Monoaminergic hypothesis of depression
The monoaminergic hypothesis of depression posits that this illness results from a deficit in serotonin (5-HT), noradrenaline, and dopamine signaling in the brain. Of these monoamines, the serotonergic system has been the one most strongly implicated in the pathophysiology and treatment of mood disorders. Although this relationship has not been proven, many findings indirectly support this hypothesis: (a) a subgroup of depressed patients have a very marked reduction of the concentration of 5-HT in plasma (Sarrias et al., 1987) and of its metabolite, 5-hydroxyindoleacetic acid, in the cerebrospinal fluid (Asberg et al., 1976) that may reflect decreased serotonergic transmission in the brain; (b) most prescribed antidepressant drugs increase serotonergic transmission in the long term; (c) a clinical study showed that in ∼50% of depressed patients that responded to selective 5-HT reuptake inhibitors (SSRIs) (see Fig. 1), depressive symptoms relapsed after depletion of the 5-HT precursor tryptophan (Delgado et al., 1999); (d) recent preclinical investigations have reported that an intact serotonergic system is required for an antidepressant response to experimental treatments such as deep brain stimulation or ketamine (Hamani et al., 2010; Gigliucci et al., 2013); and (e) optogenetic activation of the prefrontal cortex projection to the dorsal raphe (DR) nucleus produces antidepressant-like effects in rodents (Covington et al., 2010; Warden et al., 2012). Therefore, there is evidence that deficits in serotonergic transmission are associated with at least some depressive states and a poor response to antidepressant drugs (Sachs et al., 2015), whereas the activation of 5-HT neurons in the DR evokes an antidepressant response.
Figure 1.
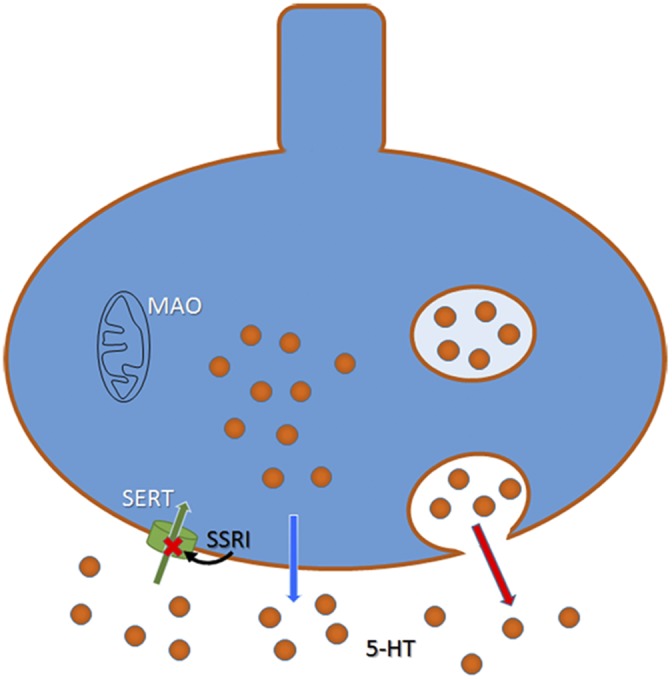
Schematic drawing of a 5-HT neuron showing exocytotic release from vesicles (red arrow) and the nonexocytotic release described by Mlinar et al. (2015) (blue arrow). Reuptake of 5-HT through SERT is depicted with a green arrow. SSRIs block SERT, thus inhibiting reuptake of 5-HT. Monoamine oxidase (MAO) enzyme is located in the external mitochondrial wall.
Role of raphe 5-HT in the effects of antidepressant drugs
Most serotonergic neurons originate in the DR nucleus found in the brainstem. This nucleus is highly enriched in 5-HT transporters (SERTs) and in inhibitory 5-HT1A autoreceptors. Although the roles of extracellular 5-HT and 5-HT1A autoreceptors in the DR have been studied extensively (Piñeyro and Blier, 1999; Adell et al., 2002), their exact function remains unclear. Some studies have shown that 5-HT1A autoreceptors function as sensors that respond only when the concentration of endogenous 5-HT in the extracellular compartment becomes excessive (Adell et al., 2002), whereas other work has provided evidence for tonic activation of 5-HT1A autoreceptors under certain experimental conditions (Haddjeri et al., 2004). It was first demonstrated in the early 90s that current antidepressant drugs predominantly increase extracellular 5-HT in the raphe region (Adell and Artigas, 1991; Invernizzi et al., 1992), thereby activating inhibitory 5-HT1A autoreceptors, reducing cell firing, and having a negative feedback influence on 5-HT release. Thus, it is likely that the rapid pharmacological action of SSRIs (and other antidepressant drugs) in the DR—desired to act like an accelerator—initially act as a brake in the therapeutic process.
