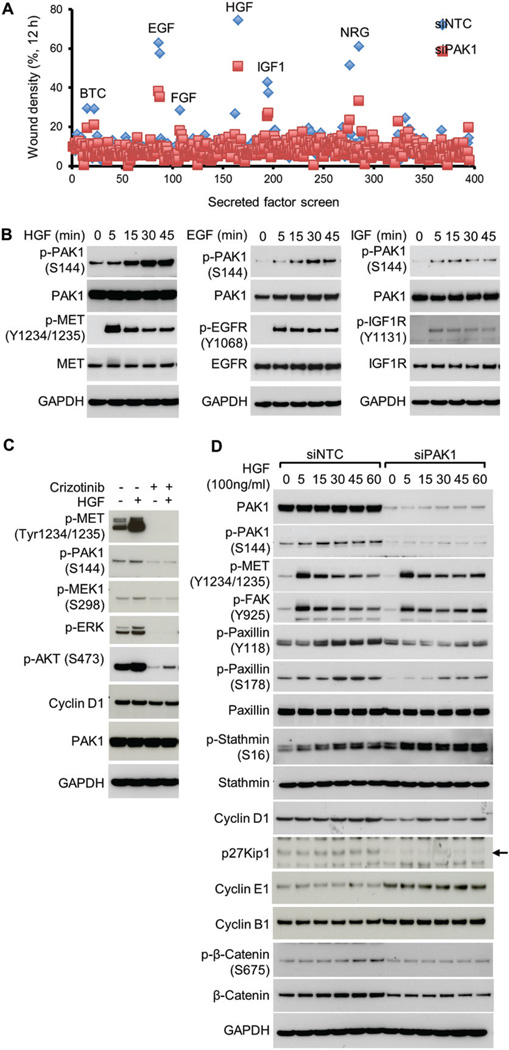
In order to characterize growth factor signalling pathways that are mediated by PAK1, a phenotypic screen was conducted using AsPC-1 pancreatic adenocarcinoma cells. AsPC-1 cells express high levels of PAK1 as well as a number of cell surface receptors whose cognate ligands were included in a custom library of 446 secreted factors (Supplementary Figure 1) [35]. Given that the most well-conserved evolutionary role of PAK1 is in the regulation of cellular motility, we used a wound migration assay and an Essen Bioscience Incucyte platform to collect and analyse relative wound densities from phase-contrast time-lapse images of cells. This method is based on creating a scratch on a confluent cell monolayer and motile cells at the leading edge close the gap until new cell–cell contacts are re-established [39]. Hepatocyte growth factor (HGF), epidermal growth factor (EGF) family ligands including EGF, beta cellulin (BTC), and neuregulin (NRG), and insulin-like growth factor 1 (IGF-1), as well as fibroblast growth factor (FGF), promoted cell motility in a PAK1-dependent manner (Figure 1A and Supplementary Figure 1). Similar results were obtained for multiple PDAC cell lines (Supplementary Figure 2). Enhanced AsPC-1 cell motility was associated with elevated PAK1 activity for these ligands, as measured by time-dependent autophosphorylation on Ser144 (Figure 1B). MET-mediated activation of PAK1 was further confirmed by treatment with HGF and/or crizotinib kinase inhibitor using additional pancreatic cancer cell lines, KP4×1.1 (KRAS mutant; Figure 1C) and BxPC3 (KRAS wild-type, Supplementary Figure 3). KP4×1.1 cells were generated by in vivo passaging of KP4 tumours (see the Materials and methods section) [38].
Figure 1.
Secreted factor library screen for PAK1-dependent motility identifies PAK1 as transducing growth factor signalling to the cytoskeleton. (A) Analysis of 446 tested secreted factors administered to AsPC-1 cells transfected with non-targeting control (siNTC) or PAK1-selective (siPAK1) siRNA for 48 h prior to wounding of the monolayer and treatment with 50 ng/ml ligand (12 h). (B) Hepatocyte growth factor (HGF), epidermal growth factor (EGF), and insulin-like growth factor-1 (IGF-1) drive activation of PAK1, as measured by autophosphorylation on Ser144. Duration of treatment is shown in minutes (min). Ligand-induced phosphorylation of receptor tyrosine kinases is shown. (C) KP4×1.1 cells were treated with either dimethyl sulphoxide (DMSO) or crizotinib (PF02341066) for 2 h and then stimulated with HGF for 15 min, and lysates were analysed for phosphorylation of PAK1 and MET signalling pathway components. (D) Assessment of PAK1-dependent signalling to cytoskeletal-associated proteins in response to HGF treatment.
Although KP4×1.1 has autocrine HGF production and basal levels of MET phosphorylation are high, this cell line can be further stimulated by exogenous HGF (Figure 1C, lane 2). Both PAK1-Ser144 and MEK1-Ser298 effector phosphorylation were dependent on MET catalytic activity in KP4×1.1 cells. Consistent with the cell motility phenotype observed for AsPC-1 cells, loss of PAK1 in KP4×1.1 cells attenuated HGF-induced signalling to cytoskeletal effector proteins, such as paxillin (Figure 1D and Supplementary Figures 4A and 4B). In order to regulate pancreatic cell motility (Supplementary Figures 4C and 4D), modest changes to the level of G1 and G2/M cell cycle regulators, such as cyclin D1, p27Kip1, cyclin E1, and cyclin B1, were observed in response to PAK1 disruption (Figure 1D), although this did not translate to dramatic changes in cell number at 72 h (< 10% decrease as measured by Cell Titer Glo) (Supplementary Figure 6A). Signalling results obtained via knockdown were consistent with downstream changes induced by treatment with an allosteric inhibitor of group I PAKs [33] (Supplementary Figure 5). Taken together, these data suggest that PAK1 may play a role in pancreatic cancer cell motility, especially downstream of HGF/MET activation.
