Abstract
Creatine kinase brain (CKB) is one of three cytosolic isoforms of creatine kinase that is predominantly expressed in the brain. The enzyme is overexpressed in a wide variety of cancers, with the exception of colon cancer, where it is downregulated. The significance of this downregulation remains poorly understood. Here, we demonstrate that overexpression of CKB-C283S, a dominant-negative construct that lacks the kinase function but retains its ability to dimerize, causes remarkable changes in cell shape, adhesion, and invasion. Furthermore, it results in increased expression of stromal cell markers such as PAGE4 and SNAIL, suggesting an epithelial-to-mesenchymal transition (EMT) in these cells. In cells transfected with a CKB-expressing construct, CKB localizes not only to the cytosol but also to the nucleus, indicating a structural or kinase role unrelated to ATP storage. Furthermore, overexpression of CFP-tagged wild-type (WT) CKB in Caco-2 colon cancer cells dramatically increased the number of cells in G2/M but had little effect on cell proliferation. Taken together, these data demonstrate that the downregulation of CKB may play an important role in colon cancer progression by promoting.
Keywords: CREATINE KINASE BRAIN, COLON CANCER, EPITHELIAL-TO-MESENCHYMAL TRANSITION, PAGE4
Creatine kinases (CKs), a family of enzymes with a highly conserved protein sequence, are involved in energy homeostasis: They reversibly catalyze the transfer of phosphate between ATP and creatine phosphate. Three cytoplasmic (non-mitochondrial) isoenzymes of CK are readily identified in human tissue. These isoenzymes are dimeric molecules with two dissociable subunits designated as M- (muscle) or B- (brain) type that can reassociate to form the electrophoretically distinct MM, BB, or MB isotypes (Bessman and Carpenter, 1985).
Previous work from our laboratory demonstrated that in nuclear matrix protein preparations from normal and colon cancer specimens, creatine kinase brain (CKB) was overexpressed in a majority of colon tumors [Balasubramani et al., 2006]. Surprisingly, upon subcellular fractionation, the overall levels of CKB were decreased rather than increased in colon tumors, suggesting that the high CKB levels observed in nuclear matrix preparations may be due to the enhanced localization of CKB to the nuclear matrix. However, the significance of the nuclear localization and overall downregulation of CKB in colon cancer remains poorly understood.
To gain additional insight into the role of CKB in tumorigenesis, we have determined the effects of overexpression of both wild-type (WT) and dominant-negative CKB in the colon cancer cell line Caco-2 [Fogh, 1975]. We demonstrate that overexpressing dominant-negative CKB, which is similar to downregulating CKB, appears to promote epithelial-to-mesenchymal transition (EMT) in colon cancer.
MATERIALS AND METHODS
PLASMIDS
The YFP empty vector and the CFP-CKB-WT and CFP-CKB-C283S vectors were described previously [Kuiper et al., 2009]. These constructs were stabily expressed in Caco-2/HCT116/Hela cells (ATCC) by transfection with Fugene HD (Roche) and selection for 1 month with Zeocin (100 µg/ml).
CELL CULTURE CONDITIONS
Cells were routinely grown in high-glucose DMEM, 1% antibiotic/antimyotic (Gibco 15240), and 10% FBS (PAA Laboratories) at 5% CO2. In some experiments, low-glucose or glucose-free DMEM was used; however, the antibiotic/antimyotic (Gibco 15240) and FBS (PAA Laboratories) were always added, even if not explicitly stated in the text.
NUCLEAR/CYTOPLASMIC PROTEIN ISOLATION
Cells (1.6 million) were plated on 10-cm dishes and isolated 24 h later by resuspending in Ablosepuffer (40-mM Hepes, pH 7.4, 10-mM EDTA, 150-mM NaCl). Cells were pelleted by centrifugation at 400 × g and the Nuclear Extract Kit (cat. 40010) from Active Motif was used essentially with the protocol devised by the manufacturer. Briefly, the cells were resuspended in hypotonic buffer and incubated for 15 min. on ice. Next, detergent was added with vortexing for 10 s. The suspension was centrifuged at 14,000 × g and the supernatant (cytosolic fraction) was stored at —80°C until further use. The pellet was resuspended in complete lysis buffer and incubated on ice for 30 min. The suspension was then centrifuged for 10 min. at 14,000 × g and the supernatant (nuclear fraction) was stored at —80°C until further use.
QPCR
Caco-2 cells (1.6 million) were plated in high-glucose DMEM, which was replaced the next morning with DMEM containg 10% fetal bovine serum with or without glucose. Cells were incubated for 48 h, then harvested using Ablosepuffer. The RNA was isolated using a Qiagen RNEASY mini kit, and cDNA was synthesized using a Bio-Rad iScript cDNA synthesis kit, according to the manufacturer’s instructions. For heat experiments, cells were incubated for 4 h at 43°C and allowed to recover for 24 h at 37°C before q-PCR. Q-PCR was performed using a Bio-Rad iQ SYBR Green Supermix kit on a Bio-Rad iCycler iQ real-time PCR detection system. PCR primers were as follows: TBP Forward: GAATATAATCCCAAGCGGTTTG, TBP Reverse: ACTTCACATCACAGCTCCCC, CKB-Forward: GGCAA-CATGAAGGAGGTGTT, CKB Reverse: ATGGGCAGGTGAGGATG-TAG, MMP7 Forward: AGATCCCCCTGCATTTCAGG, MMP7 Reverse: TCGAAGTGAGCATCTCCTCC, C-myc Forward: TGAA-AGGCTCTCCTTGCAGC, C-myc Reverse: GCTGGTAGAAGTTC-TCCTCC, PAGE4 Forward: CGTAAAGTAGAAGGTGATTG, PAGE4 Reverse: ATGCTTAGGATTAGGTGGAG, SNAIL Forward: GCGAG-CTGCAGGACTCTAAT, SNAIL Reverse: CCRCTGTCCTCATCTGACA.
FLUORESENCE MICROSCOPY
WT-CKB- and C283S–CKB-transfected CaCo-2 cells (1.6 million) were seeded on 10-cm dishes and then visualized at 40× with a Nikon Eclipse TE2000E using the GFP-BP Filter (Ex 460–500, DM 5005, DA: 510–560) and analyzed with NIS-Elements AR 3.00 Software.
PHASE CONTRAST MICROSCOPY
Caco-2 cells (1.6 million) were plated in high-glucose DMEM, which was replaced the next morning with DMEM with or without glucose. Cells were incubated for 72 h and then photographed at 10× with a Nikon QuickPix at high resolution.
CELL SIZE QUANITATION
Caco-2 cells were plated in high glucose DMEM and photographed at 40× as described above. Cell length and width were measured in arbitrary units using IMAGE-PRO PLUS Version 6.0 (Media Cybernetics, Silver Spring, MD 20910).
