Perivascular spaces may be associated with cardiovascular and dementia risks and these authors sought to determine if they are also associated with atherosclerosis. Thus, perivascular spaces were assessed in more than 700 patients and correlated with race, age, and cardiovascular risks. They were found to be more common in older hypertensive patients and the presence of carotid artery plaques strongly and consistently predicted their presence.
Abstract
BACKGROUND AND PURPOSE:
Perivascular spaces are potential spaces found between brain blood vessels and surrounding leptomeninges that have been associated with cardiovascular risk factors and dementia, but less is known about their relationship to atherosclerosis. We tested the hypothesis that perivascular spaces are associated with atherosclerosis.
MATERIALS AND METHODS:
Participants from the Northern Manhattan Study who remained stroke-free were invited to participate in an MR imaging substudy. Parenchymal hypointensities of <3 mm identified on brain axial T1-weighted MR imaging were scored as perivascular spaces. A semiquantitative score was created to express the degree of brain involvement. Generalized linear models were used to assess statistical associations with carotid plaque as a surrogate marker of atherosclerosis.
RESULTS:
The studied sample included 706 participants (mean age, 72.6 ± 8.0 years; 60% women, 61% Hispanic, 68% with hypertension, 19% with diabetes, and 57% with high cholesterol). The perivascular spaces score ranged from 0 to 19 with 52% of the sample having a perivascular spaces score of ≤4. In unadjusted analysis, perivascular spaces were associated with age (β = 0.01 per year, P = < .001), non-Hispanic black race-ethnicity (β = 0.16, P = .02), hypertension (β = 0.24, P = < .001), and carotid plaque (β = 0.22, P < .001). In multivariable analysis, only age (β = 0.01, P = .02), hypertension (β = 0.17, P = .01), and carotid plaque (β = 0.22, P = < .001) remained independently associated with perivascular spaces.
CONCLUSIONS:
Perivascular spaces were more frequently found in older participants, in those with hypertension, and in the presence of carotid plaque. These results suggest that mechanisms leading to atherosclerosis might also lead to an increased number of perivascular spaces. These results need confirmation in prospective studies.
Perivascular spaces in the brain have been described for more than a century, but their pathologic significance has not been settled.1,2 A definition of PVS can be summarized as a potential space between blood vessels created by surrounding leptomeninges as they travel through the CSF space and penetrate the brain parenchyma, acting as an isolating sheath from the CSF compartment.3 Among some physiologic functions attributed to PVS are the trafficking of inflammatory cells to and from the brain and drainage of interneuronal fluid to the systemic circulation through the lymphatic system.4,5
When these spaces dilate, they can be observed directly in postmortem brain sections or on brain MR imaging or CT.6 Pathologic and radiologic studies that have evaluated the significance of PVS have yielded conflicting results. In some cases, PVS have been described as an age-related phenomenon without pathologic associations, while in others, they are more frequently found in individuals with disease.7–9 The reasons underlying these discrepancies are various, including the heterogeneous populations studied and differing study methods. The importance of correctly identifying PVS and understanding their pathophysiology is highlighted by their association with a worse cardiovascular profile, cerebral small-vessel disease (leukoaraiosis), and worse cognition.10,11 Whether PVS are biomarkers of cardiovascular risk-factor severity and indicators of a pathophysiologic process or simply innocent bystanders remains to be clarified.
If PVS are more frequent in individuals with cardiovascular risk factors, then atherosclerosis should be more prevalent in those with PVS. However, this hypothesis has not been tested. In addition, few community-based studies have examined the prevalence of PVS in the general population or their association with vascular risk factors and atherosclerosis.6,11 We examined the prevalence and correlates of PVS in the urban Northern Manhattan population that includes Hispanic white and non-Hispanic black individuals known to be at greater risk of stroke than non-Hispanic whites.12
Materials and Methods
The Northern Manhattan Study includes a prospective population-based cohort of participants enrolled from the Northern Manhattan neighborhood between 1993 and 2001 by using random digit dialing. An overall response rate was 68% as previously described.13 Eligible participants were stroke-free at baseline, aged 40 years or older, and had resided in Northern Manhattan for at least 3 months in a household with a telephone. Between 2003 and 2008, all surviving participants remaining stroke-free were invited to participate in an MR imaging substudy. Participants were eligible if they were older than 55 years of age and had no contraindication to MR imaging. For this analysis, only subjects with MR images in DICOM format were included because the software used did not support other formats. All participants signed written informed consent, and the study was approved by the local institutional review board.
