Abstract
PTEN (phosphatase and tensin homolog on chromosome 10) is one of the most frequently lost tumor suppressor genes in human cancers and it has been described in more than two-thirds of patients with advanced/aggressive prostate cancer. Previous studies suggest that, in prostate cancer, genomic PTEN loss is associated with tumor progression and poor prognosis. Thus, we evaluated whether immunohistochemical PTEN expression in prostate cancer glands was associated with higher risk of recurrence, using a nested case–control study that included 451 men who recurred and 451 men who did not recur with clinically localized prostate cancer treated by radical prostatectomy. Recurrence was defined as biochemical recurrence (serum prostate-specific antigen >0.2 ng/ml) or clinical recurrence (local recurrence, systemic metastases, or prostate cancer-related death). Cases and controls were matched on pathological T stage, Gleason score, race/ethnicity, and age at surgery. Odds ratios of recurrence and 95% confidence intervals were estimated using conditional logistic regression to account for the matching factors and to adjust for year of surgery, preoperative prostate-specific antigen concentrations, and status of surgical margins. Men who recurred had a higher proportion of PTEN negative expression (16 vs 11%, P = 0.05) and PTEN loss (40 vs 31%, P = 0.02) than controls. Men with markedly decreased PTEN staining had a higher risk of recurrence (odds ratio = 1.67; 95% confidence intervals 1.09, 2.57; P = 0.02) when compared with all other men. In summary, in patients with clinically localized prostate cancer treated by prostatectomy, decreased PTEN expression was associated with an increased risk of recurrence, independent of known clinicopathological factors.
Keywords: biochemical recurrence, death, immunohistochemistry, metastasis, prognosis, prostate cancer, PTEN
PTEN (phosphatase and tensin homolog on chromosome 10) is one of the most frequently lost tumor suppressor genes in human cancers. Specifically, loss of PTEN in tumor cells, mostly due to genomic deletions of the 10q23 region where the gene resides, has been described in up to two-thirds of patients with prostate cancer.1,2 Moreover, several studies have suggested that genomic PTEN loss is associated with tumor progression.2–4 Also, in murine models, a clear dose-reduction relationship exists between PTEN levels and prostate oncogenesis, latency, and biological behavior.5,6 PTEN functions as a tumor suppressor protein, negatively controlling the activation of the phosphoinosite 3-kinase pathway. Loss of PTEN leads to accumulation of phosphoinosite 3,4,5-triphosphate, which in turns leads to overactivation of AKT, a key regulator of the mammalian target of rapamycin (mTOR) pathway. Activation of mTOR is associated with increased cell growth and cell proliferation, favoring the survival of cells with dysregulation of this pathway.
We have recently designed and validated a protocol for evaluating PTEN expression by immunohistochemistry.7 Using this immunohistochemistry protocol, we achieved a sensitivity of 100% and a specificity of 98% in predicting PTEN genomic status in a panel of 59 well-studied cell lines. Further, in clinical tissue samples, we found a strong concordance between PTEN loss by immunohistochemistry and loss of one or two PTEN alleles assessed by fluorescence in situ hybridization and/or high-density single-nucleotide polymorphism microarrays. We also found a correlation between loss of PTEN expression, and both increased pathological stage and increased Gleason score. In terms of patient outcome, loss of PTEN expression correlated with decreased time to metastasis development, albeit this was not independent of Gleason score. However, in that study, the role of PTEN expression in the patient outcome was evaluated in patients who all experienced biochemical recurrence. Further, many of those patients were operated on before the advent of prostate-specific antigen screening and are, therefore, less representative of patients diagnosed most commonly under present circumstances. Herein, using identical PTEN immunohistochemistry methodology, we evaluated a large nested case–control study of prostate cancer recurrence in which all patients were operated on after the onset of widespread prostate cancer screening using serum prostate-specific antigen (ie, at or after 1993). Our aim was to evaluate the prognostic role of PTEN expression as a predictor of recurrence following prostatectomy for clinically localized prostate cancer, independent of known clinicopathological factors in the prostate-specific antigen era.
