SUMMARY
Binding of insulin receptor substrate proteins 1 and 2 (IRS1/2) to the insulin receptor (IR) is essential for the regulation of insulin sensitivity and energy homeostasis. However, the mechanism of IRS1/2 recruitment to the IR remains elusive. Here, we identify adaptor protein APPL1 as a critical molecule that promotes IRS1/2-IR interaction. APPL1 forms a complex with IRS1/2 under basal conditions, and this complex is then recruited to the IR in response to insulin or adiponectin stimulation. The interaction between APPL1 and IR depends on insulin- or adiponectin-stimulated APPL1 phosphorylation, which is greatly reduced in insulin target tissues in obese mice. appl1 deletion in mice consistently leads to systemic insulin resistance and a significant reduction in insulin-stimulated IRS1/2, but not IR, tyrosine phosphorylation, indicating that APPL1 sensitizes insulin signaling by acting at a site downstream of the IR. Our study uncovers a mechanism regulating insulin signaling and crosstalk between the insulin and adiponectin pathways.
INTRODUCTION
The adaptor protein APPL1 interacts with adiponectin receptors and plays a critical role in mediating the insulin-sensitizing effect of adiponectin in muscle (Mao et al., 2006) and endothelial cells (Cheng et al., 2007). A number of studies also suggest that APPL1 has a direct effect on insulin signaling in cells. Suppression of APPL1 by RNAi impaired insulin-stimulated Akt activation and membrane translocation of GLUT4 in L6 myocytes and 3T3-L1 adipocytes (Mao et al., 2006; Saito et al., 2007). In addition, overexpression of APPL1 in mouse liver potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes, while suppressing APPL1 expression in mouse liver leads to glucose intolerance (Cheng et al., 2009). However, the underlying mechanisms remain unclear.
APPL1 contains multiple function domains, including the Bin1/amphiphysin/rvs167 (BAR) domain, the pleckstrin homology (PH) domain, the phosphotyrosine binding (PTB) domain, and the CC motif (Deepa and Dong, 2009). Accumulating data suggest that APPL1 could function as a platform orchestrating multiple signaling pathways (Deepa and Dong, 2009). Acting as an anchoring protein, APPL1 facilitates LKB1 translocation from the nucleus to the cytosol, where it phosphorylates AMP-activated protein kinase (AMPK) in response to adiponectin stimulation (Fang et al., 2010; Zhou et al., 2009). APPL1 also mediates adiponectin-stimulated p38 mitogen-activated protein kinase (MAPK) activation by scaffolding the TAK1/MKK3/p38 MAPK cascade (Xin et al., 2011). By interacting with TRB3, an endogenous Akt inhibitor, APPL1 has been shown to enhance insulin-stimulated Akt activity (Cheng et al., 2009; Mitsuuchi et al., 1999; Saito et al., 2007; Yang et al., 2003).
In the current study, we show that knockout (KO) of APPL1 in mice reduced insulin and adiponectin signaling and led to systemic insulin resistance. We found that APPL1 interacts with insulin receptor substrate proteins 1 and 2 (IRS1/2) and promotes IRS1/2 proteins to interact with the insulin receptor (IR) in response to adiponectin or insulin stimulation. In addition, we demonstrate that phosphorylation at Ser401 is critical for APPL1 to mediate the crosstalk between insulin and adiponectin pathways. Our results uncover a mechanism by which APPL1 promotes adiponectin signaling and its insulin-sensitizing effect.
RESULTS
APPL1 Promotes Insulin Sensitivity In Vivo
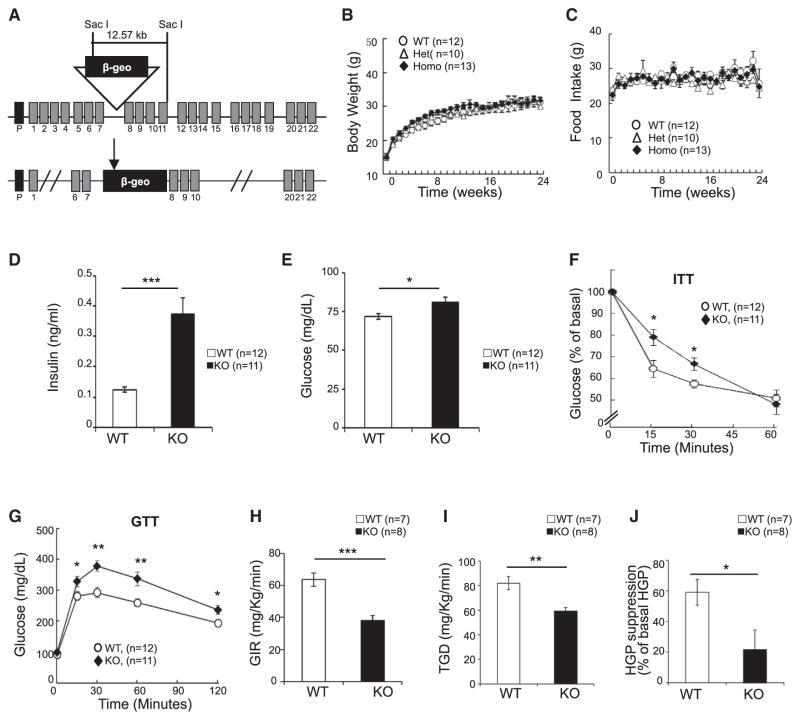
We generated APPL1 KO mice by the gene trap technique (Figures 1A and S1A–S1C). Consistent with a previous report that APPL1 is dispensable for mouse development (Tan et al., 2010b), crossing APPL1 heterozygous mice produced litters with the expected Mendelian ratios and normal body size. APPL1 KO mice are viable and fertile and have no significant differences in body weight (Figure 1B), food intake (Figure 1C), oxygen consumption (Figure S1D), tissue weights (Figure S1E), and respiratory rates (Figure S1F) compared to wild-type littermates. However, KO mice were more active (Figure S1G) and had a higher core body temperature (Figure S1H) and enhanced UCP-1 expression in their brown fat tissues (Figure S1I) compared to their wild-type littermates. KO of the appl1 gene had no significant effect on mouse insulin, adiponectin, and leptin levels as well as lipid profile under fed conditions (Figure S1J). Under fasting conditions, however, both the plasma insulin (Figure 1D) and glucose (Figure 1E) levels of KO mice were significantly higher than those of wild-type littermates. APPL1 KO mice showed impaired insulin (Figure 1F) and glucose (Figure 1G) tolerance and significant reductions in glucose infusion rate (Figure 1H), total glucose disposal (Figure 1I), and insulin-mediated suppression of hepatic glucose production (Figure 1J) during the hyperinsulinemic-euglycemic clamp compared to their wild-type littermates. These results, collectively, demonstrate that mice lacking APPL1 manifest insulin resistance.
Figure 1. Deletion of the appl1 Gene Leads to Insulin Resistance in Mice.
(A) Schematic representation of the genomic structure of the appl1 gene. Insertion of the trapping vector (pGTxf) fragment, which contains a splice acceptor (SA), a β-geo cassette, and a polyadenylation sequence (pA), between exon 7 and exon 8 leads to the disruption of the appl1 gene transcription and expression.
(B) Body weight changes of APPL1 wild-type, heterozygous, and homozygous KO mice were monitored weekly for 24 weeks under normal chow conditions.
(C) Food intake of APPL1 wild-type, heterozygous, and homozygous KO mice was monitored weekly for 24 weeks under normal chow conditions.