Several recent studies support this contention. Thus, the selective knockdown of presynaptic 5-HT1A (autoreceptors) but not postsynaptic 5-HT1A receptors by small-interfering RNA (siRNA) results in clear-cut antidepressant-like behaviors in mice (Bortolozzi et al., 2012). In contrast, mice that overexpress the DR 5-HT1A receptor exhibit increased behavioral despair, and no behavioral response to antidepressant drugs (Richardson-Jones et al., 2010). This is consistent with clinical data showing that people with increased density or activity of 5-HT1A autoreceptors are more susceptible to mood disorders and respond poorly to antidepressant treatments (Stockmeier et al., 1998; Neff et al., 2009). It is thus evident that the serotonergic transmission in projection areas is controlled, at least in part, by basal serotonergic activity on somatodendritic 5-HT1A autoreceptors on serotonergic neurons in the raphe (Sharp et al., 1989; Riad et al., 2000; Crespi, 2009). Importantly, it is of note that there is a substantial delay in the therapeutic effect of SSRIs, and it has been subsequently postulated that autoinhibitory control caused by increased extracellular raphe 5-HT might be responsible for this delay (Artigas et al., 1996). Therefore, it is critical to identify the release mechanisms that fill the extracellular compartment of the DR with 5-HT.
Mechanisms of 5-HT release in the raphe nuclei
SSRIs produce a sustained increase in extracellular 5-HT in the DR, and this leads to autoinhibition of serotonergic neurons. However, it is clear that, even though the 5-HT1A autoreceptors are activated by extracellular 5-HT, the transmitter must be released in some way (presumably independent of 5-HT cell firing) to replenish the extracellular pool. Various mechanisms have been suggested to explain the local release of 5-HT from DR neurons in the raphe nucleus. Several earlier studies suggested that extracellular 5-HT in the raphe nuclei depended on neuronal stimulation and release from storage vesicles by exocytosis (Matos et al., 1996; Portas et al., 1996; Tao et al., 1997) (see Fig. 1). The thorough study by Mlinar et al. (2015) now shows for the first time that substantial nonexocytotic 5-HT release takes place in the absence of neuronal activity, Ca2+ influx, or vesicular monoamine transporter 2–mediated vesicular accumulation of 5-HT. Furthermore, Mlinar et al. elegantly demonstrate that blockade of SERT by an SSRI does not disrupt nonexocytotic release of 5-HT, ruling out the possibility that it depends on reverse transport by SERT. Collectively, their results suggest that a prominent fraction of extracellular 5-HT in the raphe nuclei is released from a cytoplasmic pool by simple diffusion across the plasma membrane. These results contrast with those of Colgan et al. (2012), who observed action potential–independent, but vesicle-dependent release of 5-HT from dendrites. Further research is needed to determine whether the cytoplasmic 5-HT identified by Mlinar et al. (2015) represents a “functional” transmitter pool in the DR, i.e., if it has physiological and behavioral consequences.
Notably, Mlinar et al. (2015) performed their analyses in slices of brainstem exposed to the α1-adrenoceptor agonist, phenylephrine, and a cocktail of drugs indicative of a lack of glutamatergic and GABAergic tone on 5-HT release from serotonergic neurons. These findings strongly suggest that glutamatergic and GABAergic inputs to the DR do not contribute substantially to 5-HT cell autoinhibition. Moreover, the results are indicative of a relative scarcity of serotonergic transmission in the DR measured as the probability of evoking serotonergic inhibitory postsynaptic potentials (IPSC5-HT) in serotonergic neurons. Most of the recorded neurons were located in the dorsal and the ventromedial part of the DR, and higher IPSC5-HT success rates were reported in slices containing the centrocaudal extent of DR, which is the subdomain of the nucleus that projects to areas in the hippocampus that influence theta activity (Commons, 2015), thought to underlie learning and memory.
