Abstract
The feather is a complex epidermal organ with hierarchical branches and represents a multi-layered topological transformation of keratinocyte sheets. Feathers are made in feather follicles. The basics of feather morphogenesis were previously described (Lucas and Stettenheim, 1972). Here we review new molecular and cellular data. After feather buds form (Jiang et al., 2004), they invaginate into the dermis to form feather follicles. Above the dermal papilla is the proliferating epidermal collar. Distal to it is the ramogenic zone where the epidermal cylinder starts to differentiate into barb ridges or rachidial ridge. These neoptile feathers tend to be downy and radially symmetrical. They are replaced by teleoptile feathers which tend to be bilateral symmetrical and more diverse in shapes. We have recently developed a “transgenic feather” protocol that allows molecular analyses: BMPs enhance the size of the rachis, Noggin increases branching, while anti-SHH causes webbed branches. Different feather types formed during evolution (Wu et al., 2004). Pigment patterns along the body axis or intra-feather add more colorful distinctions. These patterns help facilitate the analysis of melanocyte behavior. Feather follicles have to be connected with muscles and nerve fibers, so they can be integrated into the physiology of the whole organism. Feathers, similarly to hairs, have the extraordinary ability to go through molting cycles and regenerate. Some work has been done and feather follicles might serve as a model for stem cell research. Feather phenotypes can be modulated by sex hormones and can help elucidate mechanisms of sex hormone-dependent growth control. Thus, the developmental biology of feather follicles provides a multi-dimension research paradigm that links molecular activities and cellular behaviors to functional morphology at the organismal level.
Keywords: branching, morphogenesis, skin, appendage, hair cycle, pigment pattern, sexual dimorphism, stem cell, regeneration
Introduction
Feathers are elaborate skin appendages with hierarchical branches (Fig. 1). The prototype of branch forms is the rachis, barbs and barbules. However, the prototype branching pattern is flexible, with a variable number, size, and shape of each component, thus generating a large spectrum of possible feather types. These different feather types provide diverse functions to the avian, such as maintenance of endothermy, communication, and flight. There are additional roles in specialized feathers such as tactile function in the bristle feathers and powder feathers that flake off to provide a water-repelling keratin powder. It is not too much to say that feathers are what define the Aves class. However, recent discoveries of feathered dinosaurs have challenged this dogma. The statement “All species that have feathers are birds” is no longer true, and “All skin appendages having hierarchical branching are feathers” is shaky too. A stricter definition of a feather has to be developed (Chuong et al., 2003) and these evolutionary aspects are covered in Wu et al., 2004. In this review, we will cover the developmental aspects of feather morphogenesis.
Fig. 1. The basics of feathers.

(A) A rooster with plumages. Notice the different feather tracts on different parts of the body. Ca, caudal tract; Ce, cervical tract; Fe, femoral tract; Hu, humerus tract; Sc, scale region; Sp, spinal tract. (B) Major types of feathers: radially symmetric downy feather, bilaterally symmetric contour feather, and bilaterally asymmetric flight feather (remiges). Schematic diagrams to show (C) the three basic levels of feather branches, and (D) the major zones of cellular activities of a sectioned developing feather follicle. (A) is from Green-Armytage, (2000). (B) is from Lucas and Stettenheim, (1972).
There are different forms, sizes, and colors of feathers arranged in specific patterns on the surface of a bird. Together, the plumage plays key roles in keeping the bird warm, supporting the biomechanics of flight, and radiating welcome or repulsive messages to other animals. The regional specificity can be appreciated from the chicken shown in Fig. 1A. In addition to the numerous feathers (approximately 20,000 - 80,000 feathers per bird, depending on the species), there are beaks, combs, wattles, scales, claws, etc. on the integument, serving various specialized functions. The major feather types are shown in Fig. 1B. Downy feathers are radially symmetric and fluffy and are mostly present in the ventral trunk to keep the body warm. Contour feathers are on the trunk, with the proximal part for temperature control and distal part for streamlining of the body shape and communication. Tail feathers (rectrices) are for display as well as for control of flight. Wing feathers (remiges) are for flight although birds may adopt different modes of flight (e.g., differences in the way sparrows and eagles fly). The arrangement and design of the wing feathers are different in different birds. We can also categorize feathers by the time they are produced. Neoptile feathers are those formed in embryonic development. Teleoptile feathers are those formed after this first generation of feathers (Dhouailly, 1970). The first three generation of feathers also have been refered as natal, juvenal and first basic plumages (Humphrey and Parkes, 1959)
How are such complex structures like feathers formed from the flat epidermis? Furthermore, they can regenerate and repair themselves, and generate different forms and colors of feathers in different generations, in response to sex hormones and other modulators. How do they do it? Much of the basics of feather follicles have been described in the classical book of Lucas and Stettenheim, 1972 and reviewed in Sengel, 1976. Here we draw on this background and add new progress in the molecular and cellular aspects, when available. We also take this opportunity to set down the groundwork and point out interesting questions for future exploration.
Formation of feather follicles
Early events
Using today's chicken, it is possible to dissect how the feather primordia are generated from the flat epidermis. The early events that lead to the formation of feather buds are reviewed in Jiang et al. (2004). Clearly, interactions between the dermis and epidermis play an essential role in the induction of feather follicles. First, mesenchymal cells form a layer of dense dermis under feather tract field. Then mesenchymal cells within the dense dermis form periodically arranged dermal condensations. Together with epithelial placodes they form feather primordia that are arranged in patterns (see Jiang et al., 2004). These feather primordia then differentiate further and start to express different signaling, growth and cell adhesion molecules in different parts of the feather buds, interbuds, an junction between buds and inter-buds (Chuong, 1990; Jiang and Chuong, 1992). These molecules may lead successive morphogenetic movements. Between E9-16, the feather buds undergo active cell proliferation, migration and differentiation to become the complex feather follicles. The feather follicle is an invaginated epidermis surrounding the feather filament cylinder with pulp inside (Fig. 1D).
Late events
The follicle wall epidermis has three layers: the germinative layer, the intermediate layer and the corneous layer (Fig. 2A). The feather filament epidermis also has three layers. The outermost layer of a developing feather filament is the feather sheath that disintegrates later to let the feathers pop out. The middle intermediate layer and the inner basal layer (or basilar epidermis) will form the feather rachis and barbs. At the center of the feather filament is the pulp, which is the mesenchymal part of the feather and is derived from the dermal papilla. The pulp is composed of fibroblasts and extracellular matrix including fibronectin and laminin (Chuong and Edelman, 1985a, b). The pulp is rich in blood vessels, which include a central axial artery and numerous smaller vessels and capillaries. The pulp and the surrounding pulp epithelium (or the remaining basilar epidermis) will eventually degenerate and slough away to allow the vanes of the feather to open up. Situated at the base of the feather follicle is the dermal papilla. The dermal papilla is shaped like an hourglass. The epidermis surrounding the dermal papilla is called the collar. The collar proliferates to generate the keratinocytes of the feather filaments. The bottom portion of the collar is the proliferation zone. Immediately above (more distal) the proliferation zone is the ramogenic zone. At this level, the feather filament cylinder is transformed into the barb ridges and rachidial ridges (Fig. 2A).
