Abstract
Background
Sensitive troponin assays have been developed for the evaluation of patients with suspected acute coronary syndrome (ACS). We sought to compare the performance of a commercially available sensitive troponin I (sTnI) and pre-commercial highly sensitive (hs) TnI method to conventional (c)Tn assays.
Methods
Among patients with acute chest pain but normal cTn in the emergency department of 6 centers, sTnI and hsTnI were measured at baseline, two and four hours following presentation. Diagnostic accuracy of sTnI and hsTnI relative to cTn for diagnosis during index hospitalization, as well as their associations with CAD in patients randomized to coronary computed tomographic angiography (CTA) were assessed.
Results
Overall, 322 patients were enrolled, of whom 161 had a CTA; 28 had ACS (8.7%), including 21 with UAP. Both sTnI and hsTnI values at baseline and second draw had significantly higher sensitivity for ACS and UAP than cTn, and had significantly greater area under the receiver operator characteristic curve than cTn at first and second draws. Compared to cTn, 29% of ACS cases previously categorized as UAP were reclassified to acute MI with sTnI or hsTnI. An hsTnI below limit of detection had 100% negative predictive value for ACS or significant coronary artery stenosis in those randomized to CCTA.
Conclusions
In patients with acute chest discomfort, use of sTnI and hsTnI methods led to significant improvement in the early diagnostic accuracy for ACS, reclassifying one third of UAP to MI. Very low values for hsTnI excluded underlying CAD.
Keywords: myocardial infarction, troponin, diagnosis, imaging
Introduction
For diagnosis of acute myocardial infarction (MI), the third Universal Definition of MI Global Task Force recommended use of a cardiac troponin (Tn) cut-off that is the 99th percentile of a healthy patient population (1). However, nearly all currently-used conventional (c)Tn assays in the United States are inaccurate at very low concentrations of the biomarker. Thus, cTn assays have low first-draw sensitivity due to the time required for a detectable rise in measurable Tn concentrations (leading to a “troponin blind” interval). To overcome this limitation, highly sensitive (hs) methods for Tn measurement with high precision at or below the 99th percentile have been developed and are increasingly used in many countries. These newer hsTn assays can detect measurable biomarker in the majority of normal subjects (2), and have been reported to provide superior diagnostic value when evaluating patients with suspected acute coronary syndrome (ACS) (3), with particularly higher sensitivity and negative predictive value (NPV).
While increasing assay sensitivity improves earlier recognition for acute MI greater sensitivity is accompanied by lower specificity, the consequence of which bears further scrutiny (4). Additionally, the performance characteristics of hsTn assays for diagnostic evaluation remain unclear compared to sensitive (s)Tn methods, particularly if the upper reference limit for the latter is lowered to the 99th percentile (3,5). Lastly, the ability of sTn versus hsTn methods to detect significant coronary artery disease (CAD) in patients with suspected ACS is less understood. Accordingly, among a cohort of patients with suspected ACS, we examined the ramification of increasing degrees of analytical sensitivity by comparing results from cTn results to both an sTnI and hsTnI assay in the multi-center Rule Out Myocardial Infarction Using Computer Assisted Tomography (ROMICAT) II trial (NCT01084239) (6).
Methods
The Institutional Review Board at each study center approved the procedures in this analysis; all subjects provided informed consent for inclusion, and the study protocol conforms to the Declaration of Helsinki. The study was supported by NHLBI (U01HL092040 and UO1HL092022), and in part by the American College of Radiology Imaging Network. Dr. Januzzi is supported in part by the Roman W. DeSanctis Clinical Scholar Endowment, while Dr. Truong is supported in part by NIH/NHLBI K23HL098370 and L30HL093896. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Health and Human Services.
This was a pre-specified sub-analysis from the ROMICAT II study, a multi-center, prospective comparative effectiveness study of standard care versus standard care plus coronary computed tomographic angiography (CTA) in the evaluation of chest discomfort and suspected ACS (6). The methods of the trial have been previously published (7). The primary aim of this analysis was the comparative diagnostic performance of troponin methods with increasing analytical sensitivity, while a secondary aim was to evaluate associations between more sensitive troponin methods and results from CTA.