Reduced efficacy of onartuzumab in the presence of growth factors is rescued by PAK1 inhibition
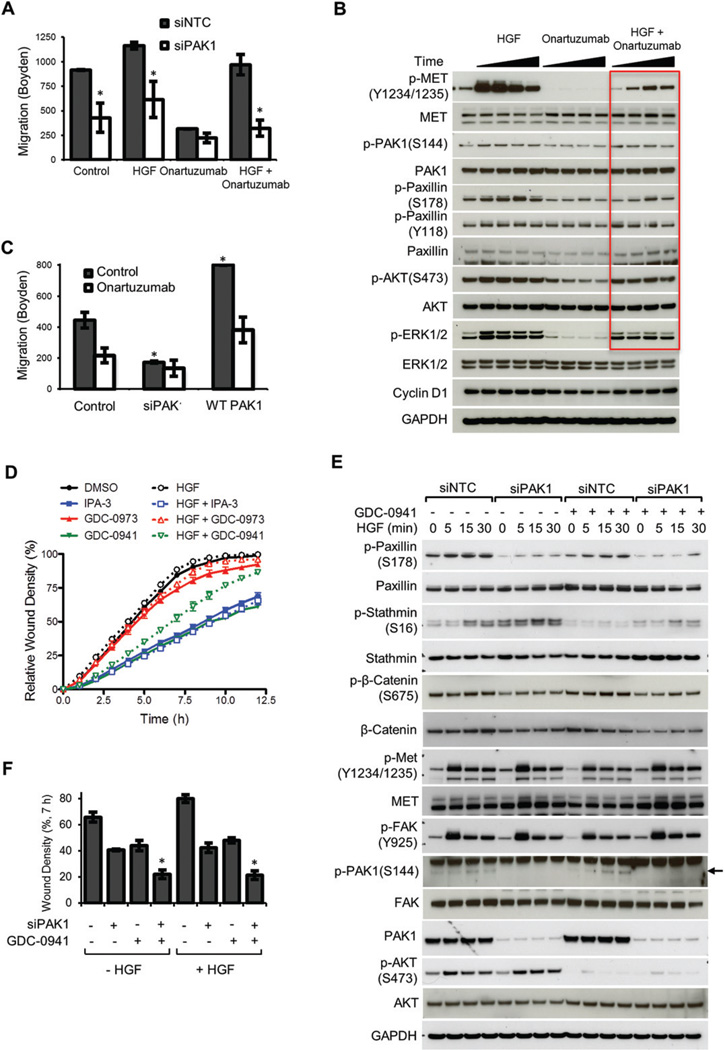
Given that HGF/MET signalling activates PAK1, we sought to compare the effect of MET and/or PAK1 inhibition in pancreatic cancer cells. The monovalent anti-MET antibody onartuzumab, which is currently in phase III clinical development [13], was utilized to interrogate MET function. Neither exogenous HGF nor onartuzumab treatment significantly altered KP4×1.1 cell proliferation (Supplementary Figure 6A), although migration in Boyden chamber trans well assays was clearly dependent on MET activity (Figure 2A). Loss of PAK1 decreased motility to an extent similar to 0.5 µM onartuzumab administration. Notably, the efficacy of onartuzumab was diminished in a dose-dependent manner by either exogenous HGF or other growth factors (Supplementary Figure 6B), although concurrent inhibition of PAK1 restored sensitivity to onartuzumab (Figure 2A, columns 7 and 8). The observed migration phenotypes were consistent with onartuzumab- and HGF-induced signalling in KP4×1.1 cells (Figure 2B). Onartuzumab treatment in the presence of 100 ng/ml HGF resulted in only a transient decrease in signalling to proximal effectors and cytoskeletal regulators, and phosphorylation rebounded over time (Figure 2B, lanes 10–13). Furthermore, KP4×1.1 cells express high levels of IGF-1 receptor and IGF-1 ligand stimulation in the presence of onartuzumab also induced reactivation of these effector pathways (Supplementary Figure 6C). To obtain further support for modulation of PAK1 as a rheostat for HGF/MET-induced migration, the efficacy of onartuzumab with either knockdown or ectopic expression of PAK1 was compared (Figure 2C). Of note, PAK1 signalling and cell migration were significantly elevated in KP4×1.1 versus KP4 parental cells (Supplementary Figures 7A and 7B) and KP4×1.1 tumour growth was resistant to MET inhibition (Supplementary Figure 7C). Elevated PAK1 expression increased motility and direct dysregulation of PAK1 could be a potential mechanism of resistance to MET inhibition (Figure 2C, columns 5 and 6).
Figure 2.
Combined inhibition of PAK1 overcomes growth factor-mediated resistance to onartuzumab. (A) Boyden chamber assay of KP4×1.1 motility towards 5% serum-containing medium in the presence or absence of 100 ng/ml HGF and 500 nM onartuzumab for 22 h. Migration assay began following 48 h transfection with non-targeting or PAK1 siRNA oligonucleotides. *p < 0.05 for siPAK1 compared with control. (B) Analysis of cell signalling under similar treatment conditions. Cells were pretreated with onartuzumab for 1 h. Time points for HGF stimulation were 0, 0.25, 0.5, 1, and 5 h. Elevated signalling by HGF-mediated resistance to onartuzumab is shown in red. *p < 0.05 for PAK1 expression modulation relative to control. (C) Boyden chamber assay comparing cell migration following either PAK1 knockdown or overexpression in the presence or absence of 100 ng/ml HGF plus 500 nM onartuzumab for 22 h. (D) Inhibition of KP4×1.1 migration by group I PAK (IPA-3) and class I PI3K small molecule inhibitors GDC-0941 (pictilisib), but not MEK inhibitor GDC-0973 (cobimetinib). Administered drug concentrations were 0.9 µM GDC-0973, 0.7 µM GDC-0941, and 40 µM IPA-3. (E) PAK1 is a central node for RTK-mediated cell motility and combination with PI3K inhibition attenuates signalling to the cytoskeleton. Pharmacodynamic analysis of 0.25 µM GDC-0941 treatment and PAK1 knockdown in the presence or absence of 100 ng/ml HGF treatment for the indicated time points (minutes). (F) Combinatorial activity of combined PAK1 and PI3K inhibition on wound healing using an IncuCyte imaging system. *p < 0.05 for combination compared with single agent treatment controls.