Materials and methods
Study Design and Population
We used a nested case–control study that we previously designed to investigate risk factors, including tissue-based, for recurrence.8 Recurrence cases and controls were selected from 4860 men who underwent radical retropubic prostatectomy for clinically localized prostate cancer at The Johns Hopkins Medical Institutions (Baltimore, MD, USA) between 1993 and 2001, and were followed through 2004. Men who received hormonal or radiation therapy before radical retropubic prostatectomy, or adjuvant therapy before recurrence, were excluded. Cases were the 524 men who had biochemical recurrence (serum prostate-specific antigen >0.2 ng/ml) or clinical recurrence (local recurrence, systemic metastases, or prostate cancer-related death). Then, for each case, we used incidence density sampling to select a control, who had not recurred by the date that the case recurred, and who was matched to the case on age at surgery, race, pathological stage, and Gleason sum. In this approach to control sampling, a man could be initially sampled as control and later be sampled as a case if he subsequently recurred. Men who remained at risk for recurrence were eligible to be sampled more than once as a control. This method of control sampling yields an odds ratio that is an unbiased estimate of the hazard ratio that would have been obtained if the entire cohort had been studied.9 Sampling controls allowed us to test a smaller number of total men than if we had used the entire cohort, making for a more time- and cost-efficient approach. We have used this set to evaluate other tissue-based biomarkers of prognosis.8,10
Tissues and Tissue Microarrays
A total of 16 tissue microarrays were built for the 524 matched cases and controls using 0.6-mm cores, following a previously described protocol.11 From each prostatectomy, paired prostate cancer and nontumor tissues were sampled three to six times each. In specimens with multifocal tumors, only the dominant tumor (with the highest Gleason score and usually with the largest diameter) was sampled. Nonprostate tissues were also included in the tissue microarrays as external control tissue. A total of 5892 tissue cores were obtained for the present study, comprising 2930 cores of tumor and 1650 cores of paired nontumor tissue from the patients with prostate cancer, plus 1312 cores of nonprostate tissue.
Immunohistochemistry for PTEN Expression
Immunohistochemistry for PTEN was carried out as previously described.7 Briefly, for each tissue microarray, 4-μm sections were deparaffinized, rehydrated, and briefly equilibrated in water. Antigen unmasking was done by steaming in EDTA buffer (pH 8.0) for 45 min. Endogenous peroxidase activity was quenched by incubation with peroxidase block for 5 min at room temperature. Nonspecific binding was blocked by incubating in 1% bovine serum albumin in Tris-HCl, pH 7.5, for 20 min at room temperature. Slides were incubated with a 1:100 dilution of rabbit monoclonal anti-PTEN antibody (clone D4.3, no. 9188, Cell Signaling Technologies, Danvers, MA, USA) overnight at 4 °C. A horseradish peroxidase-labeled polymer (PowerVision Poly-HRP anti-Rabbit IgG; Leica Microsystems, Buffalo Grove, IL, USA) was then applied for 30 min at room temperature. Signal detection was done using 3,30-diaminobenzidine tetrahydrochloride as the chromogen. Slides were counterstained with hematoxylin, dehydrated, and mounted.
Evaluation of PTEN Expression
Each cancer-containing tissue microarray spot was independently assessed by two pathologists (AC and LS), using two different approaches: a semiquantitative score (Approach 1) and a dichotomous system (Approach 2).
Approach 1
For each tissue microarray spot, an H-score was calculated as the sum of the product of the staining intensity in tumor cells (0, no staining; 1, weak staining; 2, moderate staining; 3, intense staining) and the extent of cells showing that staining intensity (0–100%). Thus, the possible H-score for a tissue microarray spot ranged from 0 to 300. For each man, we then calculated the mean H-score for all of his cancer-containing tissue microarray spots and classified the men into the following categories: H-score = 0 (no expression) vs H-score >0; and H-score o10 (minimal expression) vs H-score ≥10.
Approach 2
As PTEN expression is normally observed in stromal cells,7 these cells were used as internal positive controls to assess whether staining was markedly decreased in cancer cells. Then, each tissue microarray spot was classified as ‘markedly decreased’ or ‘not markedly decreased’. We confirmed using 306 tissue microarray spots (10% of the total number of tissue microarray spots in this study) that the agreement between two pathologists in calling a tissue microarray spot as markedly decreased was good (κ = 0.65; 95% confidence intervals 0.55, 0.74). This approach has been recently validated and found useful in detecting PTEN genomic losses.7 We then classified each man as to whether all of his cancer-containing tissue microarray spots had markedly decreased PTEN staining or not.