(D and E) Fasting serum insulin (D) and glucose (E) levels of wild-type and APPL1 KO mice. (F and G) Insulin tolerance test (ITT) (F) and glucose tolerance test (GTT) (G).
(H–J) Glucose infusion rates (GIR) (H), total glucose disposal (I), and hepatic glucose production (HGP) suppression (J) during hyperinsulinemic-euglycemic clamp.
All results are presented as mean ± SEM. p values were calculated using the Student’s t test (*p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S1.
APPL1 Mediates Insulin and Adiponectin Signaling In Vivo
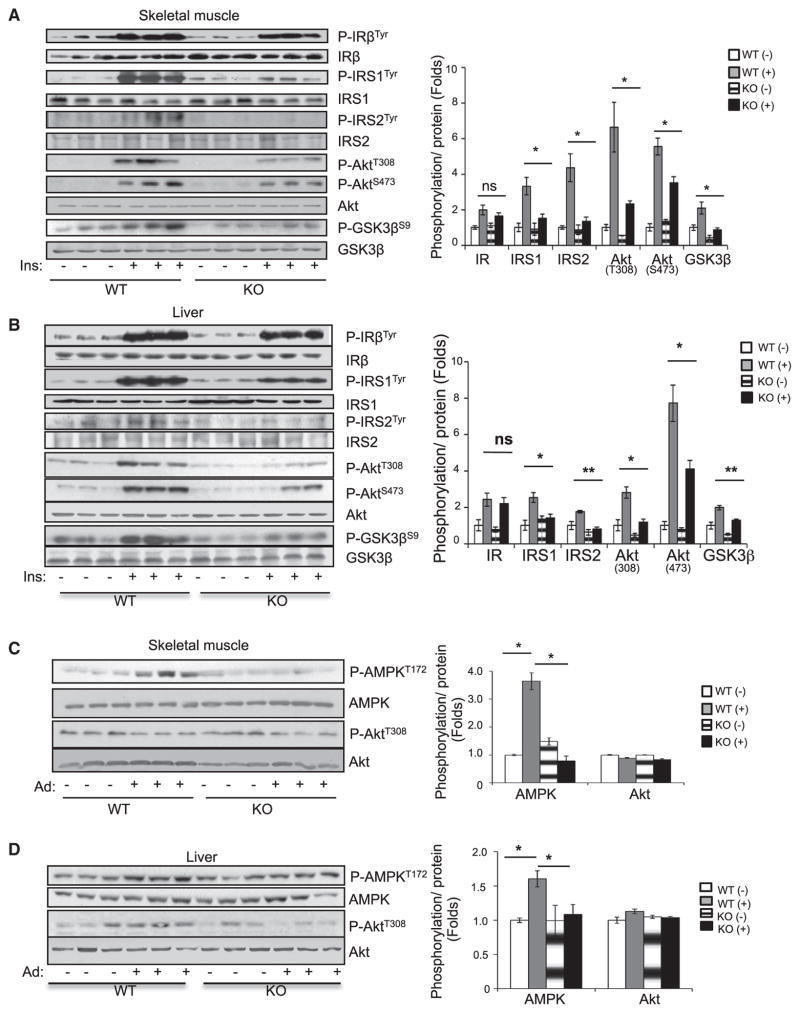
To elucidate the mechanisms underlying APPL1-deficiency-induced insulin resistance, we examined the effect of appl1 gene disruption on insulin signaling in peripheral insulin-responsive tissues. There was no significant difference in basal insulin signaling in skeletal muscle, liver, and adipose tissues between APPL1 KO mice and wild-type littermates (Figures 2A, 2B, and S2A). However, KO mice displayed a marked reduction of insulin-stimulated tyrosine phosphorylation of IRS1/2, phosphorylation of Akt (Thr308 and Ser473), and phosphorylation of glycogen synthase kinase 3 β (GSK3β) (Ser9) in skeletal muscle (Figure 2A), liver (Figure 2B), and adipose tissues (Figure S2A) compared to wild-type littermates, indicating that APPL1 plays a promoting role in insulin action in vivo. Interestingly, disruption of the appl1 gene had no effect on insulin-stimulated tyrosine phosphorylation of insulin receptor subunit β (IRβ) in all peripheral insulin target tissues (Figures 2A, 2B, and S2A), suggesting that APPL1 promotes insulin signaling by acting on a site downstream of the IR along the phosphatidylinositol 3-kinase (PI3K) signaling pathway.
Figure 2. Adiponectin and Insulin Signaling Are Impaired in APPL1 KO Mice.
(A and B) Fasted wild-type and APPL1 KO mice were injected intraperitoneally (i.p.) with insulin (0.5 U/kg of body weight) for 3 min. Insulin signaling was detected in skeletal muscle (A) and liver tissues (B) by western blot analysis.
(C and D) Overnight-fasted wild-type and APPL1 KO mice were refed for 4 hr and then injected (i.p.) with adiponectin (Ad) (5 μg/g of body weight) for 1 hr. Adiponectin and insulin signaling were detected in skeletal muscle (C) and liver tissue (D) by western blot analysis.
All results are presented as mean ± SEM. p values were calculated using the Student’s t test (*p < 0.05, **p < 0.01). See also Figure S2.
Because APPL1 plays an important role in adiponectin signaling (Cheng et al., 2007; Mao et al., 2006), we examined the effect of appl1 disruption on adiponectin action in vivo. The administration of adiponectin stimulated AMPK phosphorylation in skeletal muscle, liver, and adipose tissues of wild-type mice, and the stimulatory effect of adiponectin was significantly reduced in KO mice (Figures 2C, 2D, and S2B) but had no significant effect on Akt phosphorylation in these tissues (Figures 2C, 2D, and S2B), which is consistent with our previous findings that adiponectin alone is not sufficient to stimulate Akt phosphorylation in muscle cells (Mao et al., 2006).