CELL VIABILITY AND CELL COUNT
Caco-2 cells (1.6 million) were plated in high-glucose DMEM, which was replaced the next morning with DMEM containing 0, 1, or 4.5 g/L glucose, 1% antibiotic/antimycotic (Gibco 15240), and 10% FBS (PAA Laboratories). The cells were incubated for 48 h, harvested using Ablosepuffer, and stained with Trypan Blue Stain 0.4% (Gibco). A Cellometer Auto T4 was used to determine the cell number, viability, and diameter.
For heat experiments, 200,000 cells were plated in six-well plates, and high-glucose DMEM was used throughout the experiments. The cells were subjected to heat (43°C) for 0, 1, 2, or 4 h and then returned to the 37°C incubator for 48 h.
CELL CYCLE PROFILE BY FLUORESCENCE-ACTIVATED CELL SORTING (FACS)
Cells (400,000) were plated in each well of a six-well plate [Mooney et al., 2010]. The next day, medium was removed, and glucose-free, low-glucose, or high-glucose DMEM containing 1% antibiotic/antimyotic and 10% FBS was added. To prepare cells for propidium iodide (PI) flow cytometry analysis, the cells were harvested at 24 or 48 h using trypsin/EDTA (Gibco) and centrifuged at 1,000 × g for 5 min. They were then washed in 4 ml of ice-cold phosphate buffered saline (PBS), resuspended in 1 ml of PBS, and fixed in 9 ml of ice-cold 70% ethanol for 10 min. Cells were centrifuged again at 1,000 × g, resuspended in 1 ml of PI/RNase solution (40 µg/ml PI and 50 µg/ml RNase in PBS), and incubated in PI/RNase solution at 4°C overnight before analysis. FACS analysis was performed in PI/RNase solution on a Becton Dickinson FACScan [Acu et al., 2010].
BOYDEN CHAMBER MIGRATION ASSAY
DMEM containing 10% FBS was added to the bottom chambers of collagen I-coated Boyden chambers, while empty vector-, WT-CKB-, or C283S–CKB-transfected Caco-2 cells (50,000/chamber) in serum-free DMEM were plated in the upper chambers. Cells were incubated at 37°C for 48 h. Cells in the upper chambers were then removed, and the cells that invaded were fixed and stained with 0.5% crystal violet/25% methanol for 15 min. The chamber inserts were washed with water to remove all excess crystal violet. Random representative photographs were then taken from the middle of each chamber.
SOFT AGAR ASSAY
Six-well plates were coated with 2 ml of 0.6% agar/10%FBS/DMEM which was allowed to solidify for 1 h at room temperature. Cells were diluted in another 2 ml of 0.3% or 0.6% agar/10%FBS/DMEM and plated (3,000/well) onto six-well plates containing solidified agar. An additional 2 ml of 10% FBS/DMEM was added to each well and changed twice a week. Random representative pictures were taken after 2 weeks.
CLONOGENIC ASSAY
Cells were plated in standard high-glucose medium in 10-cm dishes, 1,000 cells for untreated controls and 10,000 cells for treatment groups. The next day, the cells were incubated with or without doxorubicin (0.25 µg/ml) for 1 h. The medium was then replaced with standard high-glucose medium and left undisturbed for 3 weeks at which point photographs were taken.
CELL ADHESION ASSAY
Uncoated or poly-lysine coated 12-well plates were incubated with 10% FBS containing DMEM high glucose for 1 h at 43°C as a blocking step. Media was then aspirated and 80,000 Caco-2 cells were plated in DMEM high glucose without FBS. Cells were incubated at 37°C for 30 min. then washed three times with PBS, trypsinized, and counted with a cellometer.
IMMUNOBLOTTING
Using standard procedures [Mooney et al., 2010], SDS-PAGE was performed, and the proteins were transferred in a semi-dry fashion from gels to nitrocellulose membranes. Antibodies were diluted in 5% milk/0.05% Tween-20 and incubated with the membranes overnight at 4°C. Then immunoblotting was performed using standard procedures and the following antibodies: rabbit polyclonal α-GFP (sc-8334), mouse monoclonal α-Tubulin (CP06), rabbit polyclonal Lamin A/C (sc-20681), rabbit monoclonal phospho-AKT ser 473 (Cell Signaling 4060), rabbit monoclonal phospho-S6 ribosomal protein ser235/236 (Cell Signaling 4858) and rabbit polyclonal α-Actin (sigma A2066).