Fig. 2. Formation of feather follicles.
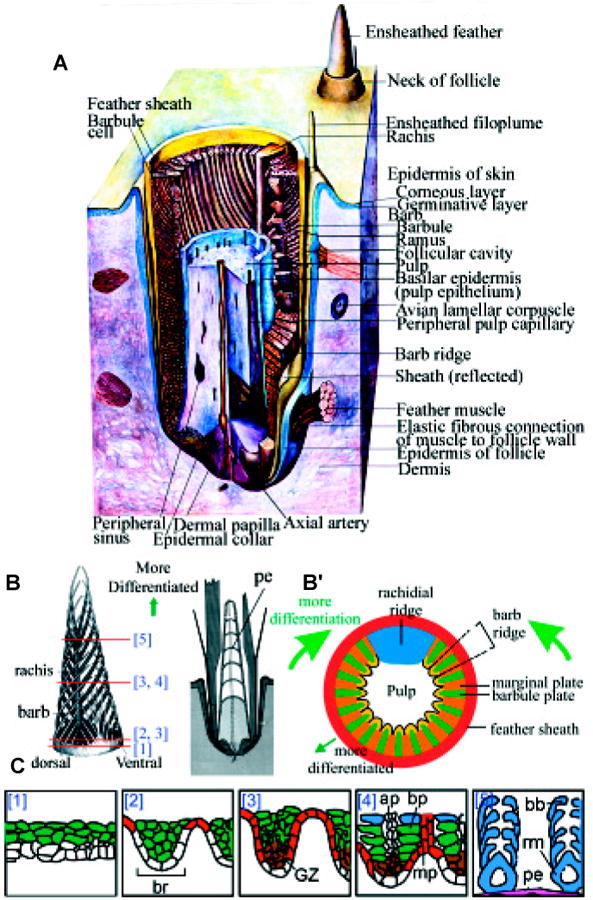
(A) Schematic three-dimensional view of feather follicles. (B) Longitudinal and cross section view of developing feather filaments, showing the three sequences of maturation within the feather follicles: proximal - distal, posterior - anterior (rachis side), center - periphery. The two longitudinal sections represent stages of developing branches and degenerating pulps. The cross sections show forming barb ridges. (C) Different stages of barb ridge formation showing how a flat layer of cells is transformed into branching structures. The basal layer is white and the pulp mesenchyme is at the bottom. The supra-basal layer is green. The feather filament epidermis then forms barb ridges (br). The marginal plate (mp) is red. The differentiated barbule plate (bp) is blue. The growth zone (GZ) is brown and later differentiates into the ramogenic zone (rm). Ap, axial plate. Pe, pulp epithelium. (A) and (B) are modified from Lucas and Stettenheim, (1972). (B′) is from Sengel, 1976. (C) is modified from Chuong and Edelman, 1985.
The cells of the germinative layer in the collar are cylindrical in shape and the nuclei are at the distal end. In chicken embryos, at E10, the feather germ starts to grow faster. Then two new morphogenetic activities take place. One activity is that the basal layer cells at the upper bud epidermal region start to form a series of ridges that are parallel to the long axis of the feather germ at E 10. In chickens, these ridges appear to be radially symmetric, while in duck they appear to be anterior - posterior (AP) asymmetric (Dhouailly, 1970; Harris et al., 2002). All the barb ridges then lengthen toward the base of the long feather bud (Prum, 1999). Epidermis surrounding the base begins to invaginate into the dermis at E 11. Further invagination leads to the formation of the feather follicle wall. As invagination and distal growth continue, the follicles are shaped into a deep, narrow pit and the feather germs look like a long cylinder sticking out of the follicles. The two events are uncoupled, and the formation of follicles is not a pre-requisite to the formation of barb ridges (Dhouailly, 1973; Sawyer and Knapp, 2003).
In the mean time, the follicle wall starts its cyto-differentiation process. A follicular cavity forms between the follicle wall and the feather sheath. At the base of the feather follicle, The epidermal collar surrounding the dermal papilla is fully formed due to active basal layer cell proliferation. The dermal papilla is formed from the previous dermal condensation and will proliferate to generate pulp at the distal end. The pulp consists of a central axial artery and many proliferating mesenchymal cells. The presence of the pulp causes the feather filament to assume a cylindrical configuration that makes many subsequent morphogenetic paths possible. Nerves start to invade into the pulp cavity. By E 14, the follicular cavities have disappeared because the feather germs completely fill their follicles. The lining of a follicle and the sheath of a feather are closely apposed and appear as a single layer.
Morphogenesis of feather filaments
During the period between E14-18, the epidermal layer has thickened and starts to differentiate to generate different cell types. The cylindrical feather filament now has a basal layer facing the pulp, an intermediate layer, and an outer layer that becomes the feather sheath. At E 13, near the tip of the feather germ, the cells of each barb ridge begin to differentiate into three longitudinal plates in sequence: the marginal plate, the barbule plates, and the axial plate. The marginal plate cells are a single-layer of cells flanking each barb ridge. The two marginal plates of two neighboring barb ridges constitute the barb septum. Within the barb ridge, cells start to rearrange and form two columns of barbule cells packed side by side. Between the two barbule plate columns, the axial plate forms (Fig. 2C).
Barb ridge morphogenesis
At E 14, at the opening of the feather follicle, a very thin follicular lining separates from the thick feather sheath. Inside the differentiating shafts of downy feathers, about 10-15 barb ridges have formed. The components of the barb ridges are more distinct in the distal part of the feather shaft. Cells of the barbule plates are compressed toward the plane of the feather sheath and start to elongate with the long axis of the barbule cells parallel to the long axis of the feather filament. Eventually these barbule plate keratinocytes will lose their nuclei and fuse together to become barbules. Imagining the cell as cuboids, they are connected to cells in the proximal and the distal side, but lose contact with barbule cells on the other four sides.
The cells in the centripetal region of the barb ridge (facing the pulp and next to the basement membrane) form the growth zone and can generate more barbule plate keratinocytes (Fig. 2C). By E 16, these growth zone cells generate a ramus at the peak of each barb ridge, and the pulp epithelium that is linked continuously as a concentric epithelial ring facing the pulp. The barb is made of barbules inserted on the ramus (Fig. 1C). Around E 15-16, rami “grow” (or are organized) toward the anterior side of the feather filament and fuse at their proximal ends. Therefore, rami insert anterior - proximally to the rachis (Fig. 2B). In bilaterally symmetric feathers, a mass of cells in the anterior side becomes the presumptive rachis, whereas a smaller mass on the opposite side becomes the presumptive hyporachis. These rachidial ridges resemble the ramus in keratinization and structures. They differentiate into a cortex and a medulla. The cortex cells are flattened at the sides. The medulla is within the cortex and cells are large with a small nucleus. Later the cells become empty, giving feathers stronger architecture.