Eligible patients were 40 to 74 years of age, and had presented to the emergency department with symptoms suggestive of ACS for at least 5 minutes duration within 24 hours before presentation. All patients were in sinus rhythm, and were judged as having intermediate likelihood for ACS by managing clinicians. Major exclusion criteria were a history of known CAD, ischemic electrocardiographic changes (defined as new ST-segment elevation or depression > 1 mm or T-wave inversion > 4 mm in more than two anatomically adjacent leads or new left bundle branch block), impaired renal function (serum creatinine > 1.5 mg/dL), hemodynamic or clinical instability, known allergy to an iodinated contrast agent, body-mass index greater than 40, or currently symptomatic asthma. Importantly, a screening cTn concentration in excess of the local laboratory upper reference limit was a major exclusion criterion (the lowest concentration associated with analytical precision <10%; assays and cut-offs are detailed in Supplemental Table 1). For cTn, blood draws were at time points determined per standard of care at each institution; results were available in 322, 252, and 64 subjects at these time points.
Overall, the ACS rate was 8% and there were no imbalances in ACS rates between the CTA or and usual care arms of the trial.
Blood samples
For subjects participating in the biomarker sub study (N=322), blood was collected independently of the timing of the local standard of care biomarkers into tubes containing ethylenediaminotetraacetic acid at pre-defined time points of study enrollment (N=309), 90–155 (N=289), and 210–300 minutes (N=231) after enrollment. Blood was immediately processed and frozen at −80 degrees C until completion of the study.
Samples were analyzed in a blinded fashion using a commercially-available sTnI (Vista, Siemens Diagnostics, Newark Delaware) and a preclinical hs method (hsVista, Siemens Diagnostics, Newark Delaware)(2). The limit of detection (LOD) and 99th percentile for sTnI are 15 ng/L and 45 ng/L respectively, versus 0.5 ng/L and 49 ng/L for hsTnI.
ACS and diagnostic evaluation of sTnI and hsTnI
As previously described (7)(7), ACS (consisting of both acute MI and unstable angina pectoris [UAP]) was determined (with local adjudication and a random sample of independent, blinded cases for confirmation) using clinical information from presentation, including the results from serial cTn and ECGs at each institution (Supplemental Table 1); UAP was defined as an ACS with a locally measured cTn below its upper reference limit.
Baseline characteristics of subjects with and without ACS were compared using the Fisher’s exact test, Student’s T test, or Wilcoxon Rank Sum test as appropriate. The performance of local cTn, as well as the sTnI and hsTnI measured for the purpose of this study was examined relative to the diagnosis of ACS (N=28) as well as UAP (N=21). Bivariate correlation between the two methods was performed using the Pearson method. Median (with interquartile range; IQR) concentrations of sTnI and hsTnI were examined at each time point as a function of diagnosis and compared using non-parametric testing. For values below the LOD, concentrations were modeled as 50% of the lowest detectable value for each method. Receiver operating characteristic (ROC) curves using the diagnoses of ACS and UAP were compared using the area under the ROC curve (AUC). Using the 99th percentile for each assay, operating characteristics were assessed, including sensitivity, specificity, positive predictive value (PPV) and NPV; sensitivities between cTn, sTnI and hsTnI were compared using the McNemar test. NPVs of the LOD for each sensitive assay were also examined, both for excluding ACS and UAP. The iterative value of serial testing at the listed time points was examined relative to incremental diagnostic yield.
Among those undergoing CTA (N=161 with available baseline samples for s or hsTnI measurement) association between TnI and CAD was assessed by categorizing subjects below the LOD for each sensitive assay, between the LOD and the 99th percentile, and above the 99th percentile at each time point, relative to the percentage of any detectable CAD by scan, as well as grades of severity (any CAD, stenosis >50%, stenosis >70%). Median coronary artery calcium scores (with IQRs) were examined in a similar fashion.
