Abstract
Different epithelial organs form as a result of epithelial - mesenchymal interactions and share a common theme modulated by variations (Chuong edit. In Molecular Basis of Epithelial Appendage Morphogenesis, 1998). One of the major modulators is the sex hormone pathway that acts on the prototype signaling pathway to alter organ phenotypes. Here we focus on how the sex hormone pathway interfaces with epithelia morphogenesis related signaling pathways. We first survey these sex hormone regulated morphogenetic processes in various epithelial organs. Sexual dimorphism of hairs and feathers has implications in sexual selection. Diseases of these pathways result in androgenic alopecia, hirsutism, henny feathering, etc. The growth and development of mammary glands, prostate glands and external genitalia essential for reproductive function are also dependent on sex hormones. Diseases affecting these organs include congenital anomalies and hormone dependent type of breast and prostate cancers. To study the role of sex hormones in new growth in the context of system biology / pathology, an in vivo model in which organ formation starts from stem cells is essential. With recent developments (Yu et al., The morphogenesis of feathers. Nature 420:308–312, 2002), the growth of tail feathers in roosters and hens has become a testable model in which experimental manipulations are possible. We show exemplary data of differences in their growth rate, proliferative cell population and signaling molecule expression. Working hypotheses are proposed on how the sex hormone pathways may interact with growth pathways. It is now possible to test these hypotheses using the chicken model to learn fundamental mechanisms on how sex hormones affect organogenesis, epithelial organ cycling, and growth related tumorigenesis.
Keywords: sexual dimorphism, feather, hair, breast cancer, prostate cancer, androgen, estrogen, morphogenesis, organogenesis
Introduction
Sexual dimorphism is the systematic difference in phenotype between individuals of different sex in the same species (Fig. 1). Sexual dimorphisms readily apparent on an animal’s body surface serve to attract members of the opposite sex. Frequently these secondary sexual characteristics involve the size, shape and color of epithelial appendages, forming the basis for sexual selection (Darwin, 1871). The formation of internal and external reproductive organs including the prostate and mammary glands are essential for reproduction. The morphogenesis of these different epithelium derived organs results from epithelial-mesenchymal interactions and are variations on top of a common theme (Chuong edit, 1998; Widelitz and Chuong, 1999; Widelitz et al, 2003). One of the major variations is the physiological response depending on the concentration of sex steroid levels, their receptors, and their co-activators. Here we will first survey sex hormone pathways and its roles on the morphogenesis of various epithelial organs in a variety of physiological and pathological conditions. We will then present some pilot data toward establishing chicken tail feathers as a model for the sex hormone regulated organ formation in the context of system biology / pathology. In the discussion, we hypothesize some mechanisms through which sex hormone pathways may interface with morphogenesis related pathways.
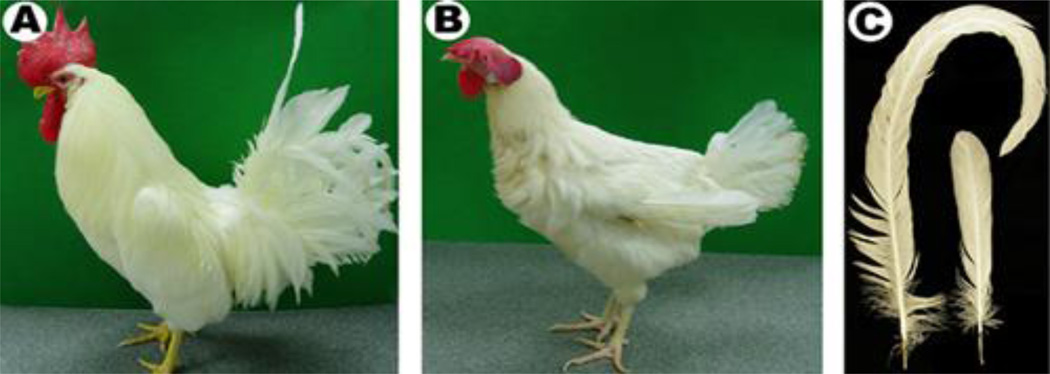
Fig. 1.
Male and female adult chickens. A) Photo of a male white leghorn. B) Photo of a female white leghorn. C) Left: male tail feather. Right: female tail feather. Both are from the midline.
Sex hormone pathways
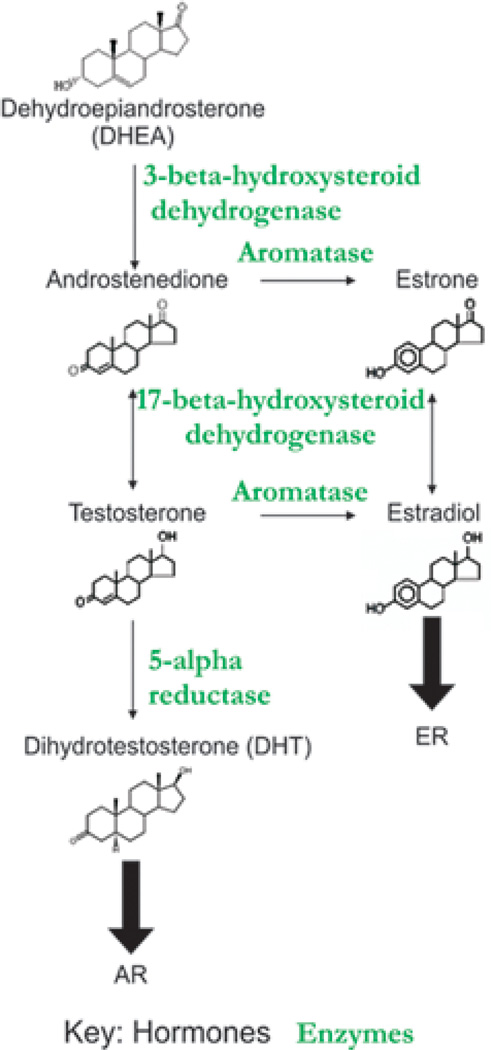
To obtain a mechanistic understanding of sexual dimorphism, we first have to understand their biochemical / molecular pathways. The sex steroid biosynthetic pathway is well established (reviewed in Chang, 2002). A simplified biosynthesis pathway is briefly described here and shown schematically in Fig. 2. Steroids are synthesized from cholesterol, which is converted to pregnenolone by the side chain cleavage enzyme, p450scc within the mitochondria. The synthesis pathways bifurcate to form progesterone and the corticosteroids or to form androgens and estrogens. Progesterone can also be converted toward androgens using similar enzymes as described below in a parallel pathway. Pregnenolone undergoes 17-α-hydroxylation by microsomal P450c17. 17-hydroxy pregnenolone is converted to dehydroepiandrosterone (DHEA) by the 17–20-lyase activity of P450c17. DHEA is then converted to androstenedione by 3-β-hydroxysteroid dehydrogenase. Androstenedione can be converted in a reversible reaction to testosterone by 17-β hydroxysteroid dehydrogenase. Testosterone can then be converted to DHT by the microsomal NADPH-dependent enzyme 5α-reductase. The non-aromatic androgens, testosterone or androstenedione, also can be converted by P450 aromatase to the aromatic estrogens, estradiol or estrone, respectively. Estrone can also be converted to estradiol in a reversible reaction by 17-β hydroxysteroid dehydrogenase. Hence, in the biosynthetic pathway, androgens are synthesized first and subsequently converted to estrogens in what is thought to be the rate limiting step in estradiol biosynthesis (Jarman et al, 1998).
Fig. 2.
Hormone biosynthetic pathway. Enzyme names are indicated in green. Products are indicated in black.
In order for the steroid hormones to exert their effects, they must bind to specific nuclear receptors, which trigger transcriptional activation events. Thus, sex hormone dependent pathways involve ligands, receptors, enzymes and co-activators (Chang, 2002). Along the pathways, different members play pivotal roles, and errors in them can have serious patho-physiological consequences. To deal with the consequences of these errors, drugs have been developed to target different steps along these pathways.
Sexual dimorphism in mammals
The roles of sex hormones on skin appendage dimorphisms have been documented in a few species. Both hormones (androgens and estrogens) are involved in determining the type of hair produced by follicles in mammals (Randall 1994). Here we introduce some concepts of hormone influence on skin appendage sexual dimorphisms.
Primates
Pelage and skin can be markedly dimorphic in primates. The brightly colored face of male mandrills as compared to that of females is one of the most well characterized examples (Setchell and Dixson, 2001). Male gelada baboons also exhibit skin and pelage differences. They have a large prominent mane, which probably serves to make males appear larger (Crook, 1972). Male orangutans possess large cheek flanges and laryngeal pouches. Many species of male and female primates differ dramatically in pelage color, while others show minor sex differences in pelage color or patterning (Crook, 1972; Hershkovitz, 1977). The reasons for these pelage and skin dimorphisms remain unclear.
Men and women differ in the amount of hair developed and the places at which hair will develop. Androgens paradoxically can stimulate and inhibit human hair growth in different regions or at different times. The hair follicle is a complicated endocrine target organ because its response to androgens is extremely complex and this paradox is unique in endocrinology (Randall et al, 2000). Low capacity, high affinity androgen receptors are found in all dermal papilla cells from androgen sensitive sites (Randall et al, 2000). Even within these androgen sensitive hair follicles there are differences. Androgens can stimulate the beard and body hair growth while inhibiting scalp hair growth. Presumably this is due to differential gene expression in the hair follicles of these different regions caused during development (Randall et al, 2000). In women, the skin is a major site of testosterone formation. Half of their testosterone production stems from the conversion of secreted 17-ketosteroids including DHEA and androstenedione (Rosenfield and Lucky, 1993). Whereas in men, the testes are the major site of testosterone production. In humans, very coarse, pigmented, sexual hairs develop during puberty from fine, unpigmented, vellus hairs. Genetic susceptibility in certain individuals allows for androgens to act upon the terminal hair follicles causing them to become vellus ones leading to androgenic alopecia (Randall et al, 2000).