Data were obtained from participants (99%) or proxies by using standardized data-collection instruments. Participants self-identified their ethnicity as Hispanic or non-Hispanic and race as white, black, or other. Standard techniques were used to measure blood pressure, height, weight, and fasting glucose levels. “Hypertension” was defined as a systolic blood pressure of 140 mm Hg or a diastolic blood pressure of ≥90 mm Hg, physician diagnosis, or self-report of a history of hypertension or antihypertensive use. “Diabetes mellitus” was defined as fasting blood glucose of ≥126 mg/dL or self-report of such a history or insulin or hypoglycemic use. “Dyslipidemia” was defined as total cholesterol of >200 mg/dL or use of cholesterol-lowering medications. Smoking was categorized as never, former, and current smoker (within 1 year). “History of cardiac disease” was defined as self-report of atrial fibrillation, myocardial infarction, angina, or coronary artery bypass grafting. Carotid plaque in any of the carotid artery segments (the common carotid artery, bifurcation, and internal carotid artery) was assessed by high-resolution B-mode carotid sonography (LOGIQ 700; GE Healthcare, Milwaukee, Wisconsin) using a standardized sonography imaging protocol with excellent inter- and intrareader reliability, as previously described.14 It was defined as an area of focal wall thickening >50% greater than the surrounding wall thickness and coded as “present” or “not.”
Imaging was performed on a dedicated 1.5T research MR imaging system (Philips Healthcare, Best, the Netherlands) by using a standardized protocol. The MR imaging sequences used in the current study were axial FLAIR and axial T1. The 3D T1 image had a section thickness of 1.5 mm with no gap, a TE of 2.1 ms, a TR of 20 ms, and a flip angle of 20°. The FLAIR images were acquired in the multi-section turbo spin-echo mode with an FOV of 250 mm, a rectangular FOV of 80%, an acquisition matrix of 192 × 133 scaled to 256 × 256 in reconstruction, a 3-mm section thickness with no gap, a TE of 144 ms, a TR of 5500 ms, an inversion recovery delay of 1900 ms, and a flip angle of 90°.
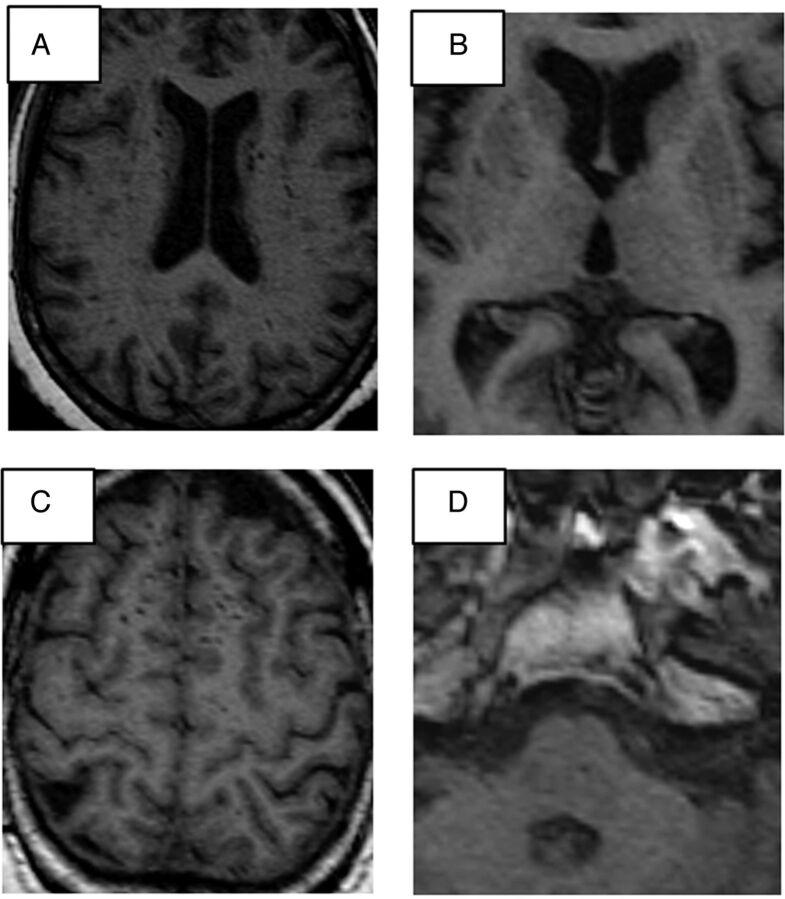
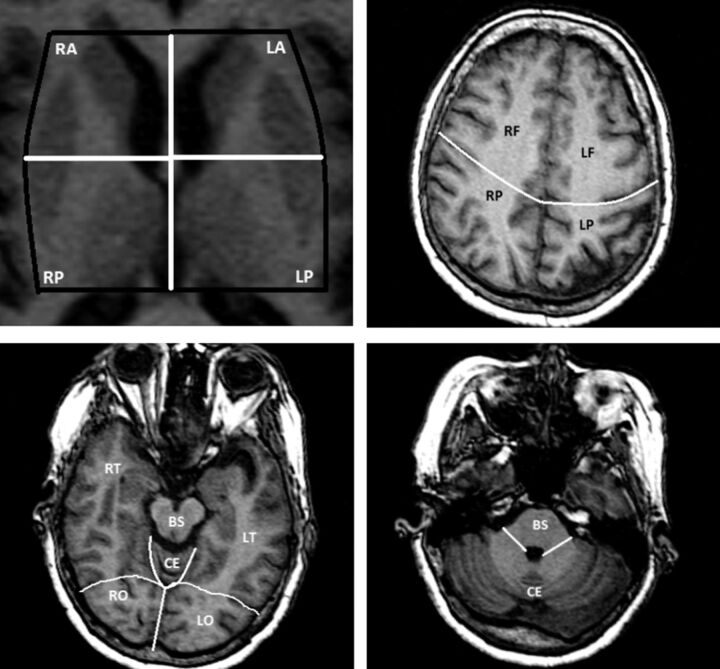
