Abstract
Purpose
Controversy exists regarding the use of continuous antibiotic prophylaxis vs observation in the management of children with vesicoureteral reflux. The reported effectiveness of continuous antibiotic prophylaxis in children with reflux varies widely. We determined whether the aggregated evidence supports use of continuous antibiotic prophylaxis in children with vesicoureteral reflux.
Materials and Methods
We searched the Cochrane Controlled Trials Register, clinicaltrials.gov, MEDLINE®, EMBASE®, Google Scholar and recently presented meeting abstracts for reports in any language. Bibliographies of included studies were then hand searched for any missed articles. The study protocol was prospectively registered at PROSPERO (No. CRD42014009639). Reports were assessed and data abstracted in duplicate, with differences resolved by consensus. Risk of bias was assessed using standardized instruments.
Results
We identified 1,547 studies, of which 8 are included in the metaanalysis. Pooled results demonstrated that continuous antibiotic prophylaxis significantly reduced the risk of recurrent febrile or symptomatic urinary tract infection (pooled OR 0.63, 95% CI 0.42–0.96) but, if urinary tract infection occurred, increased the risk of antibiotic resistant organism (pooled OR 8.75, 95% CI 3.52–21.73). A decrease in new renal scarring was not associated with continuous antibiotic prophylaxis use. Adverse events were similar between the 2 groups. Significant heterogeneity existed between studies (I2 50%, p = 0.03), specifically between those trials with significant risk of bias (eg unclear protocol descriptions and/or lack of blinding).
Conclusions
Compared to no treatment, continuous antibiotic prophylaxis significantly reduced the risk of febrile and symptomatic urinary tract infections in children with vesicoureteral reflux, although it increased the risk of infection due to antibiotic resistant bacteria. Continuous antibiotic prophylaxis did not significantly impact the occurrence of new renal scarring or reported adverse events.
Keywords: antibiotic prophylaxis, meta-analysis, pediatrics, review, vesico-ureteral reflux
Primary vesicoureteral reflux is a common condition that is present in 1% to 10% of all children in the United States.1 In the setting of a febrile urinary tract infection reflux is reportedly present in a third of children.2 In affected children reflux is associated with an increased risk of recurrent pyelonephritis and, hence, an increased risk of renal scarring.2,3 Typical interventions for children with reflux include antireflux surgery (endoscopic, laparoscopic or open) and continuous antibiotic prophylaxis. The purpose of continuous antibiotic prophylaxis is to keep the urine “sterile” so that the risk of retrograde renal infection will be decreased. Since a significant proportion of reflux cases will spontaneously resolve with time,4,5 many authors recommend a conservative approach, ie continuous antibiotic prophylaxis, as the initial management option in children, reserving surgical intervention for those in whom continuous antibiotic prophylaxis is ineffective at preventing urinary tract infection.
Significant controversy and treatment related variability still exist regarding VUR management.6 In particular the effectiveness of CAP at decreasing infections in children has recently been called into question.7 Recent RCTs investigating the effect of CAP on prevention of urinary tract infection in children with reflux have shown conflicting results.8–15 Further clouding this picture is the fact that, as noted in a recent Cochrane Review,16 many of these RCTs have significant design or reporting flaws that limit their impact. However, with the recent publication of the National Institutes of Health sponsored RIVUR trial,12 it is unclear whether the accumulated data on CAP have shifted enough to affect treatment recommendations. We evaluated the accumulated literature on the effectiveness of CAP for children with VUR and determined the extent to which reported success rates for CAP have been influenced by underlying patient or study level factors.
Patients and Methods
Search Strategy
We searched MEDLINE, EMBASE, Cochrane Controlled Trials Register, www.clinicaltrials.gov and Google Scholar electronic databases for studies published between January 2010 and May 2014 in any language based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.17 We additionally evaluated all studies previously included in systematic reviews of this topic.16 This date range was chosen to provide a contemporary selection of series. We used the search terms “vesicoureteral reflux,” “vesicoureteric reflux,” “vesico-ureteral reflux” and “vesico-ureteric reflux” (see Appendix).