APPL1 Mediates Insulin Signaling via a Direct Binding to Insulin Receptor
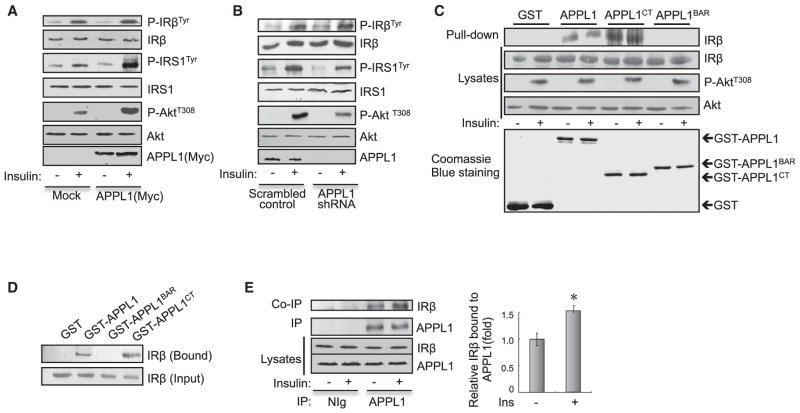
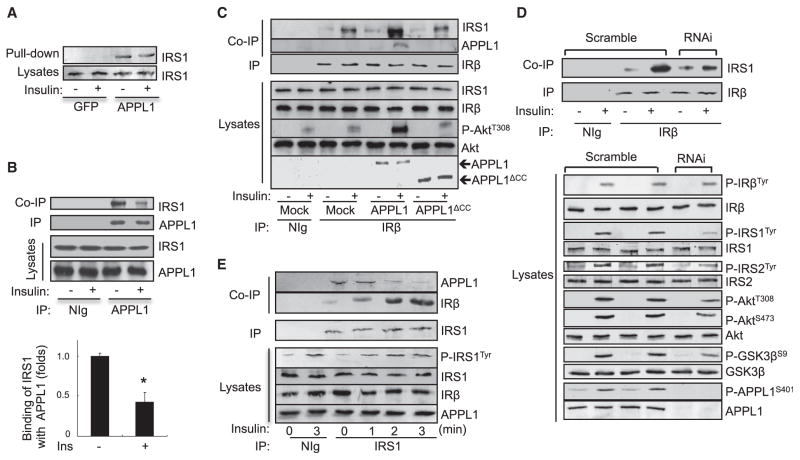
To elucidate the molecular mechanism underlying APPL1 action, we examined whether altering APPL1 expression affects insulin-stimulated phosphorylation of IRβ, IRS1, and Akt in C2C12 myocytes. Overexpression (Figure 3A) or suppression of APPL1 (Figure 3B) potentiated or impaired, respectively, insulin-stimulated tyrosine phosphorylation of IRS1 and Akt Thr308 phosphorylation in C2C12 myocytes. On the other hand, altering the expression levels of APPL1 had little effect on the tyrosine phosphorylation of IRβ (Figures 3A and 3B), confirming that APPL1 promotes insulin signaling at a site downstream of IRβ. Given that APPL1 contains several protein-protein interaction domains (Figure S3A) and acts as a scaffold protein in adiponectin signaling (Xin et al., 2011), we examined whether APPL1 interacts with insulin-signaling molecules. By in vitro pull-down studies, we found that glutathione S-transferase (GST) fused to the C terminus of APPL1 (GST-APPL1CT), but not GST alone or GST fused to the BAR domain of APPL1 (GST-APPL1BAR), interacted with IRβ (Figure 3C). To determine whether APPL1 binds directly to IRβ, we examined the interaction between affinity- purified IRβ (Figures S3B and S3C) and GST-APPL1 fusion proteins (Figure S3D). The affinity-purified IRβ interacted with the GST-APPL1 and the GST-APPL1CT fusion proteins, but not with the GST alone or GST-APPL1BAR fusion proteins (Figure 3D), indicating that APPL1 physically interacts with IRβ directly. In addition, endogenous IRβ was coimmunoprecipitated with endogenous APPL1 in C2C12 myotubes, and the interaction was stimulated by insulin (Figure 3E). Of note, insulin treatment had no effect on the interaction between IRβ and APPL1 in the in vitro pull-down assays (Figure 3C), suggesting that the stimulatory effect of insulin may be a result of insulin-stimulated post-translational modification of APPL1 rather than IR in intact cells.
Figure 3. APPL1 Interacts with IRβ.
(A) C2C12 myoblasts overexpressing Myc-tagged APPL1 were serum starved and treated with or without 100 nM insulin for 3 min. Phosphorylation of IRS1, IRβ, and Akt and protein levels were detected with specific antibodies as indicated.
(B) Scrambled control and APPL1 suppressed C2C12 myotubes were serum starved and treated with or without 10 nM insulin for 3 min. Phosphorylation of IRS1, IRβ, and Akt were detected with specific antibodies as indicated.
(C) Glutathione agarose beads linked with GST, GST-APPL1, APPL1CT, or APPL1BAR fusion proteins were incubated with cell lysates of C2C12 myotubes treated with or without 10 nM insulin for 5 min. Endogenous IRβ associated with GST-APPL1 or GST-APPL1 truncations was detected with antibodies specific for IRβ.
(D) The purified IR was incubated with GST or GST-APPL1 fusion proteins bound to glutathione agarose beads at 4°C overnight. APPL1-associated IRβ was detected by western blot analysis.
(E) Serum-starved C2C12 myotubes were treated with or without 10 nM insulin (3 min). Endogenous APPL1 was immunoprecipitated, and coimmunoprecipitated endogenous IRβ was detected by western blot analysis. Data are shown as mean ± SEM from three independent experiments. p values were calculated using the Student’s t test (*p < 0.05).
See also Figure S3.
There are six tyrosine residues on IRβ, and phosphorylation at these sites is essential for IRβ to interact with Src homology 2 (SH2) or PTB-domain-containing proteins (White and Kahn, 1994). Because APPL1 contains a PTB domain located at the C-terminal part of this protein (Figure S3A) and its interaction with IRβ is stimulated by insulin in intact cells (Figure 3E), we asked whether tyrosine phosphorylation of IRβ is essential for its binding to APPL1. By yeast two-hybrid assays, we found that mutations of the tyrosine phosphorylation sites of IRβ, which were previously shown to disrupt the interaction between IRβ and the SH2-domain-containing protein Grb10 (Dong et al., 1997), had no effect on the interaction between APPL1 and IRβ (Figure S3E), suggesting that the interaction between IRβ and APPL1 is independent of IRβ tyrosine phosphorylation.
APPL1 shares a high sequence homology with its isoform, APPL2 (Miaczynska et al., 2004; Wang et al., 2009). However, APPL1 contains a coil-coiled (CC) region that is absent in APPL2 (Deepa and Dong, 2009). Because APPL2 does not interact with IRβ (data not shown), we asked whether the CC region of APPL1 is essential for the interaction between APPL1 and IRβ. Coimmunoprecipitation experiments revealed that deletion of the CC domain disrupted the interaction between APPL1 and IRβ (Figure S3F), demonstrating that the CC domain is essential for the interaction between APPL1 and IRβ.
The Stimulatory Effect of APPL1 on Insulin Signaling Is IRS Dependent
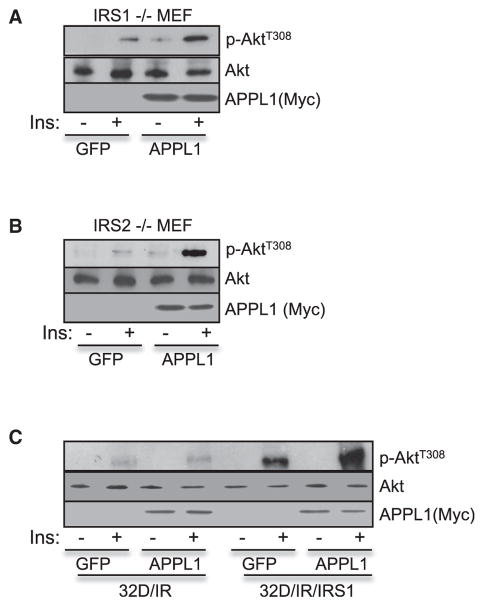
We investigated whether APPL1 is able to substitute IRS1/2 to mediate insulin signaling. While we found that overexpression of APPL1 greatly enhanced insulin-stimulated Akt activation in mouse embryonic fibroblasts (MEFs) lacking IRS1 (Figure 4A) or IRS2 (Figure 4B), overexpression of APPL1 had no effect on insulin-stimulated Akt phosphorylation in 32D/IR cells (Figure 4C), which lack both endogenous IRS1 and IRS2 (Myers et al., 1996), indicating that the effect of APPL1 on insulin signaling depends upon IRS proteins. Consistent with this, overexpression of APPL1 enhanced insulin-stimulated Akt phosphorylation in 32D/IR stably expressing exogenous IRS1 (Myers et al., 1996) (Figure 4C), and KO of APPL1 had no significant effect on epidermal growth factor-stimulated phosphorylation of epidermal growth factor receptor and Akt in vivo (data not shown).