Statistical Analysis
After excluding patients with missing or technically inadequate spots, 451 matched recurrence cases and controls were included in the statistical analysis. Differences between the cases and controls in their characteristics and PTEN expression were evaluated using the Wilcoxon sign rank test and the paired t-test. We calculated odds ratios of recurrence and 95% confidence intervals by PTEN expression (mean H-score, and markedly decreased) using conditional logistic regression, taking into account the matching factors (age, race, pathological stage, and Gleason score) and adjusting for preoperative serum prostate-specific antigen concentration, calendar year of surgery, surgical margins status, and residual difference between the cases and controls in the matching on pathological stage. All analyses were performed using SAS version 9.2(SAS Institute, Cary, NC, USA). Statistical tests were two-sided, and P-values <.05 were considered to be statistically significant.
Results
The clinicopathological features of the recurrence cases and controls are shown in Table 1. Briefly, the cases and controls were similar on the matching factors and did not differ on preoperative prostate-specific antigen concentration. Figure 1 shows patterns of PTEN expression. Mean H-score for each man’s cancer containing tissue microarray spots was not statistically significantly different between recurrence cases and controls. However, cases were more likely to have a mean H-score of 0 (16 vs 11%, P = 0.05) and to have all tissue microarrays spots with markedly decreased expression (40 vs 31%, P = 0.02) when compared with controls.
Table 1.
Characteristics of recurrence cases and controls
| Cases | Controls | P-value | |
|---|---|---|---|
| Age at surgery, years | |||
| Mean (s.d.) | 58.7 (6.1) | 58.9 (5.8) | Matched |
| Race, % | |||
| Caucasian | 85 | 88 | Matched |
| Pre-operative serum PSA, ng/ml | |||
| Mean (s.d.) | 12.3 (10.4) | 11.2 (8.5) | 0.21 |
| Median (IQR) | 9.1 (8.6) | 8.7 (7.2) | 0.22 |
| Follow-up time, years | |||
| Mean (s.d.) | 2.5 (1.9) | 5.9 (2.4) | <0.001 |
| Median (IQR) | 2 (2) | 6 (4) | <0.001 |
| Gleason score, % | Matched | ||
| ≤6 | 15 | 15 | |
| 7 | 61 | 63 | |
| ≥8 | 24 | 22 | |
| Pathological stage, % | Matched | ||
| T2 | 13 | 13 | |
| T3a | 51 | 51 | |
| T3b or N1 | 36 | 36 | |
| PTEN mean H-Score | |||
| Mean (s.d.) | 105.5 (93.6) | 112.4 (85.3) | 0.19 |
| Median (IQR) | 100 (175.8) | 102.5 (166) | 0.08 |
| PTEN expression, % | |||
| Mean H-score = 0 | 16 | 11 | 0.05 |
| All TMA spots | 40 | 31 | 0.02 |
| markedly decreaseda |
Abbreviations: IQR, interquartile range; TMA, tissue microarray.
Limited to matched pairs with three to six TMA spots evaluated per patient (N = 714).
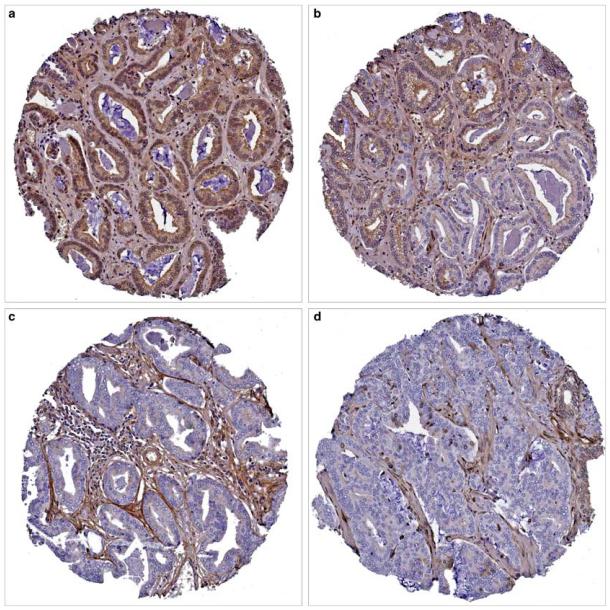
Figure 1.