Reference lists of included studies were manually screened for any additional series. We also manually searched for unpublished abstracts presented at relevant scientific meetings, including meetings of the American Urological Association, Society for Pediatric Urology, American Academy of Pediatrics Section on Urology, Pediatric Academic Societies, World Congress of Endourology, Société Internationale d’Urologie, International Pediatric Nephrology Association and the European Association of Urology. Before the formal literature search the protocol was prospectively registered at PROSPERO (No. CRD42014009639).
Selection Criteria
Inclusion criteria consisted of age 18 years or younger and history of VUR treated with CAP. Study patients were compared to individuals 18 years or younger with VUR undergoing no treatment or treatment with placebo (controls).
We chose to include only RCTs that described the number of patients treated as well as the fraction in whom treatment was successful. No study was excluded based on method of analysis, definition of success, language of publication, perceived quality or susceptibility to bias. In cases of ambiguity or where study reporting made evaluation difficult we attempted to err on the side of inclusiveness.
Data Abstraction
Two reviewers (H-HSW, JCR) independently examined all study abstracts in duplicate, with disagreements resolved by consensus. Full text articles appearing to meet selection criteria were reviewed, and study data were abstracted in the same manner. Reports published in languages other than English were translated by study authors fluent in that language and/or by institutional translation staff. Abstracted data included patient level factors (age, VUR grade, gender, UTI rates, new renal scarring on nuclear scan, adverse events) and study level factors (country of origin, year of publication, conflict of interest disclosure, study funding). COI was identified by publication of disclosure.18
Missing Data and Author Contact
In cases of missing or unreported data we attempted to contact corresponding study authors by email. Data updates/confirmations were received from 5 of 8 included studies.9,10,12–14 In cases where authors were unable to provide the missing information we excluded the study from only those analyses requiring the missing data.
Outcome Assessment
Our primary outcome was the odds ratio of having febrile or symptomatic UTI. Secondary outcomes included new renal scarring, antibiotic resistance and any adverse effects related to CAP.
Risk of Bias Assessment
Bias assessment was performed by 2 study authors (H-HSW, JCR) using the Cochrane Collaboration checklist.19 Differences were resolved by consensus discussion. Funnel plots were visually assessed for evidence of publication bias. A stratified subgroup analysis was performed between studies deemed to have a high risk and those with a low risk of bias.
Statistical Methods
Descriptive statistical analyses were performed using OR and 95% confidence intervals, as appropriate. For univariate pooling DerSimonian-Laird random effects models were constructed.20 Study heterogeneity was assessed using the Higgins-Thompson I2 method.21 Given the anticipated small number of eligible studies, metaregression was a priori planned as a series of bivariate models to evaluate whether study or patient level factors were associated with study outcomes. All statistical analyses were performed using Stata®/SE, version 11.0 and Review Manager, version 5.2.9 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark).
Results
Search Results
A total of 1,547 publications were identified using our search criteria. Of these studies 16 (67 publications) were selected for full review. Eight studies met all criteria for inclusion, and were included in the pooled qualitative review and the quantitative meta-analysis (supplementary fig. 1, http://jurology.com/).
Description of Studies
Of the 8 included studies all were randomized controlled trials.8–15 Thus far, only 1 study has been published as an abstract.9 A total of 1,594 patients were included in the final meta-analysis, nearly all of whom were diagnosed with VUR after a UTI (see table). Mean patient age ranged from 8.6 to 21.3 months (median 12 to 24). Female patients made up 37% to 93% of the study cohorts. VUR grades also varied between studies, with some including low grade only, some high grade only and some grade I to IV disease.10–15,22 Followup ranged from 1 to 3 years. Included study populations were from the United States, Italy, France, Sweden, Norway, Australia, Chile and Spain. No study author had a significant COI, although COI data were not reported for all studies.9,11,14
Patient and study characteristics.