Figure 4. APPL1 Mediates Insulin Signaling in an IRS1/2-Dependent Manner.
MEF cells derived from IRS1 KO mice (A), IRS2 KO mice (B), or 32D/IR and 32D/IR/IRS-1 cells (C) were infected by adenovirus overexpressing GFP or GFP plus APPL1. The infected cells were serum starved for 4 hr and treated with or without 100 nM insulin (5 min). Akt phosphorylation and protein expression levels were detected with specific antibodies as indicated.
APPL1 Interacts with IRS1/2, and the Interaction Is Inhibited by Insulin
To further characterize the interaction between APPL1 and IRS proteins, we performed in vitro binding studies. We found that the GST-APPL1, but not GST, interacted with IRS1 (Figure 5A). A truncation mapping study indicated that multiple domains of APPL1 (the BAR, PH, and PTB) are involved in the interaction of APPL1 with IRS1 (data not shown). Coimmunoprecipitation studies revealed that endogenous IRS1 interacted with endogenous APPL1 in C2C12 myotubes under the basal condition (Figure 5B). Interestingly, the interaction between APPL1 and IRS1 is inhibited by insulin stimulation (Figure 5B), which is opposite to the interaction between APPL1 and IRβ that is stimulated by insulin (Figure 3E).
Figure 5. APPL1 Facilitates the Recruitment of IRS1 onto IRβ.
(A) Glutathione agarose beads containing GST, GST-APPL1 fusion proteins were incubated with lysates of C2C12 myotubes treated with or without 10 nM insulin (3 min). Endogenous IRS1 associated with GST-APPL1 fusion proteins was detected with an antibody specific to IRS1.
(B) Serum-starved C2C12 myotubes were treated with or without 10 nM insulin (3 min). Endogenous APPL1 was immunoprecipitated with an antibody to APPL1, and the coimmunoprecipitated IRS1 was detected by western blot analysis using an antibody to IRS1. Data are shown as mean ± SEM from three independent experiments. p values were calculated using the Student’s t test (*p < 0.05).
(C) C2C12 myocytes transfected with or without myc-tagged APPL1 or APPL1ΔCC truncation were serum starved and treated with or without 100 nM insulin (3 min). Endogenous IRβ was immunoprecipitated, and coimmunoprecipitated endogenous IRS1 or myc-tagged APPL1 was detected with specific antibodies as indicated.
(D) Scrambled control and APPL1-suppressed C2C12 myotubes were serum starved and treated with or without 10 nM insulin (3 min). Endogenous IRβ was immunoprecipitated, and coimmunoprecipitated IRS1 was detected by western blot analysis with the antibodies as indicated.
(E) Serum-starved C2C12 myotubes were treated with or without 10 nM insulin for the indicated times. Endogenous IRS1 was immunoprecipitated, and coimmunoprecipitated endogenous IRβ and APPL1 were detected by western blot analysis with the antibodies as indicated.
The Binding of APPL1 to IRβ Is Necessary for the Stimulatory Effect of APPL1 on Insulin-Stimulated IRβ-IRS1/2 Interactions and Signaling
We investigated whether APPL1 promotes insulin signaling by facilitating the recruitment of IRS1 onto IRβ, which is a critical step for the transduction of insulin signaling to downstream targets (Taniguchi et al., 2006). We found that overexpression of APPL1 greatly enhanced the insulin-stimulated IRS1 interaction with IRβ in C2C12 cells (Figure 5C). On the other hand, suppression of APPL1 expression markedly reduced the effect of insulin on the interaction (Figure 5D). Together, these data suggest that APPL1 may exert its insulin-sensitization function by promoting IRS1’s interaction with IRβ. To determine whether the binding to IRβ is necessary for the stimulatory effect of APPL1, we examined the effect of APPL1ΔCC, which is unable to interact with IRβ (Figure S3F), on insulin signaling. Overexpression of APPL1ΔCC had no stimulatory effect on the interaction of insulin-stimulated IRS1 with IRβ and Akt phosphorylation (Figure 5C), indicating that the binding of APPL1 to IRβ is essential for the insulin-sensitizing effect of APPL1.
APPL1 Coordinates the Interaction of IRβ with IRS1
To determine if the APPL1/IR/IRS1 complex is dynamically regulated by insulin, we examined the kinetics of insulin-induced APPL1/IRS1 dissociation and IRS1/IRβ interaction. Insulin stimulation led to a time-dependent dissociation between APPL1 and IRS1 concurrently with a time-dependent increase in IRS1 tyrosine phosphorylation and the interaction between IRβ and IRS1 (Figure 5E). Together, these results suggest that APPL1 may function as a carrier to bring IRS1 to IRβ in response to insulin stimulation.
APPL1 Ser401 Phosphorylation Correlates with Insulin Sensitivity
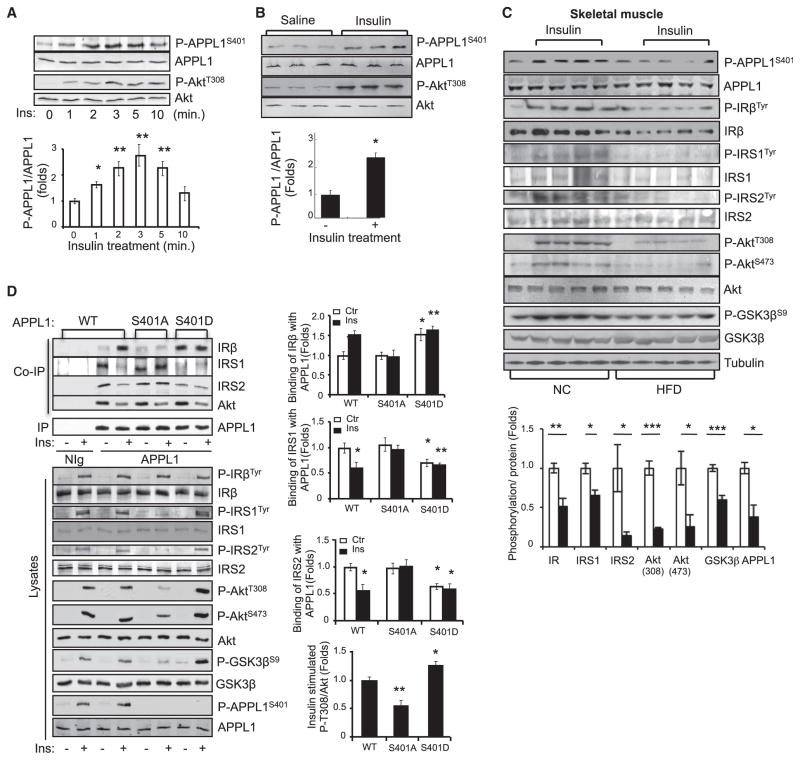
APPL1 contains several potential phosphorylation sites including Ser151, Ser401, Ser427, and Ser430 (Gant-Branum et al., 2010) that may be subjected to insulin- or adiponectin-stimulated phosphorylation. Among these potential phosphorylation sites, Ser401 is highly conserved in APPL1 among different species, and this residue is absent in the corresponding location of its isoform APPL2 (Figure S4A); the latter does not interact with the IR (data not shown). To determine the potential roles of APPL1 phosphorylation, we generated a phosphospecific antibody to Ser401 of APPL1 (Figure S4B). By western blot analysis using this antibody, we found that APPL1 phosphorylation at Ser401 is rapidly stimulated by insulin in C2C12 cells (Figure 6A) and in mouse skeletal muscle tissues (Figure 6B). The insulin-stimulated APPL1 Ser401 phosphorylation was significantly reduced in insulin target tissues of mice fed a high-fat diet compared to mice fed with normal chow, which was associated with an impaired PI3K signaling pathway (Figures 6C, S4C, and S4D). Together, these results indicate a correlation between APPL1 phosphorylation at Ser401 and insulin sensitivity in vivo.