HGF/MET signalling converges on critical downstream effectors in addition to PAK1, such as phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways [8]. GDC-0973 (cobimetinib) and GDC-0941 (pictilisib) are highly selective small molecule inhibitors of MAPK/ERK kinase (MEK) and class I PI3Ks, respectively, which are currently in advanced clinical development [40,41]. Together with IPA-3, an allosteric inhibitor of group I PAKs [33], these compounds were utilized to ascertain the relative contribution of the PAK, PI3K, and MAPK pathways to HGF-mediated pancreatic tumour cell motility (Figure 2D). The predominant pathway for HGF-induced KP4×1.1 motility was mediated by PAKs. Catalytic inhibition PI3Ks attenuated control cell migration and HGF reduced the efficacy of GDC-0941. MEK inhibition had no effect. Given the unexpected increase in stathmin phosphorylation following PAK1 knockdown (Figure 1D; potentially a result of crosstalk or compensatory signalling by other PAKs), we hypothesized that PI3K may be upstream of stathmin in PDAC cells [42] and that combined PAK and PI3K inhibition could further decrease migration. Analysis of HGF-induced signalling following PAK1 siRNA transfection and GDC-0941 treatment showed enhanced inhibition of stathmin and paxillin phosphorylation (Figure 2E). In turn, cell migration was also dramatically reduced in response to PAK1 and PI3K combined inhibition (Figure 2F). Taken together, these experiments further demonstrate the significance of PAK1 in pancreatic tumour cell motility.
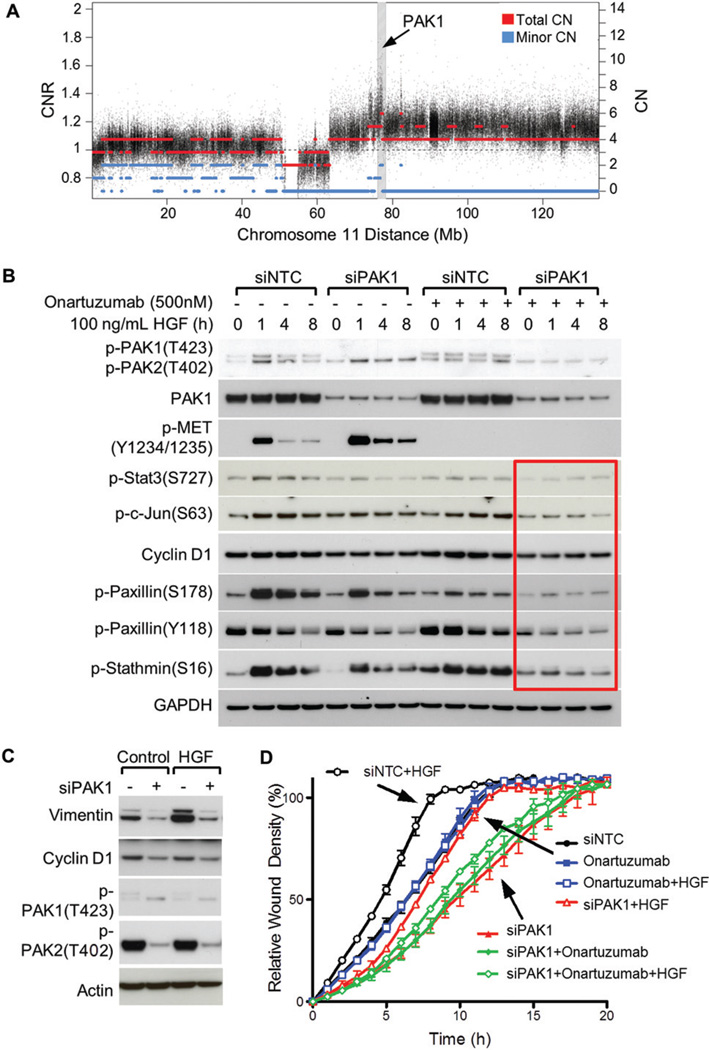
Efficacy of onartuzumab on PAK1-amplified YAPC migration is effectively enhanced by PAK1 inhibition
Focal genomic amplification of PAK1 has been reported for several tumour indications [23,25]. Although PAK1 copy number alteration is infrequent in pancreatic adenocarcinoma, YAPC cells have focal, high-level amplification of PAK1 (Figure 3A) and provide an interesting model to examine MET signalling in the context of direct dysregulation of PAK1 by the tumour [43]. An analysis of HGF/MET and PAK1 signalling was performed using exogenous HGF, onartuzumab, and PAK1 knockdown (Figure 3B) to demonstrate that combined PAK1 and MET inhibition decreased phosphorylation of c-Jun and STAT3 at sites required for transactivation of these transcription factors (Figure 3B, lanes 13–16). In addition, signalling to cytoskeletal effectors, such as paxillin and stathmin, was more effectively attenuated by the combination treatment. Of note, in the context of PAK1 amplification in YAPC cells, onartuzumab as a single agent had only a modest effect in blocking downstream signalling to these pathways (Figure 3B, lanes 9–12). Long-term activation of MET was modelled via HGF addition for 72 h (Figure 3C), and levels of vimentin, a marker of epithelial–mesenchymal transition, were elevated by HGF in a strongly PAK1-dependent manner. Although a reduction in cyclin D1 was observed following PAK1 inhibition, changes in cell proliferation were not statistically significant (data not shown). We also noted that selective knockdown of PAK1 (upper band) resulted in a compensatory increase in PAK2 phosphorylation (lower band) in this cell line (Figures 3B and 3C). This may suggest that inhibition of both group I PAK family members would be more efficacious. In terms of downstream biology and functional assays, substantial inhibition of YAPC migration resulted from PAK1 ablation, and in the presence of exogenous HGF, the combination of MET/PAK1 blockade demonstrated the maximal phenotype (Figure 3D). Taken together, these results suggest that PAK1 amplification may be a resistance factor for onartuzumab and bolster the rationale for concurrent targeting of PAK1 and MET.
