Clinical and epidemiological studies show a robust, inverse association of high-density lipoprotein (HDL) levels with cardiovascular disease (CVD) risk (1). Moreover, genetically engineered mice provide compelling evidence that HDL is atheroprotective in hypercholesterolemic animal models. These observations have triggered intense interest in targeting HDL for therapeutic intervention.
Most clinical studies have used HDL-cholesterol (HDL-C) as the metric for quantifying HDL's cardioprotective effects. However, recent evidence has raised doubts about elevating HDL-C being therapeutic (1). For example, genetic variations that associate with altered HDL-C levels do not strongly associate with altered CVD risk. Also, prospective clinical trials of niacin and cholesteryl ester transfer protein (CETP) inhibitors (two drugs that elevate HDL-C levels by different mechanisms) failed to reduce cardiac events in statin-treated subjects with established CVD (1). Moreover, when mice lack certain proteins involved in HDL metabolism—such as SRB1, the liver receptor for HDL—both HDL-C levels and atherosclerosis increase dramatically (2). Thus, quantifying HDL-C does not necessarily assess HDL's proposed ability to lower CVD risk.
Many lines of evidence indicate that one of HDL's cardioprotective tasks is to mobilize excess cholesterol from artery wall macrophages (1). For example, mouse studies demonstrate that increased hepatic expression of apolipoprotein (apo) A-I, the major HDL protein, increases cholesterol export from macrophages and retards atherosclerosis.
Two pathways for sterol export involve the membrane-associated ATP-binding cassette transporters ABCA1 and ABCG1, which are highly induced when macrophages accumulate excess cholesterol (1). Thus, atherosclerosis increases markedly in hypercholesterolemic mice when myeloid cells are deficient in ABCA1 (1). Also, humans with ABCA1 deficiency suffer from Tangier's disease and accumulate macrophages laden with cholesterol in many tissues (3). These observations support the proposal that HDL, ABCA1, and sterol efflux from cells are important regulators of sterol balance in human macrophages.
The relevance of sterol efflux from macrophages in humans has been difficult to assess because it accounts for only a small fraction of overall reverse cholesterol transport from peripheral tissues to the liver (1). To measure efflux capacity, Rothblat and colleagues pioneered the use of ‘serum HDL’ (serum depleted of the atherogenic lipoproteins LDL and VLDL, which deliver cholesterol to macrophages) with cultured J774 macrophages radiolabeled with cholesterol (4). They demonstrated that the cholesterol efflux capacity of human serum HDL varies markedly, despite similar levels of HDL-C (5). Thus, HDL-C level is not the major determinant of macrophage sterol efflux in this system.
Using the same model system (5), investigators found strong, inverse associations between the cholesterol efflux capacity of serum HDL and prevalent coronary artery disease (CAD). Differences in efflux capacity of serum HDL correlated with altered efflux by the ABCA1 pathway in macrophages (4,5). Moreover, efflux capacity remained a strong predictor of prevalent CAD after adjustment for HDL-C levels (5). This study provided the first strong clinical evidence that a proposed functional property of HDL might be more relevant to human atherosclerosis than HDL-C levels.
The efflux capacity of serum HDL with J774 macrophages can also be assessed with fluorescently labeled cholesterol, which primarily measures cholesterol export by ABCA1. A recent study of a large, population-based cohort initially free of cardiovascular disease demonstrated that sterol efflux assessed by this method associates strongly and negatively with the risk of future cardiac events (6). This association persisted after multivariate adjustment, suggesting that impaired HDL function affects incident cardiovascular risk by processes distinct from those involving HDL-C and other traditional lipid risk factors. Taken together (5,6), these observations provide strong evidence that HDL's capacity to accept cholesterol via ABCA1 is a functional metric, relevant to atheroprotection, that is independent of HDL-C.
HDL is not a homogeneous population. It is a collection of particles that range in size from <7 nm to >14 nm and contains >80 different proteins (7). Most HDL particles are spherical with a core of hydrophobic lipids (cholesteryl ester and triglycerides). However, the major initial acceptor for cholesterol excreted by cells appears to be pre-beta HDL, a quantitatively minor species of plasma HDL made of poorly lipidated apoA-I.
Pioneering studies by Oram and colleagues demonstrated that lipid-free apoA-I promotes cholesterol efflux from cells and that this pathway is defective in Tangier's disease fibroblasts (8). Other lipid-free or lipid-poor apolipoproteins can also promote cholesterol efflux by ABCA1. In contrast, lipid-free apoA-I fails to promote sterol efflux from cells by the ABCG1 pathway (1). Instead, the major acceptor for free cholesterol export by ABCG1 is spherical HDL. Efflux by ABCG1 increases as the phospholipid surface layer of spherical HDLs enlarges.