Parenchymal hypointensities observed in 3D axial T1 with an effective diameter of <3 mm and an absent FLAIR hyperintensity were considered to represent perivascular spaces (Fig 1). Due to the large number of small hypointensities observed in some cases, we used a semiquantitative score to express the degree of brain involvement per section: 0 = no voids observed in the region, 1 = 1–3 voids, and 2 = ≥4 voids. The supratentorial brain was divided into 12 sections: 1 section for the white matter of each lobe (ie, frontal, temporal, parietal, and occipital) and 4 sections in the basal ganglia. The brain stem and the cerebellum were considered as 1 separate region (14 sections, with a possible score ranging from 0 to 28; Fig 2). Intrarater reliability was assessed in a 10% random sample and was found to be excellent for the PVS score (rating by J.G., first author; intraclass correlation coefficient = 0.90).
Fig 1.
A, Multiple hyperintensities appear in both caudate heads and the corona radiata white matter tracts, suggestive of perivascular spaces. B, Small voids (hypointensities) can be observed in the lateral margins of both putamina. C, Example of multiple perivascular spaces high in the medial frontal convexity. D, A small perivascular space is observed in the pons.
Fig 2.
Division used to score perivascular spaces by anatomic regions. R indicates right; L, left; A, anterior; P, posterior; F, frontal; T, temporal; P, parietal; O, occipital; CE, cerebellum; BS, brain stem.
Statistical Analysis
Differences among participants studied in this analysis versus those excluded were evaluated with Student t tests for continuous variables and χ2 tests for categoric variables. The main outcome variable was the PVS score, but we also used the median of the PVS distribution to categorize the presence of PVS for secondary analysis. We built separate generalized linear models with the continuous PVS score as the outcome and sample demographic, clinical, and ancillary characteristics as the predictor variables. In a secondary analysis, we evaluated the continuous PVS score by anatomic region to see whether the association differed by location and the dichotomized PVS score to evaluate the effect size of predictors on the prevalence of PVS by using odds ratios. We used Poisson distributions to fit the continuous PVS score (count) distribution and binomial distributions for the dichotomized PVS score to obtain the odds ratios and their 95% confidence intervals. A P value ≤.05 was considered statistically significant by using type III effects in the generalized linear models. A random sample of 10% was used to assess intrareader reliability by using an intraclass correlation coefficient or κ value, depending on the type of variable measured. The analysis was performed with SAS software, Version 9.2 (SAS Institute, Cary, North Carolina).