Figure 3.
Combined inhibition of PAK1 and MET is efficacious in PAK1-amplified YAPC cells. (A) DNA copy number at the PAK1 locus in the YAPC cell line. Copy number estimates at each SNP are represented by black circles. Segmented total copy number is depicted by red lines. The lesser of the two allele-specific copy numbers is depicted in blue and a value of zero indicates LOH. The right vertical axis provides a copy number (CN) scale in units of absolute copy number, where 2 would be normal in a diploid cell. The left vertical axis scale shows copy number in units of copy number ratio (CNR), which is defined as the local absolute total copy number divided by the genome average for the given sample. (B) Combinatorial activity of onartuzumab and PAK1 siRNA on effector signalling to proliferation and cytoskeletal pathways. The phosphorylation-specific PAK1 antibody detects both p-PAK1(T423) (upper band) and p-PAK2(T402) (lower band) in the immunoblot. (C) HGF-mediated vimentin accumulation is PAK1-dependent. YAPC cells were treated with 50 ng/ml HGF for 72 h. (D) Wound-healing motility assay for YAPC cells treated with 100 ng/ml HGF, 10 µmol/l onartuzumab, and/or PAK1 siRNA.
PAK1 contributes to metastatic tumour spread in a preclinical model of PDAC experimental metastasis
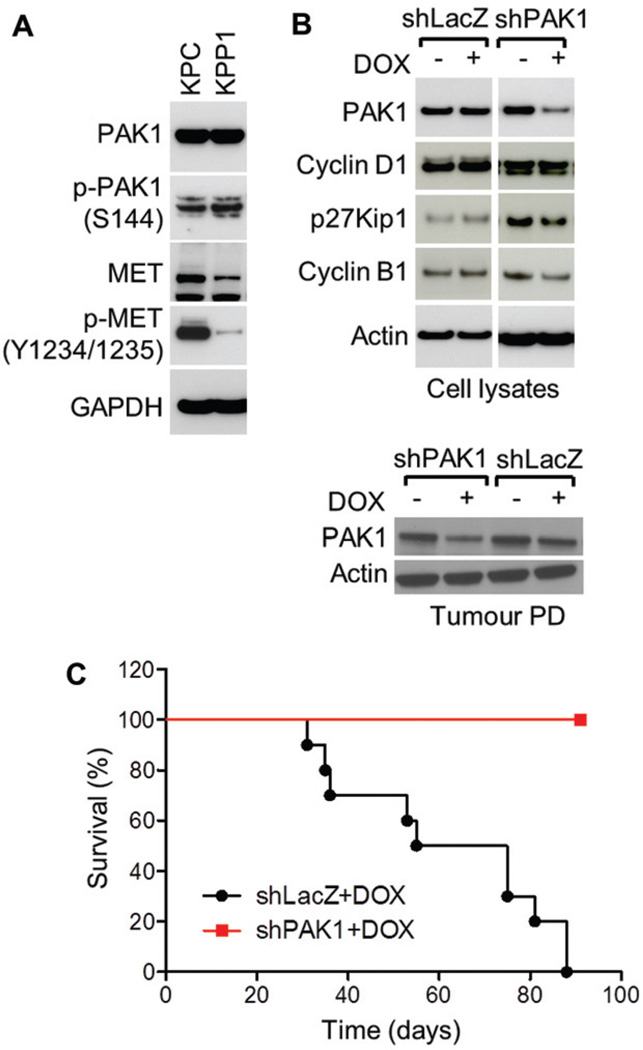
In order to evaluate the contribution of PAK1 in a model of pancreatic adenocarcinoma growth and metastasis, PAK1 and MET expression and activity were evaluated in murine cell lines, KPC and KPP1, which have been derived from genetically engineered mouse models of pancreatic adenocarcinoma [44]. KPC cells express high levels of activated PAK1 and MET (Figure 4A). A doxycycline (DOX)-inducible short-hairpin RNA (shRNA) system [23] was utilized for long-term depletion of endogenous PAK1 to further study loss-of-function effects in vivo. PAK1 or LacZ control shRNAs were introduced into KPC cells and selective knockdown was observed in cells and tumour xenografts following treatment with DOX for 3 and 7 days, respectively (Figure 4B). The extent of PAK1 knockdown in tumour lysates was less than that observed in isolated cell lines due to the presence of PAK1-expressing, infiltrating stromal cells within the tumour tissues. PAK1 ablation in KPC cells led to a reduction in migration and proliferation in shPAK1 + DOX cells, but not in either shLacZ +/-DOX or shPAK1 control groups (Supplementary Figures 8A and 8B). No apoptosis or cell senescence was observed (Supplementary Figure 8C). After confirming the knockdown of PAK1 expression, 2 × 106 cells were injected into the splenic bed prior to performing a hemisplenectomy, and mice were given 1 mg/ml DOX and 5% sucrose in the drinking water and monitored for survival. Mice injected with KPC cells engineered for inducible knockdown of PAK1 survived significantly longer than mice injected with KPC cells expressing control shRNA (p < 0.0001; Figure 4C). Necropsy was performed on all mice and assessment of the livers revealed that all mice in the control group, but none in the PAK1 shRNA group, developed liver macro-metastases (Supplementary Figure 9). Taken together, the anti-tumour efficacy of PAK1 loss-of-function in a model of experimental metastasis by pancreatic adenocarcinoma supports the conclusion that interfering with PAK1 signalling could have therapeutic efficacy in this tumour type.