RESULTS
CKB IS DOWNREGULATED IN COLON CANCER
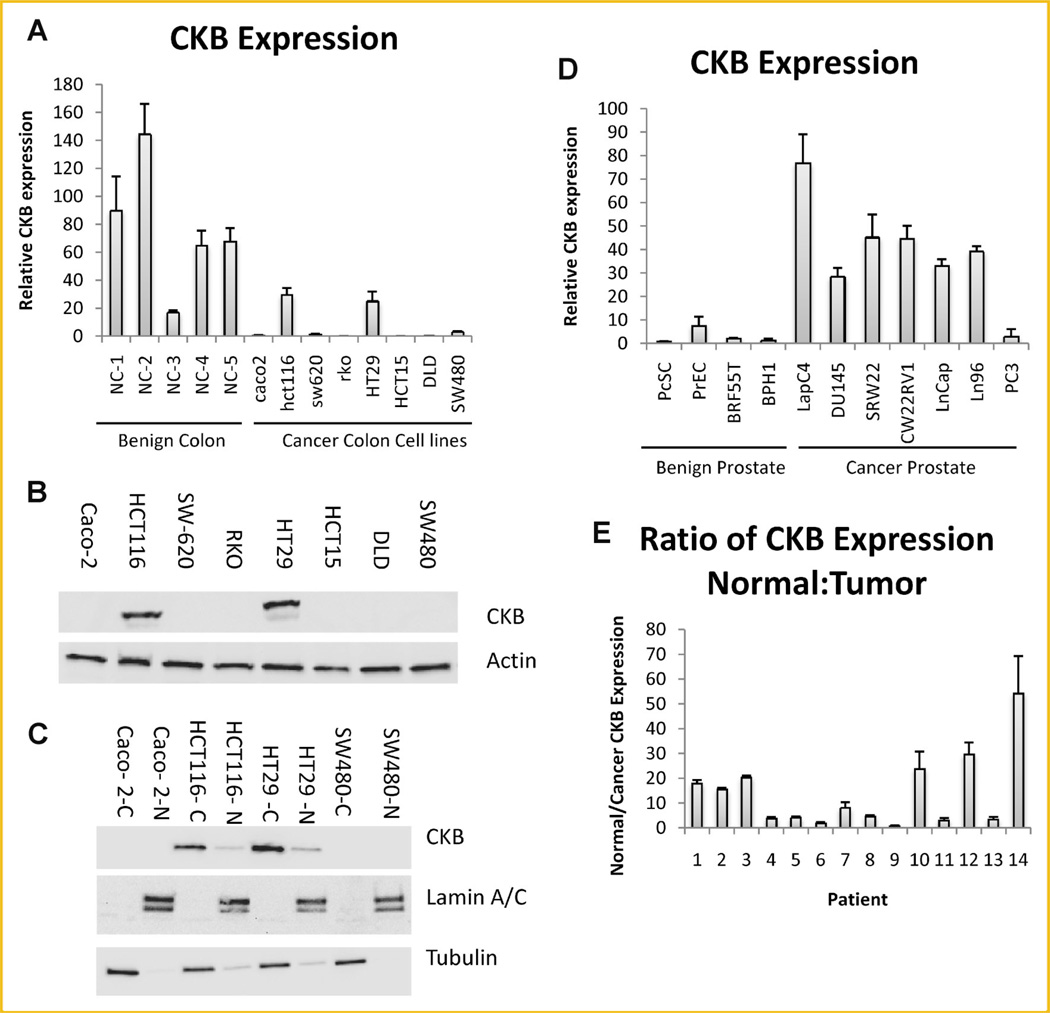
In order to determine whether the decreased levels of the CKB protein previously observed in colon cancer were a result of a decrease in mRNA abundance, a qPCR analysis was performed using benign colon tissue and colon cancer cell lines. As expected, the levels of CKB mRNA were significantly lower in most colon cancer cell lines when compared to benign colon tissue (Fig. 1A). Immunoblotting analysis demonstrated that the protein levels correlated with the mRNA levels in colon cancer cell lines and this protein localized primarily but not exclusively to the cytosol (Fig. 1B, Supplementary Fig. 1A,B). In contrast, CKB mRNA was significantly upregulated in prostate cancer but was barely detectable in the benign prostate cell lines (Fig. 1D). To further substantiate these observations, matching cancer and normal colon mucosa were analyzed. Indeed, data from 13 out of 14 individual patients showed that CKB mRNA was on average, 14-fold higher in normal colon tissue compared to the matching tumor tissue (Fig. 1E). Together with earlier observations from our laboratory [Balasubramani et al., 2006], these data indicate that CKB is downregulated both at the protein and mRNA level in colon cancer.
Fig. 1.
CKB is expressed in colon tissue as assayed by qPCR. A: Expression of CKB/TBP was assessed in benign colon (NC1–5) from five patients and compared to expression in colon cancer cell lines. B: Protein expression of CKB and actin were determined in colon cancer cell lines by immunoblotting. C: Cell lines were fractionated into cytoplasm ‘‘C’’ and nucleus ‘‘N.’’ Immunoblotting was carried out for the indicated proteins. D: Benign prostate and prostate cancer cell lines were assessed for CKB expression by qPCR. Normalization was to TBP. C: CKB/TBP expression for cancer and matching normal colon was determined by qPCR. The values for normal tissue were divided by the values for matching cancer tissue and reported.
OVEREXPRESSION OF DOMINANT-NEGATIVE BUT NOT WILD-TYPE CKB RESULTS IN A MESENCHYMAL-LIKE TRANSITION IN CACO2 CELLS
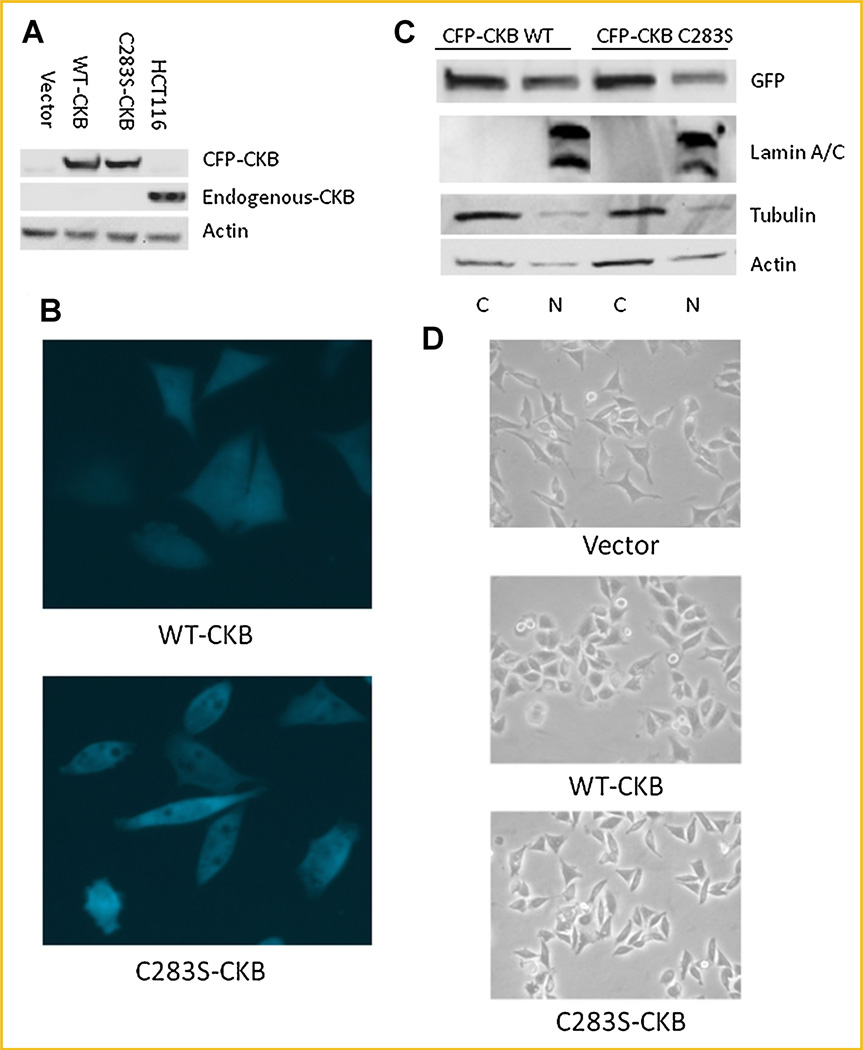
Having confirmed the downregulation of CKB in colon cancer, we next wanted to discern the effects of overexpressing CKB. Cyan fluorescence protein (CFP)-tagged CKB, or the kinase-deficient dominant-negative version, CFP-tagged CKB-C283S, was stably transfected into Caco-2 cells. Stable shRNA to CKB was not used in this cell line since levels of the endogenous protein were undetectable. As expected, both the WT and the dominant-negative CKB localized to both the nucleus and cytosol, as determined by fluorescence microscopy and immunoblotting of cell fractions (Fig. 2B,C, respectively). The CFP-tagged constructs localized similar to endogenous protein in stabily transfected HCT116 cells adding validity to our system (Supplementary Fig. 1B,C). Empty vector-transfected Caco-2 cells expressed undetectable amounts of endogenous CKB (lane 1); however, the WT-CKB- and CKB-C283S–transfected cells (lanes 2 and 3, respectively), produced CFP-CKB at levels similar to the endogenous levels in HCT116 (lane 4; Fig. 2A). Surprisingly, however, we observed a striking difference in cell shape between the WT-CKB- and the CKB-C283S–transfected Caco-2 cells: The WT-CKB-transfected cells became more spread out than the empty vector-transfected cells, while the dominant-negative-transfected cells remained spindly and narrow (Fig. 2B,D, quanitation Supplementary Fig. 3). In conclusion, CKB-C283S–transfected cells showed a more elongated mesenchymal-like appearance compared with WT-CKB transfected cells suggesting a potential EMT or an MET in the case of the WT-CKB transfected cells.