Results
Population Characteristics
Of 1290 participants who underwent brain MR imaging, we rated PVS in 706 participants (limited to data available in the DICOM format). The sample mean age was 71.6 ± 8.0 years, 60% were women, 61% were Hispanic, 20% were non-Hispanic black, and 19% were non-Hispanic white. Hypertension was present in 68%; diabetes, in 19%; hypercholesterolemia, in 57%; smoking, in 52% (36% were former smokers and 16% current smokers); and a history of cardiac disease, in 16%. There were no advanced plaques (those producing hemodynamic stenosis of >40%) among subjects included in this study. Small, nonstenotic carotid plaque was found in 55% of the participants. Those individuals included in this analysis were slightly older and more likely to be non-Hispanic black and less likely to have dyslipidemia (Table 1).
Table 1:
Characteristics of the studied sample compared with the NOMAS MRI subsample
| NOMAS MRI Study Included Sample (n = 706) | NOMAS MRI Study Excluded Sample (n = 585) | P Valuea | |
|---|---|---|---|
| Age (yr) | 71.6 ± 8.0 | 69.4 ± 9.9 | <.01 |
| Female sex (%) | 60.4 | 60.5 | .95 |
| Ethnicity (%) | |||
| Non-Hispanic white | 15.9 | 13.5 | <.01 |
| Non-Hispanic black | 20.3 | 13.7 | |
| Hispanic | 61.1 | 71.1 | |
| Hypertension (%) | 68.5 | 64.6 | .15 |
| Diabetes (%) | 18.9 | 20.0 | .61 |
| Hypercholesterolemia (%) | 56.6 | 63.2 | .02 |
| Current smoking (%) | 15.9 | 16.2 | .86 |
| Previous cardiac disease (%) | 16.3 | 16.9 | .77 |
| Carotid plaque (%) | 55.0 | 55.5 | .85 |
| Perivascular spaces score | |||
| Mean | 5.0 | Not applicable | Not applicable |
| Median | 4.0 | ||
| 25th percentile | 2.0 | ||
| 50th percentile | 4.0 | ||
| 75th percentile | 7.0 |
Note:—NOMAS indicates the Northern Manhattan Study.
Two-tailed t test was used to obtain P values for age differences, while the χ2 test was used for all other comparisons.
Perivascular Spaces Score
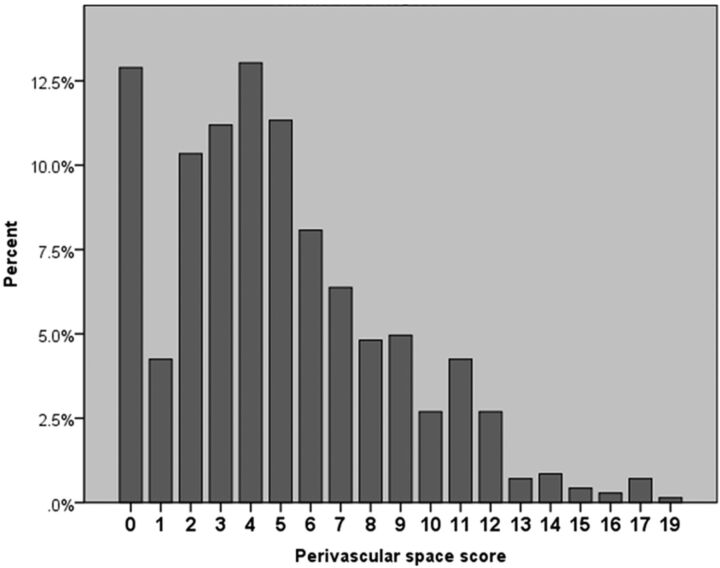
The PVS score ranged from 0 to 19, with 52% of the sample having a PVS score of ≤4 (median = 4; Fig 3, Table 1)., The percentage of participants with PVS scores of >4 increased with age. It was 39% in those between 55 and 64.9 years, 48% in those 65–74.9 years, 55% in those 75–84.9 years, and 57% in those 85 years or older. The proportion of participants who had nonstenotic carotid plaque and a PVS score of >4 was 55% compared with 40% in those without carotid plaque. In unadjusted analysis, the PVS score was higher with older age (β = 0.01, P = < .001), black race (β = 0.16, P = .02), HTN (β = 0.24, P = < .001), and the presence of carotid plaque (β = 0.22, P < .001). It was lower in participants who reported taking antihypertensive medications (β = −0.19, P = .04), antihyperglycemics (β = −0.23, P = .008), and cholesterol-lowering drugs (β = −26, P = .003). Being male was marginally associated with a lower PVS score (β = −0.11, P = .06).
Fig 3.
Distribution of PVS scores in the studied sample.
