Abstract
Background
In 2009, treatment guidelines were updated to recommend KRAS testing at diagnosis for patients with metastatic colorectal cancer (mCRC). We investigated KRAS testing rates over time and compared characteristics of KRAS-tested and not-tested patients in a community-based oncology setting.
Methods
Adult patients with a diagnosis of mCRC from 2008–2011 were selected from the ACORN Data Warehouse (ACORN Research LLC, Memphis, TN). Text mining of physician progress notes and full chart reviews identified KRAS-tested patients, test dates, and test results (KRAS status). The overall proportion of eligible patients KRAS-tested in each calendar year was calculated. Among KRAS-tested patients, the proportion tested at diagnosis (within 60 days) was calculated by year. Univariate and multivariate analyses were used to compare patient characteristics at diagnosis between tested and not-tested cohorts, and to identify factors associated with KRAS testing.
Results
Among 1,363 mCRC patients seen from 2008–2011, 648 (47.5%) were KRAS-tested. Among newly diagnosed mCRC patients, the rate of KRAS testing increased from 5.9% prior to 2008, to 13.9% in 2008, and then jumped dramatically to 32.3% in 2009, after which a modest yearly increase continued. The proportions of KRAS-tested patients who had been diagnosed in previous years but not tested previously increased from 17.7% in 2008 to 27.0% in 2009, then decreased to 19.0% in 2010 and 17.6% in 2011. Among patients who were KRAS-tested, the proportions tested at the time of diagnosis increased annually (to 78.4% in 2011). Patients more likely to have been tested included those with lung metastases, poor performance status, more comorbidities, and mCRC diagnosis in 2009 or later.
Conclusions
The frequency of KRAS testing increased over time, corresponding to changes in treatment guidelines and epidermal growth factor receptor inhibitor product labels; however, approximately 50% of eligible patients were untested during the study period.
Keywords: KRAS, KRAS testing, Metastatic colorectal cancer, mCRC
Background
Colorectal cancer (CRC) is the third leading cause of cancer deaths in the United States (US), with an estimated 142,820 new cases to be diagnosed in 2013 [1]. At the time of diagnosis, approximately 39% of patients with CRC present with localized disease (stages I-II), 37% present with regional metastases (stage III), and 19% with distant metastases (stage IV, or metastatic CRC [mCRC]) [2]. In mCRC, common sites for metastases include the liver, peritoneum, and lung. The 5-year survival rate for patients diagnosed with mCRC is approximately 10% to 12% [1,3].
Conventional chemotherapies used to treat mCRC include combinations of 5-fluorouracil, leucovorin, capecitabine, oxaliplatin, and irinotecan [4]. Bevacizumab (Avastin) is also indicated for treatment of mCRC in combination with conventional chemotherapies [5]. In addition, targeted therapies that interfere with epidermal growth factor receptor (EGFR)-mediated pathways (i.e., EGFR inhibitors) have been developed for the treatment of mCRC. In 2004, the EGFR inhibitor cetuximab (Erbitux) was approved in the US for the second-line treatment of mCRC in patients whose tumors overexpress EGFR [6], and in 2006, the EGFR inhibitor panitumumab (Vectibix) was approved for the second-line treatment of mCRC in patients with disease progression on or following conventional chemotherapies [7]. Since then, cetuximab has had its indication revised to include first- and second-line treatment of mCRC, in combination with conventional chemotherapies or as a single agent in patients who have failed oxaliplatin- and irinotecan-based therapy [6]. Similarly, in 2014, panitumumab had its indication revised to include first-line treatment of mCRC, in combination with conventional chemotherapies or as a single agent in patients who have failed fluoropyrimidine therapy [7].
KRAS is an oncogene involved with the EGFR signaling pathway; KRAS activity in mCRC is associated with increased cell proliferation, angiogenesis, migration, and survival of the cancer tissue [4,8]. In mCRC, only tumors carrying the normal (or wild-type [WT]) allele of the KRAS oncogene (i.e., KRAS mutation-negative) may have favorable responses to these EGFR inhibitors, and patients whose tumors carry certain KRAS mutations do not derive any benefit [8-10]. Approximately 60% of patients with mCRC carry the WT KRAS allele, and thus these patients may be appropriate candidates for therapy with EGFR inhibitors [4,8,9]. A recent study has suggested that KRAS mutation status is also predictive of long-term prognosis among patients with mCRC treated by conventional chemotherapy [11].