| RIVUR Trial12 | Swedish Reflux Trial8 |
PRIVENT Trial10 | Montini et al13 | Roussey-Kesler et al15 |
Garin et al11 | Pennesi et al14 | Craig et al9 | |
|---|---|---|---|---|---|---|---|---|
| No. pts | 607 | 137 | 243 | 128 | 225 | 113 | 100 | 41 |
| Followup (yrs) | 2 | 2 | 1 | 1 | 1.5 | 1 | 2 | 3 |
| COI | Grants (National Institutes of Health) | Grants (Sweden) | Grants (Australia) | Grants (Italy) | Grants (France) | Not reported | Not reported | Not reported |
| Age at randomization | Median 12 mos (range 2 –72) | Mean 21.3 mos (range 12 –24) | 0 –18 Yrs | 2 –84 Mos | Mean 11.2 mos (range 1 –36) | Median 24 mos (range 3 mose12 yrs) | Mean 8.6 mos (range 0 –30) | 0 –3 Mos |
| % Female | 92 | 93 | 62 | Not reported | 69 | 81 | 52 | 37 |
| VUR grades included | I –IV | III –IV | I –V | I –III | I –III | IV –V | II –IV | Not reported |
| Intervention group: | ||||||||
| CAP | Trimethoprim- sulfamethoxazole |
Trimethoprim, nitrofurantoin, cefadroxil |
Trimethoprim- sulfamethoxazole |
Trimethoprim- sulfamethoxazole, amoxicillin/clavulanic acid |
Trimethoprim- sulfamethoxazole |
Trimethoprim- sulfamethoxazole, nitrofurantoin |
Trimethoprim- sulfamethoxazole |
Trimethoprim- sulfamethoxazole |
| No. febrile or symptomatic UTI/total No. | 39/302 | 10/68 | 14/122 | 10/82 | 13/103 | 13/55 | 18/50 | 0/21 |
| No. new renal scarring/total No. | 18/220 | 0/68 | 0/109 | 2/187 | Not reported | 5/55 | 0/50 | 0/21 |
| No. antibiotic resistant (any pathogen)/total No. | 26/38 | 8/10 | 8/13 | Not reported | 10/13 | Not reported | 18/18 | Not reported |
| No. adverse events/total No. | 153/302 | Not reported | 2/122 | Not reported | Not reported | 0/55 | Not reported | Not reported |
| Control group: | ||||||||
| Strategy | Placebo | No treatment | Placebo | No treatment | No treatment | No treatment | No treatment | Placebo |
| No. febrile or symptomatic UTI/total No. | 72/305 | 28/69 | 21/121 | 9/46 | 19/122 | 10/58 | 15/50 | 2/20 |
| No. new renal scarring/total No. | 19/227 | 9/68 | 1/101 | 2/108 | Not reported | 2/58 | 0/50 | 0/20 |
| No. antibiotic resistant (any pathogen)/total No. | 17/69 | 9/25 | 3/19 | Not reported | 7/19 | Not reported | 0/15 | Not reported |
| No. adverse events/total No. | 165/305 | Not reported | 5/121 | Not reported | Not reported | 0/58 | Not reported | Not reported |
Risk of Bias in Included Studies
The risk of bias graph is illustrated in supplementary figure 2 (http://jurology.com/), and the risk of bias summary is illustrated in figure 1. The major bias of included studies was lack of adequate blinding. Only 2 studies provided a placebo that was similar to the active antibiotic,10,12 while the remainder provided no treatment in the control arm. Therefore, those 6 studies were deemed to have high potential for performance and detection biases. Three studies failed to provide adequate detail regarding patient randomization or allocation and/or outcome reporting.9,11,15 These series were deemed as having an unclear risk in those categories. Lastly an additional source of bias was from the source of urine samples. Two of the studies included bagged urine samples,13,15,22 which have an increased risk of contamination and falsepositive culture compared to catheterized specimens.23 There was little visual evidence of publication bias noted on funnel plots (fig. 2 and supplementary fig. 3, http://jurology.com/).
Figure 1.