Deer
In red deer sexual dimorphism is displayed in terms of size with the males being much larger in size than the females (Post et al, 1999). Mates are selected based on strategies of growth in relation to reproduction. The red deer exhibits highly coordinated seasonal coat growth with two distinct pelage types each year. During the winter breeding season the male red deer grows an androgen dependent mane due to an increase in levels of plasma testosterone (Lincoln, 1971;Thornton et al, 2000). This is then replaced with short hairs in the summer when the levels of plasma testosterone drop. Antlers are derived from the bone but also exhibit hormone dependent growth. The hairs on newly formed skin over the antler and the mane of the deer (Thornton et al., 2001) can also be used as a model to study sex hormone dependent hair growth.
Mice
In mice, sexual dimorphisms are not clearly visible. Both androgens and estrogens have been implicated in regulating the hair cycles. Estrogen receptors have been located in the dermal papillae of telogen follicles (Oh and Smart, 1996) and estradiol has a direct inhibitory effect on the hair fiber growth cycle (Mohn, 1958; Ebling and Johnson, 1964; Oh and Smart, 1996). It is important to note that it is estrogen receptor α and not estrogen receptor β that has been localized in the hair follicles of mice (Chanda et al, 2000). It is also been found that pituitary prolactin regulates seasonal hair follicle growth cycles in many mammals as well as in the non-seasonal laboratory mice pelage replacement (Craven et al, 2001). Prolactin appears to play an inhibitory role in the pelage replacement of many mammals.
Sexual dimorphism in birds
Many birds show sexual dimorphisms. Mainly, it is the male that exhibits fancy feathers or other integument appendages (e.g., comb, wattle) to attract the female. Feathers come in different sizes, shapes, colors, etc. Contour feathers are found on the body while rectrice feathers are found on the tail. The longest feathers are the tail feathers of the peacock and those of birds of paradise. While these traits make individuals spectacularly beautiful and increase their chance for mating, it also increases their visibility and hence decreases their survival due to predation. This observation puzzled Darwin in his contemplation of sexual dimorphism (Darwin, 1871).
In chickens, male and female chicks are the same weights upon hatching and do not exhibit any distinguishing secondary sexual characteristics with the exception of specific genetic variants in down color or wing feather length (Jacob & Mather, 2000). The male will develop a larger body, comb, and wattles than the female. The male white leghorn’s comb is turgid and stands erect, whereas the female’s comb upon full maturity is less turgid and lies over to one side. The male also develops a larger spur and has longer and more pointed hackle feathers than the female (Jacob & Mather, 2000).
It is widely known that male tail feathers grow longer and wider than female tail feathers and have different shapes (Aparicio et al, 2003). Here, size is measured as the length of the feather and shape is measured as the varying width of the vane and the curvature of the rachis. This difference has been attributed to a benefit to male birds with longer tails during the sexual selection process. Tail length has been negatively correlated with the density of barbs and rachis width in males but not in females (Aparacio et al, 2003). Testis size has also been positively correlated with dimorphisms in wing and tail length (Dunn et al, 2001). Hence these attributes appear to be sex dependent and thus hormone regulated. George & Wilson, (1980) suggested that female feathering patterns in chickens result from ovarian estrogens which leads us to believe that male feathering patterns result from plasma testosterone levels. We believe that the feather offers an opportunity to examine sex hormone effects on epithelial-mesenchymal interactions since the difference in feather length and diameter is sex dependent and thus hormone regulated. This hypothesis is further supported by the following observations.
Since male birds can be aggressive, small chicken farms will sometimes castrate their roosters at 2 – 4 weeks of age. This blocks androgen synthesis, making the birds docile with tender meat. The combs and wattles diminish in size and resemble those of female birds, but the hormone sensitive feathers (ie, tail feathers) grow longer. These birds are called capons (Payne, 1936; Jacob and Mather, 2000). Capons with similar feathering patterns previously were made by administering stilbestrol (an estrogen) rather than performing castrations. Caponization later in life produces a female feathering pattern. Nowadays, a combination of changes brought about by breeding and diet produce capons that mature more rapidly than in the past. Ten pound birds can be raised in 12–13 weeks as opposed to 20 weeks in years past. The feathers of 10 pound capons no longer grow as long as they used to (they mature prematurely).
On the other hand, the left ovary of female birds is functional while the right ovary regresses under natural conditions (Andrews et al, 1997). Upon ovarectomy, the right gonad can masculinize and produce androgen (Woods et al, 1966) resulting in a “male” feathering pattern (Frankenhuis and Kappert, 1980). It is unknown if this is due to a loss of estrogen or to a gain of androgen.
Problems related to sex hormone dependent skin appendage morphogenesis: alopecia, hirsutism and trans-differentiation
In humans and other animals, the growth of skin appendages can go wrong due to errors along the sex hormone pathway. The pathogenesis may be at the level of hormone concentration, receptors, enzymes, or co-activators / co-suppressors. The density and size of skin appendages may increase or decrease. Alopecia can occur in males or females (Randall, 1994; Birch et al., 2002) and likely follow different etiologies. Dermatologists are trying to identify causes of alopecia so these conditions can be corrected, perhaps by relieving a pathological blockade.
Androgenic alopecia
Commonly referred to as “male pattern baldness”, androgenic alopecia is also the most common form of hair loss in women. Androgenic alopecia is the name for male and female pattern baldness. This androgenetic alopecia is present in at least 50 % of males by age 50, 70% of males in later life and 30–40% of females over 70 years of age (Norwood, 2001). Androgenetic alopecia is characterized by the progressive replacement of terminal hairs with vellus hairs (Hoffman and Happle, 2000; Paus and Cotsarelis, 1999). This is fostered by the continually shortening anagen phase of the hair cycle causing a higher percentage of telogen phase hairs in the crown and forehead region. Furthermore, the time between telogen and a new anagen phase increases, leading to reduced hair numbers (Courtois et al, 1994). Another intriguing observation is the regional specific response of hair growth to androgen. This was found to be partially due to the differential expression of 5 α-reductase type 2 in the dermal papillae of scalp hair follicles (Thigpen et al, 1993). Inhibition of this isozyme with finasteride was shown to be significantly effective in the treatment of androgenetic alopecia (Whiting, 2001; Shapiro and Kaufman, 2003).
Females can also suffer from hirsutism or alopecia. Female type alopecia due to hyperandrogenism may be due to androgen metabolism whereas this pathway plays a very minor role if any in postmenopausal women suffering from female type alopecia (Shum et al, 2002). Treatment of women suffering from idiopathic hirsutism with anti-androgen drugs reduces the size of body and facial hair by reducing the formation of a medulla (Ebling, 1987). Still, how DHT promotes cell proliferation or suppresses hair differentiation in different hair follicles (scalp hairs vs beards vs axilla hairs) remains unknown. The interactions are further complicated by the interactions of circulating steroids and the sex hormone processing enzymes in different follicles (Hoffman and Happle, 2000).
Our nearest non-human primate relatives, orangutans and gorillas can also develop androgenetic alopecia. The stump tailed macque has been used as an in vivo model for androgenic alopecia (Brigham et al., 1988). Once hair follicles have been exposed to androgens they are fated to become androgen sensitive and androgenetic alopecia can develop. Androgenetic alopecia develops as a gradual reduction of scalp hair follicle size, accompanied by reduced time in the anagen active growth phase, leading to more hair follicles in the telogen resting stage of the hair cycle. Although periods of anagen are reduced, catagen and telogen time periods remain the same. In hair, the hormone acts first on the DP which then signals to and induces growth in the epithelium (Obana et al, 1997; Randall et al, 2001).
In chickens, a dramatic example of hormone dependent growth is the conversion of male into female feather phenotypes. In "henny feathering", a genetically transmitted constitutively active aromatase in the skin can cause roosters to exhibit "female type" tail feathers (Wilson et al., 1987). Male chickens carrying the henny feathering trait virilize normally but develop a female feathering pattern (George & Wilson, 1980). This autosomal dominant mutation causes the accumulation of aromatase mRNA and activity in extragonadal chicken tissues (Matsumine, 1991), which converts androgen to estrogen in the skin. Again, it is unknown if this is due to a decrease of androgen or an increase of estrogen.
Sex hormone dependent genetic diseases
The development of urogenital organs and external genitalia are essential to carry out reproduction function. These epithelial and mesenchymal tissues are malleable and can form the male or female type during embryonic development. As a result, patients who suffer from inborn errors of the sex hormone pathway may produce epithelial organs of the wrong sexual type.
5 α-reductase deficiency
There are two forms of 5 α-reductase which can convert testosterone to DHT. They are differentially expressed in various tissues. Androgen action in sexual organs is primarily dependent upon the type 2 isozyme (Thigpen et al, 1993) and deficiency of this isozyme form leads to pseudohermaphroditism (Andersson et al, 1991). There is only one wave of expression of the type 2 isozyme that starts at birth and ends by three years of age. The type 2 isozyme is not detected in adult skin but is found in the hair follicles of the scalp, suggesting that balding may be pre-determined early in development (Bayne et al, 1999). The major form of 5 α-reductase in the skin is the type 1 isozyme which is expressed in 2 waves. The first occurs at birth and lasts until three years of age and the second begins during puberty and lasts throughout life (Thigpen et al, 1993).
Patients with 5 α-reductase deficiency fail to metabolize testosterone into DHT. Defects in 5 α-reductase typically result in an intersex phenotype. Intersexed individuals do not develop pubic, axilla, or beard hairs normally (Griffin and Wilson, 1989), but they do exhibit normal scalp hair development (Randall et al, 1991). This suggests that the conversion of testosterone to DHT by 5 α-reductase is not essential in follicles that are androgen sensitive in both sexes but only in those that distinguish the adult male (Randall et al, 2000)
Pseudohermaphroditism
Male pseudohermaphroditism is caused by a defect in testosterone biosynthesis. Female pseudohermaphroditism is typically caused from a defect in the enzymes leading to glucocorticoids or mineralocorticoids causing a shunting of precursor molecules into the androgenic pathway (Haqq and Donahoe, 1998) or as a result of defects in estrogen formation.