Fig. 2.
WT- and C283S-CKB have similar localization in stably transfected Caco-2 cells. A: Cells were lysed and immunoblotted for expression of CKB and actin, with HCT116 whole-cell lysate used as a positive CKB control. B: The relative distributions of CFP-CKB constructs were measured in whole cells by fluorescence microscopy (40×). C: Cells were fractionated into nuclear and cytoplasmic pools. Immunoblotting was performed with a CFP antibody to show the relative distribution of transfected protein. D: Light microscopy (20×) of the three cell lines was used to determine overall cell shape. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DOMINANT-NEGATIVE CKB CAUSES AN INCREASE IN EMT-RELATED MARKERS
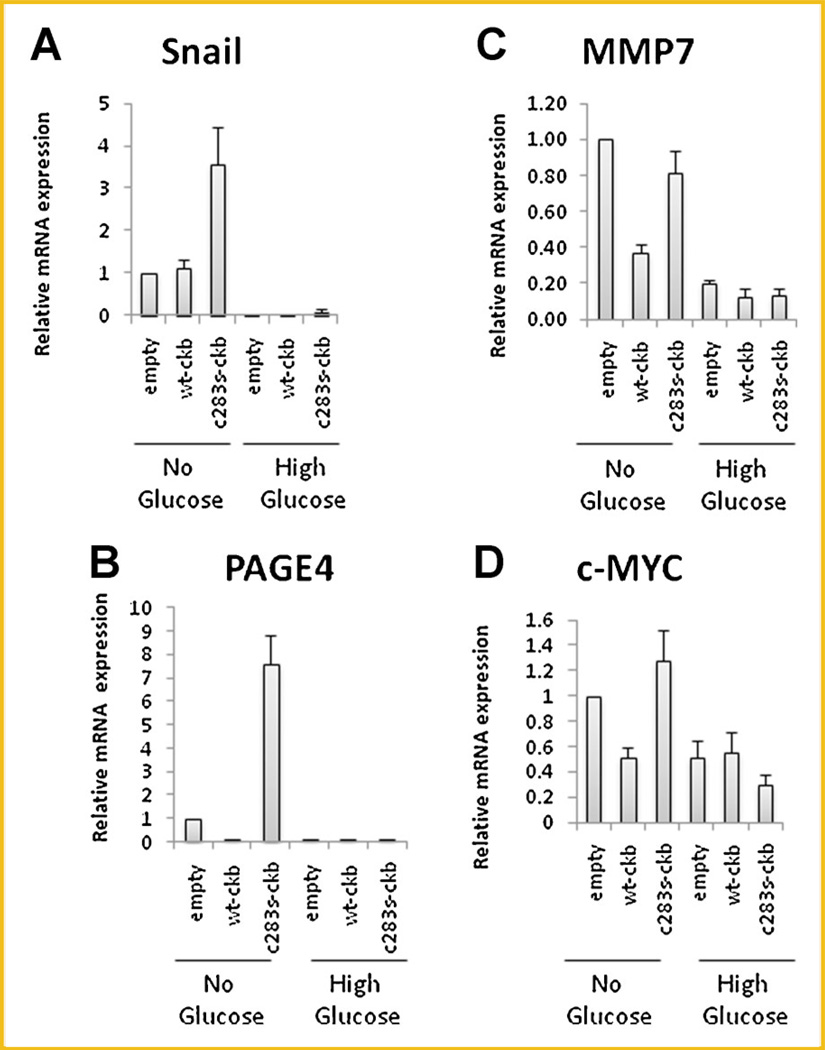
In order to confirm this hypothesis, we determined the expression of several EMT markers, including the mesenchymal markers SNAIL [Usami et al., 2008] and PAGE4 [Joyce et al., 2009], and the β-catenin-responsive genes [Kim et al., 2002], MMP7 [Hovanes et al., 2001], and c-Myc [He et al., 1998]. Since CKB is involved in energy transduction and improper vascularization causes metabolic stress within tumors, cells were grown in glucose-free medium to determine if this would make them more aggressive. We found that the mRNAs encoding SNAIL and PAGE4 were upregulated when glucose-free DMEM was used, especially in cells expressing the dominant-negative construct, which had mRNA levels that were 3.5- and 7.5-fold higher than those of empty vector-transfected cells for SNAIL and PAGE4, respectively (Fig. 3A,B, respectively). In the case of the β-catenin markers, the mRNAs encoding MMP7 and c-Myc were also upregulated when glucose-free DMEM was used; however, their expression level in the dominant-negative construct was similar to that in the empty vector-transfected cells (Fig. 3C,D for MMP7 and c-Myc, respectively). It could be argued that apoptotic pathways might cause the uprgulation of these markers however, in HCT116 cells, stabile expression of empty vector, WT-CKB, and C283S–CKB caused a similar pattern to Caco-2 in three mesenchymal markers and two β-catenin responsive genes in normal media (Supplementary Fig. 4A,B). Viability was not significantly different between the HCT116 cell lines which suggests viability differences are not an overriding factor in the gene expression (Supplementary Fig. 4C). Of further interest, when a different non-metabolic stress was used, namely heat, the results were somewhat similar to the results for glucose in that expression of the mesenchymal markers, but not the β-catenin responsive genes, was suppressed by WT-CKB (Suplementary Fig. 5). Thus, it appears that C283S–CKB can potentiate molecular changes associated with EMT while WT-CKB can repress this phenotype.
Fig. 3.
Expression of EMT markers in stably transfected Caco-2 cells. A–D: Caco-2 cells were plated in 100-mm dishes and incubated for 12 h. Medium was then replaced with 10% FBS/DMEM with or without glucose. After 48 h, mRNA was extracted and cDNA was synthesized. Various markers were assayed as indicated.