All statistical methods were performed using SAS (version 9.2; SAS Institute Inc, Cary, NC) and Stata (version 13.1, StataCorp, College Station, TX). All P values are two-sided, with values <0.05 considered significant.
Results
Of 322 subjects, all had a cTn that was normal at screening; 28 (8.7%) were subsequently diagnosed with ACS. Of these, 21 had unstable angina pectoris (UAP); of the acute MIs, none had ST segment elevation. The mean age of the sample was 53 years, and 58% were male. Table 1 details the baseline demographics and clinical characteristics of the subjects with ACS versus those without. The time from presentation to study enrollment was (mean ± standard deviation) 3.2 ± 1.1 hours overall; there was no difference in time from presentation to enrollment between those with and without ACS (3.1 ± 1.3 versus 3.2 ± 1.1 hours; P =0.74). No patients had myocarditis, heart failure, or pulmonary embolism.
Table 1.
Demographics of the ROMICAT II biomarker sub-study subjects. Of those with ACS, modest differences were present.
| ACS (N=28) | No ACS (N=294) | P value | |
|---|---|---|---|
| Age, years | 55.5 ± 9.2 | 52.6 ± 7.6 | 0.12 |
| Male sex | 92.9% | 54.8% | <0.001 |
| Caucasian | 89.3% | 72.5% | 0.07 |
| Hypertension | 46.4% | 52.0% | 0.69 |
| Diabetes | 10.7% | 15.0% | 0.78 |
| Elevated lipids | 57.1% | 40.8% | 0.11 |
| Former/current smoking | 75.0% | 47.3% | 0.005 |
| Family history of CAD | 42.9% | 36.4% | 0.54 |
| ≥2 risk factors for CAD | 75.0% | 62.6% | 0.22 |
| Aspirin use | 35.7% | 21.1% | 0.10 |
| TIMI Risk Score >2 | 7.1% | 0.3% | 0.02 |
| Heart rate, beats/minute | 74.8 ± 14.8 | 77.6 ± 14.5 | 0.34 |
| Systolic blood pressure, mm Hg | 148.1 ± 20.5 | 144.2 ± 22.7 | 0.34 |
| Diastolic blood pressure, mm Hg | 83.1 ± 12.8 | 82.7 ± 13.0 | 0.90 |
Continuous variables are expressed as mean ± standard deviation.
ACS denotes: acute coronary syndrome; CAD denotes: coronary artery disease; TIMI denotes: Thrombolysis in Myocardial Infarction.
Troponin concentrations and ACS diagnosis
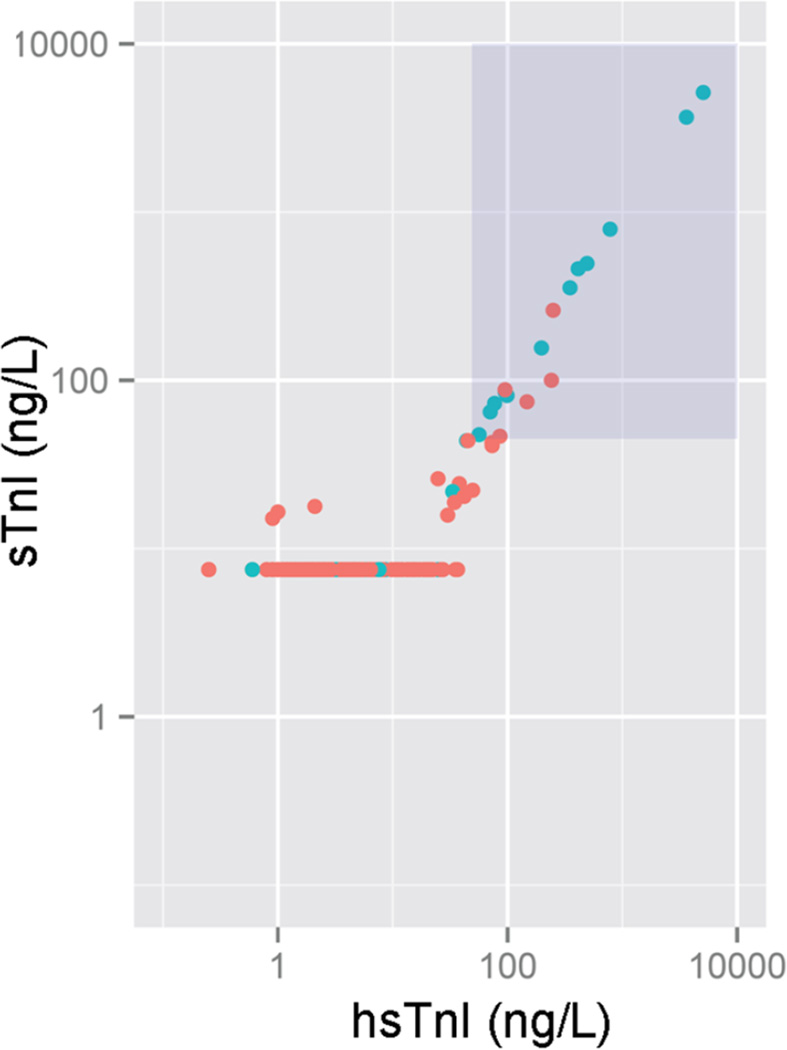
Figure 1 shows the relationship between the two high sensitive assays; 81% of results were below the LOD of the sTn assay, while 7% were below the LOD of the hsTnI method. In bivariate correlations between sTnI and hsTnI at each time point, we found respective r values of 0.999, 0.995, and 0.998 (all P <0.001) across the entire range of sTnI and hsTnI values. Focusing only on those subjects with an hsTnI value below the 99th percentile, however, the r values were considerably lower at 0.585, 0.494, and 0.535, respectively (all P values <0.001; supplemental Figure 1).
Figure 1.