Androgen insensitivity syndrome
Androgen insensitivity syndrome is also referred to as testicular feminization which is a subtype of male pseudohermaphroditism. A defect in the androgen receptor leads to an overall resistance to androgens in this disorder. Males with androgen insensitivity syndrome will typically have no hair growth in the axillary and pubic regions (Hamilton, 1950). Expression of aromatase in genital skin is androgen dependent as demonstrated by lower levels of aromatase present in cells from patients with androgen insensitivity syndromes (Stillman et al, 1991).
Sex Hormone dependent tumor growth
Cancer rates continue to rise here in the United States mainly due to increasing rates for prostate cancer among men and for breast cancers among women (Devesa et al, 1995) while mortality is steadily decreasing (Quinn, 2003). Some of these tumor types are sex hormone dependent while some are not. For those with sex hormone dependence, it is possible to treat the tumor by blocking sex hormone synthesis or receptor binding.
It has been suggested that along with breast cancer, other cancers including ovarian and endometrial cancers are a direct result of ovarian function (Pike et al, 2004). Oophorectomy then would significantly reduce the incidence of all three of these cancer types (Pike et al, 2004), but this would not be a proper solution for most women. The incidence of breast, endometrial and ovarian cancers increase with age, but show a notable slow down in relation to age around the time of menopause (Pike et al, 2004). Menopause clearly has a large protective effect against all three of these cancers. One explanation for this is that ovarian hormones stimulate cell division which is reduced after menopause. The ovaries begin producing less steroids in particular estrogen as menopause progresses and thus the relevant tissues cease to divide as a result of lack of steroid hormones. This protective effect of menopause and lack of steroid hormones, remains the central clue to the chemoprevention of various types of hormone dependent cancers including breast, endometrial, and ovarian (Pike at al, 2004).
Estrogen and breast cancer
Breast cancer is the second leading cause of cancer related deaths among women in the United States. The American Cancer Society estimates that 40,200 (39,800 females, 400 males) people will die from breast cancer in 2003. Sex steroids have been suggested to be involved in breast carcinogenesis, and may be fundamental molecules involved in the progression and growth of many carcinomas. In many breast cancer types, tumorigenesis initially is hormone dependent. Conditions associated with altered estrogen/androgen ratios predispose patients to male breast cancer (Ravandi-Kashani and Hayes, 1998). There are many risk factors associated with the onset of breast cancer. The three most important and well studied reproductive risk factors for breast cancer include early menarche, late first full-term pregnancy, and late menopause (FFTP; Kelsey and Bernstein, 1996; Colditz and Rosner, 2000).
The mammary gland is an epithelial organ derived from epidermis as a result of epithelial - mesenchymal interactions (Parmar and Cunha, 2004; Veltmaat et al., 2003). Estrogens play an important role in the growth and differentiation of normal breast epithelium acting through the estrogen receptor in embryonic development and in lactation cycle. Hormone therapy tries to modulate pathway activity at different levels. Estrogen antagonists block estrogen action in several ways. For example, tamoxifen competitively binds to the estrogen receptor (ER), displacing the natural ligand, estradiol. AhR (Aryl hydrocarbon receptor) agonists down regulate ligand bound ER (Wormke et al., 2003), but up regulate unbound ER (Ohtake et al., 2003). Aromatase inhibitors block the conversion of testosterone to estradiol (Elbrecht and Smith, 1992). Aromatase is the enzyme responsible for the conversion of non-aromatic androgens, particularly testosterone and androstenedione, to aromatic estrogens: Estrone and Estradiol. In postmenopausal women testosterone is converted to estrogens by aromatase in the skin, muscles, and liver. Thus, conversion of testosterone to estradiol is thought to be the rate-limiting step in estradiol biosynthesis.
Aromatase inhibitor in breast cancer therapy
The conversion of androgens to estrogens by the enzyme aromatase is considered the rate-limiting step of estrogen biosynthesis (Brodie and Njar, 2000). As women proceed through menopause ovarian estrogen production diminishes. In postmenopausal women, the main source of estrogen comes from the conversion of androgens to estrogens by aromatase in peripheral tissues such as adipose and muscle tissues (Brodie and Njar, 2000). Since estrogens are known to play a critical role in the development and growth of breast cancers, inhibitors of aromatase have been produced in an effort to inhibit tumor proliferation. Aromatase inhibitors can be divided into two classes: steroidal and non-steroidal inhibitors. Steroidal inhibitors such as formestane and exemestane, compete with androgens (testosterone and androstenedione) where they bind irreversibly to aromatase causing inhibition (Buzdar and Howell, 2001). Non-steroidal inhibitors including fadrozole, anastrozole and letrozole compete with androgens for the conversion of testosterone to estradiol (Buzdar and Howell, 2001). Androgenic alopecia is common in women undergoing hormone therapy as treatment for breast cancer (Budzar et al, 2001). Mutations in the aromatase gene can also cause females to develop a form of pseudohermaphroditism with androgenetic alopecia (Hoffmann and Happle, 2000).
Androgen and prostate cancer
Prostate cancer is the second leading cause of cancer related deaths among men in the United States. The American Cancer Society estimates that 28,900 men will die from prostate cancer in 2003. It is estimated that there is a one in six probability that a man will develop prostate cancer in his lifetime. Prostate cells are dependent upon androgens (testosterone and DHT) for growth and function (Anderson, 2003; Marker et al., 2003). Males who are castrated at early an early age do not develop prostate cancer. This suggests that androgens and their receptors are major risk factors for prostate cancer.
Prostate cancers initially arise as androgen dependent but following androgen ablation therapy the tumors re-surface in an androgen-independent manner. Anti-androgens can compete for androgen receptor binding sites in the nucleus of the prostate cells thus inhibiting prostate cancer growth and promoting apoptosis (Anderson, 2003). One of the treatment modalities utilizes anti-androgens (flutamide, casodex) to suppress androgen activity. Flutamide is a non-steroidal pure antiandrogen that acts as a competitive inhibitor of DHT (Grzywacz et al, 2003) but does not suppress testosterone secretion (Anderson, 2003). Hydroxyflutamide is the active metabolite of Flutamide and blocks the expression of genes containing an androgen response element in their promoters while preventing stabilization of the androgen receptor (Grzywacz et al., 2003). Other more recent treatments utilize agents such as finestaride to inhibit androgen synthesis.
The androgen receptor may serve as a link between other signaling pathways. β-catenin (Truica et al, 2000) and Her2/neu (Zhau et al, 1992) have been reported to interact with the androgen receptor in prostate cancer to elicit cell survival and growth. Members of the bone morphogenetic protein (BMP) pathway have also been implicated in regulating prostate cancer growth. BMP2 has been shown to decrease prostate cell growth (Ide et al, 1997) and others such as BMP4 (Thomas et al, 1998) and BMP6 (Barnes et al, 1995) are constitutively expressed despite castration.
The need for establishing an in vivo experimental model for sex hormone dependent growth
A novel, physiological epithelial growth model responsive to sex hormones
In order to manage the loss of new growth in alopecia, an understanding of how nature regulates normal new growth must be achieved. The mechanism of how sex hormones regulate growth is largely unknown and model systems to examine it are lacking. While mice have strong genetics, they are not good models because they lack sexual dimorphism. In humans, the hormone regulated growth control of hair is mostly studied in patients with abnormal hair formation. Thus there is a need for a good model in which both in vitro and in vivo studies can be performed to bring out these mechanisms. We have been using White Leghorn chicken feathers as an animal model to study sex hormone effects on epithelial appendage growth. Chickens have clear cut sexual dimorphism (Yu et al, 2004) and the regulation of male and female tail feathers is part of their physiological process. Roosters and hens are good experimental models because they provide a true in vivo model with an obvious sexual dimorphism in their tail feathers or “rectrices” (Fig. 1).
Materials and Methods
Housing of Birds
The birds are housed within the USC Vivaria facility. They are monitored daily and housed under a twelve hour light / dark cycle. The birds are fed lay crumble (Southwest Farms, Ca) and water at lib.
Measurement of feather growth
Quantitative differences in size and shape were assessed using a regeneration model because it allows for the synchronization of the feather growing process. Adult male and female white leghorn chickens of known ages (SPAFAS, pathogen-free chickens) were used in this experiment because they are sexually mature and should possess ER, AR and detectable levels of sex steroids. The tail feathers of these birds were measured every other day for the duration of the regeneration cycle and photographed periodically. For each bird, the two feathers closest to the midline were measured since they have the most pronounced sexual dimorphic phenotypes. The lateral feather to each side of the central pair was also measured as a comparison since they too contribute to the dimorphic phenotype. From the daily measurements, the average rate of growth as well as total length and duration of growth was quantified.
Sections and immunostaining
Specimens were fixed in 4% paraformaldehyde for 24hrs at 4° C followed by a dehydration series and embedding in paraffin wax. Cross sections, and longitudinal sections along the AP and LR axes cut with a microtome were stained with antibodies against PCNA (Chemicon, CA) to visualize proliferating cells and β-catenin (Sigma) to visualize signaling. Binding of the secondary antibody and diaminobenzidine (DAB) staining were done using the SK4100 Kit (Vector), according to the manufacturer’s protocol as described (Jiang et al, 1998). Some of these experiments use the Ventana Discovery instrument for processing.
In-situ Hybridization
In-situ hybridization was performed as described previously in Jiang et al. (1998). Probes for feather keratin A and cytokeratin II were used to visualize differentiating cells. Feather Keratin A expression was used to assess differentiation status (Yu et al, 2002). Some of these experiments use the Ventana Discovery instrument for processing.