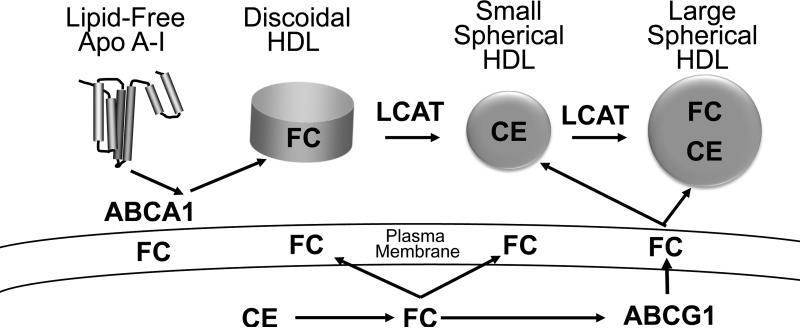
In this model, lipid-free or poorly lipidated apoA-I accepts sterol (and phospholipid) from cells by a pathway involving ABCA1 (Fig. 1A) to become discoidal HDL particles that lack a cholesteryl ester core. Lecithin-cholesterol acyltransferase (LCAT) then converts the newly accepted cholesterol to cholesteryl ester, which—being more hydrophobic—migrates into the core of the nascent HDL particle to generate a more mature spherical HDL (9). This form of HDL becomes an acceptor for cholesterol exported from cells by ABCG1, and this second wave of cholesterol enlarges the HDL particles
Figure. Current (A) and proposed (B) models for how different HDL species promote cholesterol efflux from macrophages.
Both ABCA1 and ABCG1 are transmembrane proteins but they promote sterol efflux by different mechanisms. ABCA1 exports cholesterol from the plasma membrane to cholesterol acceptors, while ABCG1 is an intracellular sterol transporter that enriches the plasma membrane with cholesterol. See the text for additional details. FC, cholesterol; CE, cholesteryl ester; LCAT, lecithin-cholesterol acyltransferase.
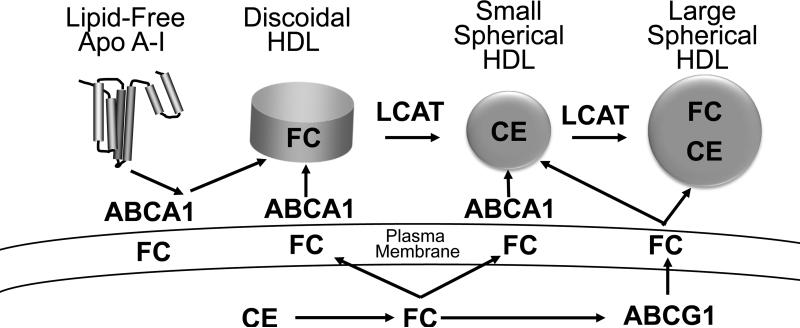
A key feature of the current model is that discoidal and more mature forms of HDL do not promote sterol efflux by the ABCA1 pathway (Fig. 1A). The model also implies that increased LCAT activity should promote sterol efflux from macrophages. In this issue, Du et al. challenge this notion by providing strong evidence that small, dense HDL particles are potent acceptors of cholesterol exported from macrophages by the ABCA1 pathway (10). Moreover, they show that the major mediator of cholesterol export from macrophages to HDL is ABCA1.
Strengths of their studies include: using carefully defined sizes of both reconstituted HDL (rHDL) particles and human HDL to examine the impact of size on sterol efflux; quantifying sterol efflux via ABCA1 and ABCG1 from multiple types of macrophages (bone marrow-derived mouse macrophages, a human macrophage cell line, and primary human monocyte-derived macrophages); and quantifying cholesterol efflux with both radiolabeled cholesterol and by sterol mass. The latter point is significant because, under certain circumstances, efflux of radiolabeled cellular cholesterol can be dissociated from changes in overall sterol balance in macrophages. In future studies it will be critical to determine the mechanistic basis for the variations in efflux capacity seen with different sizes of HDL.
The work of Du et al. suggests a new model for how HDL promotes sterol efflux from macrophages (Fig. 1B): both lipid-free/poorly lipidated apoA-I and small HDL particles promote sterol efflux by the ABCA1 pathway. As in the current model, cholesterol derived from both LCAT and ABCG1 increase the size of HDL. Because LCAT converts discoidal HDL and small HDL to larger particles, the enzyme might interfere with macrophage sterol export by the ABCA1 pathway. It is noteworthy that overexpression of LCAT in hypercholesterolemic mice increases both HDL-C and atherosclerosis (11). Moreover, humans deficient in LCAT have very low HDL-C levels but no major increase in CVD risk (7).
These observations may have important implications for therapies targeted to increase the cardioprotective subspecie(s) of HDL. Importantly, both drugs that failed to reduce cardiac risk in the clinical trials increase the concentration of HDL2, the large form of HDL (1). If smaller forms of HDL (e.g., HDL3) are indeed the main promoters of sterol efflux from macrophages by ABCA1, the drugs may have been ineffective because they failed to increase the concentration of the small HDL particles that promote efflux by this pathway.
