Abstract
HIV is an immunosuppressive virus that targets CD4+ T-lymphocytes. HIV infections cause increased susceptibility to opportunistic infections and cancer. HIV infection can also alter central nervous system (CNS) function causing cognitive impairment. HIV does not infect neurons but it does infect astrocytes and microglia in the CNS. HIV can also infect enteric glia initiating an intestinal inflammatory response which causes enteric neural injury and gut dysfunction. Part of the inflammatory response is HIV induced production of proteins including, Transactivator of transcription (Tat) which contribute to neuronal injury after release from HIV infected glial cells. A risk factor for HIV infection is intravenous drug use with contaminated needles and chronic opiate use can exacerbate neural injury in the nervous system. While most research focuses on the actions of Tat and other HIV related proteins and opiates on brain function, recent data indicate that Tat can cause intestinal inflammation and disruption of enteric neuron function, including alteration of Na+ channel activity and action potential generation. A paper published in this issue of Neurogastroenterology and Motility extends these findings by identifying an interaction between Tat and morphine on enteric neuron Na+ channel function and on intestinal motility in vivo using a Tat expressing transgenic mouse model. These new data show that Tat protein can enhance the inhibitory actions of morphine on action potential generation and propulsive motility. These findings are important to our understanding of how HIV causes diarrhea in infected patients and for the use of opioid drugs to treat HIV-induced diarrhea.
Keywords: enteric nervous system, Tat, sodium channels, enteric neuropathy, diarrhea
INTRODUCTION
Human immune deficient virus (HIV) is the viral infection responsible for acquired immune deficiency syndrome (AIDS). HIV initially infects peripheral CD4+ T-lymphocytes to cause immune suppression but HIV also gains access to the central nervous system (CNS) via the cerebral spinal fluid, perivascular macrophages or infected T-lymphocytes. In the brain, HIV infects macrophages and microglia (which express low CD4 levels) where the virus replicates.1 HIV infection of the brain causes neural injury resulting in what was initially called HIV associated dementia (HAD) but is now labelled HIV-associated neurocognitive disorders (HAND) to better describe the range of changes caused by brain HIV infections.2,3 HAND results from “by-stander” effects of proteins shed by infected macrophages, microglia and astrocytes. HIV trans-activating protein (Tat) is one of these neurotoxic proteins. Tat is a viral protein that enhances the efficiency of viral genome transcription by binding to RNA polymerase II which accelerates HIV replication.4,5 Tat is secreted by infected lymphocytes and in the brain it is secreted by infected macrophages and microglia. Tat stimulates cytokine production causing inflammation and oxidative stress that can directly damage neurons and Tat can stimulate apoptosis.6 In addition, Tat is toxic to neurons because it disrupts intracellular Ca2+ handling7 and causes excitoxicity by direct action on neurons.8
HIV AND MORPHINE DISRUPT NERVOUS SYSTEM FUNCTION
A risk factor for HIV infection is intravenous drug use. Heroin and morphine are commonly abused and addictive drugs that are self-administered intravenously. This is important as morphine (the active product of heroin metabolism in the brain) enhances the neurotoxic effects of Tat.9 The synergistic neurotoxic effects of Tat and morphine are mediated by mu-opioid receptors (MOR) and are glial cell-dependent.9,10 Morphine enhances Tat-induced Ca2+ increases in astrocytes which then secrete higher levels of the chemokines monocyte chemoattractant protein-1 (MCP-1) and regulated on activation normal T cell expressed and secreted (RANTES) and the inflammatory cytokine, interleukin-6 (IL-6).10,11 Astrocyte release of MCP-1 and RANTES attracts activated microglia to the sites of infected astrocytes. Tat does not produce these effects in the absence of morphine. Morphine and Tat also have a direct synergistic action on microglia to further enhance microglia activation.12 The end result is that the Tat/morphine combination stimulates production and release of inflammatory cytokines by astrocytes and also secrete chemokines which attract activated microglia leading to an enhanced inflammatory response at sites of HIV infected glial cells. This leads to increased neurotoxicity and HAND development.