Results
Different growth kinetics in male and female type tail feathers
Feathers are lost through seasonal molts and regrown throughout a bird’s life. This process can also be initiated by removing the feathers through plucking. After plucking, a specialized region of the mesenchyme, the DP, is left behind. The epithelia surrounding the plucked regions represent a wound. The epithelium becomes organized in the healing process so that a single layer of epithelium covers the DP by 2 days after plucking. This then forms a stratified epithelium by day 3. Plucking resets the molecular and cellular processes to a naïve state and synchronizes ensuing feather growth enabling direct comparisons between similarly aged specimens. This model was used to explore the role of hormones in feather growth.
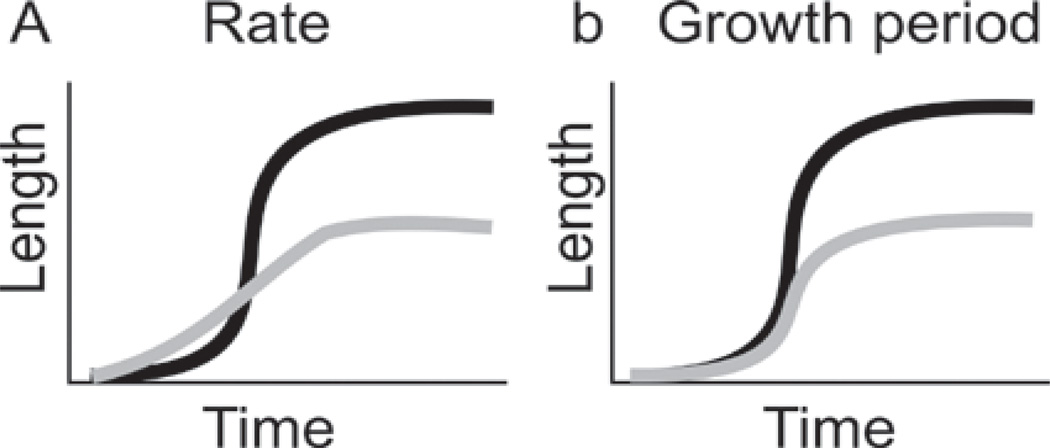
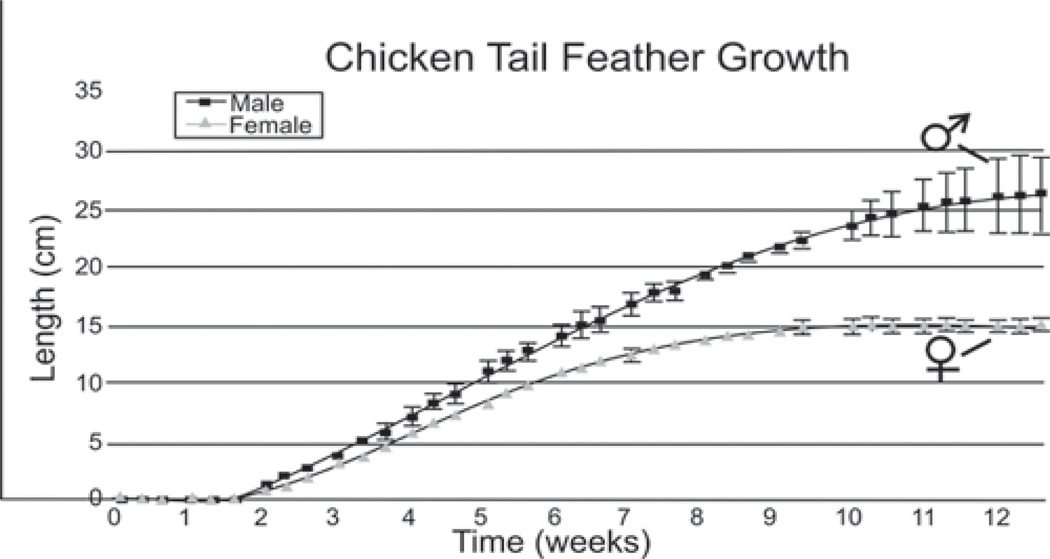
When the size of two epithelial organs A and B are different, it can be due to differences in growth rate (A>B), or that the duration of the growth period is longer in A than B (Fig. 3). The difference between male and female tail feather growth kinetics was determined by plucking the four center feathers showing the most dramatic sexually dimorphic elongation patterns. There are 14 rectrice tail feathers (Lucas and Stettenheim, 1972). For each bird, the four rectrices closest to the midline were measured since they have the most pronounced sexual dimorphic phenotypes. Both male and female tail feathers begin to emerge from the invaginated feather follicles about 10 days after plucking. Male tail feathers have a much longer growth period than female tail feathers (males - 9 weeks; females - 5 weeks), but they both exhibit similar growth rates during the elongation phase of the feather cycle (Fig. 4). Male tail feathers have a wider diameter than female tail feathers. Their calamus, the lower feather portion that remains unbranched, is longer than in female feathers.
Fig. 3.
How does the growth of male tail feathers differ from female tail feathers? Do they a) grow at different rates or b) grow at the same rate for different durations? Black, male; gray, female.
Fig. 4.
Growth kinetics of male and female tail feathers. Regenerative tail feather length after tail feathers were plucked (time 0). Note the male and female feathers grow at similar rates, but the male tail feathers grow for a longer period of time. The increased standard error detected in the male growth curves at later time points are attributed to differences in the growth period between the center (which grows longest) and lateral tail feathers.
Comparison of feather follicles in male and female rectrice feathers
We next examined the feather follicles. Following feather bud development (Widelitz and Chuong, 1999), epithelium invaginates into the dermis to form a follicle structure (Lucas and Stettenheim, 1972; Yu et al., 2004). Thus epithelium forms a cylindrical or tube structure with mesenchyme wrapped inside (Prum, 1999, Fig. 5). Cells proliferate in the proximal follicle, but start to form barb branches in the distal follicle that differentiate to become the feather filament, which is pushed further distally. We have earlier identified a shift of localized growth zones in these changing morphogenetic processes (Chodankar et al., 2003), and show that the Wnt pathway (Chang et al., 2004a), along with other pathways are involved (Widelitz et al., 2003). Those studies were done with flight feathers and body contour feathers. Here we examine rectrices (tail feathers) from mature male and female chickens.
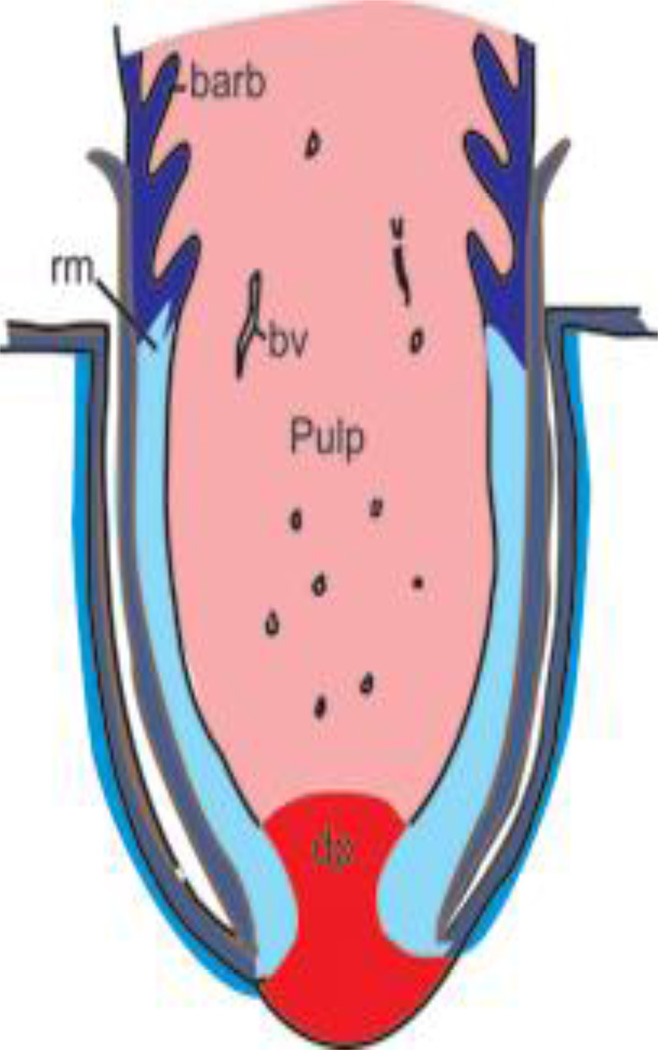
Fig. 5.
Schematic of feather follicle structure. Feathers initially form as a cylinder. The mesenchymal structures (shades of red) are the dermal papilla (dp) and the pulp. Blood vessels (bv) bring nutrients to the growing feather follicles. The epithelia (shades of blue) will form the feather. The epithelial localized growth zone (LoGZ) is near the base of the feather above the dermal papilla. Feather branching into barbs takes place in the differentiating ramogenic zone (rz). After differentiation, the pulp cells die, enabling the feather branches to open up.
The tail feathers of one year old birds were plucked and the feather follicles were removed at various time points of regeneration. H&E staining was performed on regenerating adult feathers at various time points. Differences in the size of male and female 6-week-old feather follicles can be seen (Fig 6). Data reveals that there are a few structural differences between male and female tail feathers. Male tail feathers are not only longer; they are also wider in diameter and contain a larger collar (site of active proliferation) than female feathers of the same age (Fig 6).
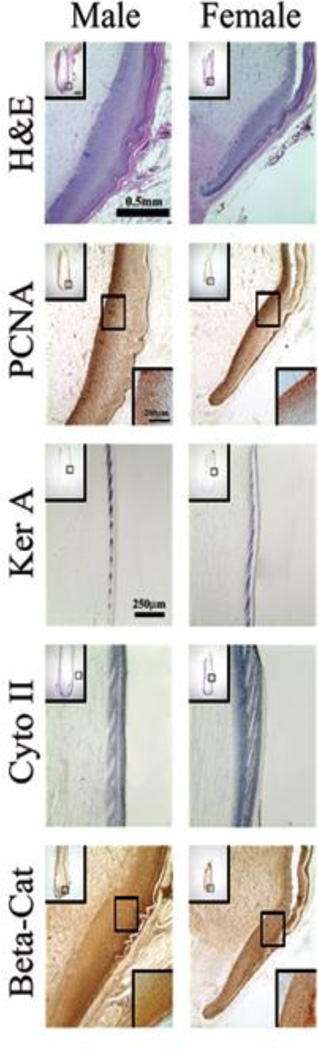
Fig. 6.