Figure 4.
Inducible shRNA knockdown of PAK1 inhibits PDAC metastasis and prolongs mouse survival. (A) Comparison of PAK1 and MET expression and activity levels in cell lines derived from genetically engineered PDAC mouse models, KPC and KPP1. (B) Inducible PAK1 knockdown in shPAK1, but not shLacZ, stable cell clones and tumours. Cells in culture or tumour-bearing mice were treated with 250 ng/ml or 1 mg/ml doxycycline (DOX), respectively, to induce knockdown. (C) Kaplan–Meier curves comparing the survival of mice receiving KPC cells carrying either control shRNA or PAK1-selective shRNA. Inhibition of PAK1 significantly prolonged survival 90 days post-implantation (p < 0.0001).
PAK1 and MET co-expression in human PDAC tissues and correlation to clinical metastasis
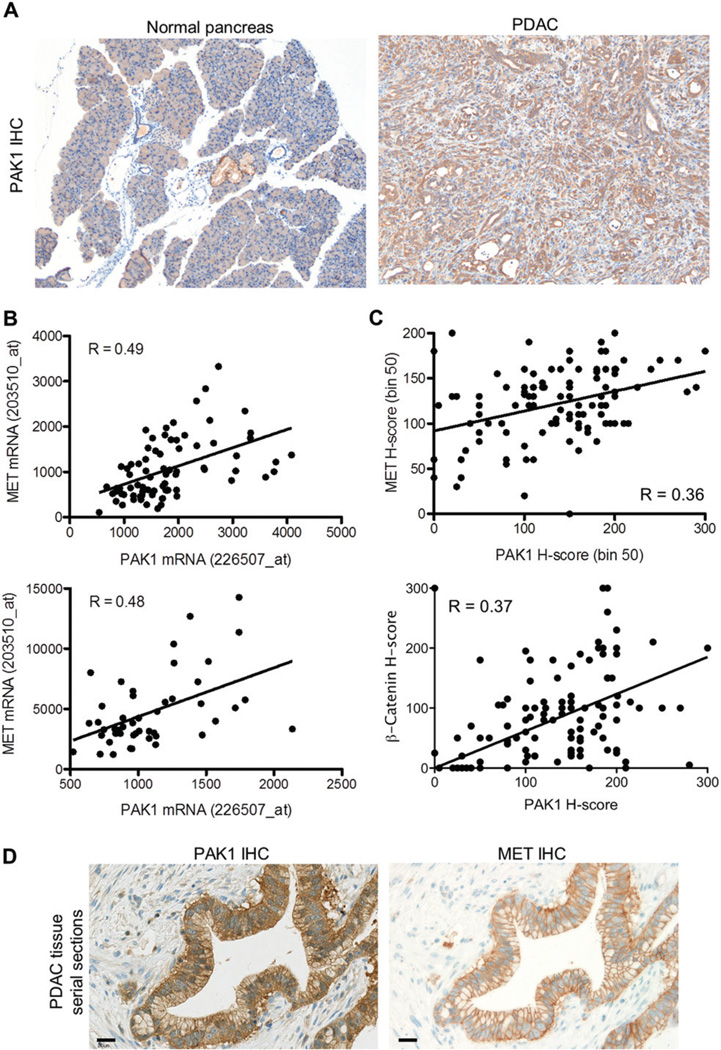
To determine the possible extent of PAK1 dysregulation in PDAC, we assayed primary human and murine tumour tissue samples from 304 patients using immunohistochemistry (IHC) (Figure 5A). Robust and selective IHC reactivity of PAK1 antibody had been previously demonstrated [23] (Supplementary Figure 10). In PDAC, 262 (86%) primary pancreatic tumour samples were positive for cytoplasmic PAK1 expression and 33.2% of all cases showed staining of moderate (2+) or strong (3+) intensity in the malignant cells (Supplementary Table 1). Nuclear localization of PAK1 was evident in approximately one-third of the samples. PAK1 was not appreciably expressed in normal pancreatic acini or islets, but was expressed at moderate levels in normal pancreatic ducts adjacent to the cancer (Figure 5A). Furthermore, mRNA was purified from two distinct cohorts of PDAC specimens (n=127) and PAK1 and MET expression were positively correlated (Figure 5B; p < 0.001). Comparable results for PAK1 and MET co-expression were also observed in publically available microarray data for PDAC (Supplementary Figure 11A). Given the observed connection between PAK1 and MET expression at the mRNA level, a subset of PDAC tumour tissues were also analysed and quantified for MET and p53 IHC (Supplementary Figure 12A and Supplementary Table 1). Notably, robust PAK1 and MET expression was seen in the same tumour tissues (Figure 5C) and also identical tumour cells in serial histological sections (Figure 5D). PDAC tissues with elevated PAK1 and MET levels also showed membranous β-catenin localization (Supplementary Figure 12B and Supplementary Table 1), which can affect cell–cell adhesion through its interactions with E-cadherin and is consistent with the immunofluorescence imaging performed using PDAC cell lines (Supplementary Figure 4A). PAK1 expression and β-catenin expression were also well correlated (Figure 5C and Supplementary Table 2). There was no evidence for nuclear accumulation of β-catenin or transcriptional up-regulation of WNT-pathway target genes such as Axin2 in these tissues (data not shown). To assess whether elevated PAK1 expression is also associated with clinical metastasis, autopsy specimens from a separate cohort of 36 American patients were analysed. PAK1 protein expression correlated with a widespread metastatic pattern (p=0.067, chi square test; Supplementary Figure 13), consistent with data obtained from experimental models (Figure 4C). Together, these data show that PAK1 is broadly up-regulated in human PDAC and that its expression is significantly associated with MET positivity and linked to tumour metastasis.
Figure 5.