Figure 6. Ser401 Phosphorylation Is Essential for the Promoting Effect of APPL1 on Insulin Signaling.
(A) C2C12 myotubes were serum starved and treated with insulin (10 nM) for the indicated times. Phosphorylation of APPL1 and Akt were detected by western blot analysis with the specific antibodies indicated.
(B) C57BL/6 mice were fasted and injected with saline or insulin (0.5 U/kg of body weight, 3 min). The phosphorylation of APPL1 and Akt and their protein expression in muscle tissues were detected by western blot analysis. n = 9 per group.
(C) Normal chow (NC) and high-fat diet (HFD)-fed male C57BL/6 mice were fasted overnight and treated with or without insulin (0.5 U/kg of body weight, 3 min). The phosphorylation of APPL1 and APPL1 protein in muscle tissues was detected by western blot analysis. Phosphorylation of IRβ, IRS1, and IRS2 was detected after immunoprecipitation with indicated antibodies, respectively. Bar graphs represent the ratios of insulin-stimulated phosphorylation over their total protein levels. n = 4 per group.
(D) APPL1-suppressed C2C12 myoblasts were transfected with myc-tagged and RNAi-resistant wild-type (WT), S401A, or S401D mutants of APPL1. The cells were serum starved and treated with or without 100 nM insulin (3 min). WT and mutants of APPL1 were immunoprecipitated with an anti-myc antibody. Bar graphs represent the binding of IRβ, IRS1, or IRS2 to APPL1 protein. Data are mean ± SEM from three independent experiments.
All results are presented as mean ± SEM. p values were calculated using the Student’s t test (*p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S4.
Ser401 Phosphorylation Regulates APPL1 Binding to IRβ and Dissociation with IRS1
To investigate the role of Ser401 phosphorylation in mediating the insulin-sensitizing role of APPL1, we overexpressed RNAi-resistant wild-type and phosphorylation mutants (S401A and S401D) of APPL1 in APPL1-suppressed C2C12 cells. Consistent with the notion that the binding of APPL1 to IRβ sensitizes insulin signaling by acting at a site downstream of the IR (Figures 2A, 2B, and S2A), overexpression of either APPL1S401A or APPL1S401D had no effect on insulin-stimulated IRβ tyrosine phosphorylation (Figure 6D). However, replacing Ser401 with alanine greatly impaired the ability of APPL1 to bind with IRβ in response to insulin stimulation, concurrent with a loss of response to insulin-stimulated dissociation from IRS1 and IRS2 (Figure 6D). Consequently, the promoting effect of APPL1 on insulin-stimulated tyrosine phosphorylation of IRS1/2, phosphorylation of Akt at both Thr308 and Ser473, and GSK3β activation was significantly reduced by mutating serine401 of APPL1 to alanine (Figure 6D). Conversely, the S401D mutant of APPL1 showed high binding affinity to IRβ, which occurs concurrently with a reduced interaction with IRS proteins and enhanced insulin signaling compared to wild-type APPL1 (Figure 6D). Taken together, these results clearly demonstrate that phosphorylation at Ser401 plays a key role in mediating the insulin-regulated binding of APPL1 with IRβ and dissociation with IRS proteins.
APPL1 has previously been shown to bind to inactive Akt (Mitsuuchi et al., 1999). Consistent with this finding, we found that APPL1 interacted with Akt under basal conditions (Figure 6D). Like IRS1/2, Akt dissociates from the APPL1-IRβ complex in response to insulin stimulation (Figure 6D). These results suggest that in addition to facilitating IRS1/2’s interaction with the IR, APPL1 also promotes Akt translocation to the plasma membrane for insulin-stimulated activation. Interestingly, neither S401A nor S401D mutant had any effect on APPL1/Akt interaction compared with wild-type protein (Figure 6D), suggesting that phosphorylation at Ser401 is not essential for regulating APPL1/Akt binding.
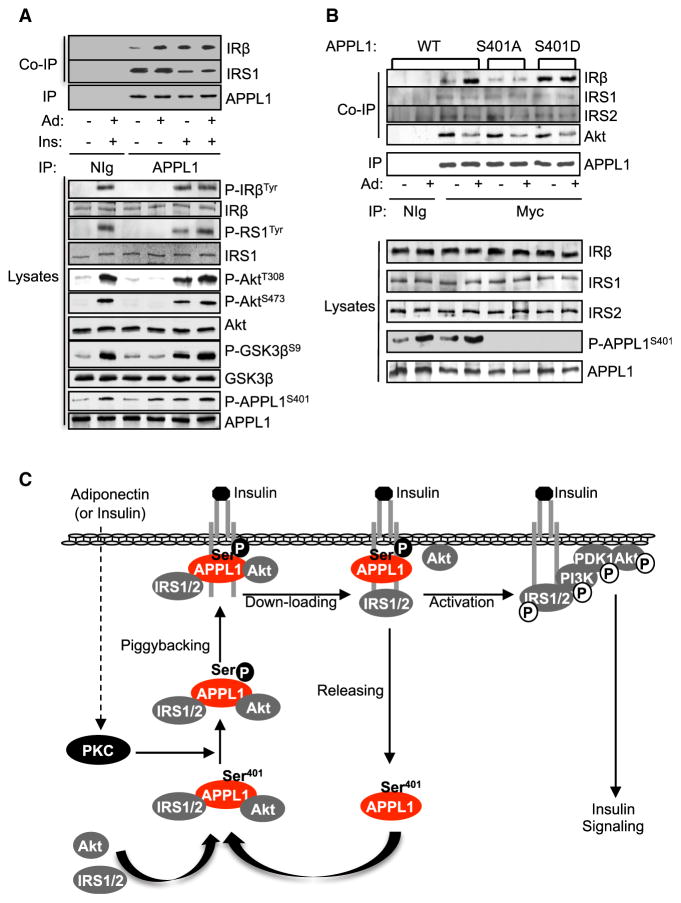
Adiponectin Promotes Ser401 Phosphorylation and APPL1-IRβ Interaction
To determine whether adiponectin has an effect on APPL1 phosphorylation and interaction with IRβ, we treated C2C12 cells with adiponectin alone or together with insulin. Adiponectin treatment greatly stimulated APPL1 phosphorylation at Ser401 and enhanced APPL1 interaction with IRβ (Figures 7A and S5A). No synergistic effect of adiponectin and insulin on APPL1 phosphorylation and its binding to IRβ was observed (Figure 7A), suggesting that a common mechanism may be used by insulin and adiponectin to induce APPL1 phosphorylation. In agreement with this view, treating C2C12 cells with the protein kinase C (PKC) inhibitor Gö6983, but not inhibitors for GSK3β (LiCl), mitogen-activated protein kinase kinase (PD98059), or PI3K (LY-294002), blocked both insulin- and adiponectin-induced Ser401 phosphorylation (Figures S5B and S5C). Taken together with the findings that phorbol ester phorbol-12-myristate-13-acetate stimulated the phosphorylation (Figure S5D) and that the insulin- and adiponectin-stimulated phosphorylation was inhibited by Gö6976 (Figure S5E), a selective inhibitor of Ca2+-dependent PKC isoforms such as PKCα and PKCβI, it is conceivable that a conventional PKC isoform may mediate both insulin- and adiponectin-stimulated APPL1 phosphorylation at Ser401.