Scatterplot of sensitive troponin I (sTnI) versus highly sensitive troponin I (hsTnI) for patients with and without ACS. The blue points are patients with ACS and the red points are patients without. The shaded box in the upper right hand corner represents the troponin 99th percentile. The horizontal line of red points at 15 ng/L indicates patients below the limit of detection on the sTnI assay.
Concerning the adjudicated gold-standard diagnosis of ACS, the median concentrations of both sensitive TnI assays were higher in those with ACS, compared to those without (Table 2). Notably, among those without ACS, the median value of was routinely below the LOD of sTnI at each time point.
Table 2.
Median (inter-quartile range) concentrations of A) sTnI and B) hsTnI as a function of presence or absence of ACS at each time point. Note median values of sTnI were frequently below the detection limit of the assay in ACS patients. P value refers to comparison of median values; in the presence of a result below the LOD, a value of 50% of the detection limit (sTnI = 7.5 ng/L; hsTnI = 0.25 ng/L) was imputed.
| A) sTnI | |||
|---|---|---|---|
| Time point | ACS | No ACS | P value |
| Baseline (N=309) | <LOD (<LOD-120.5) | <LOD | <0.001 |
| 90–155 minutes (N=289) | <LOD (<LOD-294.5) | <LOD | <0.001 |
| 210–300 minutes (N=231) | <LOD (<LOD-120.5) | <LOD | <0.001 |
| B) hsTnI | |||
|---|---|---|---|
| Time point | ACS | No ACS | P value |
| Baseline (N=309) | 29.0 (3.5–148.3) | 2.5 (1.3–5.1) | <0.001 |
| 90–155 minutes (N=289) | 28.4 (4.6–313.4) | 2.7 (1.6–6.0) | <0.001 |
| 210–300 minutes (N=231) | 24.6 (4.4–142.5) | 2.7 (1.6–5.7) | <0.001 |
LOD denotes: limit of detection
ROC analysis for the gold standard diagnosis of ACS across the three methods and three time points is depicted in Table 3. hsTnI and sTnI had similar AUC at each time point, but both had significantly greater AUC than cTn at first and second draws, which reflects significantly higher sensitivity and nominal trade-off in specificity. Illustrating this, operating characteristics of each Tn method for acute MI and ACS are detailed in Table 4. Given the inclusion criteria required a negative local cTn value, by definition, baseline concentrations of the local cTn had 0% sensitivity for acute MI and ACS. With serial cTn draws, greater sensitivity was seen. In contrast, the sTnI or hsTnI assays both had significantly higher sensitivity than cTn at first draw for ACS and UAP for both first and second blood draws (Supplemental Table 2); both sTnI and hsTnI had excellent NPV for both ACS and UAP. For the small number of acute MIs, sTnI and hsTnI were concordant.