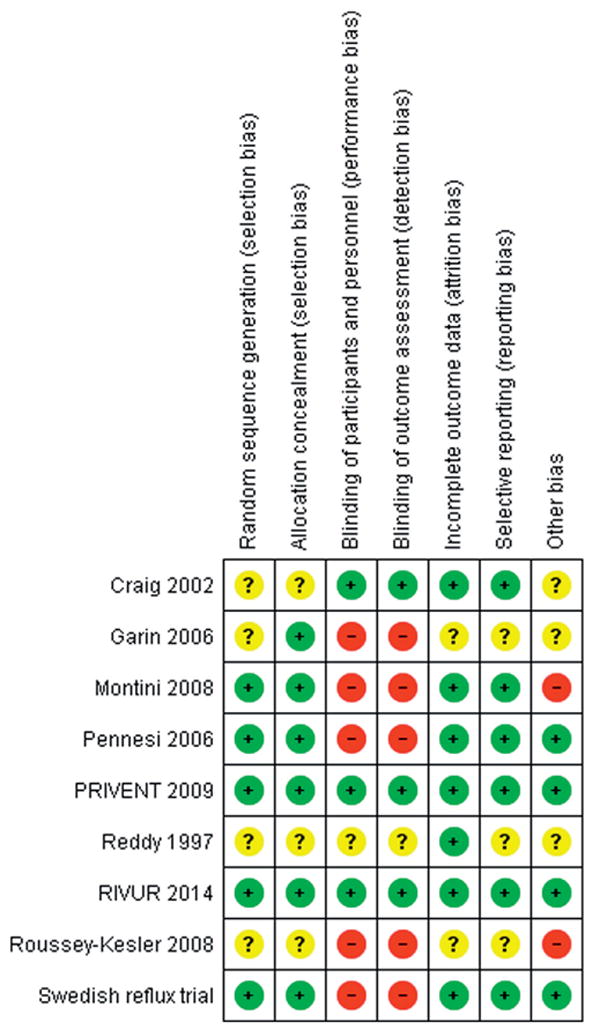
Risk of bias detail. Green circles represent positive risk of bias. Red circles indicate negative risk of bias. Yellow circles signify unknown risk of bias.
Figure 2.

Funnel plot of included studies for UTI. Circles indicate series with low risk of bias. Squares represent studies with higher risk of bias.
Effects of Interventions
CAP significantly reduced the risk of febrile or symptomatic UTI in children with reflux (pooled OR 0.63, 95% CI 0.42–0.96, p = 0.03, fig. 3). When we stratified by the susceptibility of each study to bias, those studies at lower risk of bias revealed an even more significant protective effect from CAP (pooled OR 0.51, 95% CI 0.35–0.73, p = 0.0003). There was no statistically significant impact of CAP on febrile or symptomatic UTI in studies with a higher risk of bias (p = 0.34).
Figure 3.
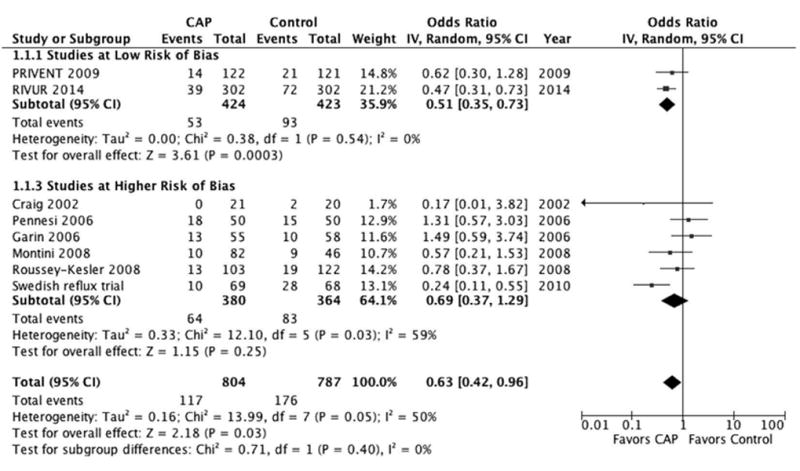
Forest plot of febrile or symptomatic UTI. Squares indicate odds ratios. Diamonds represent summary measures (center of diamond) and associated confidence intervals (lateral tips of diamond).
Regarding the development of antibiotic resistance, CAP was associated with an increased risk of resistant bacteria (pooled OR 8.75, 95% CI 3.52–21.73, p <0.0001). This risk remained significant after stratifying between studies based on risk of bias (supplementary fig. 4, http://jurology.com/). CAP failed to demonstrate any significant impact on new renal scarring or antibiotic related adverse events. Bivariate metaregression models showed no significant association between the likelihood of febrile or symptomatic UTI and duration of followup (p = 0.5), gender (p = 0.1), placebo (vs no treatment, p = 0.5) or study year (p = 0.1).