Molecular expression in male and female tail feathers. Longitudinal male and female tail feather sections (in regeneration) were stained for H&E, PCNA, Feather Keratin A (Ker A), Cytokeratin II (Cyto II) and β-catenin (Beta-Cat). The distributions of PCNA and β-catenin protein were determined using immunostaining. The distribution of Feather Keratin A and Cytokeratin II were determined using in situ hybridization. A high power view of the feather collar region is shown (H &E, PCNA, and Beta-Cat). Since there was no staining in the collar region for Ker A, the ramogenic zone is shown for Ker A and Cyto II with size bars = 250µm. A lower power view is shown in the upper insets with size bars = 0.5 mm and a higher power view is shown in the lower insets with size bars = 200µm.
Regions of proliferating cells were determined by staining for PCNA (Fig 6). PCNA staining illustrates the transiently amplified (TA) cells that give rise to the feather barbs and ridges. Proliferating cells are present at a higher concentration and are retained for a longer duration in male birds. This proliferation could be the result of continuous hormonal stimulation in the male birds resulting in a longer proliferation period and therefore longer tail feather growth. Feather keratins are not expressed until keratinocytes reach differentiation zones (ramogenic zone). Feather keratinocytes in both the collar and feather filament show expression of cytokeratin II (α keratin; Fig. 6). Based on these staining patterns, we can estimate the size of the TA cell and differentiated cell compartments in hormone sensitive “male” type and “female” type tail feathers, and hormone insensitive body or flight feathers. Furthermore, by observing the formed feather, we see that the males have a longer calamus, and the dermal ridges begin higher up in the male feather than the female which further illustrates structural differences between male and female birds.
Comparison of feather branches in male and female rectrice feathers
As feather filaments extend more distally, they form feather branches through differential apoptosis (Chang et al., 2004b). The length, angle and spacing of barbs can be different in diverse feather types (Prum and Brush, 2002). Aparicio et al, (2003) report that in some species, male tail feathers sacrifice structural integrity for enhanced length. We also noted structural changes between the tail feathers from male and female chickens and try to define these parameters as markers for male or female tail feathers for future studies (Fig. 7) Our data indicate that male and female feathers in hormone responsive and hormone insensitive regions share similar basic structures, although the size of some of the features may be increased in hormone sensitive male feathers. We compared feather diameter at the widest point, rachis diameter, total feather length, the shape of the rachis, barb density, location and number of proliferating cells using image analysis (Scion Image, NIH image software for the PC). Chickens have 7 pairs of tail feathers. Each of the female tail feathers are of approximately the same length (center feathers 19.09 +/− 0.79 cm, most lateral feathers 16.95 +/− 0.7 cm, while the male tail feathers form a growth gradient, with the longest feathers near the midline (40.49 +/− 5.96 cm) and the shortest feathers at the lateral sides (19.65 +/− 0.64 cm, n=7) (Table 1). For this reason, our focus in this study was on the four central tail feathers.
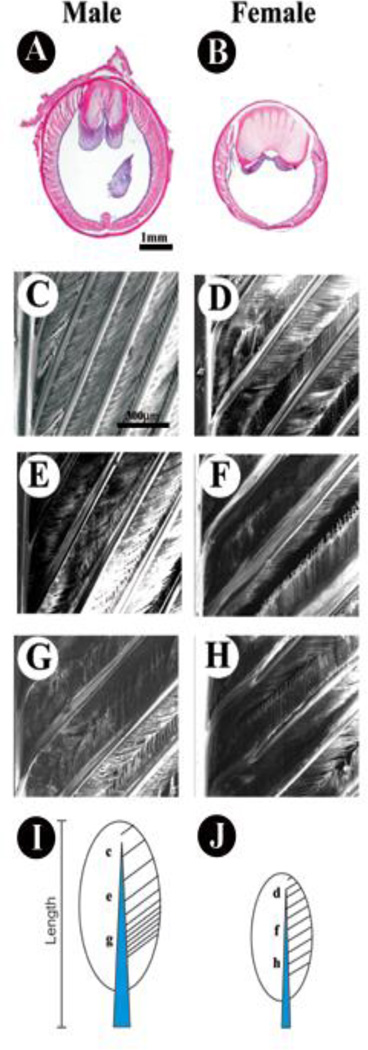
Fig. 7.
Morphological difference in male and female tail feathers. H&E staining of male (A) and female (B) feathers at the same height up from the base reveals differences in diameter, rachis width, and barb density. The barb density is distributed as a gradient in male rectrices but is constant in female feathers as seen in scanning EM photographs. In males the density is lowest near the distal tip (compare C, D), moderate in the middle (compare E, F) and highest at the base (compare G, H) as shown in scanning EM photographs. Overall, the rachis is wider at the proximal base of tail feathers, but tapers toward the distal end; however, the width of the male is larger than the width of the female feathers at equivalent locations. These differences are shown schematically for male (I) and female (J) tail feathers.
Table I.
Tail feather physical characteristics – Male vs Female
| Male tail feather | Female tail feather | |
|---|---|---|
| Length (base to tip) | 40.49 ± 5.96 cm | 19.09 ± 0.79 cm |
| Rachis diameter * | 350 ± 30 µm | 250 ± 40 µm |
| Barb density index # | 0.68 | 1 |
The difference in male and tail female feather diameter can clearly be seen at the level of the ramogenic zone.
The barb density index = barb density in the distal vane/barb density in the proximal vane
Discussion
Sex hormones in epithelial-mesenchymal interactions
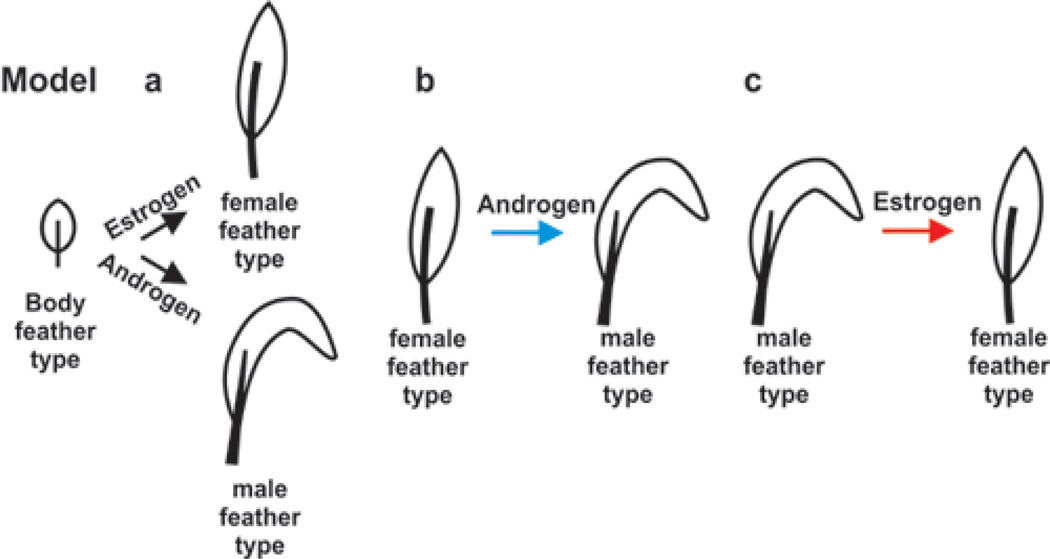
Humans show regional specificity in their response to sex hormone stimulation, i.e. androgens stimulate the growth of beards but also induce male pattern baldness in the scalp hair. In chicken, feathers also come in different sizes and shapes. Body feathers (or contour feathers) growing on the breast are small and do not respond to the sex hormones. Tail feathers grow longer in roosters than hens, suggesting that their growth is regulated by hormone dependent pathways (Fig. 2). The mechanism of their action is as yet not understood. We have devised three possibilities describing possible ways that sexual dimorphic feathers can occur (Fig. 8). In model “a”, the default tail feather prototype in the absence of hormone stimulation is a body feather. Estrogen mediated events transform the prototype to the “female type” tail feather; androgen mediated activities transform it to the “male type” tail feather. In model “b”, the default tail feather form is the “female” tail feather, which is transformed by androgen stimulation to the “male type”. In model “c”, the default tail feather type is the “male” type, which estrogen transforms to the “female type”. These possibilities will be tested in future studies by blocking or amplifying androgen and estrogen pathways in the skin with known inhibitors of the biosynthetic pathways and by blocking receptor binding with known competitive antagonists or activate them by known antagonists.
Fig. 8.
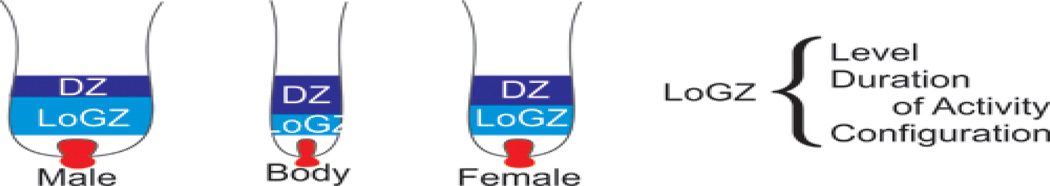
Possible models of hormone effects. Model A, the body (contour) feather is the default type. Sex hormones influence whether a “male” or “female” feather will develop. Model B, the “female” is the default feather type. Androgens cause a transition to a “male” feather type. Model C, the “male” feather is the default and estrogen converts the feather to a “female” feather type.