Most studies have focused on CNStoxicity of HIV infection and its synergistic interactions with morphine as these actions cause significant cognitive impairment. However, the gut immune system is extensive and very active and GI dysfunction is a common consequence of HIV infections. HIV infects gastrointestinal lymphocytes and macrophages causing disruption of gut immune regulation. Enterocytes are a target for HIV infection and changes in enterocyte function will alter gastrointestinal secretion and cause malabsorption.13 This results in chronic diarrhea and malabsorption and contributes to the wasting syndrome in AIDS patients.14 HIV infected patients also have disrupted upper gastrointestinal motor function that would include dyspepsia, nausea and vomiting15 and intestinal pseudo-obstruction is not an uncommon finding in HIV infected patients.16
Alterations in gut motor function have been attributed to enteric neuropathies although these are scant data to support this hypothesis. However, a recent study by Ngwainmbi and colleagues examined Tat-related intestinal inflammation and motor function in mice in vivo and on murine myenteric neurons maintained in primary tissue culture.17 These investigators used a transgenic mouse that expressed Tat under the control of a tetracycline responsive glial fibrillary associated protein (GFAP) promoter. When these mice were fed doxycycline (DOX, which binds to the tetracycline sensitive promoter) containing chow, Tat expression was induced selectively in glial cells, including enteric glia. Tat expressing mice had accelerated gastrointestinal transit and increased fecal output. This would be consistent with the diarrhea observed in HIV infected patients. Tat treatment of enteric neuron/glia co-cultures increased extracellular levels of IL-6 and RANTES but not TNF-α.s found that when Consistent with this RANTES, IL-6 and IL-1b, but not TNF-α are increased in the intestines of Tat+ transgenic mice. This is similar to the cytokine and chemokine profile detected in brain neuron/glial co-cultures treated with Tat and morphine or in the brain of mice treated with Tat and morphine.9,10,11 Ngwainmbi et al.,17 also studied the neuronal effects Tat on murine myenteric neurons using whole cell patch clamp techniques. Neurons were exposed to Tat either by in vitro incubation during tissue culture, or by in vivo exposure for 4 days in the Tat+ transgenic mice. Control neurons not exposed to Tat either in vitro or in vivo fired single action potentials during membrane depolarization from the resting potential while Tat exposed neurons fired multiple action potentials. In addition, Tat exposed neurons required smaller depolarizations (reduced rheobase) to reach action potential threshold. The increased excitability is likely due to a leftward shift in the voltage activation curve for Na+ currents meaning that Na+ channels open at more negative membrane potentials in Tat exposed compared to control neurons. The changes in Na+ current properties are linked to changes in Na+ channel isoform expression in Tat exposed neurons. Myenteric neurons maintained in tissue culture and exposed to Tat for 2 days and myenteric neurons exposed to Tat in vivo using Tat+/DOX mice exhibited increased expression of the tetrodotoxin-resistant isoforms of voltage-gated Na+ channels (NaV1.7 and NaV1.8) while expression of the tetrodotoxin sensitive isoform (NaV1.3) was unaffected by Tat. This is an interesting result as tetrodotoxin-resistant (NaV1.7 and NaV1.8) channels (particularly NaV1.8) are upregulated in primary afferent sensory neurons in many inflammatory disorders and in neuropathic pain models.18 Upregulation of tetrodotoxin-insensitive Na+ channels in sensory neurons increases their excitability and this contributes to hypersensitivity of the sensory neurons in inflammation and other chronic pain disorders. The increased expression of NaV1.7 and NaV1.8 is likely a result of the increased levels of inflammatory cytokines that occur in the Tat+/DOX mice expressing Tat selectively in glial cells. In human HIV patients intestinal inflammation can also occur in response to opportunistic infections that occur in immune compromised patients. Na+ channel expression can also be altered directly by bacterial products that leak through the compromised intestinal barrier that occurs during intestinal inflammation.19
Na+ CHANNELS ARE A TARGET FOR TAT/MORPHINE INTERACTION IN THE ENS
As discussed above, there is a synergistic interaction between the actions of morphine and Tat on central nervous system toxicity and this interaction requires glial cells. Glial cells are also abundant in the enteric nervous system20,21 so it might be expected that similar morphine-Tat synergy occurs to produce enteric neurotoxicity. Fitting et al.22 in this issue of Neurogastroenterology and Motility have begun to address this question using the glial expressing Tat+/DOXmouse model described above. Morphine is a strong antidiarrheal drug and constipation is one of the main side effects of morphine (and other opiate drugs) when used to treat chronic pain.23 Synthetic opioid receptor agonists such as loperamide (which does not cross the blood brain barrier) are effective antidiarrheal drugs.24