DOMINANT-NEGATIVE CKB RESULTS IN AN INCREASED AGGRESSIVENESS OF COLON CANCER CELLS
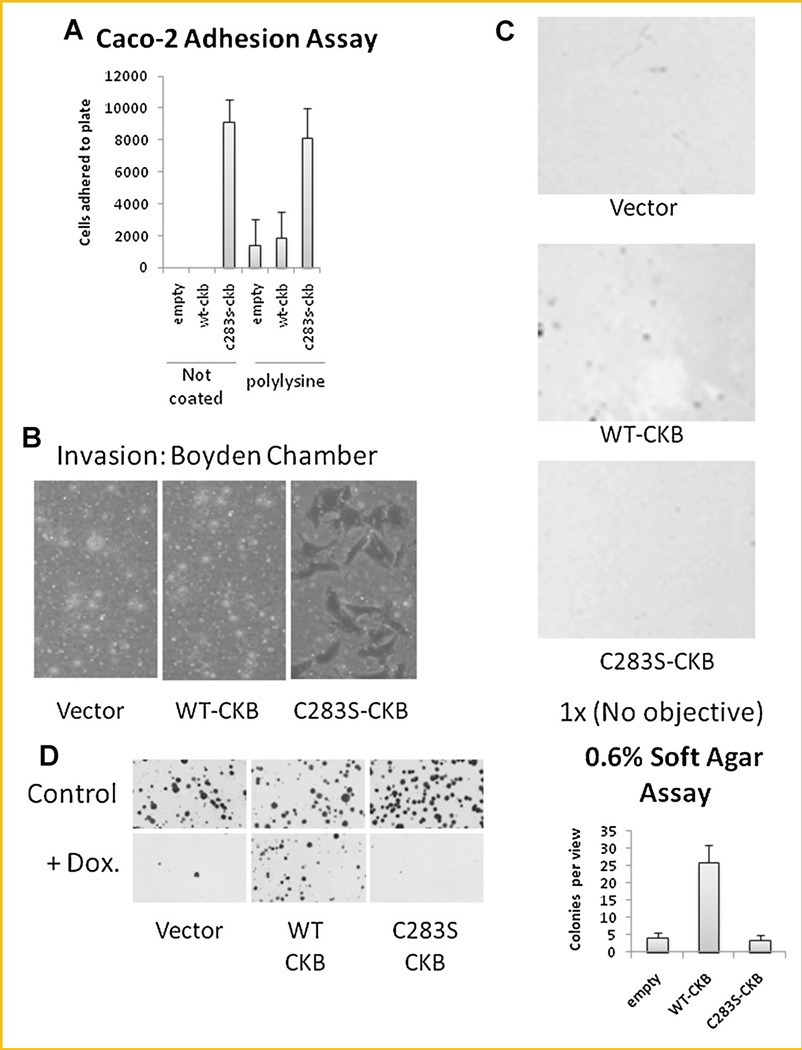
To further elucidate the ability of the dominant-negative CKB to induce an EMT-like phenotype, we evaluated additional parameters such as cell adhesion and invasion. Indeed, C283S–CKB-transfected cells demonstrated increased adhesion to plastic dishes, either uncoated or coated with poly-lysine (Fig. 4A), and increased invasion through collagen (Fig. 4B), when compared to WT-CKB-transfected cells. Thus downregulation of CKB may be necessary for cells to become invasive. Interestingly, while WT-CKB-transfected cells could grow and form colonies in the anchorage-independent growth assay (0.6% soft agar; Fig. 4C), C283S–CKB-transfected cells could not unless a lower concentration (0.3% soft agar) was used (data not shown) suggesting that WT-CKB-transfected cells may be more capable than C283S–CKB-transfected cells in forming solid tumors [Zhang et al., 2010] in vivo. Furthermore, a colony-forming assay performed after exposing cells to the DNA damaging chemotherapeutic drug, doxorubicin (0.25 ng/ml) for 1 h demonstrated that WT-CKB-transfected cells were able to form colonies more easily than empty vector- and C283S–CKB-transfected cells (Fig. 4D), highlighting the potential of WT-CKB-transfected cells to form colonies in vivo.
Fig. 4.
CKB affects the physiology of stably transfected Caco-2 cells. A: Cells were plated in 12-well plates and incubated at 37°C for 30 min. Cells were than trypsinized and counted. B: Cells were plated in collagen-coated Boyden chambers and incubated for 48 h. Filters were then stained with crystal violet and imaged by light microscopy (20×). C: Cells were suspended in 0.6% agar and incubated for 14 days at 37°C. Pictures were taken with a scanner (1 ×), and the average number of colonies per view is displayed in the bar graph. D: Caco-2 cells were plated on 10-cm plates and treated with 0.25 µg/ml doxurubicin for 1 h or left untreated. The cells were then incubated in normal medium for 2 weeks and photographed using a scanner (1 ×).
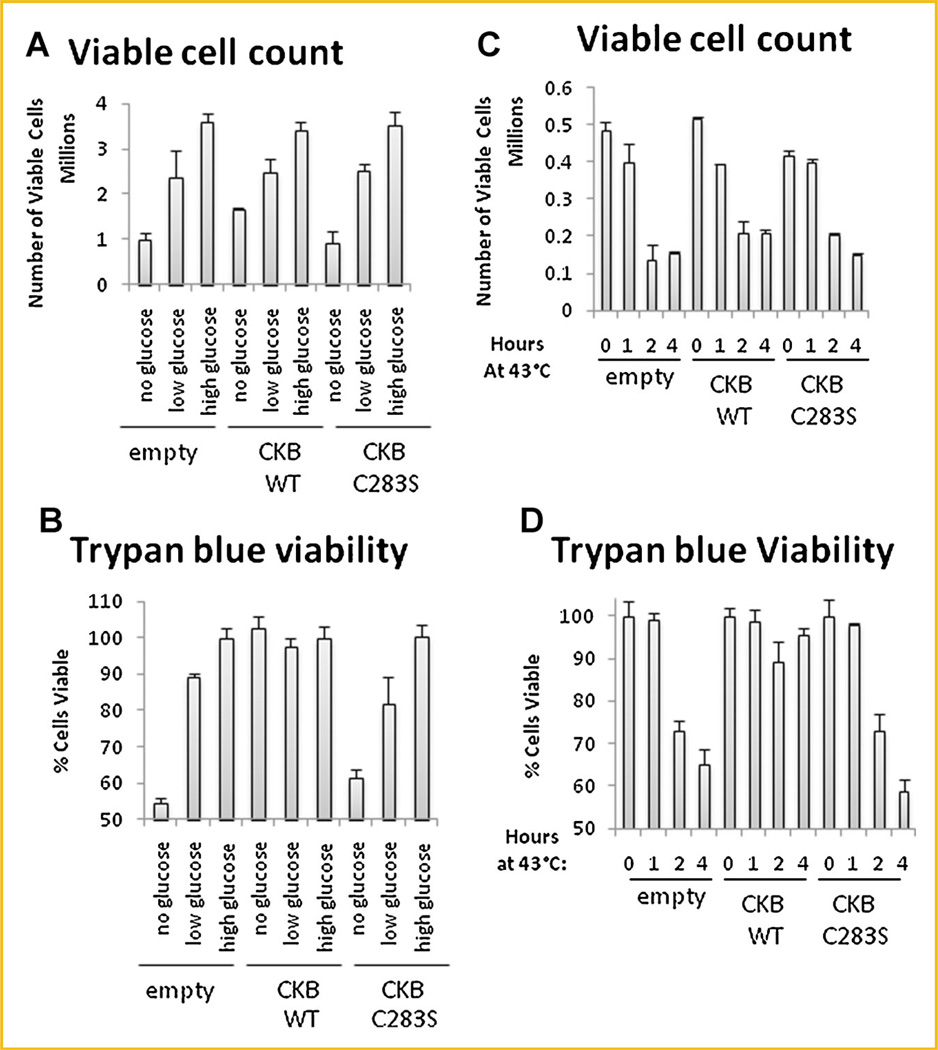
CKB ENABLES CELLS TO SURVIVE DURING METABOLIC STRESS OR HEAT
Based upon the role of CKB in energy transduction, especially during periods of high energy demand such as exercise [Wyss and Kaddurah-Daouk, 2000], we reasoned that CKB overexpression might enable cells to be more resistant to stress. To test this hypothesis, we incubated cells for 48 h in glucose-free, low-glucose, or high-glucose medium and determined the number of viable cells and percent cell viability. The number of viable cells was directly correlated to the glucose concentration (Fig. 5A). The cells were nearly 100% viable except in glucose-free medium, in which C283S–CKB- and empty vector-transfected cells were 50% viable. WT-CKB-transfected cells, on the other hand, were 100% viable even in glucose-free medium (Fig. 5B), despite a lack of proliferation under these conditions.