In light of these findings, the National Comprehensive Cancer Network (NCCN) updated its treatment guidelines for mCRC in late 2008, recommending that KRAS testing on tumors and metastases be part of the pretreatment workup for all patients with metastatic (stage IV) disease [4,12,13]. In mid-2009, NCCN updated these guidelines, stipulating that only patients with WT KRAS genotypes should receive treatment with EGFR inhibitors [13-15]. Similarly, the American Society for Clinical Oncology (ASCO) recommended that all patients with mCRC who are candidates for anti-EGFR antibody therapy have their tumors tested for KRAS mutations, and that those in whom mutations in codon 12 or 13 are detected should not receive EGFR inhibitors [15,16]. In 2009, the US Food and Drug Administration (FDA) required that the labels for both cetuximab and panitumumab be updated to restrict these agents to patients with mCRC whose tumors carry the WT KRAS genotype [6,7,17]. Most recently, in 2012, the FDA approved the first companion diagnostic test for KRAS genotyping (the therascreen® RGQ PCR Kit).
The objectives of the present study were to investigate KRAS testing rates over time and to compare the characteristics of tested and not-tested patients in a sample of community-based US oncology practices. In recent years, the clinical uptake and utilization of KRAS testing in mCRC, and the impact of KRAS testing on treatment outcomes, have been investigated in observational studies performed in Europe, Latin America, Asia [18], and the US [19]. In contrast to previous US studies, the current analysis includes patients covered by multiple healthcare payers (e.g., commercial, Medicare, Medicaid) enrolled in various plan types as well as self-pay patients.
Methods
Data source
The primary data source for this study was the ACORN EMR Warehouse (ACORN Research LLC, Memphis, TN), a database which contains information on approximately 175,000 patients seen in 12 community-based oncology practices by 120 oncologists across the US since 2004. This database links electronic medical records (EMRs), which contain patient demographics, tumor type and stage, treatments, and outcomes, with laboratory results and other clinical information. One limitation is that the results of KRAS testing are not captured in standard EMR data fields; however, this information is often found in physician progress notes, which ACORN stores electronically in the data warehouse to complement the EMR data. Deidentified data on patients meeting select criteria were provided by ACORN for analysis.
Study population
Adult patients (aged 18 years or older) with a primary diagnosis of mCRC (based on ICD-9-CM codes [CRC: ICD-9-CM 153.x-154.1; metastatic disease: ICD-9-CM 197.x-198.x] and/or listing of “stage IV” or “stage 4” in physician notes) between January 1, 2008 and December 31, 2011 were identified. Patients with evidence of other primary cancers and those with only one visit to a contributing practice were excluded. All database records for included patients were extracted through March 31, 2012. For the purposes of the present study, “baseline” was defined as the time of first diagnosis of metastatic disease.
Development and validity of KRAS testing algorithms
Algorithms for electronic text mining of physician progress notes were developed by ACORN to identify whether patients with mCRC were tested for KRAS and whether a patient’s tumor designation was WT or mutant [20]. These text mining algorithms were validated by trained oncology nurse abstractors who reviewed a random selection of 300 charts. When applied to the dataset, the best-performing algorithm (employing a random forest approach) achieved a kappa value (i.e., how often the model agreed with the chart review, accounting for chance) of 0.97, with a positive predictive value of 96.4% and a negative predictive value of 98.0% [20]. Trained oncology nurses then reviewed the medical charts of all patients identified by the model as “KRAS-tested” and recorded the results (WT, mutant, or unknown [ie, no evidence of testing results]) and the date of testing. The data were then merged with the study population data using blinded patient identifiers.
Proportion of population tested
The overall rate of KRAS testing was based on the number of patients tested at any point between diagnosis of CRC and end of follow-up, divided by the total study sample. Rate of KRAS testing by year was based on the number of patients tested in the given year divided by the number of patients eligible for the given year. Patients eligible for testing, by year, was the sum of patients newly diagnosed with mCRC within a given year and those diagnosed with mCRC in previous years (with a visit to a participating provider within the given year) but not previously KRAS-tested. Finally, the proportion of those tested at diagnosis was defined as those tested within 60 days (before or after) of their first recorded mCRC diagnosis date within a given year divided by the total number of study patients diagnosed with mCRC in that same year.