No associations were noted between the PVS score and systolic blood pressure, diastolic blood pressure, pulse pressure, dyslipidemia, low-attenuation lipoprotein cholesterol levels, diabetes, current or former smoking, body mass index, or history of cardiac disease. In multivariable analysis, age (β = 0.01, P = .02), HTN (β = 0.17, P = .01), and the presence of carotid plaque (β = 0.22, P = < .001) were significant predictors of a higher PVS score (Table 2), while taking cholesterol-lowering medications was a predictor of lower PVS scores (β = −0.23, P = .01). Using the same variables in a multivariate model with binomial distribution to predict the prevalence of PVS (ie, PVS score ≥5) reproduced the same independent association of PVS with age (OR, 1.03; 95% CI, 1.06–1.05), HTN (OR, 1.58; 95% CI, 1.05–2.38), the presence of carotid plaque (OR, 1.71; 95% CI, 1.18–2.47), and taking cholesterol-lowering medications (OR, 0.65; 95% CI, 0.44–0.94).
Table 2:
Adjusted β coefficientsa of predictors of the overall perivascular space scores and by anatomic regions
| Overall Score |
Perivascular Space Score by Anatomic Region |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subcortical White Matter |
Basal Ganglia |
Infratentorial |
||||||
| β Coefficient | P Value | β Coefficient | P Value | β Coefficient | P Value | β Coefficient | P Value | |
| Age | 0.01 | .02 | 0.008 | .15 | 0.01 | .02 | 0.02 | .19 |
| Male sex | −0.11 | .12 | −0.21 | .01 | 0.05 | .52 | −0.35 | .08 |
| Race | ||||||||
| White | Ref | Ref | Ref | Ref | ||||
| Black | 0.004 | .96 | 0.11 | .37 | −0.07 | .51 | −0.47 | .12 |
| Hispanic | −0.11 | .17 | −0.06 | .57 | −0.15 | .10 | −0.20 | .40 |
| Body mass index | −0.004 | .53 | −0.004 | .61 | −0.003 | .64 | −0.008 | .63 |
| Smoking | ||||||||
| Never | Ref | Ref | Ref | Ref | ||||
| Former | 0.02 | .35 | 0.05 | .55 | −0.05 | .52 | 0.35 | .07 |
| Current | 0.08 | .78 | 0.14 | .20 | −0.04 | .61 | 0.76 | .001 |
| Hypertension | 0.17 | .01 | 0.11 | .20 | 0.20 | .02 | 0.59 | .008 |
| Antihypertensive medications | −0.11 | .09 | −0.13 | .13 | −0.09 | .24 | −0.28 | .24 |
| Diabetes | 0.03 | .71 | 0.06 | .51 | −0.003 | .96 | −0.04 | .88 |
| Hypoglycemic medications | −0.18 | .19 | −0.19 | .10 | −0.03 | .79 | −0.31 | .40 |
| Hypercholesterolemia | −0.12 | .21 | 0.01 | .93 | −0.14 | .02 | −0.16 | .38 |
| Cholesterol-lowering medications | −0.24 | .02 | −0.22 | .08 | −0.22 | .08 | −0.76 | .04 |
| History of cardiac disease | 0.07 | .32 | −0.02 | .81 | 0.15 | .05 | 0.26 | .23 |
| Carotid plaque | 0.22 | <.001 | 0.29 | <.001 | 0.16 | 0.02 | 0.22 | .21 |
Note:—Ref indicates reference group.
Coefficients were adjusted for all other demographic variables plus medications to treat hypertension, diabetes, and hypercholesterolemia.
Perivascular Spaces by Anatomic Region
Analysis of the PVS scores demonstrated variation in the degree of involvement by anatomic region. While >73% of participants had PVS in either the subcortical white matter or basal ganglia, only 18% of the sample had infratentorial lesions. Using the same multivariable model used for the global PVS scores, we found important differences in the PVS score by anatomic locations (Table 2). Perivascular scores in the subcortical white matter were more frequent in women than in men. They were not associated with HTN or hypercholesterolemia but were associated with the presence of carotid plaque. The basal ganglia PVS were more frequent in those with a history of cardiac disease and less frequent in the context of dyslipidemia. The infratentorial PVS score was greater among current smokers and hypertensive participants. The effect estimates for the association of carotid plaque with the infratentorial PVS score had the same magnitude as in other anatomic regions but did not reach statistical significance.