Estrogens exert their effects by binding to two receptors which have been identified. ERα and ERβ are encoded by two different genes. They differ in size; ERα is 67kD and ERβ approximately 54kD (Muramatsu, 2000). The gene for ERα is localized on chromosome 6 and that encoding ERβ is found on chromosome 14 in humans (Enmark et al, 1997). ERα has a higher affinity than ERβ for 17 β-estradiol, the most active estrogen form. In human skin, ERα and ERβ have different distributions. ERα was only found in the sebaceous gland while ERβ was found in the outer root sheath, epithelial matrix and dermal papilla (Thornton et al, 2003).
The principal action of androgen is to regulate gene expression through the androgen receptor (AR), which belongs to the superfamily of nuclear receptors. Androgens (Testosterone and DHT) control the development, differentiation, and function of male reproductive and accessory sex tissues, such as the seminal vesicle, epididymis, and prostate. Other organs and tissues, such as skin, skeletal muscle, bone marrow, hair follicles, and brain, are also under the influence of androgen. Androgens are primarily synthesized in the gonads of chickens but can also be made in the epididymis following partial or complete castration (Budras and Sauer, 1975). The AR has also been mapped to a number of chicken tissues including the skin (Shanbhag and Sharp, 1996). In human skin, the AR was found in the dermal papilla of the hair follicle (Randall at al., 1992) and the basal cells of the sebaceous gland (Thornton et al, 2003, Pelletier and Ren, 2004). Androgen receptors are found in higher levels in balding dermal papilla cells of the hair follicle than those in the non-balding scalp (Hibberts et al, 1998). The growth of sexual hairs is dependent upon the binding of testosterone or DHT to the AR. The area of the hair cortex within facial follicles is correlated with both the volume of the dermal papilla per cell and the number of cells in the dermal papilla (Elliott at al., 1999).
Potential cellular/molecular mechanisms in sexual dimorphic feathers
It has long been known that male tail feathers grow longer than female tail feathers but how they do this is unknown, particularly at the cellular/molecular level. Presumably, female chicken feathering patterns result from ovarian estrogens and male feathering patterns from androgens. In our recent work, we used the concept of a localized growth zone to explain the shape of feather/scale (Chuong et al, 2000), chicken/duck beaks (Wu et al, 2004), and liver (Suksaweang et al, 2004) etc. successfully. Mainly, the duration, size, and number of localized growth zones can shape a growing organ. While it is not clear which hormones are causing the effects, here we show that the sex steroids do have a different impact on the levels of activity and duration of localized growth zones in male and female tail feathers (Fig. 9). These differences also occur elsewhere within the integument, including the comb, wattle, spurs, hackle feathers and saddle feathers. In each case, these features are larger in males than females. Sexual dimorphisms appear developmentally early in the comb, wattle and tail feathers and later in the spurs, hackle feathers and saddle feathers (Jacob and Mather, 2000). Since the tail feathers and other secondary characteristics grow more robust in the male we presume that these differences are due to hormonal control. Results of the growth kinetics study indicate that male tail feathers grow at a similar rate but possess a considerably longer growth period than those of the female. This change in duration must mean that particular molecules controlling this growth phase are influenced by sex hormones.
Fig. 9.
Sex hormones influence on feather diameter and length. The dermal papilla (red), LoGZ (light blue) and differentiation zone (DZ, or ramogenic zone, dark blue) are shown.
One thought for the difference in growth is that the males sacrifice structural integrity for length. This would mean that there would be differences in the feather morphology of the feathers, which we noted. By careful observation of the tail feathers, we noted that male tail feathers are longer, have a larger degree of curvature, have a wider feather diameter, a longer calamus, and the dermal ridges begin higher up in the feather than in the female. These structural differences may reflect differences in signaling pathways. We tested molecules known to be involved in the growth pathways of other tissues as well as molecules that would identify possible differences in proliferation and differentiation. Results indicate that proliferating cells are present at a higher level and are retained for a longer duration in male birds. This proliferation could be the result of continuous hormonal stimulation in the male birds resulting in a longer proliferation period and therefore longer tail feather growth.
Sexual dimorphism of feathers may result from modulating the shared stem and transient amplifying (TA) cell configurations and underlying molecular pathways. TA cells might have the ability to mediate differences in proliferation causing the sexual dimorphism of chicken tail feathers. Since males and females have different levels of hormones present throughout development, a comparison of the size and distribution of TA cell populations and dermal papilla cells will help to identify which cell groups may be the target of sex hormones. These observed paradoxical effects of sex hormones on feather growth suggest that there are complex cross talk mechanisms and highlight the importance of having an in vivo model so no components are missed, as well as a normal model so we know how they are regulated normally.
How do hormones exert their effects on growth? We believe that they modulate growth regulatory pathways that are also present in hormone insensitive tissues. A number of growth pathways involved in feather morphogenesis have been described. Our previous work has focused on the role of the canonical Wnt – β-catenin signaling pathway in feather growth. Wnts, which invoke the canonical β-catenin pathway, induce new feather growth from scales and apteric regions (Noramly et al, 1999; Widelitz et al, 2000). In feather producing regions, these Wnts induce enlarged and distorted feather phenotypes (Chodankar et al, 2003). Blocking Wnt signaling with Dkk, suppressed feather growth (Chang et al, 2004). Additionally, β-catenin can bind directly to the androgen receptor to synergistically enhance transcription (Truica et al, 2000). We recently found that β-catenin can synergistically act as a co-activator or the androgen receptor with co-activator-associated arginine methyltransferase (CARM) 1 (Koh et al, 2002). Therefore, there is good reason that sex hormones may modulate growths via the β-catenin pathway. Another candidate is the bone morphogenetic pathway (BMP) pathway. BMP7 has been shown to be highly regulated with estrogen receptor levels, which is an important prognostic marker for breast cancer (Schwalbe et al, 2003). BMPs and their interaction with type II BMP receptors have also been shown to contribute to the proliferation of human breast cancer cells (Pouliot et al, 2003). A direct interaction between Smads and ER was shown to cause an inhibitory action on Smad activity, which suggests cross talk between BMP and steroid hormone pathways, particularly the ER pathway (Yamamoto et al, 2002). We are currently examining the role of β-catenin in mediating hormone dependent growth to determine how hormones exert there effects on normal growth mediating processes.
Regional specificity of sex hormone effects
Why do hormones exert different effects in different regions of the integument? Why do androgens induce facial hair growth (beard) but suppress scalp hair growth? In our model system, the question can be rephrased as why do the tail feathers respond differently to hormone action than the comb and wattle and why are the body feathers seemingly unaffected? These differential responses are due to regional specificity. How is this regional specific hormone response established? Different molecular machinery in cells within each region may cause different responses to the same hormonal stimuli. While steroid receptor co-activators and co-suppressors have made much progress in mammals (Chang, 2002), these studies are still at a primitive stage in birds. The skin Hox code may be involved in setting up such different competence in skin regional specificity (Chuong et al, 1990).
Many aspects of hormone metabolism take place in the peripheral tissues. Hence the local concentrations of hormone forms are dependent on the levels of circulating precursors within the blood and the availability of local metabolizing enzymes. A schematic of hormone biosynthesis indicating enzymes (green) and products (black) is shown in Fig. 2. 3β-hydroxy-steroid dehydrogenase converts DHEA to androstenedione. It is also involved in converting androstenediol to testosterone. 17β-hydroxy-steroid dehydrogenase converts DHEA to androstenediol, androstenedione to testosterone and estrone to estradiol. Aromatase converts androstenedione to estrone and testosterone to estradiol.
The sex hormone biosynthetic pathways are highly conserved across species. In each case the steroids must bind to their receptors, which interact with co-activators and co-suppressors to modulate transcriptional activation. Many of the molecular aspects of transcriptional activation have been elegantly demonstrated using tissue culture cells (reviewed in Chang, 2002). It is thought that similar pathways are active in mediating similar events in tissues and modulate epithelial-mesenchymal interactions. Since these processes are so important to diseases of skin appendages, it is critical to have a model in which to assess hormone effects in a physiological situation in vivo. We have observed that the chicken tail feathers respond to hormone stimulation by altering its growth and molecular expression patterns. This experimental model can now be exploited to obtain new understandings that may in the future assist patients suffering from hormone dependent growth related diseases affecting other skin appendages, such as hair growth related diseases, breast cancer or prostate cancer.
Summary
Hair, mammary glands, feathers, etc. are all skin appendages derived from epidermis as a result of epithelial - mesenchymal interactions. Pelage and skin can be markedly dimorphic in many mammals including deer and primates. The growth of these epithelial organs is regulated by fundamental morphogens including BMP, SHH, Wnt/β-catenin, etc (reviewed in Chuong, 1998; Widelitz et al, 2003). Mutation or de-regulation of these morphogens has been identified in many human diseases including tumors and genetic diseases. Sex hormones also play a major role in regulating the growth and phenotypes of these epithelial organs differently in male and female animals (sexual dimorphism). How the sex hormones are coupled to the morphogens is mostly unknown, hence, β-catenin, BMPs, etc., are candidates that may couple the two pathways. It has been suggested that female chickens have feathering patterns resulting from ovarian estrogens (George & Wilson, 1980), which leads us to believe that the male feathering pattern is a result of plasma testosterone levels. Another way of analyzing the role of hormones in skin appendage growth is to assess them in various diseases. Examples include androgenic alopecia, 5 α-reductase deficiency, henny feathering, hyperactive mutated aromatase, etc. The feather offers a unique opportunity to do experiments involving sex hormones and study their effects in epithelial-mesenchymal interactions, in vivo.
Acknowledgement
We thank Dr. Valerie Randall for discussion. JAM is supported by the USC/Norris Breast Cancer Research Program Training Fellowship. This work is supported by grants from NCI (RB Widelitz) and NIAMS (CM Chuong).