The MOR is the predominant subtype of opioid receptor expressed by enteric neurons and all opioid receptors are G-protein coupled receptors.25 MOR agonists produce constipation by inhibiting enteric neuron function by linking to inhibition of adenylate cyclase and protein kinase A (PKA) activation26 and by linking to inhibition of Ca2+ and Na+ channels and to activation of K+ channels.27,28,29 Inhibition of nerve terminal Ca2+ channels reduces neurotransmitter release from enteric nerves. K+ channel activation causes membrane potential hyperpolarization and inhibition of action potential firing.30,31 Both effects are mediated by G-protein coupling to the Ca2+ or K+ channel either decreasing (Ca2+) or increasing (K+) the opening probability of the channel.27,29,32 Opioid agonist induced inhibition of enteric neurotransmitter release and decreased excitability of enteric neurons disrupts the function of the enteric nerve circuitry responsible for propulsive motility patterns and for control of intestinal secretion.33
Fitting and co-workers22 studied Na+ currents in small intestinal myenteric neurons from Tat−/DOX and Tat+/DOX mice described above. Neurons were placed in primary culture and Na+ currents were recorded using whole cell patch clamp methods. Na+ currents were more sensitive to morphine inhibition in neurons from Tat+/DOXmice vs.Tat−/DOX mice where a concentration of morphine (0.03 μmol−1) that did not affect Na+ currents in Tat−/DOX neurons inhibited Na+ currents in Tat+/DOX neurons by ~50%. This was due to a reduction in Na+ channel density (channels available to open per unit area of cell membrane). A reduction in the number of Na+ channels in the membrane or a functional change in the Na+ channels that result in a lower likelihood that channels open at a given membrane potential could account for these results. This question was addressed by applying Tat protein to myenteric neurons for >6 h in primary culture. This treatment also enhanced Na+ channel sensitivity to the inhibitory effects of morphine. This time course would be long enough to cause either changes in Na+ membrane expression or Na+ function. Additional voltage clamp studies suggest that morphine produced changes in the function of available channels. Na+ channels inactivate (some isoforms inactivate rapidly, others more slowly but this still occurs in <1s) and inactivation depends on membrane potential. More Na+ channels become unavailable to open when the membrane potential is less negative. Morphine alone studied in myenteric neurons from Tat−/DOX mice treated shifted the Na+ activation curve to more negative potentials. This effect was markedly enhanced in Tat+/DOX mice. The functional consequence of this shift is that there will be fewer Na+ channels available to produce action potentials when the membrane potential reaches the threshold for Na+ activation and action potential generation. The threshold for Na+ channel activation was not altered by morphine alone or in morphine-treated neurons from Tat+/DOX mice. These data indicate that morphine and Tat protein collaborate to alter the functional properties of Na+ channels. It is unlikely that Tat changes the expression of MOR as mRNA for this receptor was similar in Tat−/DOX and Tat+/DOX mice. MOR agonist drugs including buprenorphine, fentanyl and tramadol can block Na+ channels using a mechanism similar to local anesthetics where the drugs enter the ion pore from the inside of the cell membrane. However, morphine does not inhibit Na+ channels via this mechanism.34 Morphine can reduce the voltage sensitivity of Na+ channels (stronger depolarizations were required to open the channels) and this effect does not require G-protein dependent signaling mechanisms.35 Perhaps, inflammatory cytokines released by glial cells in vivo in morphine treated Tat+/DOX mice or glial cells that are present in the primary cultures of enteric neurons activate intracellular signaling pathways that modify the functional properties of Na+ channels.22 The interaction of morphine and Tat protein on Na+ channel function is interesting but the significance of this interaction is highlighted by in vivo data showing that Tat+/DOX mice were more sensitive to the constipating actions of morphine (reduced fecal pellet output) compared to Tat−/DOX mice.
The studies of Fitting et al.22 provide provocative new information about potential interactions between inflammation and drugs on enteric neuron function. The details of the mechanisms by which morphine alone modulates Na+ channel function and how HIV infection interacts with the cellular effects of morphine on enteric neurons remain to be determined. Morphine modulation of Na+ channel function differs from how MOR couples to modulation of Ca2+ and K+ channels as the Na+ channel effect does not appear to be linked directly to G-protein activation. As shown previously, Tat+ mice have increased intestinal levels of a number of important inflammatory cytokines that could contribute to the increased GI transit and diarrhea that occurs in HIV infected patients. Another potential molecular link may be through glycogen synthase kinase-3β (GSK3β). GSK3β contributes to neuronal signaling involved in neural plasticity,36 HIV neurotoxicity37 and neuropathology caused by chronic opiate abuse.38 Morphine and supernatants from HIV infected THP-1 cells (a monocytic leukemia cell line) produced synergistic neurotoxic effects on murine striatal neurons maintained in tissue culture. This effect was blocked by GSK3β inhibitors.39 Studies of the effects of GSK3β inhibitors on morphine/Tat interactions in enteric neurons would be interesting.