Figure 7. Adiponectin Stimulates the Interaction between APPL1 and IRS1.
(A) Serum-starved C2C12 myotubes were pre-treated with or without adiponectin (1 μg/ml, 10 min) and then with or without insulin (10 nM, 3 min). Endogenous APPL1 was immunoprecipitated. Coimmunoprecipitated IRβ or IRS1 and phosphor-ylated IR, IRS1, Akt, GSK3β, or APPL1 and their protein levels in cell lysates were detected by western blot analysis with specific antibodies as indicated.
(B) APPL1-suppressed C2C12 myocytes were transfected with myc-tagged and RNAi-resistant WT, S401A, or the S401D mutant form of APPL1. Transfected cells were serum starved and treated with adiponectin (1 μg/ml, 10 min). WT and mutant APPL1 were immunoprecipitated with an anti-myc antibody. Coimmunoprecipitated IRβ, IRS1, or IRS2 and their protein levels and phosphorylated APPL1 in the cell lysates were determined by western blot analysis using specific antibodies to these proteins.
(C) The model of the sensitization role of APPL1 in insulin signaling.
See also Figure S5.
To investigate the role of adiponectin-stimulated APPL1 phosphorylation in regulating APPL1/IRβ interaction, we overexpressed RNAi-resistant wild-type and phosphorylation mutants (S401A and S401D) of APPL1 in APPL1-suppressed C2C12 cells. Replacing Ser401 with alanine greatly impaired adiponectin-stimulated APPL1 binding with IRβ (Figure 7B). Conversely, the S401D mutant of APPL1 displayed an enhanced ability to bind with IRβ (Figure 7B). Taken together, these results indicate that phosphorylation at Ser401 provides a key mechanism by which adiponectin regulates the binding of APPL1 with IRβ and IRS1/2.
While insulin treatment triggered the dissociation between APPL1 and IRS1/2 (Figures 6D and 7A), we found that adiponectin had no effect on APPL1/IRS1 dissociation (Figure 7A). Time course studies also revealed that adiponectin treatment did not affect the interaction between IRS1 and APPL1 or IRβ (Figure S5F). These results are consistent with our previous finding that adiponectin by itself does not stimulate Akt phosphorylation in cells (Mao et al., 2006). In agreement with these results, adiponectin treatment alone did not stimulate the tyrosine phosphorylation of IRβ and IRS1 or the serine/threonine phosphorylation of Akt and GSK3β (Figure 7A).
DISCUSSION
In this study, we demonstrate that APPL1 interacts with both IRβ and IRS1/2 and acts as an adaptor protein to facilitate the insulin-stimulated recruitment of IRS1/2 onto IRβ (Figure 7C). Under basal conditions, APPL1 forms a complex with IRS1/2 and Akt in the cytosol. The insulin- or adiponectin-induced APPL1 Ser401 phosphorylation promotes APPL1 binding directly to the IR, allowing APPL1 to piggyback IRS proteins onto the IR. Insulin stimulation further induces the dissociation of IRS proteins and Akt from the APPL1-IR complex, facilitating the binding of IRS1/2 to the activated IR near the plasma membrane. Our study thus reveals a mechanism underlying the role of APPL1 in sensitizing the insulin-stimulated PI3K pathway.
APPL1 has previously been shown to be phosphorylated at Ser401 in human skeletal muscle (Holmes et al., 2011). However, the role of this phosphorylation is unknown. We found that Ser401 phosphorylation is significantly impaired in skeletal muscle, liver, and adipose tissues of mice fed a high-fat diet, suggesting that impairment of this phosphorylation may have a pathophysiological role in metabolism. Consistent with this view, Ser401 phosphorylation plays a key role in regulating the interaction between APPL1 and IRβ or IRS1/2. Residue Ser401 is located in a region between the PH domain and the C terminus of APPL1. Of note, the C-terminal part of APPL1 is essential for the interaction of APPL1 with its multiple binding partners, including AdipoR1, DCC, and follicle-stimulating hormone receptor. Interestingly, all of these interactions appear to be independent of the tyrosine phosphorylation of the receptors (Liu et al., 2002; Mao et al., 2006; Nechamen et al., 2004). Our current study shows that APPL1 binds to wild-type and tyrosine phosphorylation site mutants of IRβ to a similar extent in the yeast two-hybrid system. In addition, the pull-down study reveals that APPL1 binds with a similar affinity to IRβ expressed in C2C12 myotubes treated with or without insulin. These data suggest that the regulation of APPL1/IR interaction is via postmodification of APPL1 rather than tyrosine phosphorylation of IR. It is interesting to note that APPL2, which does not contain Ser401, did not interact with IRβ in cells (data not shown). Because IR binds to the CC region of APPL1, phosphorylation at Ser401 could induce a conformational change, which makes the C-terminal region more accessible for the interaction with the receptor. Further studies on the structure of the APPL1 Ser401 mutant or the complex between the CC region of APPL1 and its binding partners may shed light on the molecular mechanism regulating this type of interaction.
Adiponectin promotes APPL1 Ser401 phosphorylation but does not stimulate insulin signaling in C2C12 cells or in vivo, demonstrating that this phosphorylation alone is not sufficient to activate IR downstream signaling events. One possible explanation is that while phosphorylated APPL1 is able to deliver IRS1/2 onto the IRβ, the tyrosine phosphorylation of IRS proteins and the activation of downstream signaling events depend on the kinase activation of IRβ, which is stimulated by insulin. Consistent with this view, adiponectin treatment alone has no stimulatory effect on the insulin-stimulated activation of IRS1 and Akt in cells from muscle, liver, and heart (Fang et al., 2010; Mao et al., 2006; Wang et al., 2007a; Yamauchi et al., 2001). These results reveal distinctive roles of adiponectin and insulin in regulating the interaction between IRS1/2 and APPL1 and provide a mechanism by which adiponectin sensitizes insulin signaling.
In addition to the IR and IRS1/2, APPL1 has previously been shown to interact with other components of the insulin-signaling pathway, including the p110 subunit of PI3K and Akt (Mitsuuchi et al., 1999). The interaction between APPL1 and Akt has been shown to prevent Akt from binding to its inhibitor, TRB3, thus promoting the membrane translocation of Akt (Cheng et al., 2009). Interestingly, insulin has been found to cause the dissociation of APPL1 from Akt (Mitsuuchi et al., 1999; Saito et al., 2007). Our study indicated that both insulin and adiponectin could induce dissociation of the APPL1-Akt complex. However, the binding of APPL1 with Akt is independent of Ser401 phosphorylation, suggesting that APPL1 interacts with Akt or IRS1/2 via distinct mechanisms. It is currently unknown whether APPL1 functions as a carrier to facilitate the interaction between Akt and its binding partners/substrates. Nevertheless, these findings imply that APPL1 may promote insulin signaling by orchestrating the essential protein-protein interactions in the signaling pathway, thus controlling the efficiency and specificity of the insulin-signaling transduction.