Histological analysis of PAK1 and MET expression in PDAC tissues. (A) Representative images of PAK1 immunohistochemistry in normal and PDAC murine tissues. (B) Significant correlation of PAK1 and MET mRNA expression in two distinct cohorts of human PDAC tissues (n=79 and n=48 samples). Affymetrix probes for PAK1 and MET quantification are indicated. (C) Analysis of PAK1 and MET immunoreactivity by a semi-quantitative histological score (H-score). PAK1 and MET expression were significantly associated (Spearman R = 0.3549, p = 0.0002), as were PAK1 and β-catenin expression (Spearman R = 0.3744, p = 0.0001). (D) IHC analysis of adjacent, serial tissue sections of a human PDAC tumour to demonstrate PAK1 and MET co-expression. Both cytoplasmic and membranous immunohistochemical staining is observed for PAK1, and only membranous staining is detected for MET. Scale bar=20µm.
Discussion
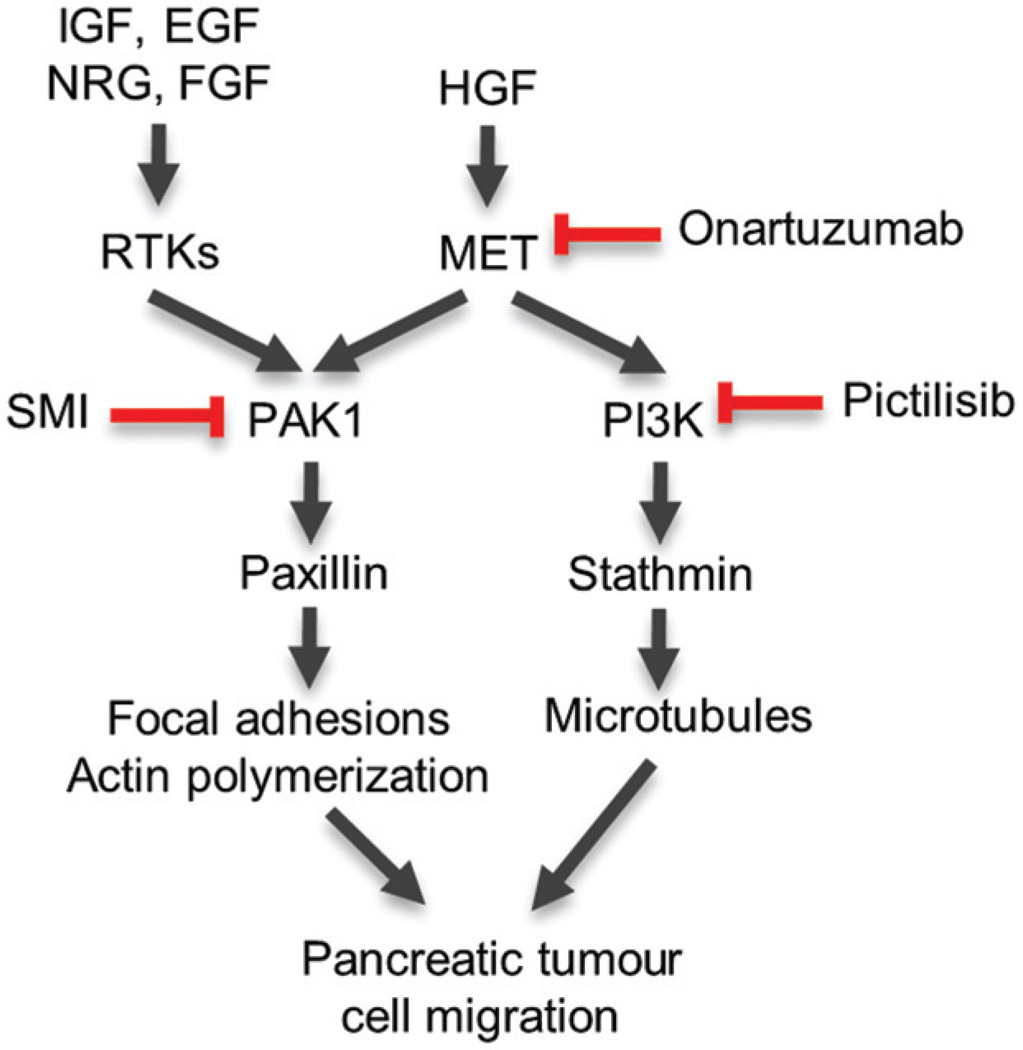
The majority of PDAC cases are characterized by alteration in only four genes that are not easily tractable targets for pharmacological intervention. Mutations in the KRAS oncogene occur in more than 90% of cases and tumour suppressors CDKN2A, p53, and DPC4/SMAD4 are altered in approximately 95%, 50–75%, and 55% of tumours, respectively [7]. Hence, given the poor treatment options and 5-year survival rate for PDAC patients, there is a need to identify effective and druggable targets for this disease. Herein, we report that PAK1 is highly expressed in human PDAC (Figure 5) and is a key effector of several receptor tyrosine kinases, including MET, to regulate the motility of pancreatic adenocarcinoma cells (Figure 1). Combination of PAK1 inhibition with MET antagonists currently in clinical development, such as onartuzumab, was essential for maximizing blockade of cell migration (Figures 2 and Figure 3). PAK1 inhibition also resulted in anti-tumour efficacy in a liver metastasis model of PDAC (Figure 4C). We demonstrated that combined inhibition of PAK1 and class I PI3K, via GDC-0941 (pictilisib) [41], phenocopied direct MET inhibition with respect to PDAC cell migration and signalling to the cytoskeleton (Figure 6). Given that GDC-0941 is currently in phase II trials, this combination represents another option for further preclinical and clinical validation.
Figure 6.