Differentiation and maturation
Barbules continue to differentiate and assume different characteristics depending on different types of feathers. The columns of barbule cells have each lengthened, differentiated, and fused to ramus, forming a base and a pennulum. The two rows of barbules are named distal and proximal barbules. The one closest to the rachis (anterior) is the distal barbule. In plumulaceous barbules, both barbules are of the same shape and form a fluffy structure. In pennaceous barbules, the distal barbules form hooklets, while the proximal barbules form cilia. Thus the distal barbule hooklets interlock with the superjacent proximal barbule cilia to form a vane in a Velcro-like mechanism.
By E19, differentiation of cell shape and keratinization in the neoptile feather is finishing, starting from the tip of the feather (Haake et al., 1984). Nuclei and boundaries of the cells have disappeared in different parts of the feather. The feather sheath outside is thinner here than at a lower level and has one or two disjunctive layers. They are ready to flake off. Inside the feather filament, the basal layer of the epidermis on the inner surfaces of the barb ridges fuses into a tube around the pulp. This structure is known as the “pulp epithelium”. The pulp epithelium then differentiates into inner and outer layers. As the tip of the pulp is absorbed, the inner layer produces a series of pulp caps at periodic intervals, like the bamboo shoot. The pulps are confined in each segment and start to degenerate and be absorbed from the distal end of the rachis. The bases of barbules are more flattened and the pennula are more nearly oval or round in cross section than earlier. The cells of the axial and marginal plates have nearly vanished, but the tube of basilar epidermis around the pulp has grown thicker. The cortex of each ramus has thickened and the cells of the medulla become enucleated (Fig. 2C).
In the proximal end of the feather, development of barb ridges has stopped, signaling the end of the vane. Further proliferation of cells in the epidermal collar builds the homogenously differentiated epidermal tube that becomes the calamus. As the building process of a feather nears completion, the pulp becomes entirely absorbed, and a final pulp cap is formed over the dermal papilla. This cap closes the inferior umbilicus of the final calamus. Thus, the making of a feather is indeed a complex interplay between epidermis and dermis, and involves a transition of many cell types in both components. The lineage of both feather epidermal and dermal stem cells are shown here (Fig. 3).
Fig. 3. Lineage of feather epidermal and dermal cells.
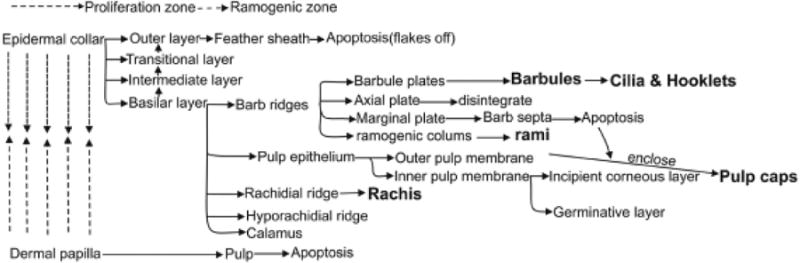
The epithelial and mesenchymal lineage and their potential interactions are summarized. (Modified from Lucas and Stettenheim, 1972).
Shortly after a chick hatches, the top of each feather sheath flakes away and the feather with all its barb branches now emerges from its sheath and pops out. The outer (more peripheral) side of the rami and rachis will form the dorsal surface of the vane, and the previous basal cell side (which faces the previous pulp) will form the ventral surface of the vane. The body of the chick is now mostly covered with downy feathers, except for the apteria regions, where there are no feathers. In the chick wings, flight teloptile feathers develop faster. The second cycle of teleoptile primary remiges are already growing, with the first cycle of neoptile feathers still attached at their tips. These time sequences are different in different birds.
Perturbation of feather branching patterns
Molecular expression
How are the above complex events that occur within the feather follicles regulated? Feather branching is fundamental to feather filament morphogenesis and probably evolved to maintain temperature, and later evolved for communication and flight (Wu et al., this volume; Chuong et al., 2003). Here we try to study the molecular mechanisms of feather branching. In situ hybridization and immunostaining revealed molecular expression patterns in different cell types. Using the BMP pathway as an example, BMP4 was mainly expressed in the dermal papilla and the pulp, with expression lower toward the distal end. BMP2 was first in the marginal plate, and then switched to the barbule plate. SHH was also expressed in the marginal plate. Both BMP2 and BMP4 appeared in the barbule plate when these cells started to form and differentiate. On the other hand, noggin was in the pulp at the level of the ramogenic zone, where barb ridges start to form (Fig. 4). The expression of several major signaling molecules has been mapped in the developing feathers including NCAM, LCAM (Chuong and Edelman, 1985a, b), SHH (Ting-Berreth and Chuong, 1996), the Wnt pathway (Clogdancer et al., 2003), and the Notch pathway (Chen et al., 1997).
Fig. 4. Examples of molecular expression in feather follicles.
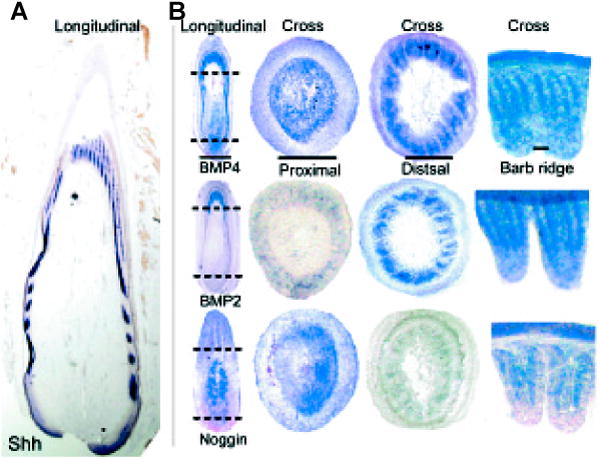
(A) Longitudinal sections with SHH in situ hybridization. (B) In situ hybridizations of BMP4, BMP2 and Noggin (From Yu et al., 2002).
Molecular perturbation
How do we test the function of molecules in feather morphogenesis? Recent progress in molecular and developmental biology has allowed us to dig into the molecular basis of these complex morphogenetic mechanisms. We have recently developed a novel powerful model to analyze feather follicle morphogenesis. RCAS viruses carrying candidate genes or dominant negative genes are added to plucked feather follicles. Feathers are allowed to regenerate. The regenerated feathers carry the mis-expressed genes and may exhibit abnormalities if the tested genes are involved in morphogenesis (Yu et al., 2003) (Fig. 5).
Fig. 5. Retroviral-mediated gene transduction in regenerating feathers.