Table 3.
Comparison of ROC results for each method for A) ACS and B) UAP. Both sTnI and hsTnI had superior area under the curve (AUC) to cTn for diagnosis or exclusion of each outcome at first and second draw; no difference in AUC was observed between sTnI and hsTnI.
| A) | |||||
|---|---|---|---|---|---|
| Time point | AUC cTn | AUC sTnI | AUC hsTnI | P value cTn vs sTnI |
P value cTn vs hsTnI |
| Baseline | 0.50 | 0.71 | 0.70 | <0.001 | <0.001 |
| 90–155 minutes | 0.50 | 0.73 | 0.72 | <0.001 | <0.001 |
| 210–300 minutes | 0.60 | 0.69 | 0.69 | 0.24 | 0.24 |
| B) | |||||
|---|---|---|---|---|---|
| Time point | AUC cTn | AUC sTnI | AUC hsTnI | P value cTn vs sTnI |
P value cTn vs hsTnI |
| Baseline | 0.50 | 0.63 | 0.63 | 0.008 | 0.01 |
| 90–155 minutes | 0.50 | 0.65 | 0.64 | 0.01 | 0.02 |
| 210–300 minutes | 0.55 | 0.63 | 0.63 | 0.40 | 0.40 |
Table 4.
Performance of cTn versus that of sTnI and hsTnI at each time point, for A) ACS (both acute MI and UAP) and B) UAP alone. Compared to cTn, the two more sensitive methods had greater sensitivity for acute MI at first drawn and substantially greater sensitivity for ACS at all time points. Additionally, hsTnI had 100% NPV at baseline to exclude all ACS.
| A) ACS (acute MI plus UAP) | |||||
|---|---|---|---|---|---|
| Operating characteristics: ACS | |||||
| Time point | Sens | Spec | PPV | NPV, 99th % | NPV, LOD |
| cTn, baseline (N=322) | 0% | 100% | N/A | 91% | N/A |
| cTn, second draw (N=252) | 14% | 100% | 100% | 93% | N/A |
| cTn, third draw (N=64) | 21% | 100% | 100% | 93% | N/A |
| sTnI, baseline (N=309) | 43% | 98% | 71% | 95% | 95% |
| sTnI, 90–155 minutes (N=289) | 46% | 98% | 69% | 95% | 95% |
| sTnI, 210–300 minutes (N=231) | 43% | 99% | 82% | 95% | 95% |
| hsTnI, baseline (N=309) | 43% | 97% | 60% | 95% | 100% |
| hsTnI, 90–155 minutes (N=289) | 46% | 97% | 58% | 95% | 100% |
| hsTnI, 210–300 minutes (N=231) | 43% | 98% | 64% | 95% | 86% |
| B) UAP (excluding acute MI) | |||||
|---|---|---|---|---|---|
| Operating characteristics: ACS | |||||
| Time point | Sens | Spec | PPV | NPV, 99th % | NPV, LOD |
| cTn, baseline (N=315) | 0% | 100% | N/A | 93% | N/A |
| cTn, second draw (N=245) | 0% | 100% | N/A | 93% | N/A |
| cTn, third draw (N=58) | 5% | 100% | 100% | 94% | N/A |
| sTnI, baseline (N=302) | 29% | 98% | 55% | 95% | 95% |
| sTnI, 90–155 minutes (N=282) | 29% | 98% | 50% | 96% | 95% |
| sTnI, 210–300 minutes (N=226) | 31% | 99% | 71% | 95% | 95% |
| hsTnI, baseline (N=302) | 29% | 97% | 43% | 95% | 100% |
| hsTnI, 90–155 minutes (N=282) | 29% | 97% | 39% | 96% | 100% |
| hsTnI, 210–300 minutes (N=226) | 31% | 98% | 50% | 95% | 86% |
*NPV for cTn is at the lowest cut-off providing 10% imprecision.