References
- Anderson J. The role of antiandrogen monotherapy in the treatment of prostate cancer. B.J.U. Int. 2003;91:455–461. doi: 10.1046/j.1464-410x.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JE, Smith CA, Sinclair AH. Sites of estrogen receptor and aromatase expression in the chicken embryo. Gen. Comp. Endocrinol. 1997;108:182–190. doi: 10.1006/gcen.1997.6978. [DOI] [PubMed] [Google Scholar]
- Aparicio JM, Bonal R, Cordero PJ. Evolution of the structure of tail feathers: implications for the theory of sexual selection. Evolution Int. J. Org. Evolution. 2003;57:397–405. doi: 10.1111/j.0014-3820.2003.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Barnes J, Anthony CT, Wall N, Steiner MS. Bone morphogenetic protein-6 expression in normal and malignant prostate. World J. Urol. 1995;13:337–343. doi: 10.1007/BF00191214. [DOI] [PubMed] [Google Scholar]
- Bayne EK, Flanagan J, Einstein M, Ayala J, Chang B, Azzolina B, Whiting DA, Mumford RA, Thiboutot D, Singer I, Harris G. Immunohistochemical localization of types 1 and 2 5alpha-reductase in human scalp. Br. J. Dermatol. 1999;141:481–491. doi: 10.1046/j.1365-2133.1999.03042.x. [DOI] [PubMed] [Google Scholar]
- Birch MP, Lalla SC, Messenger AG. Female pattern hair loss. Clin. Exp. Dermatol. 2002;27:383–388. doi: 10.1046/j.1365-2230.2002.01085.x. 2002. [DOI] [PubMed] [Google Scholar]
- Brigham PA, Cappas A, Uno H. The stumptailed macaque as a model for androgenetic alopecia: effects of topical minoxidil analyzed by use of the folliculogram. Clin. Dermatol. 1988;6:177–187. doi: 10.1016/0738-081x(88)90084-3. [DOI] [PubMed] [Google Scholar]
- Brodie AM, Njar VC. Aromatase inhibitors and their application in breast cancer treatment. Steroids. 2000;65:171–179. doi: 10.1016/s0039-128x(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Budras KD, Sauer T. Morphology of the epididymis of the cock (Gallus domesticus) and its effect upon the steroid sex hormone synthesis. II. Steroid sex hormone synthesis in the tubuli epididymidis and the transformation of the ductuli aberrantes into hormone producing noduli epididymidis in the capsule of the adrenal gland of the capon. Anat. Embryol. (Berl.) 1975;148:197–213. doi: 10.1007/BF00315269. [DOI] [PubMed] [Google Scholar]
- Buzdar A, Douma J, Davidson N, Elledge R, Morgan M, Smith R, Porter L, Nabholtz J, Xiang X, Brady C. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J. Clin. Oncol. 2001;19:3357–3366. doi: 10.1200/JCO.2001.19.14.3357. [DOI] [PubMed] [Google Scholar]
- Buzdar A, Howell A. Advances in aromatase inhibition: clinical efficacy and tolerability in the treatment of breast cancer. Clin. Cancer Res. 2001;7:2620–2635. [PubMed] [Google Scholar]
- Chanda S, Robinette CL, Couse JF, Smart RC. 17beta-estradiol and ICI-182780 regulate the hair follicle cycle in mice through an estrogen receptor-alpha pathway. Am. J. Physiol. Endocrinol. Metab. 2000;278:E202–E210. doi: 10.1152/ajpendo.2000.278.2.E202. [DOI] [PubMed] [Google Scholar]
- Chang C. Androgens and androgen receptor: Mechanisms, functions and clinical applications. Norwell, MA: Kluwer Academic Publishers; 2002. [Google Scholar]
- Chang C-H, Jiang T-X, Lin C-M, Burrus L, Chuong C-M, Widelitz RB. Distinctive Phenotypes Generated by WNT Member misexpression During Hierarchical Morphogenesis of Dermis, Skin Regions and Individual Feathers. Mech. Develop. 2004a;121:157–171. doi: 10.1016/j.mod.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Yu M, Wu P, Jiang T-X, Yu H-S, Widelitz RB, Chuong C-M. Sculpting Skin Appendages Out of Epidermal Layers Via Temporally and Spatially Regulated Apoptotic Events. J. Invest. Dermato. 2004b;122:1348–1355. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodankar R, Chang C-H, Yue Z, Suksaweang S, Burrus L, Chuong C-M, Widelitz RB. Shift of Localized Growth Zones (LoGZ) Contributes to the Morphogenesis of Skin Appendages: Association with Wnt/B-catenin activities. J. Invest. Dermatol. 2003;120:19–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM. Feather Morphogenesis: A Model of the Formation of Epithelial Appendages. In: Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Austin: Landes Bioscience; 1998. pp. 3–14. [Google Scholar]
- Chuong CM, Oliver G, Ting SA, Jegalian BG, Chen HM, De Robertis EM. Gradients of homeoproteins in developing feather buds. Development. 1990;110:1021–1030. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am. J. Epidemiol. 2000;152:950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- Courtois M, Loussouarn G, Hourseau C, Grollier JF. Hair cycle and alopecia. Skin Pharmacol. Whiting, D.A. Advances in the treatment of male androgenetic alopecia: a brief review of finasteride studies. Eur. J. Dermatol. 1994;7:84–89. doi: 10.1159/000211279. [DOI] [PubMed] [Google Scholar]
- Craven AJ, Ormandy CJ, Robertson FG, Wilkins RJ, Kelly PA, Nixon AJ, Pearson AJ. Prolactin signaling influences the timing mechanism of the hair follicle: analysis of hair growth cycles in prolactin receptor knockout mice. Endocrinology. 2001;142:2533–2539. doi: 10.1210/endo.142.6.8179. [DOI] [PubMed] [Google Scholar]
- Crook JH. Sexual selection, dimorphism, and social organization in the primates. In: Campbell B, editor. Sexual selection and the descent of man 1871–1971. Chicago: Aldine; 1972. pp. 231–281. [Google Scholar]
- Darwin C. The Descent of Man and Selection in Relation to Sex. Reprint by Princeton. 1871 1981. [Google Scholar]
- Devesa SS, Blot WJ, Stone BJ, Miller BA, Tarone RE, Fraumeni JF., Jr Recent cancer trends in the United States. J. Natl. Cancer Inst. 1995;87:175–182. doi: 10.1093/jnci/87.3.175. [DOI] [PubMed] [Google Scholar]
- Dunn PO, Whittingham LA, Pitcher TE. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution Int. J. Org. Evolution. 2001;55:161–175. doi: 10.1111/j.0014-3820.2001.tb01281.x. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Johnson E. The control of hair growth. Symp. Zool. Soc. Lond. 1964;12:97–130. [Google Scholar]
- Ebling FJG. The biology of hair. Dermatol. Clinics. 1987;5:467–481. [PubMed] [Google Scholar]
- Elbrecht A, Smith RG. Aromatase enzyme activity and sex determination in chickens. Science. 1992;255:467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- Elliott K, Stephenson TJ, Messenger AG. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J. Invest. Dermatol. 1999;113:873–877. doi: 10.1046/j.1523-1747.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Frankenhuis MT, Kappert HJ. Experimental transformation of right gonads of female fowl in to fertile testes. Biol. Reprod. 1980;23:526–529. doi: 10.1095/biolreprod23.3.526. [DOI] [PubMed] [Google Scholar]
- George FW, Wilson JD. Pathogenesis of the henny feathering trait in the Sebright bantam chicken. Increased conversion of androgen to estrogen in skin. J. Clin. Invest. 1980;66:57–65. doi: 10.1172/JCI109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JE, Wilson JD. The resistance syndromes: 5•-reductase deficiency, testicular feminisation and related disorders. In: Scriver CR, Sly WL, editors. The Metabolic Basis of Inherited Disorders. New York: McGraw-Hill; 1989. pp. 1919–1944. [Google Scholar]
- Grzywacz MJ, Yang JM, Hait WN. Effect of the multidrug resistance protein on the transport of the antiandrogen flutamide. Cancer Res. 2003;63:2492–2498. [PubMed] [Google Scholar]
- Hamilton JB. Quantitative measurement of a secondary sex character, axillary hair. Ann. N.Y. Acad. Sci. 1950;53:585–599. doi: 10.1111/j.1749-6632.1951.tb31960.x. [DOI] [PubMed] [Google Scholar]
- Haqq CM, Donahoe PK. Regulation of sexual dimorphism in mammals. Physiol. Rev. 1998;78:1–33. doi: 10.1152/physrev.1998.78.1.1. [DOI] [PubMed] [Google Scholar]
- Hershkovitz P. Living New World monkeys (Platyrrhini) Vol. 1. Chicago: University of Chicago Press; 1977. [Google Scholar]
- Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J. Endocrinol. 1998;156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Happle R. Current understanding of androgenetic alopecia. Part I: etiopathogenesis. Eur. J. Dermatol. 2000;10:319–327. [PubMed] [Google Scholar]
- de H, Yoshida T, Matsumoto N, Aoki K, Osada Y, Sugimura T, Terada M. Growth regulation of human prostate cancer cells by bone morphogenetic protein-2. Cancer Res. 1997;57:5022–5027. [PubMed] [Google Scholar]
- Jacob J, Mather FB. Sex reversal in chickens. Factsheet PS-53. Department of Animal Sciences, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida; 2000. [Google Scholar]
- Jarman M, Smith HJ, Nicholls PJ, Simons C. Inhibitors of enzymes of androgen biosynthesis: cytochrome P450(17) alpha and 5 alpha-steroid reductase. Nat. Prod. Rep. 1998;15:495–512. doi: 10.1039/a815495y. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Stott S, Widelitz RB, Chuong CM. Current methods in the study of avian skin appendages. In: Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Austin: Landes Bioscience; 1998. pp. 395–408. [Google Scholar]
- Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu. Rev. Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- Koh SS, Li H, Lee YH, Widelitz RB, Chuong CM, Stallcup MR. Synergistic coactivator function by coactivator-associated arginine methyltransferase (CARM) 1 and β-catenin with two different classes of DNA-binding transcriptional activators. J. Biol. Chem. 2002;277 doi: 10.1074/jbc.M110865200. 26031-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln GA. Puberty in a seasonally breeding male, the red deer stag (Cervus elaphus L.) J Reprod. Fertil. 1971;25:41–54. doi: 10.1530/jrf.0.0250041. [DOI] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR, editors. Avian Anatomy: Integument. Agriculture Handbook 362: Agricultural Research Services. Washington DC: US Department of Agriculture; 1972. [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 2004;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Matsumine H, Herbst MA, Ou SH, Wilson JD, McPhaul MJ. Aromatase mRNA in the extragonadal tissues of chickens with the henny-feathering trait is derived from a distinctive promoter structure that contains a segment of a retroviral long terminal repeat. Functional organization of the Sebright, Leghorn, and Campine aromatase genes. J. Biol. Chem. 1991;266:19900–19907. [PubMed] [Google Scholar]
- Mohn MP. The effects of different hormonal states on the growth of hair in rats. In: Montagna W, Ellis RA, editors. The Biology of Hair Growth. New York: Academic Press; 1958. pp. 335–398. [Google Scholar]
- Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem. Biophys. Res. Commun. 2000;270:1–10. doi: 10.1006/bbrc.2000.2214. [DOI] [PubMed] [Google Scholar]
- Noramly S, Freeman A, Morgan BA. beta-catenin signaling can initiate feather bud development. Development. 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- Norwood OT. Incidence of female androgenetic alopecia (female pattern alopecia) Dermatol. Surg. 2001;27:53–54. [PubMed] [Google Scholar]
- Obana N, Chang C, Uno H. Inhibition of hair growth by testosterone in the presence of dermal papilla cells from the frontal bald scalp of the postpubertal stumptailed macaque. Endocrinology. 1997;138:356–361. doi: 10.1210/endo.138.1.4890. [DOI] [PubMed] [Google Scholar]
- Oh HS, Smart RC. An estrogen receptor pathway regulates the telogenanagen hair follicle transition and influences epidermal cell proliferation. Proc. Natl. Acad. Sci., USA. 1996;93:12525–12530. doi: 10.1073/pnas.93.22.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr. Relat. Cancer. 2004;11:437–458. doi: 10.1677/erc.1.00659. [DOI] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N. Engl. J. Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Payne LF. Capon production. Topeka, Kansas: W.C. Austin; 1936. [Google Scholar]
- Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histol. Histopathol. 2004;19:629–636. doi: 10.14670/HH-19.629. [DOI] [PubMed] [Google Scholar]
- Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23:6379–6391. doi: 10.1038/sj.onc.1207899. [DOI] [PubMed] [Google Scholar]
- Post E, Langvatn R, Forchhammer MC, Stenseth NC. Environmental variation shapes sexual dimorphism in red deer. Proc. Natl. Acad. Sci., USA. 1999;96:4467–4471. doi: 10.1073/pnas.96.8.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot F, Blais A, Labrie C. Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Res. 2003;63:277–281. [PubMed] [Google Scholar]
- Prum RO. Development and evolutionary origin of feathers. J. Exp. Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q. Rev. Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- Quinn MJ. Cancer trends in the United States--a view from Europe. J. Natl. Cancer Inst. 2003;95:1258–1261. doi: 10.1093/jnci/djg063. [DOI] [PubMed] [Google Scholar]
- Randall VA. Androgens and human hair growth. Clin. Endocrinol. (Oxf) 1994;40:439–457. doi: 10.1111/j.1365-2265.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Randall VA, Hibberts NA, Thornton MJ, Hamada K, Merrick AE, Kato S, Jenner TJ, de Oliveira I, Messenger AG. The hair follicle: a paradoxical androgen target organ. Horm. Res. 2000;54:243–250. doi: 10.1159/000053266. [DOI] [PubMed] [Google Scholar]
- Randall VA, Hibberts NA, Thornton MJ, Merrick AE, Hamada K, Kato S, Jenner TJ, de Oliveira I, Messenger AG. Do androgens influence hair growth by altering the paracrine factors secreted by dermal papilla cells? Eur. J. Dermatol. 2001;11:315–320. [PubMed] [Google Scholar]
- Randall VA, Thornton MJ, Hamada K, Redfern CFP, Nutbrown M, Ebling JG, Messenger AG. Androgens and the hair follicle. Ann. N.Y. Acad. Sci. 1991;642:355–375. [PubMed] [Google Scholar]
- Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of scalp. J. Endocrinol. 1992;133:141–147. doi: 10.1677/joe.0.1330141. [DOI] [PubMed] [Google Scholar]
- Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur. J. Cancer. 1998;34:1341–1347. doi: 10.1016/s0959-8049(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Lucky AW. Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess. Endocrinol. Metab. Clin. North Am. 1993;22:507–532. [PubMed] [Google Scholar]
- Schwalbe M, Sanger J, Eggers R, Naumann A, Schmidt A, Hoffken K, Clement JH. Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int. J. Oncol. 2003;23:89–95. [PubMed] [Google Scholar]
- Setchell JM, Dixson AF. Circannual changes in the secondary sexual adornments of semifree-ranging male and female mandrills (Mandrillus sphinx) Am. J. Primatol. 2001;53:109–121. doi: 10.1002/1098-2345(200103)53:3<109::AID-AJP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Shanbhag BA, Sharp PJ. Immunocytochemical localization of androgen receptor in the comb, uropygial gland, testis, and epididymis in the domestic chicken. Gen. Comp. Endocrinol. 1996;101:76–82. doi: 10.1006/gcen.1996.0009. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss) J. Investig. Dermatol. Symp. Proc. 2003;8:20–23. doi: 10.1046/j.1523-1747.2003.12167.x. [DOI] [PubMed] [Google Scholar]
- Shum KW, Cullen DR, Messenger AG. Hair loss in women with hyperandrogenism: four cases responding to finasteride. J. Am. Acad. Dermatol. 2002;47:733–739. doi: 10.1067/mjd.2002.124608. [DOI] [PubMed] [Google Scholar]
- Stillman SC, Evans BA, Hughes IA. Androgen dependent stimulation of aromatase activity in genital skin fibroblasts from normals and patients with androgen insensitivity. Clin. Endocrinol (Oxf) 1991;35:533–538. doi: 10.1111/j.1365-2265.1991.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Suksaweang S, Lin C-M, Jiang T-X, Hughes MW, Widelitz R, Chuong C-M. Morphogenesis of chicken liver: Identification of localized growth zones and The role of β-catenin / Wnt in size regulation. Dev. Biol. 2004;266:109–122. doi: 10.1016/j.ydbio.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J. Clin. Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Anderson WA, Raman V, Reddi AH. Androgen-dependent gene expression of bone morphogenetic protein 7 in mouse prostate. Prostate. 1998;37:236–245. doi: 10.1002/(sici)1097-0045(19981201)37:4<236::aid-pros5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Thornton MJ, Hibberts NA, Street T, Brinklow BR, Loudon AS, Randall VA. Androgen receptors are only present in mesenchyme-derived dermal papilla cells of red deer (Cervus elaphus) neck follicles when raised androgens induce a mane in the breeding season. J. Endocrinol. 2001;168:401–408. doi: 10.1677/joe.0.1680401. [DOI] [PubMed] [Google Scholar]
- Thornton MJ, Taylor AH, Mulligan K, Al-Azzawi F. Oestrogen receptor beta is not present in the pilosebaceous unit of red deer skin during the non-breeding season. Horm. Res. 2000;54:259–262. doi: 10.1159/000053268. [DOI] [PubMed] [Google Scholar]
- Thornton MJ, Taylor AH, Mulligan K, Al-Azzawi F, Lyon CC, O'Driscoll J, Messenger AG. The distribution of estrogen receptor β is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J. Investig. Dermatol. Symp. Proc. 2003;8:100–103. doi: 10.1046/j.1523-1747.2003.12181.x. [DOI] [PubMed] [Google Scholar]
- Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- Whiting DA. Advances in the treatment of male androgenetic alopecia: a brief review of finasteride studies. Eur. J. Dermatol. 2001;11:332–334. [PubMed] [Google Scholar]
- Widelitz RB, Chuong C-M. Early events in skin appendage formation: induction of epithelial placodes and condensation of dermal mesenchymal cells. J. Invest. Dermatol. 1999;4:302–306. doi: 10.1038/sj.jidsp.5640234. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang T-X, Yu M, Wu P, Yue Z, Chuong C-M. Molecular biology of feather morphogenesis: A testable model of Evo-Devo research. J. Exp. Zool. 2003;298B:109–122. doi: 10.1002/jez.b.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz RB, Jiang T-X, Lu J-F, Chuong C-M. Beta catenin in epithelial morphogenesis: Conversion of part of avian foot scales into feather buds with a mutated beta catenin. Dev. Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Leshin M, George FW. The Sebright bantam chicken and the genetic control of extraglandular aromatase. Endocr. Rev. 1987;8:363–376. doi: 10.1210/edrv-8-4-363. [DOI] [PubMed] [Google Scholar]
- Woods JE, Domm LV. A histochemical identification of the androgen-producing cells in the gonads of the domestic fowl and albino rat. Gen. Comp. Endocr. 1966;7:559–570. doi: 10.1016/0016-6480(66)90076-1. [DOI] [PubMed] [Google Scholar]
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell Biol. 2003;23:1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Saatcioglu F, Matsuda T. Cross-talk between bone morphogenic proteins and estrogen receptor signaling. Endocrinology. 2002;143:2635–2642. doi: 10.1210/endo.143.7.8877. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Yue Z, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM. The biology of feather follicles. Int. J. Dev. Biol. 2004;48:181–191. doi: 10.1387/ijdb.031776my. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhau HE, Wan DS, Zhou J, Miller GJ, von Eschenbach AC. Expression of c-erb B-2/neu proto-oncogene in human prostatic cancer tissues and cell lines. Mol. Carcinog. 1992;5:320–327. doi: 10.1002/mc.2940050413. [DOI] [PubMed] [Google Scholar]