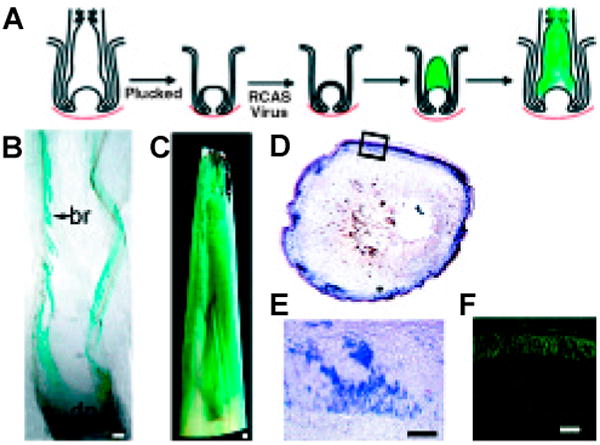
(A) Strategy showing feathers plucked, infected with RCAS retroviral vectors and allowed to regenerate. (B,C) X-gal staining of sections from feather follicles infected with RCAS-LacZ (From Yu et al., 2002). (D,E) Detection of Noggin transcripts in cross sections of feather follicles infected with RCAS-Noggin (From Yu et al., 2002). (F) UV light view of a cross section of feather follicles infected with RCAS-GFP (unpublished data).
Having developed this experimental model, we first try to perturb feather branching. Here we showed that overexpression of BMP2 and BMP4 caused the formation of a giant rachis and barb fusions. Overexpression of Noggin, a BMP antagonist, caused splitting of the rachis and excessive branching of barb ridges. Suppression of SHH altered the fate of marginal plates and lead to a webbed membrane remnant between the barbs (Yu et al., 2002; Fig. 6). Thus a balance between BMPs and their antagonist, Noggin, plays essential roles in regulating branching morphogenesis. Appropriate BMP and SHH expression in the marginal plate is essential for its fate specification and subsequent apoptosis to ensure branch separation. Using chicken and duck embryos, interactions between Shh and BMP2 were also suggested to be involved in feather branching morphogenesis (Harris et al., 2002).
Fig. 6. Altering feather branch patterns with molecular misexpression.
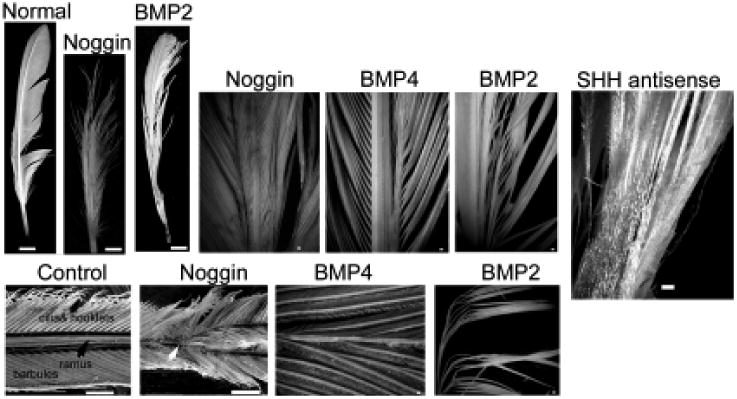
Feathers regenerated from follicles injected with RCAS-BMP2; RCAS-BMP4; RCAS-noggin and RCAS-Shh antisense showed altered rachis, barb and bar-bule conformation. While misexpression of BMP2 or BMP4 caused barb fusions and ectopic rachis-like structures, Noggin caused further branching of barbs and rachis. Blocking of Shh by RCAS-Shh antisense caused a failure of barb separation to form a web-like epithelial sheet (modified from Yu et al., 2002).
A large variety of feathers can be seen in evolution and nature today, carrying out distinct functions. They can also be appreciated in the many feather variants selected by fancy bird breeders (Bartels, 2003). In development, the neoptile natal down feathers have little variations. The second generation, or teleoptile feathers start to show more variations. The variety of feathers can be formed by modulating some basic elements, such as the size, arrangement, and characteristics of the rachis, barbs, barbules, and calamus. These different feather forms have been simulated by computer modeling using several basic parameters (Prum and Williamson, 2001; Streit and Heidrich, 2002). However, the cellular and molecular mechanisms remain unknown. The model we now set up (Yu et al., 2002) opens up the new possibility to study these issues.
The color of feathers
Feathers have evolved diverse colors. Feathers exhibit an extraordinary variety of colors that are distributed in striking patterns. The color can be formed by chemical color, physical color or both in combination. Pigments are chemical compounds that absorb light at certain wavelengths. In birds, there are three major kinds of pigments: melanins, carotenoids, and porphyrins (Lucas and Stettenheim, 1972). Melanin and eumelanin are synthesized by melanocytes and are black or lighter. Carotenoids and porphyrins are obtained from diet and are lipid soluble. They are deposited into different parts of a feather at different times. They are yellow, bright red, or magenta. Structural color is the result of light interference and scattering. Iridescence is produced by light interference from the production of regular air spaces, melanin tubes, the arrangement of keratin filaments, etc. and changes in hue are due to spacing changes when the feathers are viewed from different angles. These colors tend to be of short wave length (metal bluish or greenish). Light scattering is produced by tiny melanin granules. The color can also be combined, such as the epidermal physical blue color and dermal yellow carotenoids can give a green color, as seen in the feet of a duck.
Melanin
Among the different types of colors described above, melanin is the major one and here we will describe this system further. Melanocytes are derived from melanoblasts that come from neural crest cells in early embryos. The melanoblasts are not pigmented. They migrate into the epidermal and dermal region of many tissues, and the process may involve c-Kit and stem cell factors (Lecoin et al., 1995). Melanoblasts in the epidermis and the dermis of the skin multiply from E4 to E7 in chick embryo. First they are evenly distributed. Then they become localized in the newly formed feather primordia where they persist and multiply, but disappear from the inter-primordia epidermis. These melanocyte precursors begin to synthesize melanin at about E 7-8. Melanins are synthesized in melanosomes, which are granule-like organelles formed inside melanoblasts that undergo progressive differentiation during the formation of the melanocytes. Cytologically, the nuclei of the melanocytes become spherical, and the cytoplasm sends out several branched processes. With the appearance of pigment color and the cell morphological changes, the melanoblasts become the fully differentiated melanocytes.
Soon after the melanin production in the fully differentiated melanocytes, transfer of melanins to keratinocytes takes place (Lucas and Stettenheim, 1972). This happens in particular locations and during particular periods of feather filament development, thus creating unique pigmented patterns in different mature feathers. While melanocytes are randomly arranged in the proximal feather germ, they gradually become aligned in parallel longitudinal rows as barb ridges form. At about E 11, melanocytes send processes outward to the cells of the barbule plates and transfer the melanosomes to them, starting from the outermost barbule cells and gradually withdraw and “feed” the more centrally located cells and the rami. After the transfer of melanosomes, the melanocytes will retract their processes and degenerate at further distal locations. As the feather germ grows, additional melanoblasts in the dermis will continue to differentiate to form new melanocytes.
During the regenerative process in feather follicles, new melanocytes are probably derived from a reservoir of melanocyte stem cells located at the base of the follicle, in the base of the epidermal collar and near the apex of the dermal papilla (Lucas and Stettenheim, 1972). Some suggest that new melanocytes may also come from extra-follicular melanoblasts. Melanocytes are supplied to the pulp and epidermis at the base of the blastema. They transfer pigments to the newly formed feather filament. The pigment granules formed in each melanocyte are nearly uniform in size, shape and color, and if different melanin pigments are present in one barbule keratinocyte, they probably were produced in different melanocytes. The number of melanocytes in each barb ridge varies from one to four. Distal barbules are usually more heavily pigmented with melanin than the proximal barbules. The process is specific, as axial plate cells, marginal plate cells, or feather sheaths do not receive pigments.