Two key unresolved issues are the concentrations of the different sizes of HDL subspecies in blood, and how the different subspecies in blood contribute to the sterol efflux capacity of “serum HDL” with macrophages. Here, the report by Du et al. strongly implies that different sized HDLs have distinct functional capacities and furthermore that small, cholesterol-poor particles play important roles in mobilizing cholesterol from macrophages. HDL levels are generally measured indirectly as the cholesterol content of HDL. Because HDL varies widely in size, the cholesterol content of an HDL particle can vary over 10-fold (7). At a given level of HDL-C, it is theoretically possible to have many small cholesterol-poor particles or far fewer large, cholesterol-rich particles. Thus, a key issue confounding the interpretation of HDL-C as a measure of HDL concentration and function is the relative balance between large and small HDL particles. An additional complexity is the difference in the stoichiometry of apoA-I in large and small HDL particles, which makes interpretation of apoA-I levels (or HDL protein content, the method used by Du et al.) problematic for assessing HDL particle size and concentration. These issues highlight the need for new HDL metrics which are not biased towards certain subpopulations, such as large cholesterol rich HDL.
We recently developed a method to measure the concentration and size of HDL particles in plasma. Termed, calibrated ion mobility analysis (IMA), the method employs protein standards to convert IMA signal intensity, a relative measurement, to a metric that quantifies the absolute concentration of HDL particles (12). Using this method, we found the total concentration of HDL particles in plasma to be 13.4 μM, with a mean plasma apoA-I value of 48.8 μM, indicating that each HDL particle contains ~3.6 molecules of apoA-I (assuming that all HDL particles contain apoA-I). This value is in excellent agreement with the stoichiometry of human HDL determined by other methods (13). Moreover, calibrated IMA also yielded values for the size of the HDL subspecies that agree well with those obtained by orthogonal methods (7,12). As expected, both HDL-C and apoA-I levels, were poor predictors of HDL particle number, both metrics underrepresented small HDLs. In future studies, quantification of HDL size and concentration should be a valuable tool for investigating the roles of specific HDL subspecies and pathways in regulating the efflux capacity of serum HDL.
Recent clinical and genetic studies clearly demonstrate that elevating HDL-C does not necessarily reduce CVD risk (1). Therefore, it is time to end the clinical focus on HDL-C and to understand—at the mechanistic level—how HDL's functional properties contribute to that risk. It will also be important to link changes in HDL's size and function to genetics and HDL-targeted therapies. The development of new metrics for quantifying HDL function, based on a better understanding of macrophage reverse cholesterol transport, is essential for achieving these goals.
Acknowledgements
The author's work was supported by awards from the National Institutes of Health (HL112625, HL108897, P30 DK017047, P01 HL092969).
References
- 1.Rader DJ, Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–6. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 2.Trigatti B, Rayburn H, Viñals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A. 96:9322–7. doi: 10.1073/pnas.96.16.9322. 19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer EJ, Zech LA, Schwartz DE, Brewer HB., Jr Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease). Ann Intern Med. 1980 Aug;93(2):261–6. doi: 10.7326/0003-4819-93-2-261. [DOI] [PubMed] [Google Scholar]
- 4.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenson RS, Brewer HB, Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 57:392–410. doi: 10.1373/clinchem.2010.155333. 201. [DOI] [PubMed] [Google Scholar]
- 8.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glomset JA, Norum KR, King W. Plasma lipoproteins in familial lecithin:cholesterol acyltransferase deficiency: lipid composition and reactivity in vitro. J Clin Invest. 1970;49:1827–37. doi: 10.1172/JCI106400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du X, Kim MJ, Hou L, Le Goff W, Chapman MJ, van Eck M, Curtiss LK, Burnett J, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. HDL Particle Size is a Critical Determinant of ABCA1-Mediated Macrophage Cellular Cholesterol Export. Circ Res. 2015;116:xxx–xxx. doi: 10.1161/CIRCRESAHA.116.305485. [in this issue] [DOI] [PubMed] [Google Scholar]
- 11.Bérard AM, Föger B, Remaley A, Shamburek R, Vaisman BL, Talley G, Paigen B, Hoyt RF, Jr, Marcovina S, Brewer HB., Jr Santamarina-Fojo S. High plasma HDL concentrations associated with enhanced atherosclerosis in transgenic mice overexpressing lecithin-cholesteryl acyltransferase. Nat Med. 1997;3:744–9. doi: 10.1038/nm0797-744. [DOI] [PubMed] [Google Scholar]
- 12.Hutchins PM, et al. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clinical Chemistry. 2014;60:1393–1401. doi: 10.1373/clinchem.2014.228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang R, Silva RA, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ, Davidson WS. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol. 2011;18:416–22. doi: 10.1038/nsmb.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]