Patterns of PTEN (phosphatase and tensin homolog on chromosome 10) expression in prostate carcinoma. (a) Diffuse cytoplasmic PTEN expression. (b) Reduced PTEN expression, more obvious in the glands at the lower right. (c, d) Markedly decreased to negative PTEN expression in all glands. Note the PTEN positivity in the stromal cells. Tissue microarray spots at (b–d) were classified as ‘markedly decreased PTEN expression’.
PTEN Expression and Risk of Recurrence
Table 2 shows the estimated risk of recurrence for patients who had biochemical recurrence first, clinical recurrence first, or either biochemical or clinical recurrence first. Patients with a mean H-score = 0 had a statistically nonsignificant higher risk of recurrence (either biochemical or clinical first) when compared with all other patients. Using a cutoff point of 10 for the mean H-score, the odds ratio of recurrence was 2.20 (95% confidence intervals 1.33, 3.63). Risk of recurrence was higher in men with markedly decreased expression, especially if all tissue microarrays spots had markedly decreased expression. For scenarios in which the endpoint was biochemical recurrence first or clinical recurrence first, all associations followed the same trend we expected to see given the overall analysis, including either biochemical or clinical recurrence first.
Table 2.
PTEN expression and risk of recurrence after prostatectomy for clinically localized prostate cancer
| Biochemical or clinical recurrence |
Biochemical recurrence first |
Clinical recurrence first |
||||
|---|---|---|---|---|---|---|
| PTEN expression | Odds ratioa | P-value | Odds ratioa | P-value | Odds ratioa | P-value |
| N = 902 (452 pairs) | N = 574 (287 pairs) | N = 328 (164 pairs) | ||||
| Mean H-score = 0 | 1.41 (0.81, 2.45) | 0.22 | 1.34 (0.63,= 2.85) | 0.44 | 2.52 (1.07,= 5.95) | 0.03 |
| Mean H-score<10 | 2.20 (1.33, 3.63) | 0.002 | 2.09 (1.10, 3.96) | 0.02 | 1.50 (0.65, 3.47) | 0.34 |
| Any spot markedly decreasedb | 1.32 (0.90, 1.94) | 0.15 | 1.26 (0.76, 2.09) | 0.37 | 1.36 (0.74, 2.50) | 0.32 |
| N = 714 (357 pairs) | N = 462 (231 pairs) | N = 252 (126 pairs) | ||||
| All spots markedly decreasedc | 1.67 (1.09, 2.57) | 0.02 | 1.33 (0.78,= 2.26) | 0.30 | 2.19 (0.98,= 4.91) | 0.06 |
Adjusted for year of surgery, preoperative serum PSA concentration, surgical margin status, and the residual difference in pathological stage between the cases and controls. Values in parenthesis correspond to 95% confidence intervals.
Irrespective of the number of TMA spots evaluated per patient.
Limited to matched pairs with 3 to 6 spots evaluated per patient. The number of matched pairs is included in parenthesis.
Discussion
In this study, we evaluated PTEN expression as a factor for predicting recurrence in 451 matched cases and controls of patients with clinically localized prostate carcinoma treated by radical prostatectomy in the post prostate-specific antigen era. To the best of our knowledge, this is the largest study to date evaluating PTEN in association with recurrence in patients with prostate cancer. Decreased or loss of PTEN expression was associated with higher risk of recurrence, independent of established clinicopathological prognostic factors. We used two approaches to classify the patients with respect to PTEN expression, mean H-score, and markedly decreased expression. Although the two approaches yielded similar inferences, assessment of markedly decreased expression was substantially less labor intensive and had good inter-observer agreement in this study and in a previous one at our institution,7 supporting its use in future studies evaluating the prognostic utility of PTEN expression.