Pigment pattern
What makes the color of birds so interesting is not only that the types of colors are more diverse than mammals, but also that the arrangement pattern is unique. The plumage color patterns of a bird can be based on differences displayed by individual feathers (intra-feather patterning) and differences displayed by different body regions. Here we show some examples of color patterns on a single feather vane (Fig. 7 A,B). The color can be asymmetrically restricted to the left or right vane. It can also be on and off along the proximal-distal axis, thus generating horizontal stripes or chevrons, which can be out of phase in the left and right vanes. The stripes can “bend”, generating curves that may appear to be bordering the vane. If the process becomes periodic, wave-like curved patterns appear. The stripes can also break, generating arrays of offset spots. One can speculate that the extreme curved stripes may form an elliptic or a circular spot. It follows that paired spots and concentric rings can form. Amazingly, the above patterns (e.g., bars and spots) can coexist, suggesting that they are under independent control.
Fig. 7. Pigment patterns of feathers.

(A) Representative pigment patterns within a feather. Feathers are from chicken, zebra finch and peacock. (B) From these, some basic patterns such as barbs, chevrons, circles, dots, etc. are deduced. Note pigments in the left and right vane are under different control. Please see text. (C) There are also pigment patterns at the level of the whole body.
The types and levels of pigmentation vary among the cells according to their location in the feather and the location of the feathers on the body. The molecular and cellular mechanisms that control the pigment pattern formation are still unknown. A reaction diffusion model was proposed to simulate the formation and transition of various feather color patterns (Prum and Williamson, 2002), which suggested that the feather pigmentation patterning was probably determined by antagonistic interactions among various molecular expression gradients within the feather follicles. However, the molecular identities are entirely unknown. Taking the melanin based pigmentation patterns on the plumage as an example, one does not know whether the regulation is exerted at the level of the presence or absence of melanocytes, formation of melanosomes, activities of melanosomes, or the transfer of melanosomes to keratinocytes. By transplanting the limb bud and constructing quail/guinea fowl chimeric embryos, it was suggested that prepattern “cues” were present in the feathers that can control melanoblast patterns, even if the melanoblasts are derived from different species (Richardson et al., 1991). However, they also stated that there might be exceptions. Much more experimental work will be required before we can understand the mechanisms of the beautiful colors of the feathers.
Connections of feather follicles with the organism for higher-level functions
Our earlier data showed that feather epithelia and mesenchyma could set up periodic patterns, resulting in the bud and interbud domain without the involvement of muscles or nerves (Jiang et al., 1999; 2004). Subsequently the interbud mesenchyma become more heterogeneous. Muscles, tendons, blood vessels, and other connective tissues that connect feather follicles with each other and with other parts of the body gradually form. These tissues are invaded by axon terminals for sensory and motor innervation. Functionally, these neuro-muscular connections make feathers more than simple skin appendages, but unique motile and sensory organs essential for flight.
Muscle connections
Mature feathers are richly connected with muscles, nerves and blood vessels in the dermis (Stettenheim and Lucas, 1972). A network of muscles lies in the dermis that encircles each feather follicle (Fig. 8). These muscles include erector muscles; depressor muscles and retractor muscles that are arranged in antagonistic quadrilaterals for neighboring follicles to serve the purpose of pulling the feather in various directions that are critical for a birds ability to fly (Homberger and De Silva, 2003). During development, they start from apparently homogenous inter-primordial dermis. Muscle precursors then gradually emerge and form specific connections between feather follicles.
Fig. 8. Connection of feather follicles with muscles.
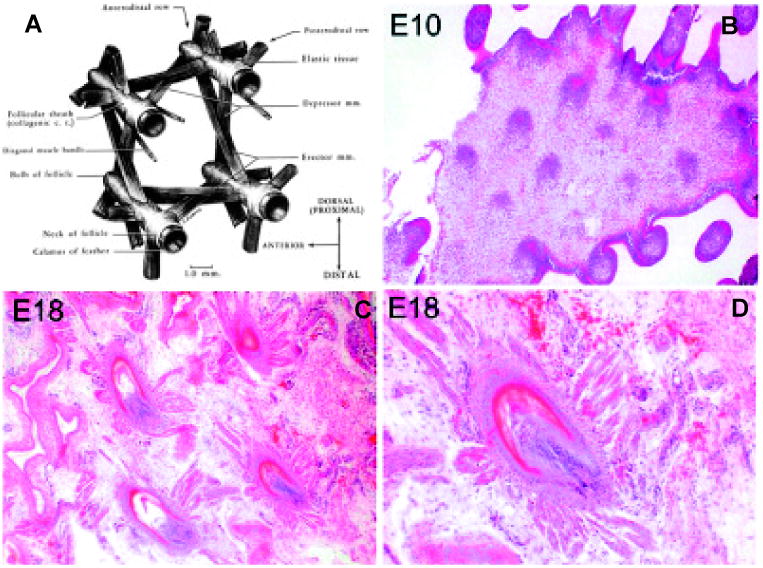
(A) The exquisite muscle connections of mature feather follicles (from Lucas and Stettenheim, 1972). (B-D) During development, the interbud mesenchyma are at first homogenous (E10), but gradually form specifically arranged muscle and tendon fibers which link feather follicles (E18).
Innervation
Most feathers also are innervated by an arcade of sensory nerve fibers, which form ring-like structures under the skin (Fig. 9), with the rings encircling each follicle (Saxod, 1978; Saxod et al., 1995). The arcade may serve the purpose of sensory innervation for feather. The extensive sensory nerve arcade appears at the time of the emergence of feather follicles during skin development (Pays et al., 1997). First, the dorsal branches of spinal nerve reach the skin at around embryonic day 6 as large axon bundles (Verna and Saxod, 1979). These nerve bundles then further branch out and form ring-like axon terminals around each feather follicle. Many molecules in the extracellular matrix, i.e., tenascin-C (Jiang and Chuong, 1992), chondroitin sulfate proteoglycans (Fichard et al., 1991; Pays et al., 1997; Cahoon and Scott, 1999), and brain-derived neurotrophic factor (Cahoon-Metzger et al., 2001), etc. are suggested to be involved in guiding the ingrowing axons in forming the arcade. In addition, certain neural pathfinding molecule pairs may play roles in nerve arcade formation at various stages.
Fig. 9. Innervations of feather follicles.