Fig. 5.
Cellular stress is modulated by CKB in Caco-2 cells. Stably transfected Caco-2 cells were plated in six-well plates. Medium was replaced with 10% FBS/DMEM containing the stated amounts of glucose, and the cells were incubated for 48 h at 37°C. Cells were then trypsinized and stained with trypan blue. Counting was done with a hemocytometer. The number of viable cells (A) and the percentage of viable cells (B) are shown. In (C) and (D), cells were incubated for 1, 2, or 4 h at 43°C and then moved to 37°C for 48 h. Cells were then trypsinized, stained, and counted as in (A) and (B).
In order to determine whether WT-CKB could also protect the cells against non-nutrient stress, cells were incubated at 43°C for 1, 2, or 4 h and then allowed to recover for 48 h. While CKB overexpression did not alter cell proliferation (Fig. 5C), it did prevent cells from dying (Fig. 5D). These results suggest that CKB confers resistance to various stresses.
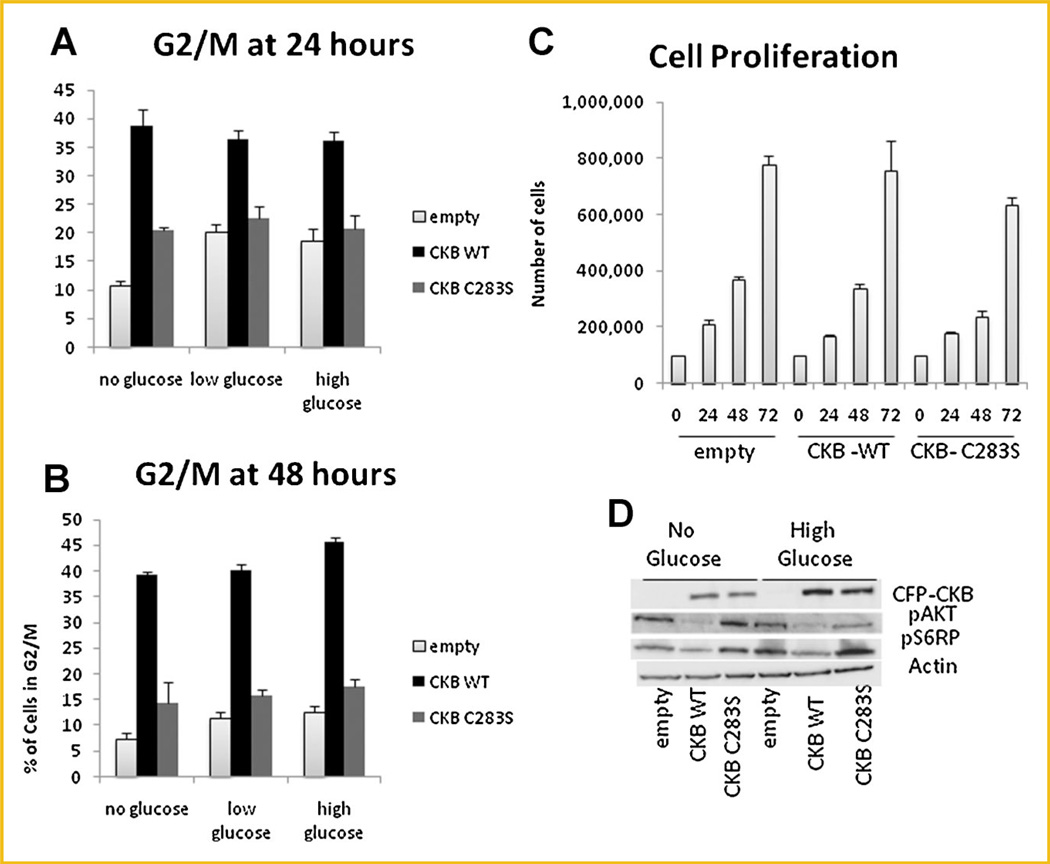
CKB OVEREXPRESSION CAUSES AN INCREASE IN THE G2/M PHASE IN CACO-2 CELLS
Next, to determine whether the protective effect seen was due to an aberrant cell cycle profile, propidium iodide staining of cells followed by FACS analysis was performed after incubation in various glucose concentrations. Interestingly, the WT-CKB-transfected cell population had significantly more cells arrested at the G2/Mphase, both at 24 (Fig. 6A) and 48 h (Fig. 6B) after the medium was replaced, however, this difference was surprisingly irrespective of glucose content. Furthermore, despite the accumulation of WT-CKB-transfected cells in G2/M, proliferation of these cells was not affected (Fig. 6C). Since CKB is involved in energy storage and energy transduction pathways are well known to be altered in cancer [Vander Heiden et al., 2010], we reasoned that the AKT pathway which is involved in both glycolysis and glucose uptake might be altered by CKB expression [Elstrom et al., 2004; Matsui et al., 2006]. An immunoblotting analysis of proteins activated in energy transduction pathways revealed that the AKT and mTOR pathways were downregulated in cells overexpressing WT-CKB (Fig. 6D).
Fig. 6.