The association between PTEN status and prostate cancer has been studied before. PTEN deletions, either homozygous or hemizygous, are reported in 44–68% of men with prostate carcinoma.1,2 Decrease or loss of PTEN expression, detected either by immunohistochemistry or fluorescence in situ hybridization, has been consistently associated with higher Gleason grade, larger tumors, advanced pathological stage, extraprostatic extension, and seminal vesicle invasion.2,4,12–14 In addition, PTEN status has also been linked to outcome, either alone or in combination with other biomarkers. Han et al3 identified PTEN deletions in 54% of patients with metastases, a proportion significantly higher than the 17% found in patients with localized prostate cancer. Yoshimoto et al2 found that PTEN deletions were associated with an earlier onset of biochemical recurrence, with homozygous deletions carrying a worse prognosis than hemizygous deletions. Halvorsen et al4 linked decreased PTEN expression with increased risk of biochemical recurrence. Finally, in a recently published study,7 we found that loss of PTEN expression was associated with decreased time to metastasis in patients with prostate cancer. Our current study provides further support for the role of PTEN expression as a predictor of biochemical recurrence in patients with prostate cancer. However, in other studies, PTEN expression was not predictor of biochemical recurrence when evaluated alone, but it did when associated with other biomarkers. Bedolla et al15 found that decreased PTEN combined with high phos-AKT expression predicted biochemical recurrence better than PTEN or phos-AKT alone. Also, considering the role of TMPRSS2-ERG fusions in prostate oncogenesis,10 the association between PTEN loss and ERG rearrangement as a predictor of outcome was also investigated by two groups of researchers. In the first study on the topic, Yoshimoto et al16 found that neither PTEN loss nor TMPRSS2-ERG fusions predicted outcome when evaluated separately. However, both events in combination predicted early biochemical recurrence. Opposite results were obtained by Reid et al17 the second study on the topic. Although in their study, as in the previous one, neither PTEN nor ERG/ETV1 rearrangements predict biochemical recurrence separately, PTEN loss without ERG/ETV1 rearrangements was associated with poorer cancer-specific survival. Clearly, more studies evaluating the joint ability of PTEN and ERG in predicting prostate cancer outcome are required to settle this issue. Finally, in a recently published study, Li et al18 found that neither heme oxygenase-1 overexpression nor PTEN deletions alone were associated with biochemical recurrence. However, the combined status of both markers correlated with disease progression.
Emergence of hormone-resistant disease is a crucial step during prostate cancer progression. Evidence suggests that this event is linked to activation of the phosphoinosite 3-kinase/AKT pathway,6,19 which is in turn controlled by PTEN. In this context, tumor cells with PTEN loss would gain a survival advantage over other cells that are still sensitive to androgen-deprivation therapy. Recent evidence also suggests that PTEN loss is associated with repression of androgen receptor signaling and a bypass of the requirement for high-level androgen receptor signaling, providing a new mechanism for androgen-resistance in prostate cancer.20,21 Identifying patients who have not progressed yet (ie, with clinically localized disease), and who might respond better to androgen-deprivation therapy is clearly crucial for proper clinical management. Patients with PTEN deficiency would not only be at greater risk of biochemical recurrence (suggesting more rigorous surveillance), but may also be less likely to respond to androgen-deprivation therapy. Inhibitors of the mTOR pathway could be beneficial in these situations, as suggested by murine models of prostate cancer.22 Furthermore, Carver et al20 have recently shown a greater tumor regression when both the mTOR and the androgen receptor pathways were inhibited, as opposed to the tumor regression observed with mTOR inhibitors alone. Moreover, Mulholland et al21 found that androgen-deprivation therapy may be more effective in combination with mTOR inhibitors when PTEN is lost.
In summary, consistent with prior studies on genomic loss of PTEN, a decrease or loss of PTEN immunohistochemical expression was associated with higher risk of recurrence in men with clinically localized prostate cancer, who were treated by radical prostatectomy, independent of established clinicopathological prognostic factors.
Acknowledgements
We acknowledge the contributions of Helen L. Fedor from the Brady Urological Research Institute Prostate Specimen Repository at the Johns Hopkins School of Medicine for the generation of the TMAs for this nested case–control study, funded in part by the Prostate SPORE Pathology Core (P50 CA58236) and a DOD grant (DAMD 17-03-0273).