Statistical analyses
Demographic information, disease characteristics, and clinical history at first diagnosis of metastatic disease were compared between KRAS-tested and not-tested patients using chi-squared and Student’s t-tests. A logistic regression model was used to identify baseline factors associated with ever being KRAS-tested and with being tested at diagnosis. The level of statistical significance for all tests was 0.05 and results were not adjusted for multiple comparisons. All statistical analyses were performed using SAS version 9.2, on a PC platform.
Results
Among 12,420 patients with a CRC diagnosis between 2008 and 2011, 2,080 were 18 years of age or older with documented distant metastases. Of these patients, 686 were excluded because they had evidence of other cancers, and an additional 31 were excluded because they only had one visit to the contributing practice, yielding a study population of 1,363 patients (Table 1). Of these 1,363 patients, 648 (47.5%) were KRAS-tested at some point in their disease. Of these 648 tested patients, 312 patients (48.1%) were identified as WT, 274 patients (42.3%) as mutant, and 62 patients (9.6%) as unknown (no evidence of testing results). Proportions of those patients KRAS-tested were similar between males and females, but KRAS testing was more frequent among patients aged 65 years and older than among younger patients (p = 0.002). Patients tested for KRAS were also less likely to be self-pay (3.9% vs. 8.1% for not-tested patients, p = 0.040), were more likely to be enrolled in a clinical trial (13.7% vs. 8.7%, p = 0.003), had more comorbid conditions (mean, 3.7 vs. 2.7, p < 0.001), and had poorer performance status (ECOG 2–4: 41.9% vs. 34.0%, p = 0.042) at diagnosis. No significant differences were found between tested and nontested cohorts for race, gender, cancer location (colon, rectum, or both), tumor histologic grade, or whether patients were newly diagnosed versus having recurrent mCRC.
Table 1.
Demographic characteristics of the eligible study population
| Parameter | Number of patients ( N = 1,363) |
|---|---|
| Age, years | |
| 18-34 | 26 (1.9%) |
| 35-54 | 357 (26.2%) |
| 55-74 | 724 (53.1%) |
| ≥75 | 256 (18.8%) |
| Gender | |
| Female | 640 (47.0%) |
| Male | 723 (53.0%) |
| Race | |
| Caucasian | 724 (53.1%) |
| African American | 182 (13.4%) |
| Hispanic | 8 (0.6%) |
| Other | 23 (1.7%) |
| Not recorded | 426 (31.3%) |
| Insurance type | |
| Commercial | 663 (48.6%) |
| Medicare | 461 (33.8%) |
| Medicaid | 105 (7.7%) |
| Self-pay | 83 (6.1%) |
| Other | 51 (3.7%) |
| Death reported | 653 (47.9%) |
| Enrolled in a clinical trial | 151 (11.1%) |
| Location of cancer | |
| Colon | 915 (67.1%) |
| Rectum | 397 (29.1%) |
| Both | 51 (3.7%) |
| Course of mCRC | |
| Newly diagnosed | 482 (35.4%) |
| Recurrent | 43 (3.2%) |
| Other | 4 (0.3%) |
| Not reported | 834 (61.2%) |
| Consolidated/Combined ECOG/KPS score | |
| ECOG = 0 to 1/KPS = 80 to 100 | 466 (34.2%) |
| ECOG = 2-4/KPS = 10-70 | 274 (20.1%) |
| Not reported | 623 (45.7%) |
| Histologic grade | |
| Well differentiated | 40 (2.9%) |
| Moderately differentiated | 265 (19.4%) |
| High grade/poorly differentiated | 89 (6.5%) |
| Undifferentiated | 3 (0.2%) |
| Cannot be assessed | 30 (2.2%) |
| Not reported | 936 (68.7%) |
| KRAS genotype | |
| Wild-type | 312 (22.9%) |
| Mutant | 274 (20.1%) |
| Unknown | 62 (4.5%) |
| Not tested | 715 (52.5%) |
Abbreviations: ECOG Eastern Cooperative Oncology Group, KPS Karnofsky performance score, mCRC metastatic colorectal cancer.
The proportions of eligible patients ever KRAS-tested increased from 5.9% to 16.1% in the calendar year 2008 (prior to the inclusion of testing recommendations in the NCCN guidelines) and continued to increase to 29.1% in 2009 (coincident with NCCN and ASCO guidance updates and US labeling changes regarding KRAS genotype for cetuximab and panitumumab), after which the proportions of tested patients remained fairly consistent. However, as shown in Figure 1A, when looking separately at the two populations of eligible patients, the proportions of newly diagnosed mCRC patients KRAS-tested increased from 13.9% in 2008 to 40.5% in 2011, while patients who were previously diagnosed, but not tested, tended to remain not tested over time. Among patients who were KRAS-tested, the proportions of those tested at the time of diagnosis increased greatly over the four years testing was widely available (27.4% in 2008 to 78.4% in 2011) (Figure 1B).