Sens denotes: sensitivity; Spec denotes: specificity; PPV denotes: positive predictive value; NPV denotes: negative predictive value; LOD denotes: limit of detection; N/A denotes: not applicable.
Notably, 29% of ACS cases previously categorized as UAP using local cTn methods would have been reclassified to acute MI with sTnI or hsTnI in the present analysis. Interestingly, serial blood draws for sTnI or hsTnI did not add substantially to diagnostic accuracy, no matter whether examined for a rise and/or fall in subsequent levels (results not shown).
When examining patients as a function of the LOD for the sTnI or hsTnI methods, NPV of sTnI for ACS was 95% at each time point, however NPV of hsTnI <LOD remained 100% at baseline and 90–155 minutes.
Troponin concentrations, CTA results, and coronary calcium score
There were 92 participants with any CAD at the time of CTA. Using a comparison of ROC curves for sTnI and hsTnI, we found hsTnI had modest, albeit significantly higher AUC compared to sTnI for the presence of CAD (0.62 [95% CI=0.53–0.71] versus 0.54 [95% CI=0.49–0.58]; P for difference in AUC=0.05).
With higher values for either sTnI and hsTnI, a higher presence and greater extent of coronary calcium and CAD were observed, regardless of the presence or absence of ACS (Table 5). As the majority of these subjects were in a range between LOD and 99th percentile, the use of the 99th percentile as the lower decision limit resulted in substantial patients “missed” for the presence of CAD (Supplemental Table 3), indicating the importance of values between the LOD and 99th percentile. Among those with a hsTnI below the LOD of 0.5 ng/L, we observed a median calcium score of zero and 100% NPV for significant coronary stenoses at first draw.
Table 5.
Associations between baseline concentrations of A) sTnI and B) hsTnI and presence or extent of epicardial CAD and coronary artery calcium score in patients undergoing CTA. Extremely low concentrations of TnI using both methods (but particularly hsTnI) were associated with less epicardial CAD and absence of coronary artery calcium.
| A) sTnI | |||
|---|---|---|---|
| sTnI <LOD | sTnI LOD to 99th percentile | sTnI ≥99th percentile | |
| Calcium score, median (IQR) | 0 [0–28] | 0.1 [0–3] | 213 [38–521] |
| Any CAD | 55.6% | 50.0% | 90.0% |
| Stenosis >50% | 18.8% | 16.7% | 80.0% |
| Stenosis >70% | 12.5% | 0% | 70.0% |
| B) hsTnI | |||
|---|---|---|---|
| hsTnI <LOD | hsTnI LOD to 99th percentile | hsTnI ≥99th percentile | |
| Calcium score, median (IQR) | 0 [0-0] | 0 [0–29] | 111 [0.2–480] |
| Any CAD | 11.1% | 58.3% | 83.3% |
| Stenosis >50% | 0% | 19.4% | 75.0% |
| Stenosis >70% | 0% | 13.0% | 58.3% |
LOD denotes: limit of detection; IQR denotes: interquartile range; CAD denotes: coronary artery disease
Discussion
We examined the information gained beyond cTn results by measuring two incrementally sensitive TnI assays in a cohort of patients evaluated with symptoms considered intermediate in likelihood for cardiac chest pain. These subjects were relatively young—though demographically consistent with those commonly seen in the emergency department setting (8)—and had to be suitable for CTA. By definition they also had a normal cTn at baseline. Compared to cTn, both sensitive assays had higher first-draw sensitivity and NPV for acute MI, and were particularly superior to cTn for detection of ACS. This is due to the fact that both assays reclassified 29% of patients thought to have UAP (using cTn) to a new diagnosis of acute MI a finding consistent with other reports (3,4,9). This “detection” of UAP suggests a significant percentage of patients previously thought not to have myocardial necrosis in the setting of ACS indeed have an MI. Notably, substantial loss of specificity for ACS was not seen with the more sensitive methods. Further, with substantially greater sensitivity, hsTnI had 100% NPV for ACS or significant epicardial CAD if below the LOD of 0.5 ng/L at baseline. Our results demonstrate the effect of incremental sensitivity of troponin assays on both clinical application as well as CTA results.