Future prospects
Depression is the most common neuropsychiatric disorder and, according to World Health Organization, is predicted to be the second leading cause of global disability burden by 2020. Most existing antidepressant drugs target the serotonergic system but, unfortunately, they fall short of being adequate for a large subset of patients. One of the main drawbacks of such therapy is the therapeutic delay of SSRIs, for which the increase in extracellular 5-HT in the raphe nuclei is postulated to be a key factor. To overcome this problem, it is important to characterize the precise mechanism(s) of transmitter release in this structure. Previous work has shown that there are multiple ways to induce 5-HT release in raphe nuclei, as also observed in the forebrain (Adell et al., 1989). The study of Mlinar et al. (2015) uses an in vitro preparation to describe in detail a new mechanism of nonexocytotic and nonvesicular release of 5-HT in the raphe nuclei. An important feature of this process is that it is not performed by reversal of SERT, which means that it cannot be inhibited by SSRIs. The in vitro nature of the study is a clear limitation and further research is needed to determine its therapeutic potential. Should this nonexocytotic release of 5-HT in the raphe nuclei occur in vivo, it would represent an important target for antidepressant drugs inasmuch as raphe 5-HT exerts a fine tuning of the control of serotonergic transmission throughout the brain.
Acknowledgments
Elizabeth M. Adler served as editor.
References
- Adell A., and Artigas F.. 1991. Differential effects of clomipramine given locally or systemically on extracellular 5-hydroxytryptamine in raphe nuclei and frontal cortex. Naunyn Schmiedebergs Arch. Pharmacol. 343:237–244. 10.1007/BF00251121 [DOI] [PubMed] [Google Scholar]
- Adell A., Sarna G.S., Hutson P.H., and Curzon G.. 1989. An in vivo dialysis and behavioural study of the release of 5-HT by p-chloroamphetamine in reserpine-treated rats. Br. J. Pharmacol. 97:206–212. 10.1111/j.1476-5381.1989.tb11943.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell A., Celada P., Abellán M.T., and Artigas F.. 2002. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Brain Res. Rev. 39:154–180. 10.1016/S0165-0173(02)00182-0 [DOI] [PubMed] [Google Scholar]
- Artigas F., Romero L., de Montigny C., and Blier P.. 1996. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 19:378–383. 10.1016/S0166-2236(96)10037-0 [DOI] [PubMed] [Google Scholar]
- Asberg M., Thorén P., Träskman L., Bertilsson L., and Ringberger V.. 1976. “Serotonin depression”—a biochemical subgroup within the affective disorders? Science. 191:478–480. 10.1126/science.1246632 [DOI] [PubMed] [Google Scholar]
- Bortolozzi A., Castañé A., Semakova J., Santana N., Alvarado G., Cortés R., Ferrés-Coy A., Fernández G., Carmona M.C., Toth M., et al. 2012. Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol. Psychiatry. 17:612–623. 10.1038/mp.2011.92 [DOI] [PubMed] [Google Scholar]
- Colgan L.A., Cavolo S.L., Commons K.G., and Levitan E.S.. 2012. Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J. Neurosci. 32:15737–15746. 10.1523/JNEUROSCI.0020-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons K.G. 2015. Two major network domains in the dorsal raphe nucleus. J. Comp. Neurol. 10.1002/cne.23748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington H.E. III, Lobo M.K., Maze I., Vialou V., Hyman J.M., Zaman S., LaPlant Q., Mouzon E., Ghose S., Tamminga C.A., et al. 2010. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 30:16082–16090. 10.1523/JNEUROSCI.1731-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi F. 2009. Apamin increases 5-HT cell firing in raphe dorsalis and extracellular 5-HT levels in amygdala: A concomitant in vivo study in anesthetized rats. Brain Res. 1281:35–46. 10.1016/j.brainres.2009.05.021 [DOI] [PubMed] [Google Scholar]
- Delgado P.L., Miller H.L., Salomon R.M., Licinio J., Krystal J.H., Moreno F.A., Heninger G.R., and Charney D.S.. 1999. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol. Psychiatry. 46:212–220. 10.1016/S0006-3223(99)00014-1 [DOI] [PubMed] [Google Scholar]