Figure 1.
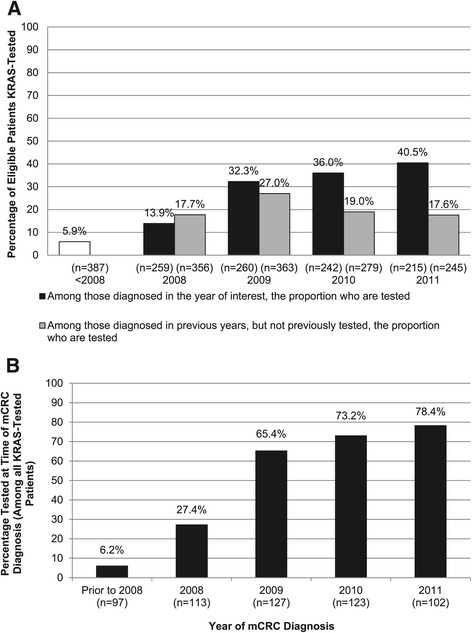
KRAS testing by calendar year. Proportion of newly diagnosed patients with mCRC tested for KRAS genotype and proportion of patients diagnosed in a prior year but not previously tested for KRAS genotype by calendar year (A), and among KRAS-tested patients, proportion tested at time of mCRC diagnosis by calendar year (B). Abbreviation: mCRC = metastatic colorectal cancer.
Baseline characteristics believed to be associated with ever being KRAS-tested, and with being KRAS-tested at diagnosis (among those tested), were modeled in a multivariable model (Figure 2A and B, respectively). Among eligible patients, those more likely to have been KRAS-tested (Figure 2A) included those with lung metastases (OR: 1.58, 95% CI: 1.11 to 2.26; ref: those with no lung metastases), those with poor performance status (OR: 1.45, 95% CI: 1.04 to 2.03; ref: those with good performance status), those with more comorbid conditions (OR: 1.10, 95% CI: 1.06 to 1.14), those diagnosed during the first three quarters of 2009 (OR: 1.46, 95% CI: 1.04 to 2.03; ref: those diagnosed in 2008 or earlier), and those diagnosed in the fourth quarter of 2009 or later (OR: 1.65, 95% CI: 1.12 to 2.43; ref: those diagnosed prior to the fourth quarter of 2009). Those less likely to have been tested included self-pay patients (OR: 0.35, 95% CI: 0.17 to 0.72; ref: those with Medicaid), and those with peritoneal metastases (OR: 0.37, 95% CI: 0.17 to 0.78; ref: those without peritoneal metastases), ovarian metastases (OR: 0.32, 95% CI: 0.11 to 0.98; ref: those without ovarian metastases), or other metastases (OR: 0.47, 95% CI: 0.25 to 0.87; ref: those without other metastases).
Figure 2.
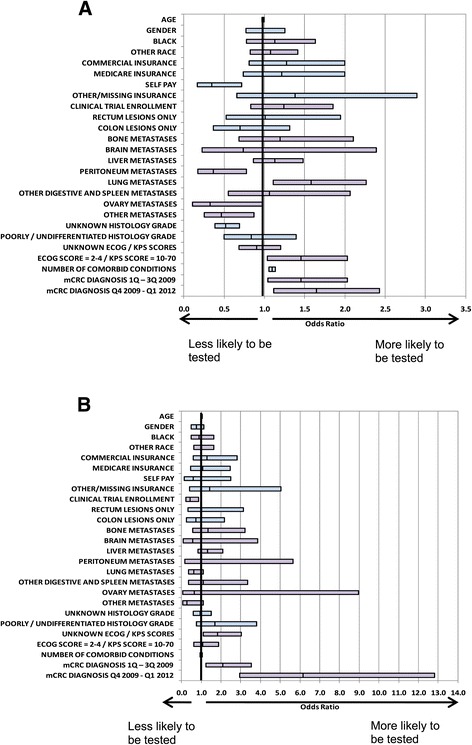
Baseline characteristics associated with KRAS testing. From multivariate logistic regression modeling among all eligible patients (A) and among KRAS-tested patients, those tested at time of mCRC diagnosis (B). Reference groups: gender-male, race-Caucasian, insurance-Medicaid, location of lesions-both, histology grade-high/moderately differentiated, ECOG/KPS score-(0-1/80-100), mCRC diagnosis-2008 or earlier. Abbreviations: ECOG = Eastern Cooperative Oncology Group; KPS = Karnofsky performance score; mCRC = metastatic colorectal cancer; Q = quarter.