The general experience with hsTn assays has been that first-draw sensitivity is improved compared to cTn and sTn methods, particularly in patients presenting <2 hours from symptom onset (3,9–13); our results confirm this clinically meaningful superiority. Additionally, compared to conventional assays, hsTn methods provide excellent ability to exclude acute MI at an earlier time frame through rapid serial assessment (10–13). Beyond these characteristics, hsTn assays appear to routinely increase the number of ACS patients identified as having acute MI when cTn results were not abnormal (3,4,9,14). While a considerable advance, areas of uncertainty exist regarding hsTn. Given the many reasons for low-level myocardial necrosis in states other than acute MI, reduction in specificity has been observed in more medically complex patients than ours (15); the use of serial measurement to detect a rise and/or fall of Tn, together with application of clinical judgment and the knowledge of the differential diagnosis of diagnoses associated with the Tn concentration detected, may help to mitigate this challenge (1,10–12,16).
Consistent with the work by Body and colleagues (10), values below the LOD for hsTnI at each time point excluded ACS (including UAP) with 100% NPV at first and second draws. Surprisingly, with higher sensitivity, we did not see the expected trade off in lower specificity; this offers considerable reassurance that when measured in certain circumstances such as in patients resembling ours, the diagnostic performance of more highly sensitive Tn methods will be acceptable.
Of interest, we found that sTnI and hsTnI concentrations were associated with results from CTA: elevated values for both assays were more likely observed in those with elevated CAC scores or with significant epicardial CAD, and Tn values were higher in those with more diffuse disease. These results are consistent with those we reported with hsTnT in the ROMICAT I biomarker sub-study (4). Beyond the obvious link to acute MI, predictors of circulating hsTn concentrations include a wide range of variables including the presence of hemodynamic, myocardial, and metabolic abnormalities (17–19); perhaps most prominently however has been the link between hsTn values with presence, severity, and biological instability of underlying coronary atherosclerosis (4,20). This suggests a potential role for hsTn to provide quantification of atherothrombotic risk, particularly in those without prevalent ACS. It is worth mention we found that hsTnI below the LOD had a strikingly high NPV for exclusion of significant CAD. Conversely, those without ACS with measurable (but not overtly elevated hsTnI) were more likely to have non-obstructive but nonetheless clinically important atherosclerosis. We are currently performing more analyses with more detailed plaque characterization to better understand the link(s) between hsTnI and coronary atherosclerosis.
As has been argued, interpretation of hsTn values should be on the basis of absolute values (21), rather than simply “positive” or “negative”. In this light, the results from our and other studies make it tempting to speculate that concentrations of hsTn might be leveraged for more accurate patient care beyond their higher sensitivity for MI diagnosis; an hsTnI above the 99th percentile indicates myocardial injury and in appropriate context, may more rapidly diagnose acute MI. Beyond this, however, an hsTn <LOD might also identify patients eligible for more rapid discharge in appropriate context, while in those with a measurable hsTnI without ACS, hsTn may be useful to detect signal of underlying atherosclerosis or heart muscle disease to trigger follow up secondary prevention.