APPL1 is located in the nucleus, endosome, and cytosol in various cells (Miaczynska et al., 2004; Saito et al., 2007). The APPL1-positive endosomes, which reside at the cell edge (Zoncu et al., 2009), have been suggested to be involved in cargo trafficking (Urbanska et al., 2011) and Akt substrate selection (Schenck et al., 2008). Because the IR and its downstream molecules, including IRS1, are found in endosomes (Kublaoui et al., 1995; Wang et al., 1996), it is possible that the APPL1-associated endosomes play a role in regulating insulin signaling. We previously found that APPL1 selectively promotes the PI3K/Akt, but not the ERK1/2, singling pathway in C2C12 cells (Mao et al., 2006). In addition, APPL1 interacts with IRS1/2 in the cytosol, and the interaction is regulated by APPL1 Ser401 phosphorylation. Interestingly, this phosphorylation has no effect on the interaction between APPL1 and Akt, which has been shown to occur in the endosome (Schenck et al., 2008). Thus, there seem to be distinct cytosolic and endosomal pools of APPL1 that may play important roles in regulating the substrate selectivity and signaling specificity in cells. One possible model is that cytosolic APPL1 carries IRS1/2 onto IR on the cell surface, facilitating insulin-stimulated IR-IRS1/2 interaction. APPL1-positive endosomes, however, may contribute to IRS1/2 endocytosis after rapid activation by plasma membrane IR. Because internalized IR is highly active (Wang et al., 1996), APPL1-positive endosomes may bring activated IRS proteins in close proximity to the intercellular IR to further sensitize insulin signaling. Consistent with this view, internal-membrane-associated IRS1 has been found to undergo insulin-stimulated tyrosine phosphorylation (Kublaoui et al., 1995). It is possible that adiponectin sensitizes insulin signaling by promoting IRS1 endocytosis via APPL1-positive endosomes, thus providing a synergistic effect on insulin-stimulated downstream events. Further studies are needed to test this possibility.
Our results show that KO of the appl1 gene in mice reduced insulin sensitivity and impaired insulin and adiponectin signaling in vivo. While these results are in agreement with the finding that APPL1 is essential for insulin action in mouse adipose and liver tissue (Cheng et al., 2009; Saito et al., 2007), it should be noted that some studies showed that disruption of the appl1 gene in mice had no significant effect on insulin sensitivity (Tan et al., 2010a, 2010b). The reason for the discrepancy between our results and those of Tan et al. remains unknown but could be related to the differences in the KO animal models used (gene trapping versus exon 1 or 5 KO), tissues investigated (liver, skeletal muscle, and adipose tissue versus brain, lung, and thymic T cells), and approaches used to detect the effect of APPL1 on insulin signaling (insulin-stimulated versus basal Akt phosphorylation). To the last point, it is interesting to note that in the study by Tan et al. (Tan et al., 2010a), Akt phosphorylation in brain, lung, and thymic T cells was measured under basal conditions. In a subsequent study (Tan et al., 2010b), the effect of APPL1 on insulin-stimulated Akt phosphorylation was measured only in MEFs and not in APPL1 KO mice. On the other hand, we have demonstrated that disruption in appl1 gene expression in mice had a marked effect not only on insulin-stimulated serine/threonine phosphorylation of Akt and GSK3β but also on insulin-stimulated tyrosine phosphorylation of IRS1/2 in different insulin target tissues such as liver, skeletal muscle, and adipose tissue. These results are consistent with our findings and those of others showing that APPL1 plays an important role in regulating in vivo insulin signaling and action in various animal models (Cheng et al., 2009; Cleasby et al., 2011; Schenck et al., 2008; Wang et al., 2011, 2013; Wen et al., 2010).
The mechanisms underlying the insulin-sensitizing effects of adiponectin remain to be fully elucidated. Our results demonstrate that APPL1 potentiates the effect of insulin on IRS1/2, but not IRβ, tyrosine phosphorylation in cells and in vivo, which is consistent with our previous finding that adiponectin, by suppressing the negative effect of S6K on IRS1, promotes insulin-stimulated tyrosine phosphorylation of IRS1, but not the IR (Wang et al., 2007a). Together, these results indicate a direct crosstalk between the adiponectin- and insulin-signaling pathways. Under the in vivo condition, however, adiponectin may sensitize insulin signaling via both direct and indirect mechanisms. Consistent with this view, adiponectin has been shown to reduce the levels of triglycerol and diglycerol and increase insulin secretion in vivo (Holland et al., 2011; Kim et al., 2007; Yamauchi et al., 2001), which may lead to enhanced tyrosine phosphorylation of the IR.
In summary, our study has identified APPL1 as a binding partner of IR and IRS proteins. We have also demonstrated that APPL1 phosphorylation at Ser401, which is downregulated in obesity, plays a key role in regulating the interaction between APPL1 and the IR or IRS1/2 and thus insulin signaling. The finding that Ser401 phosphorylation is stimulated by adiponectin also uncovers a mechanism underlying the crosstalk between the adiponectin- and insulin-signaling pathways.
EXPERIMENTAL PROCEDURES
Plasmids, Adiponectin, and Antibodies
The cDNAs of full-length and truncations of human APPL1 were generated as described previously (Wang et al., 2009). RNAi-resistant APPL1 construct was generated by mutating the codon for Cys368 from TgT to Tgc by PCR and cloned into pcDNA vector. The recombinant globular adiponectin and antibody specific to APPL1 were generated in our lab (Mao et al., 2006; Wang et al., 2009). All other antibodies were obtained from Cell Signaling Technologies. The phosphospecific antibody against APPL1 Ser401 was homemade (Figure S4B). The pan phosphotyrosine antibody (4G10) against tyrosine phosphorylation of IRβ and IRS1/2 proteins was purchased from Millipore.
Generation of APPL1 Knockout Mice
The APPL1-deficient mouse embryonic stem cell (ESC) line, in which the appl1 gene was trapped by the insertion of a cDNA fragment encoding β-geo (containing β-galactosidase and neomycin gene) and a polyadenylation signal between exons 7 and 8, was obtained from Bay Genomics (cell line no. RRS819). To generate APPL1 KO mice, the APPL1 gene-trapped ESCs were microinjected into C57BL/6 blastocysts and chimeras were crossed with C57/BL6 mice. Animals were maintained on a 12 hr light/dark cycle and had free access to standard normal chow and water. All protocols for animal use and euthanasia were reviewed and approved by the University of Texas Health Science Center Animal Care and Use Committee.
Animal Studies
Male mice were used in this study. For the glucose-tolerance test, mice were starved for 16 hr and then injected intraperitoneally with glucose (2 g/kg). Tail vein blood glucose was checked using an automatic glucometer (Rightest GM300; Bionime) at the indicated time. For the insulin-tolerance test, 4-hr-fasted mice were injected intraperitoneally with 0.75 U/kg insulin (Humulin R, Eli Lilly) and tail vein blood glucose was then measured at the indicated time. For the in vivo insulin-stimulation assay, male mice 10 to 12 weeks of age were fasted overnight and injected intraperitoneally with insulin (0.5 U/kg of body weight) or an equal volume of saline. After 3 min, the mice were sacrificed via cervical dislocation. For the high-fat diet treatment, 4-week-old wild-type C57/BL6 mice were fed a high-fat diet (45 kcal% from fat; Research Diets) for 12 weeks. Hyperinsulinemic-euglycemic clamp studies were performed on animals fed with regular chow at 10 weeks of age (n = 7 [wild-type], n = 8 [KO]) as described previously (Wang et al., 2007b). Plasma concentrations of metabolic factors were measured with the Insulin (Mouse) Ultrasensitive EIA (ALPCO Diagnostics), Triglyceride Assay Kit (Cayman Chemical Company), Cholesterol Assay Kit (Cayman Chemical Company), Free fatty Acid Quantification Kit (Biovision) and NEFA-HR(2) (Wako), Adiponectin (Mouse) ELA (ALPCO Diagnostics), HDL and LDL/VLDL Cholesterol Quantification Kit (Biovision), and Leptin (mouse) ElA Kit (Assay Designs).