Among patients who were KRAS-tested, the only factor significantly associated with being tested at diagnosis (Figure 2B) was the calendar time when testing was conducted. Testing at diagnosis was 2.1 times more likely to occur in the first three quarters of 2009 (OR: 2.09, 95% CI: 1.24 to 3.52; ref: those diagnosed in 2008 or earlier) and 6.2 times more likely to occur from the fourth quarter of 2009 onward (OR: 6.15, 95% CI: 2.95 to 12.81; ref: those diagnosed prior to the fourth quarter of 2009) compared to the period before 2009.
Discussion
Using text-based documents (e.g., physician progress notes) electronically stored within the ACORN Data Warehouse, a text-mining algorithm and a corresponding statistical model was developed to accurately identify patients with mCRC who were and were not tested for KRAS genotype. This study is the first to investigate the uptake of KRAS testing using EMR data from community-based oncology practices across the US representing all insurance types and multiple health plan types. A standard field to capture whether a patient was KRAS-tested and the results of testing is not available in EMR data. In the present study, this was addressed by text mining physician notes and subsequent chart review of those patients identified as KRAS-tested. As advances in biomarker identification and testing in cancers of all types continue, oncology-based EMR systems will need to accommodate the capture of this information.
Corresponding to changes in treatment guidelines and US product labels for anti-EGFR agents, KRAS testing increased annually between 2008 and 2009 and remained constant through 2011. Nevertheless, despite the increasing frequency of testing appropriate patients for KRAS genotype, slightly more than half the patients (52.5%) show no evidence in their medical records of having been KRAS-tested. These patients may include those whom physicians consider inappropriate candidates for anti-EGFR therapies, those diagnosed prior to the adoption of KRAS testing guidelines, and those with tumors progressing too quickly for KRAS genotype-directed treatment decisions. Given that data supporting first-line use of an EGFR inhibitor (cetuximab) over first-line bevacizumab [21] have emerged more recently, most physicians were likely using EGFR inhibitors between 2008 and 2011 in later lines of therapy, and thus, may have waited to test for KRAS status (rather than testing at the time of diagnosis). As such, it is possible that patients diagnosed between 2008 and 2011 had not progressed to the point of considering EGFR inhibitor treatment (and accordingly KRAS-tested) during the follow-up period included in the dataset. If this is true, then our findings may support a practice of KRAS testing at the time of potential EGFR inhibitor use rather than physicians’ lack of knowledge about or uptake of KRAS testing in their practice. Indeed, only a fraction (approximately 50%) of patients with mCRC who receive first-line therapy go on to receive second-line therapy and beyond [22,23]. Additionally, the study period for this analysis (2008–2011) occurred before the availability of an FDA-approved KRAS diagnostic test in 2012; the availability of such tests may affect the proportion of patients who are KRAS-tested in the future.
This analysis suggests that certain patient characteristics may influence whether or not a patient is KRAS-tested, but that once a clinician decides to test for KRAS, patient characteristics play less of a role in influencing the timing of the decision. In this study population, a substantial proportion of patients were aged 75 years and older; as age increases, patients may be less likely to ever have been KRAS-tested. In the model, when the age variable was changed from a quasi-continuous variable to a discrete binary variable (i.e., patients aged 75 and older), the model results were not dramatically different (data not shown).
Although the proportion of tested patients identified as KRAS-WT (48%) was slightly lower than previously reported (57%) [18], the 10% of patients with KRAS-status unknown (i.e., no evidence of testing) is consistent with what is seen in clinical practice. Unknown status is more likely the result of the pathologist having too little sample to conduct KRAS testing rather than the result being unavailable in the chart.
Access to reimbursement (for the costs of both KRAS testing and of anti-EGFR therapies) may be a factor affecting KRAS testing for appropriate patients, particularly prior to the adoption of the updated treatment guidelines. Additionally, it appears that healthier patients are less likely to be tested than less healthy patients (i.e., those with poor performance status or more comorbidities). One consideration is that patients with poorer performance status and more comorbidities may be better candidates for single-agent EGFR inhibitor therapy than other combination regimens, necessitating KRAS testing to determine course of treatment.