Footnotes
Disclosure/conflict of interest This study was partially supported by the Johns Hopkins Medicine–Patana Fund for Research, NIH-NCI P50 CA58236 Grant–Johns Hopkins University SPORE in Prostate Cancer, and the Patrick C Walsh Research Fund. Dr Alcides Chaux was partially supported by an award granted by the Consejo Nacional de Ciencia y Tecnologia, CONACYT (National Council of Science and Technology) dependent of the Presidency of the Republic of Paraguay, as an Active Researcher of Level 1 of the Programa Nacional de Incentivo a los Investigadores, PRONII (National Incentive Program for Researchers).
References
- 1.Yoshimoto M, Cutz JC, Nuin PA, et al. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet. 2006;169:128–137. doi: 10.1016/j.cancergencyto.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimoto M, Cunha IW, Coudry RA, et al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer. 2007;97:678–685. doi: 10.1038/sj.bjc.6603924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Mehra R, Lonigro RJ, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22:1083–1093. doi: 10.1038/modpathol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halvorsen OJ, Haukaas SA, Akslen LA. Combined loss of PTEN and p27 expression is associated with tumor cell proliferation by Ki-67 and increased risk of recurrent disease in localized prostate cancer. Clin Cancer Res. 2003;9:1474–1479. [PubMed] [Google Scholar]
- 5.Carracedo A, Alimonti A, Pandolfi PP. PTEN level in tumor suppression: how much is too little? Cancer Res. 2011;71:629–633. doi: 10.1158/0008-5472.CAN-10-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trotman LC, Niki M, Dotan ZA, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotan TL, Gurel B, Sutcliffe S, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–6573. doi: 10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toubaji A, Albadine R, Meeker AK, et al. Increased gene copy number of ERG on chromosome 21 but not TMPRSS2-ERG fusion predicts outcome in prostatic adenocarcinomas. Mod Pathol. 2011;24:1511–1520. doi: 10.1038/modpathol.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang MH, Shugart YY, Cole SR, et al. A simulation study of control sampling methods for nested case-control studies of genetic and molecular biomarkers and prostate cancer progression. Cancer Epidemiol Biomarkers Prev. 2009;18:706–711. doi: 10.1158/1055-9965.EPI-08-0839. [DOI] [PubMed] [Google Scholar]
- 10.Chaux A, Albadine R, Toubaji A, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol. 2011;35:1014–1020. doi: 10.1097/PAS.0b013e31821e8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89–101. doi: 10.1385/1-59259-780-7:089. [DOI] [PubMed] [Google Scholar]
- 12.McMenamin ME, Soung P, Perera S, et al. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 13.Koksal IT, Dirice E, Yasar D, et al. The assessment of PTEN tumor suppressor gene in combination with Gleason scoring and serum PSA to evaluate progression of prostate carcinoma. Urol Oncol. 2004;22:307–312. doi: 10.1016/j.urolonc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Dreher T, Zentgraf H, Abel U, et al. Reduction of PTEN and p27kip1 expression correlates with tumor grade in prostate cancer. Analysis in radical prostatectomy specimens and needle biopsies. Virchows Arch. 2004;444:509–517. doi: 10.1007/s00428-004-1004-6. [DOI] [PubMed] [Google Scholar]
- 15.Bedolla R, Prihoda TJ, Kreisberg JI, et al. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin Cancer Res. 2007;13:3860–3867. doi: 10.1158/1078-0432.CCR-07-0091. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto M, Joshua AM, Cunha IW, et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol. 2008;21:1451–1460. doi: 10.1038/modpathol.2008.96. [DOI] [PubMed] [Google Scholar]
- 17.Reid AH, Attard G, Ambroisine L, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer. 2010;102:678–684. doi: 10.1038/sj.bjc.6605554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Su J, DingZhang X, et al. PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J Pathol. 2011;224:90–100. doi: 10.1002/path.2855. [DOI] [PubMed] [Google Scholar]
- 19.Shen MM, Abate-Shen C. Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res. 2007;67:6535–6538. doi: 10.1158/0008-5472.CAN-07-1271. [DOI] [PubMed] [Google Scholar]
- 20.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castrationresistant prostate cancer growth. Cancer Cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]