Discussion
In this study pooled RCT results reveal that CAP was associated with a 37% decrease in the odds of febrile or symptomatic urinary tract infection in children with reflux (pooled OR 0.63). This effect estimate differs from the most recent Cochrane Review,16 largely due to the completion of the recent RIVUR trial. The fact that a subset analysis of the only 2 RCTs noted to have a low risk of bias10,12 demonstrated an even stronger effect of CAP (49% decrease, pooled OR 0.51) would seem to lend further support to the argument that CAP does, indeed, have a protective effect against the development of UTI in children with reflux.
Despite this positive effect, rates of new renal scarring were not significantly associated with CAP. There are many potential reasons for this finding, most of which ultimately depend on the impressively low rate of renal scarring in all included studies. First, the rather short-term followup of all included studies may preclude detection of renal scarring formation. In addition, the “healthy volunteer” phenomenon may have a role, ie patients who are enrolled in a RCT may be less likely to have renal scars develop due to parental/provider vigilance and care at the first signs of UTI. Furthermore, most of these studies, including the PRIVENT (Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts) and RIVUR trials, contained patients recruited after the first or second UTI. Given that the risk of renal scarring after only 1 to 2 UTIs is quite low,24 it is not surprising that renal scarring events were uncommon. As the risk of renal scarring has been found to increase exponentially with an increasing number of UTIs, it stands to reason that if CAP prevents UTI, it should also prevent renal scarring. However, this supposition cannot be proved based on the data in this metaanalysis. It is noteworthy that recent studies have shown higher rates of renal scarring (approximately 15%),25,26 suggesting that these RCT participants may have had lower renal scar rates than the general population.
Although long-term CAP was associated with a higher rate of UTIs due to antibiotic resistant bacteria, there was no increase in associated adverse events reported in the CAP group compared to controls. Given its overall protective effect against UTI, coupled with the low rate of adverse events, CAP seems to be a reasonable management option to prevent recurrent febrile or symptomatic UTI in children with reflux. These benefits should be weighed against the obvious trade-off in terms of increased antibiotic resistance. Some authors have reported successful discontinuation of antibiotic prophylaxis in children with persistent reflux without subsequent UTI,27,28 implying that there may be certain populations at lower risk for UTI in whom the benefit of long-term CAP may not be adequate to justify the risks. Unfortunately, to our knowledge it is not yet feasible to reliably identify such a population.
One potential impact of our results involves the use of radiological imaging, particularly voiding cystourethrography, in children after a first febrile UTI. Given that the recent AAP guidelines were formulated based on data suggesting a lack of protective effect of CAP against UTIs,23 our findings may prompt reconsideration of those guidelines. We would tend to agree with the RIVUR authors, who noted, “Decision analysis and cost-effectiveness analysis may help to clarify the clinical and financial trade-offs, which may help clinicians and families reach more informed decisions about the advisability of imaging in young children.”12
This series should be interpreted in light of its limitations. As all of these studies included patients diagnosed with VUR after an initial UTI, these results may not apply to children diagnosed with VUR for other reasons (eg prenatal hydronephrosis and sibling VUR). As with any systematic review, our analyses were limited by the available data from the included studies. As seen in supplementary figure 2, many published studies of CAP were judged to have a risk or potential risk of significant bias. Selection bias could not be ruled out in studies lacking a clear description of recruitment, exclusion, randomization and/or allocation methodology. Many of the included studies were not blinded, which may introduce performance and detection bias. Thus, as with any meta-analysis, we must rely on a potentially imperfect data set. Nevertheless, to our knowledge this data set also represents the best data currently available.
Additionally it is noteworthy that we conducted our risk analyses at the study level instead of at the patient level. This is a form of ecological bias, where the aggregate results across studies are different from the raw results within studies. Similarly our analysis of author factors such as COIs are highly dependent on the veracity of author disclosures. While we have no reason to suspect that groups were not wholly forthright in their disclosures, it is noteworthy that the omission or false report of COI in even a small number of groups could have significantly impacted our findings.