Diagram depicting the mechanism of action for PAK1-and PI3K–mediated regulation of the cytoskeleton and PDAC cell migration following HGF and growth factor stimulation. Targets of onartuzumab and pictilisib (GDC-0941) are indicated. In the context of growth factor-mediated resistance to onartuzumab, combined inhibition of MET and PAK1, or PAK1 and PI3K, may be effective strategies to limit tumour migration and invasion.
The most well-conserved, evolutionary roles for PAK1 and HGF/MET are in the regulation of cell shape, motility, and regulation of the actin and microtubule cytoskeleton [8,17]. There are previous reports that PAK family members are activated downstream of HGF to drive migration in other indications, such as prostate cancer cell lines [45,46]. Regulation of tumour cell motility by this pathway could play a role in promoting the invasive component that contributes to the growth of primary pancreatic adenocarcinomas. Tumour cell invasion, like the proliferative index, is a histological parameter that is generally associated with poor prognosis and can lead to surgical intractability and decreased quality of life for patients. For example, the pancreas is proximal to neural plexi in the retroperitoneum, and perineural invasion is a frequent source of pancreatic cancer recurrence, even after apparently curative surgery [6]. Perineural invasion is also associated with the extreme pain that is frequently observed in pancreatic cancer. Inhibition of MET and its associated effectors may also have a role in these settings and could delay further tumour invasion and growth.
Additional signalling mechanisms have been suggested for MET and PAK1 signalling, including activation of NF-κB [47] and PAK1-mediated feedback to MET activation via the regulation of merlin [48]. In our study, the dominant effectors for PAK1 in PDAC cells are related to cytoskeletal signalling; however, there are intriguing effectors, such as phosphorylation of the c-Jun transcription factor on key residues that are required for transactivation (Figure 3B), that warrant further investigation. In addition, characterization of PAK1 knockout mice has also allowed investigation of the role of this kinase in glucose homeostasis [49]. In pancreatic β-cells, PAK1 participates in insulin granule localization and vesicle release via the regulation of actin filament assembly and MAPK pathway signalling [50]. Given this physiological role for PAK1 in insulin secretion, glucose intolerance should be monitored for PDAC patients potentially treated with future PAK inhibitors. Together with our findings, these areas for future investigation may have important implications for the development of new strategies and agents for the treatment of PDAC.
Supplementary Material
Acknowledgments
We thank Debra Dunlap, Shari Lau, Linda Rangell, Suchit Jhunjhunwala, Maike Schmidt, Jeffrey Settle-man, and our immunohistochemistry facility for providing insightful discussions, suggestions, and technical assistance.
Footnotes
Conflict of interest statement: Some of the authors are employees and shareholders of Genentech/Roche.
Author contribution statement
WZ, AMJ, and HK conceived and designed the project. WZ, KL, and CCO performed in vitro experiments. QX, MR, and KT performed in vivo efficacy studies. MM and LZ conceived and supervised in vivo studies. GF, PMH, QS, and WFF conducted bioinformatics and statistical analyses. DA, RG, CP, AdJ-A, EMJ, and LZ provided tumour tissues. RD, LF, YY, YW, RR, and CP performed molecular analyses on tumour tissues. AMJ, CP, and HK conducted histological analyses. RMN, MB, and LSF provided key reagents. The manuscript was written by WZ and KPH. AMJ, PMH, CP, LZ, and KPH revised the manuscript. All authors approved the submitted version.
SUPPORTING INFORMATION ON THE INTERNET
The following supporting information may be found in the online version of this article:
Supplementary materials and methods.
Figure S1. Raw data for secreted protein library screen of PAK1-dependent motility using AsPC-1 cells.
Figure S2. Quantification of PAK1-dependent migration is shown for (A) AsPC-1, (B) KP4x1.1, (C) PSN-1, and (D) Capan-2 cells.
Figure S3. Combined inhibition of PAK1 overcomes HGF-mediated resistance to onartuzumab in KRAS wild-type BxPC-3 cells.
Figure S4. Subcellular localization and contribution to cell motility of PAK1 effectors.
Figure S5. Group I PAK inhibitor, IPA-3, modulates signalling to cytoskeletal-associated proteins similar to RNAi-mediated PAK1 knockdown in KP4x1.1 cells.
Figure S6. Characterization of growth factor-mediated resistance to onartuzumab in PDAC cells.
Figure S7. Comparison of KP4 and KP4x1.1 cell signalling and efficacy.
Figure S8. Inducible shRNA knockdown of PAK1 inhibits KPC cell motility (A) and proliferation (B).
Figure S9. Haematoxylin and eosin staining of liver showing metastatic tumour formation from mice implanted with KPC-shLacZ PDAC tumour cells.
Figure S10. Representation images of PAK1 immunochemistry of PDAC tissues showing (A) negative, (B) low, (C) moderate, and (D) high staining intensity.
Figure S11. Bioinformatics analysis of MET and PAK1 mRNA expression in PDA and non-small-cell lung cancer (NSCLC) adenocarcinoma.
Figure S12. Representative images of SP44 anti-MET and anti-β-catenin immunohistochemistry.
Figure S13. Elevated PAK1 expression may be associated with clinical metastasis.
Table S1. IHC and ISH data for PAK1, PAK4, MET, and p53 in PDAC.
Table S2. MET, PAK1, and β-catenin mRNA and protein expression are correlated in pancreatic adenocarcinoma.
References
- 1.Cancer Facts & Figures. [Accessed 1 March 2014];American Cancer Society. 2013 Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2013/index.