Discussion
Our study demonstrates that PVS were more frequent in older participants, in the presence of HTN, and in those with carotid atherosclerotic plaques. Carotid plaque was found to be among the strongest and most consistent predictors of PVS, suggesting a biologic link between PVS and atherosclerosis, though we cannot evaluate causality in this observational study. It seems unlikely that the development of PVS causes atherosclerosis, but the biophysics are poorly understood. For example, it is not known whether a tendency to form PVS might influence the response of an arterial tree to variations in blood flow or wall shear stress that might encourage local atherosclerotic plaque formation. It also seems unlikely that small, nonstenotic carotid plaques observed in our study had an impact on cerebral hemodynamics that might have affected PVS formation because flow is not thought to be altered until the degree of stenosis reaches >80%.15 Even in the case of carotid stenosis of >80%, it is unlikely that dampening of cerebral blood flow underlies the association between PVS and HTN.16,17 The association of PVS with carotid plaque may represent a shared association with atherosclerotic risk factors. Another explanation may be that PVS are an epiphenomenon of the presence of atherosclerosis in other vessels. Other possible mediators in the development of PVS may be arterial stiffness and pulse-wave reflection, because both increase with age and atherosclerosis. Increased arterial stiffness in large arteries can be associated with increased pulsatility to richly vascularized organs, such as the brain.18,19 In turn, greater pulsatility in brain penetrator arteries could lead to PVS as a consequence of atherosclerosis and aging.16 This hypothesis requires further investigation.
Hypertension seems to be one of the most consistent cardiovascular risk factors associated with the presence and number of PVS.10,20,21 In a similar population-based MR imaging study of dementia and stroke-free participants, the presence and severity of PVS were associated with HTN.10 Additionally, the severity of their PVS score was associated with other markers of small-vessel disease such as white matter hyperintensities and male sex. Other studies, though from more selected populations, have also reproduced the association of PVS and HTN.20,21 The reasons underlying this association are difficult to explain with cross-sectional data, but it is plausible that an increase in intraluminal pressure might lead to greater extravasation of fluid through the small arteries into these spaces. This hypothesis is supported by rat experiments in which sustained HTN led to increased permeability of endothelial cells and fluid-induced damage to surrounding brain parenchyma.22 Due to the close proximity of PVS to brain parenchyma, elevated pulsatility in these areas could lead to enlargement of perivascular spaces.16 Also, small penetrating arteries receive their flow from large arteries, with a large drop in caliber that might expose the smaller vessels to greater pulsatility and mechanical forces. For example, from an average MCA diameter of 3.0 mm, the diameter of lenticulostriate arteries drops to 0.5–0.9 mm.23,24 Furthermore, a bifurcation angle between 70 and 110° is associated with increased wall shear stress in the branching vessel (the lenticulostriate arteries leave the MCA at an angle of approximately 90°).25 This greater angle could partially explain why one of the most common anatomic locations of PVS is the basal ganglia, at the origin of the lenticulostriates.10,26–28
However, other factors might be involved. The association of HTN with PVS has not always been reproduced, and the brain stem is the least affected region, even though a similar drop in diameter occurs from the basilar artery to small penetrators.6,27,28 An alternate explanation for the predisposition of the basal ganglia to develop PVS might be the existence of the more complex, doubled-layered microstructure of the PVS compartments in this region compared with the cortex.29 More studies are needed to assess this hypothesis.
The prevalence of PVS in our sample increased with age after adjusting for other covariates; this finding suggests that PVS might be a manifestation of aging. The high prevalence of PVS in older individuals compared with the lower prevalence in younger groups supports this finding.10,30 However, PVS are also found in children, in whom their presence has been associated with behavioral and neurologic symptoms.9,31 Perivascular spaces associated with neuropsychiatric abnormalities in children are most likely of a different etiology than those of middle-aged and older individuals with cardiovascular risk factors. While serotonin has been invoked to explain the relationship between PVS and migraine in children, inflammation has been cited as the leading process in the presence of PVS in patients with multiple sclerosis and other brain inflammatory processes.7,31–33 Although low-attenuation lipoprotein cholesterol levels were not associated with PVS, taking cholesterol-lowering medications was a strong negative predictor of PVS in this study. We found that taking antihypertensive or hypoglycemic medications was also associated with a lower PVS score. These findings taken together might suggest that controlling vascular risk factors leads to a reduction in PVS prevalence. A role of inflammation in the development of PVS is also possible in adults with cardiovascular risk factors. The link between PVS and inflammation will be pursued in our cohort in the future.