Finally, we noted fairly significant heterogeneity in our pooled analysis, especially in the outcome group of febrile or symptomatic UTI (I2 55%, p = 0.04, fig. 3). This heterogeneity was principally driven by RCTs judged to have a higher risk of bias. Not surprisingly, in addition to the various biases due to study design and execution, many of the potentially important cohort characteristics such as age, VUR grade, CAP regimen and definition of outcomes also varied greatly among these studies. However, the 2 RCTs with a low risk of bias revealed consistently minimal heterogeneity (I2 0%) across all outcome measures.
Conclusions
Compared to no treatment or placebo, CAP significantly reduced the risk of febrile and symptomatic urinary tract infections in children with VUR, although it increased the risk of infection due to antibiotic resistant bacteria. The protective effect of CAP was more prominent in studies deemed to have a low risk of bias. CAP did not significantly impact the rate of new renal scarring or reported treatment related adverse events.
Supplementary Material
Abbreviations and Acronyms
- AAP
American Academy of Pediatrics
- CAP
continuous antibiotic prophylaxis
- COI
conflict of interest
- RCT
randomized controlled trial
- RIVUR
Randomized Intervention for Children with Vesicoureteral Reflux
- UTI
urinary tract infection
- VUR
vesicoureteral reflux
Appendix
Search Terms and Strategy
Medline
(“vesico-ureteral reflux” OR “vesicoureteral reflux” OR “vesico-ureteric reflux” OR “vesicoureteric reflux” OR “kidney reflux”) AND “ “2010/01/01”[PDat] : “2014/05/09”[PDat]”
Embase
(vesico-ureteral reflux)/exp AND (2010:py OR 2011:py OR 2012:py OR 2013:py OR 2014:py) AND ‘randomized controlled trial (topic)’/de
Cochrane Controlled Trials Register
((“vesicoureteral reflux” (title, abstract, keywords) OR “vesicoureteric reflux” (title, abstract, keywords) OR “vesico-ureteral reflux” (title, abstract, keywords) OR “vesico-ureteric reflux” (title, abstract, keywords)) and limited to 2010-2014
Clinicaltrials.gov
“vesico-ureteral reflux” OR”vesicoureteral reflux” OR “vesico-ureteric reflux” OR “vesicoureteric reflux”
Google Scholar
(“vesico-ureteral reflux” OR”vesicoureteral reflux” OR “vesico-ureteric reflux” OR “vesicoureteric reflux”) AND “randomized controlled trial”
Contributor Information
Hsin-Hsiao S. Wang, Division of Urologic Surgery, Duke University Medical Center, Durham, North Carolina
Rasheed A. Gbadegesin, Division of Pediatric Nephrology, Duke University Medical Center, Durham, North Carolina
John W. Foreman, Division of Pediatric Nephrology, Duke University Medical Center, Durham, North Carolina
Shashi K. Nagaraj, Division of Pediatric Nephrology, Duke University Medical Center, Durham, North Carolina
Delbert R. Wigfall, Division of Pediatric Nephrology, Duke University Medical Center, Durham, North Carolina
John S. Wiener, Division of Urologic Surgery, Duke University Medical Center, Durham, North Carolina
Jonathan C. Routh, Division of Urologic Surgery, Duke University Medical Center, Durham, North Carolina
References
- 1.Sargent MA. What is the normal prevalence of vesicoureteral reflux? Pediatr Radiol. 2000;30:587. doi: 10.1007/s002470000263. [DOI] [PubMed] [Google Scholar]
- 2.Hoberman A, Charron M, Hickey RW, et al. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195. doi: 10.1056/NEJMoa021698. [DOI] [PubMed] [Google Scholar]
- 3.Montini G, Toffolo A, Zucchetta P, et al. Antibiotic treatment for pyelonephritis in children: multicentre randomised controlled noninferiority trial. BMJ. 2007;335:386. doi: 10.1136/bmj.39244.692442.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwab CW, Jr, Wu HY, Selman H, et al. Spontaneous resolution of vesicoureteral reflux: a 15-year perspective. J Urol. 2002;168:2594. doi: 10.1016/S0022-5347(05)64225-5. [DOI] [PubMed] [Google Scholar]
- 5.Tamminen-Möbius T, Brunier E, Ebel KD, et al. Cessation of vesicoureteral reflux for 5 years in infants and children allocated to medical treatment. The International Reflux Study in Children. J Urol. 1992;148:1662. doi: 10.1016/s0022-5347(17)36997-5. [DOI] [PubMed] [Google Scholar]