Our study has limitations. A major drawback is its small size, which undermines the fidelity of our findings; our results are nonetheless consistent with other analyses, and provide useful hypothesis-generating data. Additionally, enrollment of patients in our study was subject to inclusion and exclusion criteria, which may have influenced our findings; indeed our subjects had a low to intermediate risk profile. However, the demographics and ACS rate in our study subjects are consistent with those reported in larger reports (8). Notably, sTnI had similar performance to hsTnI for diagnosis of ACS; this is not surprising given the sensitive nature of the assay (and the fact the two methods are based on similar antibodies). However, given higher analytical sensitivity, superior NPV of hsTnI at its LOD was seen for diagnosis of ACS and CAD. The performance of both sTnI and hsTnI was compared to lesser sensitive cTn methods, which were used to judge diagnosis (i.e. UAP versus acute MI); in order to avoid penalizing the more sensitive methods, we thus elected to analyze the performance of these markers for “ACS”, rather than focus on acute MI only. The relatively wide windows of time at each blood draw could theoretically have affected sensitivity results; perhaps this explains the fact serial blood draws did not add substantially to diagnostic accuracy. Including patients with shorter times to presentation would likely have affected the performance characteristics of both sTnI and hsTnI. Uncommonly, ACS may occur with normal coronary arteries; a normal CTA might not entirely exclude this forms of ACS, but in these circumstances, clinical judgment, hsTn as well as ECG evidence would assist to confirm the diagnosis.
In conclusion, with increasing analytical sensitivity, we observed a greater ability to identify or exclude ACS, without unacceptable trade-off in specificity for the diagnosis. Further, with highest sensitivity, the ability to not only exclude ACS but also underlying structural heart disease when below the LOD of the assay was a compelling application, suggesting those patients in this category may be eligible for rapid, safe discharge. Our results are illustrative as to the expected clinical benefits of increased sensitivity provided from novel Tn assays.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 2.Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clinical chemistry. 2011;58:54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. The New England journal of medicine. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 4.Januzzi JL, Jr, Bamberg F, Lee H, et al. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation. 2010;121:1227–1234. doi: 10.1161/CIRCULATIONAHA.109.893826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clinical chemistry. 2010;56:941–943. doi: 10.1373/clinchem.2010.143958. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. The New England journal of medicine. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann U, Truong QA, Fleg JL, et al. Design of the Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography: a multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. American heart journal. 2012;163:330–338. 338 e1. doi: 10.1016/j.ahj.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. The New England journal of medicine. 2000;342:1163–1170. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 9.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. The New England journal of medicine. 2009;361:868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 10.Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. Journal of the American College of Cardiology. 2011;58:1332–1339. doi: 10.1016/j.jacc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 12.Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124:136–145. doi: 10.1161/CIRCULATIONAHA.111.023937. [DOI] [PubMed] [Google Scholar]
- 13.Cullen L, Mueller C, Parsonage WA, et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. Journal of the American College of Cardiology. 2013;62:1242–1249. doi: 10.1016/j.jacc.2013.02.078. [DOI] [PubMed] [Google Scholar]
- 14.Lipinski MJ, Baker NC, Escarcega RO, et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: A collaborative meta-analysis. American heart journal. 2015;169:6 e6–16 e6. doi: 10.1016/j.ahj.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Januzzi JL, Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. European heart journal. 2012;33:2265–2271. doi: 10.1093/eurheartj/ehs191. [DOI] [PubMed] [Google Scholar]
- 16.Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- 17.Gaggin HK, Szymonifka J, Bhardwaj A, et al. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart failure. 2014;2:65–72. doi: 10.1016/j.jchf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Lazo M, Chen Y, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130:1374–1382. doi: 10.1161/CIRCULATIONAHA.114.010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aeschbacher S, Schoen T, Bossard M, et al. Relationship Between High-Sensitivity Cardiac Troponin I and Blood Pressure Among Young and Healthy Adults. American journal of hypertension. 2014 doi: 10.1093/ajh/hpu226. [DOI] [PubMed] [Google Scholar]
- 20.Seifarth H, Schlett CL, Lehman SJ, et al. Correlation of concentrations of high-sensitivity troponin T and high-sensitivity C-reactive protein with plaque progression as measured by CT coronary angiography. Journal of cardiovascular computed tomography. 2014;8:452–458. doi: 10.1016/j.jcct.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller C. Use of high-sensitivity troponin for the diagnosis of acute myocardial infarction. Coronary artery disease. 2013;24:710–712. doi: 10.1097/MCA.0000000000000049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.