The analysis of PVS by brain anatomic areas revealed consistent findings across all locations for most of the included variables except smoking. Current smoking was associated with greater infratentorial PVS scores. Rather than suggesting a biologic relationship of smoking with PVS in the posterior circulation, this association might represent misclassification of small infarcts as PVS in the brain stem and cerebellum. Although PVS are present in the brain stem, the occurrence of infarcts is considered more likely.23,28,34 The lack of a significant association between carotid plaque and infratentorial PVS might be related to the lower prevalence of PVS in this region.
This study has noteworthy limitations. Our study included a stroke-free, unselected population with a high prevalence of cardiovascular risk factors, but it may not be generalizable to other nonurban US populations and further studies are needed in other groups. Using axial images as the only plane for measuring PVS diameters could underestimate the volumes of thin, vertically oriented voids of >3 mm, but the use of FLAIR to look for evidence of gliotic changes suggestive of infarction minimizes misclassification. The lack of prospective data hampers inferences about the cause of PVS. More important, it remains unknown whether PVS have a role as a biomarker for identifying individuals at high risk of developing cerebrovascular disease that would require a different diagnostic and treatment approach. Additional limitations to this study include the survivor bias of the MR imaging cohort and a lack of data on the inter-rater reliability of our PVS scoring system, though the intrarater reliability was high.
Conclusions
In this population-based race/ethnically diverse sample, we found that PVS were associated with age and several vascular risk factors and were strongly related to carotid plaque. Prospective data are needed to confirm the importance of these subclinical MR imaging findings in relation to cardiovascular risk, dementia, and other clinical outcomes.
ABBREVIATIONS:
- PVS
perivascular spaces
- HTN
hypertension
Footnotes
Disclosures: Jose Gutierrez—UNRELATED: Grants/Grants Pending: American Heart Association. Tatjana Rundek—UNRELATED: Grant: National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS),* Comments: R01 and K24. Mitchell S.V. Elkind—RELATED: Grant: Bristol-Myers Squibb (BMS)-Sanofi Partnership,* diaDexus Inc,* Comments: research on stroke biomarkers, Consulting Fee or Honorarium: BMS-Pfizer Partnership, Comments: use of anticoagulants for stroke prevention, Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees, and the Like: Jarvik Heart, Comments: Clinical Event Committee for an artificial heart trial, Other: Organon, Boehringer-Ingelheim, Comments: NuvaRing and stroke; Pradaxa and hemorrhage, UNRELATED: Board Membership: American Heart Association, American Stroke Association, Comments: board membership, New York City and Founders Affiliate, Expert Testimony: Organon, Boehringer-Ingelheim, Comments: Nuvaring and stroke, PRADAXA and hemorrhage, Payment for Development of Education Presentations: QuantiaMD, Comments: cholesterol and stroke prevention, Other: American Academy of Neurology, Comments: Associate Editor of society journal, Neurology. Ralph L. Sacco—RELATED: Grant: NIH,* Comments: Northern Manhattan Study NINDS grants (NINDS R37 NS029993). Clinton B. Wright—RELATED: NIH,* Comments: grants (K02 NS 059729; R01 HL108623), UNRELATED: Consultancy: Merck, Comments: stroke adjudication for clinical trial, Grants/Grants Pending: NIH, American Heart Association, Royalties: UpToDate, Comments: author of 2 chapters on vascular dementia. *Money paid to the institution.
This study is supported by the NIH/NINDS (grant: R37 NS 29993).