- 2.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed WB, Ng J, Wazer DE, et al. New tools and novel approaches in treating locally advanced pancreatic adenocarcinoma. JOP. 2012;13:354–357. doi: 10.6092/1590-8577/938. [DOI] [PubMed] [Google Scholar]
- 5.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th edn. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 6.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nature Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 9.Di Renzo MF, Poulsom R, Olivero M, et al. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995;55:1129–1138. [PubMed] [Google Scholar]
- 10.Li C, Wu JJ, Hynes M, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–2227. doi: 10.1053/j.gastro.2011.08.009. e5. [DOI] [PubMed] [Google Scholar]
- 11.Otte JM, Schwenger M, Brunke G, et al. Expression of hepatocyte growth factor, keratinocyte growth factor and their receptors in experimental chronic pancreatitis. Eur J Clin Invest. 2001;31:865–875. doi: 10.1046/j.1365-2362.2001.00894.x. [DOI] [PubMed] [Google Scholar]
- 12.Martens T, Schmidt NO, Eckerich C, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res. 2006;12:6144–6152. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 13.Merchant M, Ma X, Maun HR, et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci U S A. 2013;110:E2987–2996. doi: 10.1073/pnas.1302725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:4105–4114. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catenacci DV, Henderson L, Xiao SY, et al. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov. 2011;1:573–579. doi: 10.1158/2159-8290.CD-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royal I, Lamarche-Vane N, Lamorte L, et al. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao ZS, Manser E. PAK family kinases: physiological roles and regulation. Cell Logist. 2012;2:59–68. doi: 10.4161/cl.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whale A, Hashim FN, Fram S, et al. Signalling to cancer cell invasion through PAK family kinases. Front Biosci. 2011;16:849–864. doi: 10.2741/3724. [DOI] [PubMed] [Google Scholar]
- 19.He H, Shulkes A, Baldwin GS. PAK1 interacts with beta-catenin and is required for the regulation of the beta-catenin signalling pathway by gastrins. Biochim Biophys Acta. 2008;1783:1943–1954. doi: 10.1016/j.bbamcr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Arias-Romero LE, Villamar-Cruz O, Huang M, et al. PAK1 kinase links ErbB2 to beta-catenin in transformation of breast epithelial cells. Cancer Res. 2013;73:3671–3682. doi: 10.1158/0008-5472.CAN-12-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–116. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eswaran J, Li DQ, Shah A, et al. Molecular pathways: targeting p21-activated kinase 1 signaling in cancer - opportunities, challenges, and limitations. Clin Cancer Res. 2012;18:3743–3749. doi: 10.1158/1078-0432.CCR-11-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong CC, Jubb AM, Haverty PM, et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2011;108:7177–7182. doi: 10.1073/pnas.1103350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow HY, Jubb AM, Koch JN, et al. p21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012;72:5966–5975. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong CC, Jubb AM, Jakubiak D, et al. P21-activated kinase 1 (PAK1) as a therapeutic target in BRAF wild-type melanoma. J Natl Cancer Inst. 2013;105:606–615. doi: 10.1093/jnci/djt054. [DOI] [PubMed] [Google Scholar]
- 26.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Mancera PA, Rust AG, van der Weyden L, et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486:266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koeppen H, Yu W, Zha J, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib {+/−} onartuzumab in advanced non-small-cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res. 2014;20:4488–4498. doi: 10.1158/1078-0432.CCR-13-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jubb AM, Chalasani S, Frantz GD, et al. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene. 2006;25:3445–3457. doi: 10.1038/sj.onc.1209382. [DOI] [PubMed] [Google Scholar]
- 31.Geyer FC, Lacroix-Triki M, Savage K, et al. Beta-catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Lee W, Jiang Z, et al. Genome and transcriptome sequencing of lung cancers reveal diverse mutational and splicing events. Genome Res. 2012;22:2315–2327. doi: 10.1101/gr.140988.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deacon SW, Beeser A, Fukui JA, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodig SJ, Shapiro GI. Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr Opin Investig Drugs. 2010;11:1477–1490. [PubMed] [Google Scholar]
- 35.Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain A, Slansky JE, Matey LC, et al. Synergistic effect of a granulocyte-macrophage colony-stimulating factor-transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Ann Surg Oncol. 2003;10:810–820. doi: 10.1245/aso.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura K, Jain A, Allen HE, et al. Selective targeting of antitumor immune responses with engineered live-attenuated Listeria monocytogenes. Cancer Res. 2006;66:1096–1104. doi: 10.1158/0008-5472.CAN-05-2307. [DOI] [PubMed] [Google Scholar]
- 38.Jin H, Yang R, Zheng Z, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360–4368. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- 39.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 40.Hoeflich KP, Merchant M, Orr C, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72:210–219. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]
- 41.Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H–indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 42.Wallin JJ, Guan J, Edgar KA, et al. Active PI3K pathway causes an invasive phenotype which can be reversed or promoted by blocking the pathway at divergent nodes. PloS One. 2012;7:e36402. doi: 10.1371/journal.pone.0036402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada T, Okajima F, Adachi M, et al. Growth dependency of a new human pancreatic cancer cell line, YAPC, on autocrine interleukin-1α stimulation. Int J Cancer. 1998;76:141–147. doi: 10.1002/(sici)1097-0215(19980330)76:1<141::aid-ijc22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Zheng L, Foley K, Huang L, et al. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PloS One. 2011;6:e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells CM, Whale AD, Parsons M, et al. PAK4: a pluripotent kinase that regulates prostate cancer cell adhesion. J Cell Sci. 2010;123:1663–1673. doi: 10.1242/jcs.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bright MD, Garner AP, Ridley AJ. PAK1 and PAK2 have different roles in HGF-induced morphological responses. Cell Signal. 2009;21:1738–1747. doi: 10.1016/j.cellsig.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Fan S, Gao M, Meng Q, et al. Role of NF-κB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005;24:1749–1766. doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- 48.Shrestha Y, Schafer EJ, Boehm JS, et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene. 2012;31:3397–3408. doi: 10.1038/onc.2011.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiang YT, Jin T. p21-Activated protein kinases and their emerging roles in glucose homeostasis. Am J Physiol Endocrinol Metab. 2014;306:E707–E722. doi: 10.1152/ajpendo.00506.2013. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Oh E, Clapp DW, et al. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem. 2011;286:41359–41367. doi: 10.1074/jbc.M111.291500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.