Statistical Analysis
All results are presented as mean ± SEM, and p values were calculated using the Student’s t test (*p < 0.05, **p < 0.01, and ***p < 0.001).
Supplementary Material
Acknowledgments
This work was supported in part by a Career Development Award from the American Diabetes Association (to L.Q.D.) and NIH grants (R01 DK080344 to L.Q.D., R01 DK76902 to F.L., and NIA T32 AG021890 to A.K.G.).
References
- Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- Cheng KK, Iglesias MA, Lam KS, Wang Y, Sweeney G, Zhu W, Vanhoutte PM, Kraegen EW, Xu A. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417–427. doi: 10.1016/j.cmet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Cleasby ME, Lau Q, Polkinghorne E, Patel SA, Leslie SJ, Turner N, Cooney GJ, Xu A, Kraegen EW. The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signalling pathway. J Endocrinol. 2011;210:81–92. doi: 10.1530/JOE-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009;296:E22–E36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LQ, Farris S, Christal J, Liu F. Site-directed mutagenesis and yeast two-hybrid studies of the insulin and insulin-like growth factor-1 receptors: the Src homology-2 domain-containing protein hGrb10 binds to the autophosphorylated tyrosine residues in the kinase domain of the insulin receptor. Mol Endocrinol. 1997;11:1757–1765. doi: 10.1210/mend.11.12.0014. [DOI] [PubMed] [Google Scholar]
- Fang X, Palanivel R, Cresser J, Schram K, Ganguly R, Thong FS, Tuinei J, Xu A, Abel ED, Sweeney G. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab. 2010;299:E721–E729. doi: 10.1152/ajpendo.00086.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant-Branum RL, Broussard JA, Mahsut A, Webb DJ, McLean JA. Identification of phosphorylation sites within the signaling adaptor APPL1 by mass spectrometry. J Proteome Res. 2010;9:1541–1548. doi: 10.1021/pr901043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RM, Yi Z, De Filippis E, Berria R, Shahani S, Sathyanarayana P, Sherman V, Fujiwara K, Meyer C, Christ-Roberts C, et al. Increased abundance of the adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif (APPL1) in patients with obesity and type 2 diabetes: evidence for altered adiponectin signalling. Diabetologia. 2011;54:2122–2131. doi: 10.1007/s00125-011-2173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublaoui B, Lee J, Pilch PF. Dynamics of signaling during insulin-stimulated endocytosis of its receptor in adipocytes. J Biol Chem. 1995;270:59–65. doi: 10.1074/jbc.270.1.59. [DOI] [PubMed] [Google Scholar]
- Liu J, Yao F, Wu R, Morgan M, Thorburn A, Finley RL, Jr, Chen YQ. Mediation of the DCC apoptotic signal by DIP13 alpha. J Biol Chem. 2002;277:26281–26285. doi: 10.1074/jbc.M204679200. [DOI] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, Testa JR. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18:4891–4898. doi: 10.1038/sj.onc.1203080. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Zhang Y, Aldaz GA, Grammer T, Glasheen EM, Yenush L, Wang LM, Sun XJ, Blenis J, Pierce JH, White MF. YMXM motifs and signaling by an insulin receptor substrate 1 molecule without tyrosine phosphorylation sites. Mol Cell Biol. 1996;16:4147–4155. doi: 10.1128/mcb.16.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechamen CA, Thomas RM, Cohen BD, Acevedo G, Poulikakos PI, Testa JR, Dias JA. Human follicle-stimulating hormone (FSH) receptor interacts with the adaptor protein APPL1 in HEK 293 cells: potential involvement of the PI3K pathway in FSH signaling. Biol Reprod. 2004;71:629–636. doi: 10.1095/biolreprod.103.025833. [DOI] [PubMed] [Google Scholar]
- Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem. 2007;282:32280–32287. doi: 10.1074/jbc.M704150200. [DOI] [PubMed] [Google Scholar]
- Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Tan Y, You H, Coffey FJ, Wiest DL, Testa JR. Appl1 is dispensable for Akt signaling in vivo and mouse T-cell development. Genesis. 2010a;48:531–539. doi: 10.1002/dvg.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, You H, Wu C, Altomare DA, Testa JR. Appl1 is dispensable for mouse development, and loss of Appl1 has growth factor-selective effects on Akt signaling in murine embryonic fibroblasts. J Biol Chem. 2010b;285:6377–6389. doi: 10.1074/jbc.M109.068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Urbanska A, Sadowski L, Kalaidzidis Y, Miaczynska M. Biochemical characterization of APPL endosomes: the role of annexin A2 in APPL membrane recruitment. Traffic. 2011;12:1227–1241. doi: 10.1111/j.1600-0854.2011.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Balba Y, Knutson VP. Insulin-induced in situ phosphorylation of the insulin receptor located in the plasma membrane versus endosomes. Biochem Biophys Res Commun. 1996;227:27–34. doi: 10.1006/bbrc.1996.1462. [DOI] [PubMed] [Google Scholar]
- Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007a;282:7991–7996. doi: 10.1074/jbc.M700098200. [DOI] [PubMed] [Google Scholar]
- Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, et al. Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol Cell Biol. 2007b;27:6497–6505. doi: 10.1128/MCB.00679-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, Chen H, Mao X, Kikani CK, Liu F, Dong LQ. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009;284:31608–31615. doi: 10.1074/jbc.M109.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng KK, Lam KS, Wu D, Wang Y, Huang Y, Vanhoutte PM, Sweeney G, Li Y, Xu A. APPL1 counteracts obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of nitric oxide and endothelin-1 in mice. Diabetes. 2011;60:3044–3054. doi: 10.2337/db11-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li X, Mu K, Li L, Wang S, Zhu Y, Zhang M, Ryu J, Xie Z, Shi D, et al. Deficiency of APPL1 in mice impairs glucose-stimulated insulin secretion through inhibition of pancreatic beta cell mitochondrial function. Diabetologia. 2013;56:1999–2009. doi: 10.1007/s00125-013-2971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Yang Y, Wang Y, Xu A, Wu D, Chen Y. Appl1 is essential for the survival of Xenopus pancreas, duodenum, and stomach progenitor cells. Dev Dyn. 2010;239:2198–2207. doi: 10.1002/dvdy.22356. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- Xin X, Zhou L, Reyes CM, Liu F, Dong LQ. APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3-p38 MAPK pathway. Am J Physiol Endocrinol Metab. 2011;300:E103–E110. doi: 10.1152/ajpendo.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yang L, Lin HK, Altuwaijri S, Xie S, Wang L, Chang C. APPL suppresses androgen receptor transactivation via potentiating Akt activity. J Biol Chem. 2003;278:16820–16827. doi: 10.1074/jbc.M213163200. [DOI] [PubMed] [Google Scholar]
- Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009;284:22426–22435. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.