Among KRAS-tested patients, testing at the time of mCRC diagnosis increased annually from 27.4% in 2008 to 78.4% in 2011, corresponding to current recommendations that patients be KRAS-tested at diagnosis of metastatic disease to aid in informing their overall treatment plan. It appears that the community-based oncologists serving this patient population were not only aware of the changes in clinical guidelines (and corresponding changes to EGFR inhibitor product labels), but quickly began implementing KRAS testing as part of the workup for most newly diagnosed patients with mCRC.
A limitation of this study is the fact that the contributing oncology practices used one of three EMR systems that feed the Acorn Data Warehouse; this may add variability to which data elements are captured in addition as to how they are captured. An additional data limitation is missing data on care provided outside of the ACORN-contributing practices. The results presented may not be generalizable to community-based oncology practices at large.
Conclusions
In conclusion, it appears that although there has been an increase in awareness of the need for KRAS testing among patients with mCRC (i.e., an increase in the proportion of eligible patients tested; and among those tested, an increase in the proportion of those tested at first diagnosis of metastatic disease), the overall proportion of patients KRAS-tested (approximately 50%) could be increased further to improve therapy planning for patients and ultimately improve patient outcomes. Testing rates have likely improved since the period examined within this study (2008–2011) due to further label changes and availability of an FDA-approved test, thus increasing awareness and improving access to testing. Continued education and awareness to promote the importance of KRAS testing is warranted, especially as new biomarkers emerge in the treatment of mCRC. As the continuum of care for mCRC evolves and the optimal sequence of available agents is better characterized, maximizing knowledge about predictive and prognostic biomarkers at the time of diagnosis will help clinicians develop short- and long-term individualized therapeutic strategies for their patients.
Acknowledgments
The authors wish to acknowledge the patients and healthcare providers who contributed information to the ACORN Data Warehouse; Mark S. Walker and Paul J.E. Miller of ACORN for the design and conduct of physician note text mining and interpretation of overall study results; and Jeffrey Walter and Teri Tucker of inVentiv Health Clinical, for assistance writing and editing this manuscript, respectively.
Abbreviations
- ASCO
American Society for Clinical Oncology
- CRC
Colorectal cancer
- ECOG
Eastern Cooperative Oncology Group
- EGFR
Epidermal growth factor receptor
- EMR
Electronic medical record
- FDA
Food and Drug Administration
- KPS
Karnofsky performance score
- mCRC
Metastatic colorectal cancer
- NCCN
National Comprehensive Cancer Network
- OR
Odds ratio
- WT
Wild type
Footnotes
Competing interests
This study was supported by Eli Lilly and Company. Authors Cuyun Carter, Nicol, and Li are employees and stockholders of Eli Lilly and Company, Indianapolis, IN. Authors Landsman-Blumberg, Johnson, and Juneau are employees of Truven Health Analytics, and are consultants on behalf of Eli Lilly and Company. Author Shankaran has no conflicts of interest to declare.
Authors’ contributions
Authors GCC, PLB, BHJ, and SJN designed the study and collected data. Authors LL and SJN performed statistical analyses. All authors contributed to interpretation of the results, drafting and revision of the manuscript, and approval of its final content.
Contributor Information
Gebra Cuyun Carter, Email: cuyun_carter_gebra@lilly.com.
Pamela B Landsman-Blumberg, Email: pamela.blumberg@xcenda.com.
Barbara H Johnson, Email: barbara.h.johnson@truvenhealth.com.
Paul Juneau, Email: paul.juneau@truvenhealth.com.
Steven J Nicol, Email: nicol_steven@lilly.com.
Li Li, Email: li_li_x1@lilly.com.
Veena Shankaran, Email: vshank@U.WASHINGTON.EDU.
References
- 1.American Cancer Society: Cancer Facts & Figures 2013 [http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Monzon FA, Ogino S, Hammond ME, Halling KC, Bloom KJ, Nikiforova MN. The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Arch Pathol Lab Med. 2009;133:1600–6. doi: 10.5858/133.10.1600. [DOI] [PubMed] [Google Scholar]
- 4.Benson AB III: Epidemiology, disease progression, and economic burden of colorectal cancer. J Manag Care Pharm 2007,13 (6 Suppl C):S5–S18. [DOI] [PMC free article] [PubMed]
- 5.Avastin [prescribing information]. South San Francisco, CA: Genentech, Inc; 2013.