CONCLUSION
Diarrhea is a common co-morbidity associated with HIV infection.13,14,40 Prior to the widespread use of cART this was caused largely by opportunistic infections but in the cART era diarrhea is most often a consequence of the effects of antiretroviral drugs. However, HIV can directly affect gut function through infection of enterocytes causing increasing intestinal permeability and also stimulation of Cl− secretion.13,14 HIV infection of the enteric nervous system, including enteric glia, is also likely to contribute to disruption of gut motility and secretion by activating inflammatory signaling pathways. Treatment of HIV-associated diarrhea includes the use of the blood brain barrier impermeant meperidine analog, loperamide.40 The synergistic interactions between morphine, Tat and inflammation in the brain and in the gut suggest that further studies are needed to determine if these interactions are morphine specific or if they extend to other opioid drugs that activate enteric MORs. It would also be interesting to study potential interactions between endogenous opioid peptides released by myenteric neurons41 and Tat protein on Na+ channel expression and activity.
Fig. 1.
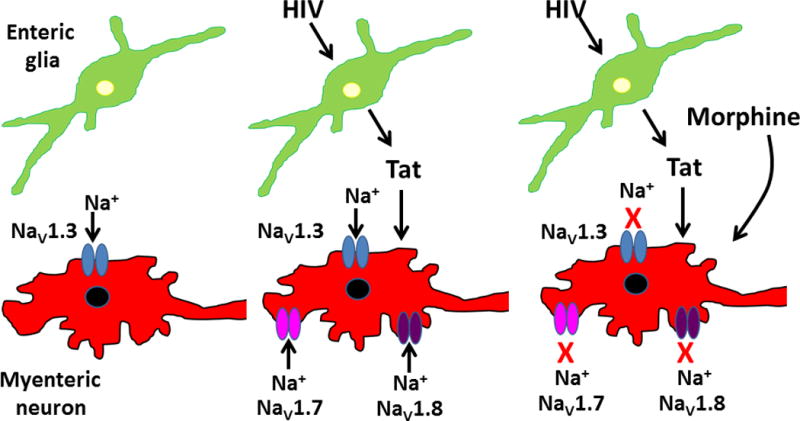
Tat-induced changes in Na+ channel expression and morphine inhibition of enteric neuronal activity. Studies were done using myenteric neurons and glia from transgenic mice that express Tat protein under the control of a doxycycline (DOX) sensitive glial fibrillary acid protein (GFAP) promoter. Under normal conditions, myenteric neurons express the tetrodotoxin-sensitive Na+ channel isoform, NaV1.3. Glial cells in mice treated with DOX produce and release Tat protein which stimulates neuronal expression of tetrodotoxin-insensitive Na+ isoforms, NaV1.7 and NaV1.8. Morphine induced inhibition of myenteric neuronal NaV1.3, NaV1.7 and NaV1.8 is enhanced after Tat exposure. The effects of Tat exposure in vivo following DOX treatment can be mimicked in vitro by Tat treatment of myenteric neurons maintained in tissue culture.
Key message.
HIV disrupts nervous system function by infecting glial cells. Infected glia release the HIV genome derived protein, Tat, which alters enteric neuron action potentials function by increasing Na+ channel expression. Morphine alterns neuronal activityin part by inhibiting Na+ channel function and this response is potentiated by Tat. Synergistic interactions between opioids and Tat may worsen GI dysfunction in HIV infected i.v. narcotic users and in HIV infected patients using opioid drugs to treat diarrhea.
Acknowledgments
The author is supported by RDK103759, DK094932 and HL070687 from the National Institutes of Health.