- Gigliucci V., O’Dowd G., Casey S., Egan D., Gibney S., and Harkin A.. 2013. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl.). 228:157–166. 10.1007/s00213-013-3024-x [DOI] [PubMed] [Google Scholar]
- Haddjeri N., Lavoie N., and Blier P.. 2004. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology. 29:1800–1806. 10.1038/sj.npp.1300489 [DOI] [PubMed] [Google Scholar]
- Hamani C., Diwan M., Macedo C.E., Brandão M.L., Shumake J., Gonzalez-Lima F., Raymond R., Lozano A.M., Fletcher P.J., and Nobrega J.N.. 2010. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry. 67:117–124. 10.1016/j.biopsych.2009.08.025 [DOI] [PubMed] [Google Scholar]
- Invernizzi R., Belli S., and Samanin R.. 1992. Citalopram’s ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug’s effect in the frontal cortex. Brain Res. 584:322–324. 10.1016/0006-8993(92)90914-U [DOI] [PubMed] [Google Scholar]
- Matos F.F., Urban C., and Yocca F.D.. 1996. Serotonin (5-HT) release in the dorsal raphé and ventral hippocampus: Raphé control of somatodendritic and terminal 5-HT release. J. Neural Transm. 103:173–190. 10.1007/BF01292626 [DOI] [PubMed] [Google Scholar]
- Mlinar B., Montalbano A., Baccini G., Tatini F., Berlinguer Palmini R., and Corradetti R.. 2015. Nonexocytotic serotonin release tonically suppresses serotonergic neuron activity. J. Gen. Physiol. 145:225–251. 10.1085/jgp.201411330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff C.D., Abkevich V., Packer J.C., Chen Y., Potter J., Riley R., Davenport C., DeGrado Warren J., Jammulapati S., Bhathena A., et al. 2009. Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Mol. Psychiatry. 14:621–630. 10.1038/mp.2008.8 [DOI] [PubMed] [Google Scholar]
- Piñeyro G., and Blier P.. 1999. Autoregulation of serotonin neurons: Role in antidepressant drug action. Pharmacol. Rev. 51:533–591. [PubMed] [Google Scholar]
- Portas C.M., Thakkar M., Rainnie D., and McCarley R.W.. 1996. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J. Neurosci. 16:2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M., Garcia S., Watkins K.C., Jodoin N., Doucet E., Langlois X., el Mestikawy S., Hamon M., and Descarries L.. 2000. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 417:181–194. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones J.W., Craige C.P., Guiard B.P., Stephen A., Metzger K.L., Kung H.F., Gardier A.M., Dranovsky A., David D.J., Beck S.G., et al. 2010. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 65:40–52. 10.1016/j.neuron.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs B.D., Ni J.R., and Caron M.G.. 2015. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc. Natl. Acad. Sci. USA. 112:2557–2562. 10.1073/pnas.1416866112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrias M.J., Artigas F., Martínez E., Gelpí E., Alvarez E., Udina C., and Casas M.. 1987. Decreased plasma serotonin in melancholic patients: A study with clomipramine. Biol. Psychiatry. 22:1429–1438. 10.1016/0006-3223(87)90100-4 [DOI] [PubMed] [Google Scholar]
- Sharp T., Bramwell S.R., Clark D., and Grahame-Smith D.G.. 1989. In vivo measurement of extracellular 5-hydroxytryptamine in hippocampus of the anaesthetized rat using microdialysis: Changes in relation to 5-hydroxytryptaminergic neuronal activity. J. Neurochem. 53:234–240. 10.1111/j.1471-4159.1989.tb07319.x [DOI] [PubMed] [Google Scholar]
- Stockmeier C.A., Shapiro L.A., Dilley G.E., Kolli T.N., Friedman L., and Rajkowska G.. 1998. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J. Neurosci. 18:7394–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R., Ma Z., and Auerbach S.B.. 1997. Influence of AMPA/kainate receptors on extracellular 5-hydroxytryptamine in rat midbrain raphe and forebrain. Br. J. Pharmacol. 121:1707–1715. 10.1038/sj.bjp.0701292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden M.R., Selimbeyoglu A., Mirzabekov J.J., Lo M., Thompson K.R., Kim S.Y., Adhikari A., Tye K.M., Frank L.M., and Deisseroth K.. 2012. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 492:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]