- 6.Routh JC, Nelson CP, Graham DA, et al. Variation in surgical management of vesicoureteral reflux: influence of hospital and patient factors. Pediatrics. 2010;125:e446. doi: 10.1542/peds.2009-1237. [DOI] [PubMed] [Google Scholar]
- 7.Routh JC, Bogaert GA, Kaefer M, et al. Vesicoureteral reflux: current trends in diagnosis, screening, and treatment. Eur Urol. 2012;61:773. doi: 10.1016/j.eururo.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Brandström P, Jodal U, Sillén U, et al. The Swedish Reflux Trial: review of a randomized, controlled trial in children with dilating vesicoureteral reflux. J Pediatr Urol. 2011;7:594. doi: 10.1016/j.jpurol.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Craig J, Roy L, Sureshkumar P, et al. Long-term antibiotics to prevent urinary tract infection in children with isolated vesicoureteric reflux: a placebo-controlled randomized trial. Nephrology. 2002;7(suppl):44. abstract. [Google Scholar]
- 10.Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361:1748. doi: 10.1056/NEJMoa0902295. [DOI] [PubMed] [Google Scholar]
- 11.Garin EH, Olavarria F, Garcia Nieto V, et al. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006;117:626. doi: 10.1542/peds.2005-1362. [DOI] [PubMed] [Google Scholar]
- 12.Hoberman A, Greenfield SP, Mattoo TK, et al. RIVUR Trial Investigators: Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370:2367. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montini G, Rigon L, Zucchetta P, et al. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics. 2008;122:1064. doi: 10.1542/peds.2007-3770. [DOI] [PubMed] [Google Scholar]
- 14.Pennesi M, Travan L, Peratoner L, et al. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics. 2008;121:e1489. doi: 10.1542/peds.2007-2652. [DOI] [PubMed] [Google Scholar]
- 15.Roussey-Kesler G, Gadjos V, Idres N, et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: results from a prospective randomized study. J Urol. 2008;179:674. doi: 10.1016/j.juro.2007.09.090. [DOI] [PubMed] [Google Scholar]
- 16.Nagler EV, Williams G, Hodson EM, et al. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2011;6:CD001532. doi: 10.1002/14651858.CD001532.pub4. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Fenton JJ, Mirza SK, Lahad A, et al. Variation in reported safety of lumbar interbody fusion: influence of industrial sponsorship and other study characteristics. Spine (Phila Pa 1976) 2007;32:471. doi: 10.1097/01.brs.0000255809.95593.3b. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Egger M, Moher P. Addressing reporting biases. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, New Jersey: John Wiley & Sons Inc; 2012. pp. 297–334. chapt 10. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Brandstrom P, Esbjorner E, Herthelius M, et al. The Swedish Reflux Trial in Children: I. Study design and study population characteristics. J Urol. 2010;184:274. doi: 10.1016/j.juro.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 23.Roberts KB, Downs SM, Finnell SM, et al. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management: Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 24.Jodal U. The natural history of bacteriuria in childhood. Infect Dis Clin North Am. 1987;1:713. [PubMed] [Google Scholar]
- 25.Shaikh N, Craig JC, Rovers MM, et al. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr. 2014;168:893. doi: 10.1001/jamapediatrics.2014.637. [DOI] [PubMed] [Google Scholar]
- 26.Snodgrass WT, Shah A, Yang M, et al. Prevalence and risk factors for renal scars in children with febrile UTI and/or VUR: a cross-sectional observational study of 565 consecutive patients. J Pediatr Urol. 2013;9:856. doi: 10.1016/j.jpurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchens DM, Herndon A, Joseph DB. Outcome after discontinuing prophylactic antibiotics in children with persistent vesicoureteral reflux. J Urol. 2010;184(suppl):1594. doi: 10.1016/j.juro.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Leslie B, Moore K, Salle JL, et al. Outcome of antibiotic prophylaxis discontinuation in patients with persistent vesicoureteral reflux initially presenting with febrile urinary tract infection: time to event analysis. J Urol. 2010;184:1093. doi: 10.1016/j.juro.2010.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