References
- 1.Grill MF, Price RW. Central nervous system HIV-1 infection. Handb Clin Neurol. 2014;123:487–505. doi: 10.1016/B978-0-444-53488-0.00023-7. [DOI] [PubMed] [Google Scholar]
- 2.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harbor Perspect Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zayyad Z, Spudich S. Neuropathogenesis of HIV: From Initial Neuroinvasion to HIV-Associated Neurocognitive Disorder (HAND) Curr HIV/AIDS Rep. 2015 doi: 10.1007/s11904-014-0255-3. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbonye U, Karn J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology. 2014:454–455. 328–339. doi: 10.1016/j.virol.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Rana TM. A bimolecular mechanism of HIV-1 Tat protein interaction with RNA polymerase II transcription elongation complexes. J Mol Biol. 2002;320:925–942. doi: 10.1016/s0022-2836(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 6.Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26:661–70. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem. 1999;73:1363–74. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience. 1998;82:97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- 9.Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–63. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–46. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15:219–28. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotler D. HIV infection and the gastrointestinal tract AIDS. 2005;19:107–117. doi: 10.1097/00002030-200501280-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kotler DP. Human immunodeficiency virus-related wasting: malabsorption syndromes. Semin Oncol. 1998;25(Suppl 6):70–75. [PubMed] [Google Scholar]
- 15.Konturek JW, Fischer H, van der Voort IR, Domschke W. Disturbed gastric motor activity in patients with human immunodeficiency virus infection. Scand J Gastroenterol. 1997;32:221–225. doi: 10.3109/00365529709000198. [DOI] [PubMed] [Google Scholar]
- 16.Victorino RM, Lucas M, Neto F, de Moura MC. HIV infection and intestinal pseudo-obstruction AIDS. 1990;4:599–601. [PubMed] [Google Scholar]
- 17.Ngwainmbi J, De DD, Smith TH, El-Hage N, Fitting S, Kang M, Dewey WL, Hauser KF, Akbarali HI. Effects of HIV-1 Tat on enteric neuropathogenesis. J Neurosci. 2014;34:14243–51. doi: 10.1523/JNEUROSCI.2283-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Ann Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- 19.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 21.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitting S, Ngwainmbi J, Kang M, Khan F, Stevens DL, Dewey WL, Knapp PE, Hauser KF, Akbarali HI. Sensitization of enteric neurons to morphine by HIV-1 Tat protein. Neurogastroenterol Mot. 2015 doi: 10.1111/nmo.12514. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorn S, Lembo A, Cremonini F. Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol. 2014;2:31–37. doi: 10.1038/ajgsup.2014.7. [DOI] [PubMed] [Google Scholar]
- 24.Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7(Suppl 3):S11–18. [PubMed] [Google Scholar]
- 25.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu JG, Anand KJ. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev. 2001;38:1–19. doi: 10.1016/s0165-0173(01)00057-1. [DOI] [PubMed] [Google Scholar]
- 27.North RA, Williams JT, Surprenant A, Christie MJ. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA. 1987;84:5487–91. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith TH, Grider JR, Dewey WL, Akbarali HI. Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS One. 2012;7:e45251. doi: 10.1371/journal.pone.0045251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatsumi H, Costa M, Schimerlik M, North RA. Potassium conductance increased by noradrenaline, opioids, somatostatin, and G-proteins: whole-cell recording from guinea pig submucous neurons. J Neurosci. 1990;10:1675–1682. doi: 10.1523/JNEUROSCI.10-05-01675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherubini E, Morita K, North RA. Opioid inhibition of synaptic transmission in the guinea-pig myenteric plexus. Br J Pharmacol. 1985;85:805–817. doi: 10.1111/j.1476-5381.1985.tb11079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherubini E, North RA. Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci USA. 1985;82:1860–1863. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galligan JJ, Akbarali HI. Molecular Physiology of Enteric Opioid Receptors. Am J Gastroenterol. 2014 Sep 10;2(1):17–21. doi: 10.1038/ajgsup.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haeseler G, Foadi N, Ahrens J, Dengler R, Hecker H, Leuwer M. Tramadol, fentanyl and sufentanil but not morphine block voltage-operated sodium channels. Pain. 2006;126:234–244. doi: 10.1016/j.pain.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Krylov BV, Derbenev AV, Podzorova SA, Lyudyno MI, Kuz’min AV, Izvarina NL. Morphine decreases the voltage sensitivity of slow sodium channels. Neurosci Behav Physiol. 2000;30:431–419. doi: 10.1007/BF02463098. [DOI] [PubMed] [Google Scholar]
- 36.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 37.Sui Z, Sniderhan LF, Fan S, Kazmierczak K, Reisinger E, Kovacs AD, Potash MJ, Dewhurst S, Gelbard HA, Maggirwar SB. Human immunodeficiency virus-encoded Tat activates glycogen synthase kinase-3β to antagonize nuclear factor-kappa B survival pathway in neurons. Eur J Neurosci. 2006;23:2623–2634. doi: 10.1111/j.1460-9568.2006.04813.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramage SN, Anthony IC, Carnie FW, Busuttil A, Robertson R, Bell JE. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol Appl Neurobiol. 2005;31:439–448. doi: 10.1111/j.1365-2990.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 39.Masvekar RR, El-Hage N, Hauser KF, Knapp PE. GSK3β-activation is a point of convergence for HIV-1 and opiate-mediated interactive neurotoxicity. Mol Cell Neurosci. 2015 Jan 20; doi: 10.1016/j.mcn.2015.01.001. pii: S1044-7431(15)00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clay PG, Crutchley RD. Noninfectious diarrhea in HIV seropositive individuals: a review of prevalence rates, etiology, and management in the era of combination antiretroviral therapy. Infect Dis Ther. 2014;3:103–122. doi: 10.1007/s40121-014-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]