CKB increases the percentage of cells in G2/M. Cells were plated in 100-mm dishes, and medium was replaced the next day with 10% FBS/DMEM containing various amounts of glucose. Fixed cells were stained with propidium iodide at 24 h (A) or 48 h (B), and the percentage of cells in G2/M is shown. C: Cells were incubated in high-glucose DMEM for 24, 48, or 72 h and counted. D: Cells were incubated in glucose-free or high-glucose 10% FBS/DMEM for 48 h and then lysed. Immunoblotting for various proteins was performed.
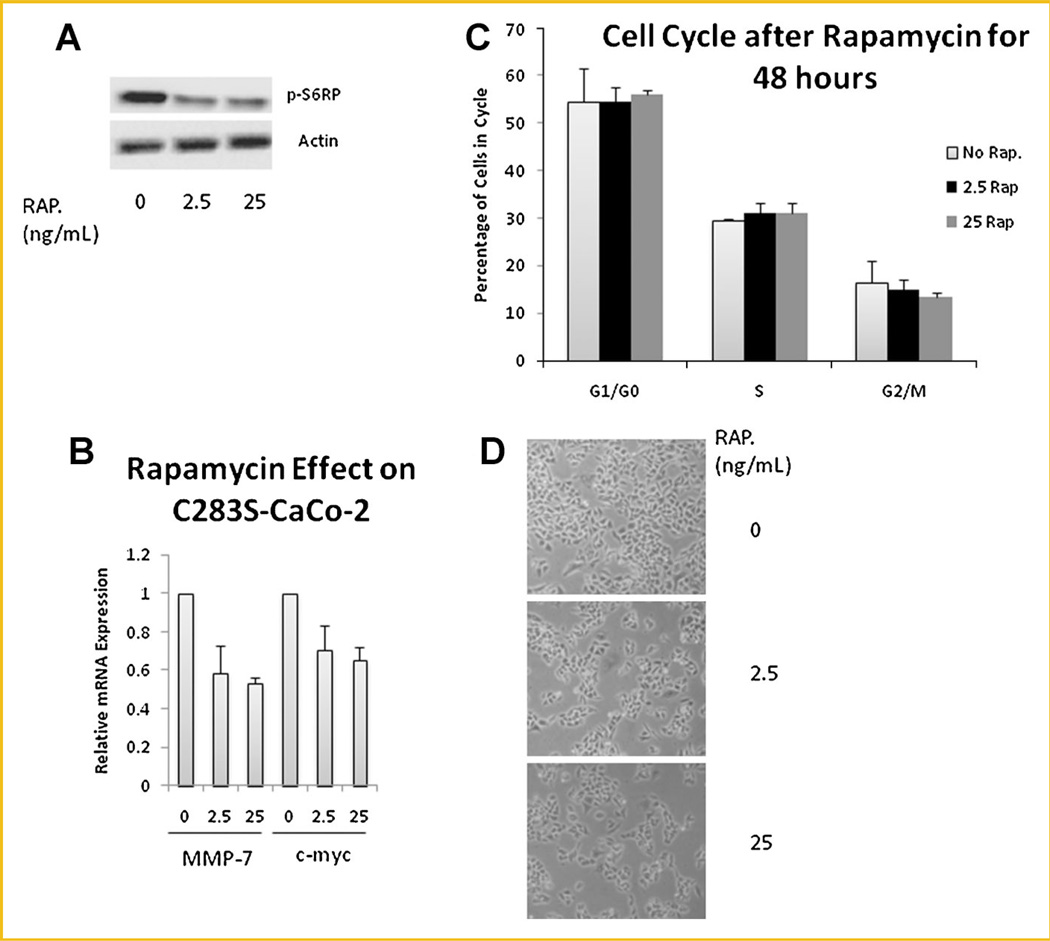
INHIBITING THE MTOR PATHWAY INHIBITS THE B-CATENIN PATHWAY
In order to determine the effects of inhibiting the mTOR pathway Caco-2 cells were treated with rapamycin (2.5 or 25 ng/ml) in glucose-free medium. Both concentrations were adequate to alter the pathway as demonstrated by decreased phosphorylation of the ribosomal protein S6 (Fig. 7A). The inhibition was enough to cause a 40% decrease in expression of MMP7 and c-Myc in C283S–transfected CaCo-2 cells (Fig. 7B). However, it could not by itself alter the number of cells in G2/M (Fig. 7C). Inhibition of the mTOR pathway, in fact, decreased the total number of cells (Fig. 7D), while overexpression of WT-CKB did not affect proliferation (Fig. 6C). These data indicate that increased mTOR activity in empty vector-transfected Caco-2 cells compared to WT-CKB-transfected cells may partially explain the expression of β-catenin responsive genes but does not explain the cell cycle changes caused by overexpression of CKB (Fig. 6A,B).
Fig. 7.
mTOR activation causes an increase in β-catenin responsive genes. C283S–CKB-transfected Caco-2 cells were grown in medium containing 0, 2.5, or 25 ng of the mTOR inhibitor rapamycin. After 48 h, (A) immunoblotting for the mTOR target p-S6RP was done to ascertain the effective dosage. B: qPCR was conducted on β-catenin target genes MMP-7 and c-myc, with TBP as a control. C: Identically treated cells were stained with propidium iodide in preparation for cell cycle analysis (D) and photographed by light microscopy at 20×.
DISCUSSION
Despite the use of CK activity as a marker for tissue injury, its functions within the cell, especially the nucleus, have not been fully elucidated. Our laboratory previously demonstrated that CKB is downregulated in colon cancer at the protein level in both the cytoplasm and nucleus but was significantly enriched in the nuclear matrix [Balasubramani et al., 2006]. This was a novel finding, since the nuclear matrix affects the structure and function of the nucleus, which is drastically altered in cancer cells [Getzenberg et al., 1991]. In fact, pathologists look to the nucleus for proper staging of cancer specimens [Coffey, 2002], which led us to hypothesize that CKB might be acting in the nucleus in a role not involving creatine phosphorylation. As far as we are aware, the expression of CKB in the nucleus has not been studied and its function remains unknown. To date, the only known substrates for CKB are creatine and ATP. However, there could be additional (protein) substrates that have yet to be identified. This may account for the changes in cell cycle seen in Caco-2 cells overexpressing CKB. Before transfection, Caco-2 cells have a very low level of CKB and ectopic CKB expression has a pronounced effect with respect to resistance to stressful conditions and cell cycle.