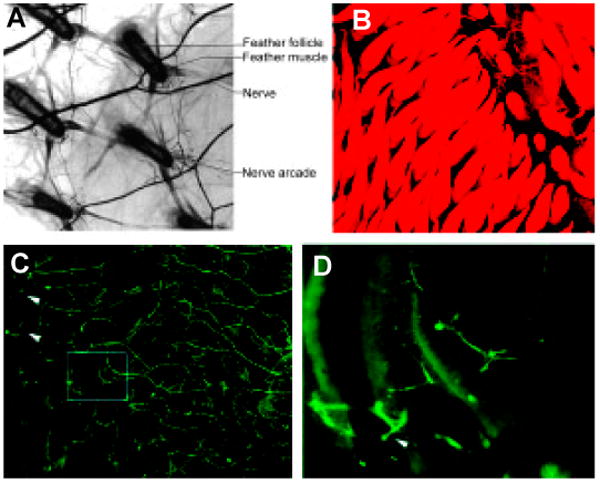
(A) The nerve network around the feather follicles (Lucas and Stettenheim, 1972). (B) During development, nerves gradually grow in and form arcades surrounding the feather buds (visualized by DiI labeling). (C,D) Nerves are labeled green by antibody to neural filaments.
The death and rebirth of feathers: feather cycling
Stages of feather cycle
Similar to the hair cycle, feather follicles also go through repetitive molting throughout the life of birds (Fig. 10A). Feathers can regenerate naturally (molting), or artificially (plucking). When a feather is plucked or lost accidentally, a new feather will usually generate from the old follicle within two weeks.
Fig. 10. Feather molting cycle.
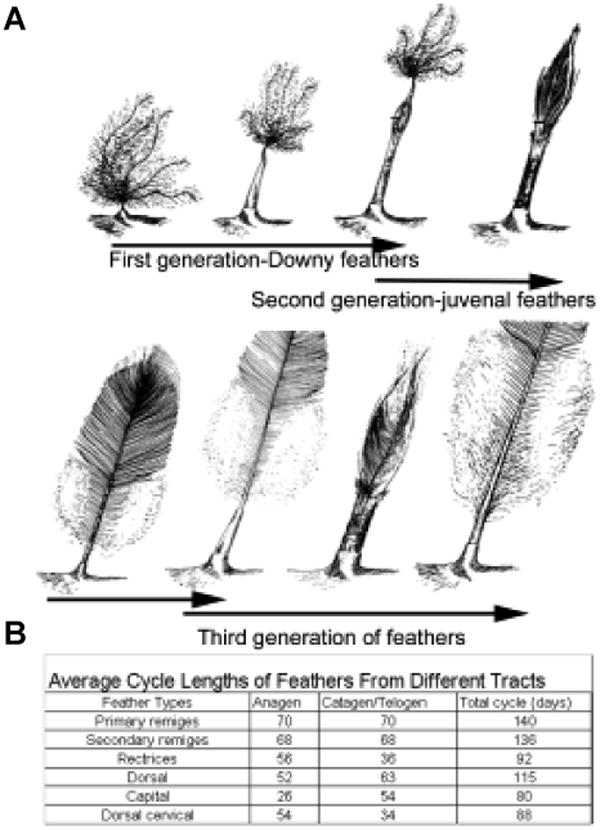
(A) Diagram showing the first three feather cycles. (B) Table showing cycling time of different feathers. (Modified from Lucas and Stettenheim, 1972).
The cell and molecular biology changes in the cycle of feather follicles are not well studied. The feather cycle is crudely divided into growth and resting phases (Lucas and Stettenheim, 1972). The growth phase can be as short as several days in some feathers to months or even years, as seen in long tail chickens. The resting phase can range from 2 days to 14 months. The dynamic changes in growth phase are described above. In the resting phase, a papilla shrinks down to a small size. Cells in the basal layer are small, round, and closely packed. Those in the apical region are larger, loosely packed, and oriented transversely to the long axis of the follicle. The dermal papilla, its apical cap, and the thin covering of epithelial cells then constitute the new feather blastema. In the case of plucking, the ectoderm of the papilla and some epidermal collar cells are left behind. These remaining cells and the dermal papilla also form the blastema. The blastema is composed of feather stem cells that will give rise to a new feather upon appropriate signaling input from the dermal papilla.
The newly generated feathers are frequently bigger and better, giving a bird an opportunity to re-engineer its integument. Upon regeneration, the dermal papilla induces the epidermal precursor cells above it to resume growing. Barbs of a juvenal feather begin to develop from the basal layer of the new collar. They project inside the old calamus, where they become continuous with the edge of the last pulp cap. At the other extreme of calamus formation, the rachis and the barbs do not fuse. This is the condition in the natal remiges. The barb ridges dedifferentiate only partially, and they continue to produce separate barbs. These lack barbules and their rami are solid, without a medulla. The barbs are held together by a short length of the sheath.
Topographic sequence
The timing and sequence of natural molting are well coordinated with physiological conditions of the organism and are regulated at the local level, organism level, and modulated by the environment. During normal molting, the first generation of the feather is called the natal down. The second generation of the feather is called the juvenal feather. The juvenal feather begins to form in the follicle late in embryonic life. As it grows, it pushes the natal down out of the follicle on its tip. This occurs faster in certain remiges and rectrices at the time of hatching (for chicken), but not until several days later in most other feathers on the trunk. A new round of molting begins after the mature juvenal feathers have been held for a period of 1 week to a few months. The newly formed feathers are the 3rd generation feathers. The 3rd generation feathers usually display the distinct texture, color and pattern of an adult chicken. From now on, the feathers usually molt at regular intervals (about two times a year as the prototype, with variations).
The timing and order of molting in different feather tracts are well coordinated in a bird to ensure no acute disruption of function (Fig. 10B). The environment is believed to modulate feather cycling though some systematic coordination, possibly hormones. Molting is usually coupled to their seasonal behavior and reproductive life. Normally, feathers undergo replacement at regular intervals and in the same sequence as found in development. For example, in the primary remiges of chickens, molting proceeds from the carpal region and from the innermost to the outermost feathers. Thus, a maturation gradient exists from the primary remiges at the carpal region to the lateral (or distal) regions. During the first molt, feathers in each tract are replaced in much the same order as their primordia were initially formed in embryonic time. This sequence is repeated in at least the next three molts. However, the sequences of primodium development and of molting can also differ noticeably as seen in the humeral and crural tracts. In the humeral tract, the first group of follicles that arises is in a longitudinal row near the lateral margin. Subsequent rows arise laterally and medially. During the first molt, however, feather replacement starts from the posterior - lateral corner of the tract and proceeds antero-medially. The original sequence reappears in the second molt, when new feathers first emerge in a longitudinal zone in the middle of the tract. Thus, the sequence in which feathers complete their cycling may or may not be the same as the sequence of primordium development. This may be due to functional requirements and/or size variations. Furthermore, in flight feather molting, feathers in the left and right wing molt in turn, so the birds can keep balance in flight. Molting has been observed to stop in the beginning of migration and resume once the bird has arrived at its destination. The mechanisms underlying these aspects are not understood.
Sexual dimorphism of feathers
By now we can appreciate that feathers in the same organism come in different sizes, shapes and colors. One of the most remarkable things is that these feather phenotypes can be modified by sex hormones. Sexual dimorphism is most distinct in the rectrices, less in flight feathers, but they can also appear in feathers from the head, chest, saddle region, etc. A rooster and hen are shown in Fig. 11. The tail feathers of the rooster are long and curved. The tail feathers of the hen are much shorter and assume a fan shape. The phenotype of female chicken rectrices is likely related to ovarian estrogens. Male chicken rectrices likely form in response to androgen hormones. However, it is not known which is the prototype, which will be dominant, and whether this phenotype may become committed at certain stages.