REFERENCES
- 1.Durand-Fardel M. Traite Clinique et Pratique des Maladies des Vieillards. Paris, France: Germer Bailliere; 1854 [Google Scholar]
- 2.Poirier J, Derouesne C. The concept of cerebral lacunae from 1838 to the present [in French]. Rev Neurol (Paris) 1985;141:3–17 [PubMed] [Google Scholar]
- 3.Greenfield JG, Blackwood W, Corsellis JA. Greenfield's Neuropathology. 3rd ed. London: E. Arnold; 1976:239 [Google Scholar]
- 4.Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol 1981;240:F329–36 [DOI] [PubMed] [Google Scholar]
- 5.Millen JW, Woollam DH. The reticular perivascular tissue of the central nervous system. J Neurol Neurosurg Psychiatry 1954;17:286–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pullicino PM, Miller LL, Alexandrov AV, et al. Infraputaminal ‘lacunes’: clinical and pathological correlations. Stroke 1995;26:1598–602 [DOI] [PubMed] [Google Scholar]
- 7.Wuerfel J, Haertle M, Waiczies H, et al. Perivascular spaces–MRI marker of inflammatory activity in the brain? Brain 2008;131:2332–40 [DOI] [PubMed] [Google Scholar]
- 8.Cumurciuc R, Guichard JP, Reizine D, et al. Dilation of Virchow-Robin spaces in CADASIL. Eur J Neurol 2006;13:187–90 [DOI] [PubMed] [Google Scholar]
- 9.Rollins NK, Deline C, Morriss MC. Prevalence and clinical significance of dilated Virchow-Robin spaces in childhood. Radiology 1993;189:53–57 [DOI] [PubMed] [Google Scholar]
- 10.Zhu YC, Tzourio C, Soumare A, et al. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41:2483–90 [DOI] [PubMed] [Google Scholar]
- 11.Maclullich AM, Wardlaw JM, Ferguson KJ, et al. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 2004;75:1519–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 1998;147:259–68 [DOI] [PubMed] [Google Scholar]
- 13.Boden-Albala B, Cammack S, Chong J, et al. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS). Diabetes Care 2008;31:1132–37 [DOI] [PubMed] [Google Scholar]
- 14.Rundek T, Arif H, Boden-Albala B, et al. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology 2008;70:1200–07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann A, Mast H, Thompson JL, et al. Transcranial Doppler waveform blunting in severe extracranial carotid artery stenosis. Cerebrovasc Dis 2000;10:33–38 [DOI] [PubMed] [Google Scholar]
- 16.Hughes W. Hypothesis. Lancet 1965;286:19–21 [Google Scholar]
- 17.Hughes W, Dodgson MC, Maclennan DC. Chronic cerebral hypertensive disease. Lancet 1954;267:770–74 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004;43:1239–45 [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res 2009;3:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awad IA, Johnson PC, Spetzler RF, et al. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke 1986;17:1090–97 [DOI] [PubMed] [Google Scholar]
- 21.Cole FM, Yates PO. Comparative incidence of cerebrovascular lesions in normotensive and hypertensive patients. Neurology 1968;18:255–59 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Masawa N, Takatama M. Pathogenesis of état criblé in experimental hypertensive rats. J Stroke Cerebrovasc Dis 2001;10:106–12 [DOI] [PubMed] [Google Scholar]
- 23.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology 1965;15:774–84 [DOI] [PubMed] [Google Scholar]
- 24.El-Barhoun EN, Gledhill SR, Pitman AG. Circle of Willis artery diameters on MR angiography: an Australian reference database. J Med Imaging Radiat Oncol 2009;53:248–60 [DOI] [PubMed] [Google Scholar]
- 25.Noren D, Palmer HJ, Frame MD. Predicted wall shear rate gradients in T-type arteriolar bifurcations. Biorheology 2000;37:325–40 [PubMed] [Google Scholar]
- 26.Patankar TF, Mitra D, Varma A, et al. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol 2005;26:1512–20 [PMC free article] [PubMed] [Google Scholar]
- 27.Groeschel S, Chong WK, Surtees R, et al. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology 2006;48:745–54 [DOI] [PubMed] [Google Scholar]
- 28.Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol 1998;245:116–22 [DOI] [PubMed] [Google Scholar]
- 29.Pollock H, Hutchings M, Weller RO, et al. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat 1997;191(pt 3):337–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jungreis CA, Kanal E, Hirsch WL, et al. Normal perivascular spaces mimicking lacunar infarction: MR imaging. Radiology 1988;169:101–04 [DOI] [PubMed] [Google Scholar]
- 31.Schick S, Gahleitner A, Wober-Bingol C, et al. Virchow-Robin spaces in childhood migraine. Neuroradiology 1999;41:283–87 [DOI] [PubMed] [Google Scholar]
- 32.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics 2007;27:1071–86 [DOI] [PubMed] [Google Scholar]
- 33.Achiron A, Faibel M. Sandlike appearance of Virchow-Robin spaces in early multiple sclerosis: a novel neuroradiologic marker. AJNR Am J Neuroradiol 2002;23:376–80 [PMC free article] [PubMed] [Google Scholar]
- 34.Elster AD, Richardson DN. Focal high signal on MR scans of the midbrain caused by enlarged perivascular spaces: MR-pathologic correlation. AJR Am J Roentgenol 1991;156:157–60 [DOI] [PubMed] [Google Scholar]