- 6.Erbitux [prescribing information]. Branchburg, NJ: ImClone LLC a wholly-owned subsidiary of Eli Lilly and Company, and Bristol-Myers Squibb Company; 2013.
- 7.Vectibix [prescribing information]. Thousand Oaks, CA: Amgen Inc; 2014.
- 8.Soulières D, Greer W, Magliocco AM, Huntsman D, Young S, Tsao MS, et al. KRAS mutation testing in the treatment of metastatic colorectal cancer with anti-EGFR therapies. Curr Oncol. 2010;17(Suppl 1):S31–40. doi: 10.3747/co.v17is1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lièvre A, Artru P, Guiu M, Laurent-Puig P, Merlin JL, Sabourin JC, et al. The KRAS mutation detection within the initial management of patients with metastatic colorectal cancer: a status report in France in 2011. Eur J Cancer. 2013;49:2126–33. doi: 10.1016/j.ejca.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Tonini G, Imperatori M, Vincenzi B, Frezza AM, Santini D. Rechallenge therapy and treatment holiday: different strategies in management of metastatic colorectal cancer. J Exp Clin Cancer Res. 2013;32:92. doi: 10.1186/1756-9966-32-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu JM, Liu XJ, Ge FJ, Lin L, Wang Y, Sharma MR, et al. KRAS mutations in tumor tissue and plasma by different assays predict survival of patients with metastatic colorectal cancer. J Exp Clin Cancer Res. 2014;33:104. doi: 10.1186/s13046-014-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network . Clinical practice guidelines in oncology (NCCN Guidelines), colon cancer. Fort Washington: NCCN; 2008. [Google Scholar]
- 13.Landsman-Blumberg PB, Carter GG, Johnson BH, Sedgley R, Nicol SJ, Li L, et al. Metastatic colorectal cancer treatment patterns according to kirsten rat sarcoma viral oncogene homolog genotype in U.S. Community-based oncology practices. Clin Colorectal Cancer. 2014;13:178–84. doi: 10.1016/j.clcc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network . Clinical practice guidelines in oncology (NCCN Guidelines), colon cancer. Fort Washington: NCCN; 2012. [Google Scholar]
- 15.Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. Word J Gastroenterol. 2012;18:5171–80. doi: 10.3748/wjg.v18.i37.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American society of clinical oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–6. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 17.Abrams TA, Meyer G, Schrag D, Meyerhardt JA, Moloney J, Fuchs CS: Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst 2014, 106:djt371. [DOI] [PubMed]
- 18.Ciardiello F, Tejpar S, Normanno N, Mercadante D, Teague T, Wohlschlegel B, et al. Uptake of KRAS mutation testing in patients with metastatic colorectal cancer in Europe, Latin America and Asia. Target Oncol. 2011;6:133–45. doi: 10.1007/s11523-011-0181-x. [DOI] [PubMed] [Google Scholar]
- 19.Webster J, Kauffman TL, Feigelson HS, Pawloski PA, Onitilo AA, Potosky AL, et al. KRAS testing and epidermal growth factor receptor inhibitor treatment for colorectal cancer in community settings. Cancer Epidemiol Biomarkers Prev. 2013;22:91–101. doi: 10.1158/1055-9965.EPI-12-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller PJ, Walker MS, Landsman-Blumberg P, Cuyun Carter G: Using text mining of electronic medical records to identify KRAS testing status in mCRC patients [abstract]. Value Health 2013, 16:A21.
- 21.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmueller J, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Mueller S, Schaefer B, Modest DP, Jung A, Stintzing S: Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS wild-type metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3) [abstract]. J Clin Oncol 2013, 31(18 suppl):Abstract LBA3506.
- 22.Hess GP, Wang PF, Quach D, Barber B, Zhao Z. Systemic therapy for metastatic colorectal cancer: patterns of chemotherapy and biologic therapy use in US medical oncology practice. J Oncol Pract. 2010;6:301–7. doi: 10.1200/JOP.2010.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim GP, Sargent DJ, Mahoney MR, Rowland KM, Jr, Philip PA, Mitchell E, et al. Phase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil, and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841. J Clin Oncol. 2009;27:2848–54. doi: 10.1200/JCO.2008.20.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]