Fig. 11. Sexual dimorphism of feathers.

(A) Left, male chicken. Right, female chicken. There are several differences in the integument between the rooster and the hen. These include the erect comb, bigger wattle, long and slender saddle feathers, bigger and curved caudal feathers. (B) Comparison of caudal feathers (rectrices). Male feathers are longer, wider, and have a curvature. Images in (A) are from www.feathersite.com. The male picture is courtesy of Andy Vardy, Melbourne National 1995.
Steroids bind to feather follicles (Kovacs et al., 1986) and may directly exert their effects. Complete left-ovarectomized females can produce androgen from the masculinized right gonad (Wallenburg, 1982) and their feather patterning is phenotypically similar to that of the male type (Frankenhuis and Kappert, 1980). Similarly, the feathers of castrated males also known to the food industry as “capons”, take on a feminized appearance. Male chickens carrying the henny feathering trait virilize normally but develop a female feathering pattern (George, 1990). This is due to the autosomal dominant mutation that causes the accumulation of aromatase mRNA and activity in extragonadal chicken tissues (Matsumine, 1991). Androgen is normally converted to estrogen by aromatase. In chickens with a henny feathering mutation, increased conversion takes place in the skin and causes roosters to have a female feathering morphology (George, 1990).
Another sex dependent difference in birds is the color. Male birds tend to be more colorful than females, and much research has been done to identify this sexual dichromatism (McGraw, 2002). Males and females deposit the same ratio of carotenoid type pigments into the feathers when they were fed with the same diet, but male gold finches deposit more carotenoids in their feathers than females (McGraw, 2002). This color difference only occurs in feathers at certain locations and in certain patterns. How this is regulated is as yet another interesting unknown.
Since feathers can regenerate from a few stem cells (Yu et al., 2002), chicken rectrices may be a good model to study how sex hormones modulate the growth of epithelial cells. The effect of sex hormones on growth control has major clinical implications as seen in breast cancer, prostate cancer as well as androgenic type alopecia. In hair and prostate, the effect of sex hormones is said to be mediated through the mesenchyme (Randall et al., 1993; Timms et al., 1999). In feathers, we do not know the mechanism but it also offers an opportunity to examine the effects of sex hormones on epithelial-mesenchymal interactions.
Conclusion
Feather follicles are unique epidermal structures invaginating into the dermis. Structurally, they are similar to hair follicles, but they are of different evolutionary origin (Wu et al; this volume). Compared to hair follicles, feather follicles are more complex. Follicles in different tracts can generate feathers with different forms, sizes and colors. The most distinct feature of feathers is that they are highly branched, and follow a hierarchical order. The rachis branches into barbs. The rami (the shaft of the barbs) branch into barbules and barbules branch into hooklets. However, during development, these branches are sculpted from a feather filament cylinder with differential cell proliferation and death. The classical descriptive work was done in Avian Integument (Lucas & Stettenheim, 1972), However, much of the molecular basis and cellular mechanism remains unknown. Feather follicles can be a good “Rosetta stone” for various studies, such as epithelial-mesenchymal interactions, epithelial branching morphogenesis, cell cycling, pigmentation pattern, etc. Most importantly, the study of feather morphogenesis provides valuable insights to the study of feather evolution, since the evolutionary biologists have to build their theories on fossils and speculations. In the past few years, some research about the early stage of chicken feather follicle development has generated valuable information about epithelial and mesenchymal interactions and pattern formation (Chuong et al., 1996; Chuong et al., 2000; Noramly and Morgan, 1998). Most recently, we have started to study the post hatch chicken feather follicles (Yu et al., 2002). These works can help us test various models for the origin and evolution of feathers (Prum, 1999; Chuong et al., 2003; Widelitz et al., 2003). The information generated has made the feather follicle a rich area of cell biology research that inspires many new perspectives.
Acknowledgments
We thank all authors for the paper/book materials used in our figures (referred in figure legends), particularly Dr. Lucas and Dr. Stettenheim for their classical volume which we refer to a lot. This work is supported by grants from NIAMS (CMC), NCI (RW), and Argyros Foundation (DYW). We thank Ms Fiona McCulloch for help in manuscript preparation.
References
- Bartels T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J Exp Zool Part B Mol Dev Evol. 2003;298:91–108. doi: 10.1002/jez.b.28. [DOI] [PubMed] [Google Scholar]
- Cahoon SM, Scott SA. Multiple mechanisms contribute to the avoidance of avian epidermis by sensory axons. Dev Biol. 1999;208:502–12. doi: 10.1006/dbio.1999.9220. [DOI] [PubMed] [Google Scholar]
- Cahoon-Metzger SM, Wang G, Scott SA. Contribution of BDNF-mediated inhibition in patterning avian skin innervation. Dev Biol. 2001;232:246–54. doi: 10.1006/dbio.2001.0172. [DOI] [PubMed] [Google Scholar]
- Chen CW, Jung HS, Jiang TX, Chuong CM. Asymmetric expression of Notch/Delta/Serrate is associated with the anterior-posterior axis of feather buds. Dev Biol. 1997;188:181–7. doi: 10.1006/dbio.1997.8643. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Edelman GM. Expression of cell-adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. J Cell Biol. 1985a;101:1009–26. doi: 10.1083/jcb.101.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Edelman GM. Expression of cell-adhesion molecules in embryonic induction. II. Morphogenesis of adult feathers. J Cell Biol. 1985b;101:1027–43. doi: 10.1083/jcb.101.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM. Adhesion molecules (N-CAM and tenascin) in embryonic development and tissue regeneration. J Craniofac Genet Dev Biol. 1990;10:147–61. [PubMed] [Google Scholar]
- Chuong CM, Widelitz RB, Ting-Berreth S, Jiang TX. Early events during avian skin appendage regeneration: dependence on epithelial-mesenchymal interaction and order of molecular reappearance. J Invest Dermatol. 1996;107:639–46. doi: 10.1111/1523-1747.ep12584254. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Chodankar R, Widelitz RB, Jiang TX. Evo-devo of feathers and scales: building complex epithelial appendages. Curr Opin Genet Dev. 2001;10:449–56. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L. Adaptation to the sky: Defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J Exp Zool Part B Mol Dev Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhouailly D. The determination of specific differentiation of neoptile and teleoptile feathers in the chick and the duck. J Embryol Exp Morphol. 1970;24:73–94. French. [PubMed] [Google Scholar]
- Dhouailly D. Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. J Embryol Exp Morphol. 1973;30:587–603. French. [PubMed] [Google Scholar]