In most cancers studied, CKB is upregulated, with colon cancer being the exception. The reason for the high CKB levels in normal colon tissue is probably due to the high demand for ATP by colon ion channels coupled with a high proliferation rate. Cancer cells, too, often require large amounts of ATP for cell proliferation; therefore, the reason that colon cancer cells downregulate their CKB is unclear [Hsu and Sabatini, 2008]. The data here seem to indicate that reduced CKB levels in colon cancer may make cells more aggressive. On the other hand, it also renders them susceptible to heat shock and glucose deprivation. This observation has potential therapeutic implications since there is great hope for the use of heat and caloric restriction as adjuvants to cancer therapy. Caloric restriction is already well known as an effective way to extend lifespan and decrease cancer susceptibility in a wide variety of organisms, including primates [Fontana et al., 1991]. As for heat, the colon is an ideal environment for heat therapy since it is easily accessible for local heating. Regional heating of the colon as an adjuvant to chemotherapy has been shown to be effective with very little toxicity in numerous studies including Phase III clinical trials, where survival went from 1 to over 2 years. Heat is also being used in many other cancers and is the new standard of care in appendiceal cancer and peritoneal mesothelioma [Coffey et al., 2006; Sugarbaker, 2007; Cho et al., 2008].
With regard to ATP regeneration, work by Dr. Be Wieringa elegantly demonstrates that local ATP generation by CKB at the leading edge of cancer cells can facilitate motility [Kuiper et al., 2009]. This may explain why WT-CKB-transfected cells spread out more than the C283S–CKB-transfected cells, making them appear more epithelial (Fig. 2B,D). It might also be anticipated that this local ATP regeneration would make colon cells more motile; however, this was not observed in Caco-2 cells. In fact, it was the C283S–CKB-transfected cells that were able to invade collagen-coated Boyden chambers which is consistent with the notion that an EMT took place (Fig. 4B). Also it was the C283S–CKB-transfected cells that were able to adhere to plastic dishes faster (Fig. 4A). It is not clear from our data whether it is the ATP regeneration capacity of CKB that allowed cells to survive in chemotherapy, glucose deprived conditions and heat. However since DNA damage caused by chemotherapy and the denaturation of proteins caused by heat are non-metabolic stresses, we might conjecture that it is not only ATP storage that is the most important function of CKB. At the moment, CKB has only one known substrate: creatine. Further experimentation needs to be conducted to ascertain whether it phosphorylates other substates or has other binding partners.
In conclusion, while CKB expression may be advantageous in the formation of a solid tumor, it appears to be a hindrance to the metastatic potential of colon cancer cells.
ACKNOWLEDGMENTS
Colon tissue samples were provided by Dr. Bert Vogelstein of Johns Hopkins University. pLZRS-CK-B, pLZRS-CK-B(C283S), and pLZRS-EYFP were provided by Dr. Be Wieringa of the Radboud University Nijmegen Medical Centre. Thanks also go to Dr. Robert Veltri for help with morphometry and Dr. Michelle Jones for manuscript review.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- Acu ID, Liu T, Suino-Powell K, Mooney SM, D’Assoro AB, Rowland N, Muotri AR, Correa RG, Niu Y, Kumar R, Salisbury JL. Coordination of centrosome homeostasis and DNA repair is intact in MCF-7 and disrupted in MDA-MB 231 breast cancer cells. Cancer Res. 2010;70:3320–3328. doi: 10.1158/0008-5472.CAN-09-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani M, Day BW, Schoen RE, Getzenberg RH. Altered expression and localization of creatine kinase B, heterogeneous nuclear ribonucleoprotein F, and high mobility group box 1 protein in the nuclear matrix associated with colon cancer. Cancer Res. 2006;66:763–769. doi: 10.1158/0008-5472.CAN-05-3771. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–62. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Cho CH, Wust P, Hildebrandt B, Issels RD, Sehouli J, Kerner T, Deja M, Budach V, Gellermann J. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: Evaluation of two annular-phased-array applicators. Int J Hyperthermia. 2008;24:399–408. doi: 10.1080/02656730801929915. [DOI] [PubMed] [Google Scholar]
- Coffey DS. Nuclear matrix proteins as proteomic markers of preneo-plastic and cancer lesions: Commentary re: G. Brunagel et al., nuclear matrix protein alterations associated with colon cancer metastasis to the liver. Clin Cancer Res. 2002;8:3039–3045. 2002.Clin Cancer Res 8: 303–3. [PubMed] [Google Scholar]
- Coffey DS, Getzenberg RH, DeWeese TL. Hyperthermic biology and cancer therapies: A hypothesis for the ‘‘Lance Armstrong effect’’. JAMA. 2006;296:445–448. doi: 10.1001/jama.296.4.445. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Fogh J. Human tumor cells in vitro. New York: Plenum Press; 1975. p. 557. [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—From yeast to humans. Science. 1991;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzenberg RH, Pienta KJ, Huang EY, Coffey DS. Identification of nuclear matrix proteins in the cancer and normal rat prostate. Cancer Res. 1991;51:6514–6520. [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Joyce T, Cantarella D, Isella C, Medico E, Pintzas A. A molecular signature for epithelial to mesenchymal transition in a human colon cancer cell system is revealed by large-scale microarray analysis. Clin Exp Metastasis. 2009;26:569–587. doi: 10.1007/s10585-009-9256-9. [DOI] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Kuiper JW, van Horssen R, Oerlemans F, Peters W, van Dommelen MM, te Lindert MM, ten Hagen TL, Janssen E, Fransen JA, Wieringa B. Local ATP generation by brain-type creatine kinase (CK-B) facilitates cell motility. PLoS One. 2009;4:e5030. doi: 10.1371/journal.pone.0005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Nagoshi T, Hong EG, Luptak I, Hartil K, Li L, Gorovits N, Charron MJ, Kim JK, Tian R, Rosenzweig A. Effects of chronic Akt activation on glucose uptake in the heart. Am J Physiol Endocrinol Metab. 2006;290:E789–E797. doi: 10.1152/ajpendo.00564.2004. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Goel A, D’Assoro AB, Salisbury JL, Janknecht R. Pleiotropic effects of p300-mediated acetylation on p68 and p72 RNA helicase. J Biol Chem. 2010;285:30443–30452. doi: 10.1074/jbc.M110.143792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SM, Grande JP, Salisbury JL, Janknecht R. Sumoylation of p68 and p72 RNA helicases affects protein stability and transactivation potential. Biochemistry. 2010;49:1–10. doi: 10.1021/bi901263m. [DOI] [PubMed] [Google Scholar]
- Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia. 2007;23:431–442. doi: 10.1080/02656730701455318. [DOI] [PubMed] [Google Scholar]
- Usami Y, Satake S, Nakayama F, Matsumoto M, Ohnuma K, Komori T, Semba S, Ito A, Yokozaki H. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol. 2008;215:330–339. doi: 10.1002/path.2365. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Zhang M, Atkinson RL, Rosen JM. Selective targeting of radiation-resistant tumor-initiating cells. Proc Natl Acad Sci USA. 2010;107:3522–3527. doi: 10.1073/pnas.0910179107. [DOI] [PMC free article] [PubMed] [Google Scholar]