- Fichard A, Verna JM, Olivares J, Saxod R. Involvement of a chondroitin sulfate proteoglycan in the avoidance of chick epidermis by dorsal root ganglia fibers: a study using beta-D-xyloside. Dev Biol. 1991;148:1–9. doi: 10.1016/0012-1606(91)90312-q. [DOI] [PubMed] [Google Scholar]
- Frankenhuis MT, Kappert HJ. Experimental transformation of right gonads of female fowl into fertile testes. Biol Reprod. 1980;23:526–9. doi: 10.1095/biolreprod23.3.526. [DOI] [PubMed] [Google Scholar]
- George FW, Matsumine H, Mcphaul MJ, Somes RGJR, Wilson JD. Inheritance of the henny feathering trait in the golden Campine chicken: evidence for allelism with the gene that causes henny feathering in the Sebright bantam. J Hered. 1990;81:107–10. doi: 10.1093/oxfordjournals.jhered.a110938. [DOI] [PubMed] [Google Scholar]
- Haake AR, Konig G, Sawyer RH. Avian feather development: relationships between morphogenesis and keratinization. Dev Biol. 1984;06:406–413. doi: 10.1016/0012-1606(84)90240-9. [DOI] [PubMed] [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294:160–76. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Homberger DG, De Silva KN. The role of mechanical forces on the patterning of the avian feather-bearing skin: A biomechanical analysis of the integumentary musculature in birds. J Exp Zool Part B Mol Dev Evol. 2003;298:123–39. doi: 10.1002/jez.b.30. [DOI] [PubMed] [Google Scholar]
- Humphrey, Parkes An approach to the study of molts and plumages. Auk. 1959;76:1–31. [Google Scholar]
- Jiang TX, Chuong CM. Mechanism of skin morphogenesis. I. Analyses with antibodies to adhesion molecules tenascin, n-cam, and integrin. Dev Biol. 1992;150:82–98. doi: 10.1016/0012-1606(92)90009-6. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Widelitz RB, Shen WM, Will P, Wu DY, Lin CM, Jung HS, Chuong CM. Integument pattern formation involves genetic and epigenetic controls: feather arrays simulated by digital hormones. Int J Dev Biol. 2004;48:117–135. doi: 10.1387/ijdb.041788tj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Kovacs K, Peczely P, Szelenyi Z. Steroid binding to feather follicles in the chicken. J Endocrinol. 1986;109:187–91. doi: 10.1677/joe.0.1090187. [DOI] [PubMed] [Google Scholar]
- Lecoin L, Lahav R, Martin FH, Teillet MA, Le Douarin NM. Steel and c-kit in the development of avian melanocytes: a study of normally pigmented birds and of the hyperpigmented mutant silky fowl. Dev Dyn. 1995;203:106–18. doi: 10.1002/aja.1002030111. [DOI] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR, editors. Avian Anatomy – Integument Agricultural Handbook 362: Agricultural Research Services. US Department of Agriculture; Washington DC: 1972. [Google Scholar]
- Matsumine H, Herbst MA, Ou SH, Wilson JD, Mcphaul MJ. Aromatase mRNA in the extragonadal tissues of chickens with the henny-feathering trait is derived from a distinctive promoter structure that contains a segment of a retroviral long terminal repeat. Functional organization of the Sebright, Leghorn, and Campine aromatase genes. J Biol Chem. 1991;266:19900–7. [PubMed] [Google Scholar]
- Mcgraw KJ, Hill GE, Stradi R, Parker RS. The effect of dietary carotenoid access on sexual dichromatism and plumage pigment composition in the American goldfinch. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:261–9. doi: 10.1016/s1096-4959(01)00500-0. [DOI] [PubMed] [Google Scholar]
- Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development. 1998;125:3775–3787. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- Pays L, Charvet I, Hemming FJ, Saxod R. Close link between cutaneous nerve pattern development and feather morphogenesis demonstrated by experimental production of neo-apteria and ectopic feathers: implication of chondroitin sulphate proteoglycans and other matrix molecules. Anat Embryol (Berl) 1997;195:457–66. doi: 10.1007/s004290050065. [DOI] [PubMed] [Google Scholar]
- Prum RO. Development and evolutionary origin of feathers. J Exp Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- Prum RO, Williamson S. Theory of the growth and evolution of feather shape. J Exp Zool. 2001;291:30–57. doi: 10.1002/jez.4. [DOI] [PubMed] [Google Scholar]
- Prum RO, Williamson S. Reaction-diffusion models of within-feather pigmentation patterning. Proc R Soc Lond B Biol Sci. 2002;269:781–92. doi: 10.1098/rspb.2001.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall VA, Thornton MJ, Messenger AG, Hibberts NA, Loudon AS, Brinklow BR. Hormones and hair growth: variations in androgen receptor content of dermal papilla cells cultured from human and red deer (Cervus elaphus) hair follicles. J Invest Dermatol. 1993;101(Suppl):114S–120S. doi: 10.1111/1523-1747.ep12363039. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Hornbruch A, Wolpert L. Pigment patterns in neural crest chimeras constructed from quail and guinea fowl embryos. Dev Biol. 1991;143:309–19. doi: 10.1016/0012-1606(91)90082-e. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J Exp Zoolog Part B Mol Dev Evol. 2003;298:57–72. doi: 10.1002/jez.b.26. [DOI] [PubMed] [Google Scholar]
- Saxod R. Combination of cholinesterase staining of nerves and stereoscopic viewing for three-dimensional study of skin innervation on whole mounts. Invest Dermatol. 1978;70:95–7. doi: 10.1111/1523-1747.ep12541229. [DOI] [PubMed] [Google Scholar]
- Saxod R, Pays L, Hemming FJ. Development of the cutaneous nervous system. Pathol Biol (Paris) 1996;44:838–48. [PubMed] [Google Scholar]
- Sengel P. Morphogenesis of skin. Cambridge University Press; Cambridge, UK: 1976. [Google Scholar]
- Streit L, Heidrich W. A Biologically-Parameterized Feather Model. Eurographics. 2002;21 [Google Scholar]
- Timms BG, Petersen SL, Vom Saal FS. Prostate gland growth during development is stimulated in both male and female rat fetuses by intrauterine proximity to female fetuses. J Urol. 1999;161:1694–701. [PubMed] [Google Scholar]
- Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev Dyn. 1996;207:157–170. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verna JM, Saxod R. Development of cutaneous innervation in the chick: ultrastructural and quantitative analysis (article in French, author's transl) Arch Anat Microsc Morphol Exp. 1979;68:1–16. [PubMed] [Google Scholar]
- Wallenburg J. Macroscopy, light and electron microscopy studies on the genesis and function of the gonads after experimental sex-reversal following left- side ovariectomy of hen chicks (Gallus domesticus) Gegenbaurs Morphol Jahrb. 1982;128:463–529. [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Yu M, Shen T, Shen JY, Wu P, Yu Z, Chuong CM. Molecular biology of feather morphogenesis: a testable model for evo-devo research. J Exp Zoolog Part B Mol Dev Evol. 2003;298:109–22. doi: 10.1002/jez.b.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JE, Domm LV. A histochemical identification of the androgen- producing cells in the gonads of the domestic fowl and albino rat. Gen Comp Endocrinol. 1966;7:559–570. doi: 10.1016/0016-6480(66)90076-1. [DOI] [PubMed] [Google Scholar]
- Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz RB, Jiang TX, Chuong CM. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:248–267. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–12. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]


