Abstract
Metabolic syndrome (MetS) is a clustering of vascular risk factors and is associated with increased risk of cardiovascular disease. Less is known about the relationship between MetS and cognition. We examined component vascular risk factors of MetS as correlates of different cognitive domains. The Northern Manhattan Study (NOMAS) includes 1290 stroke-free participants from a largely Hispanic multi-ethnic urban community. We used structural equation modeling (SEM) to model latent variables of MetS, assessed at baseline and an average of 10 years later, at which time participants also underwent a full cognitive battery. The two four-factor models, of the metabolic syndrome (blood pressure, lipid levels, obesity, and fasting glucose) and of cognition (language, executive function, psychomotor, and memory), were each well supported (CFI = 0.97 and CFI = 0.95, respectively). When the two models were combined, the correlation between metabolic syndrome and cognition was −.31. Among the metabolic syndrome components, only blood pressure uniquely predicted all four cognitive domains. After adjusting for age, gender, race/ethnicity, education, smoking, alcohol, and risk factor treatment variables, blood pressure remained a significant correlate of all domains except memory. In this stroke-free race/ethnically diverse community-based cohort, MetS was associated with cognitive function suggesting that MetS and its components may be important predictors of cognitive outcomes. After adjusting for sociodemographic and vascular risk factors, blood pressure was the strongest correlate of cognitive performance. Findings suggest MetS, and in particular blood pressure, may represent markers of vascular or neurodegenerative damage in aging populations.
Keywords: Cognition, Dementia, Hypertension, Aging, Metabolic syndrome, Cardiovascular, Vascular markers
INTRODUCTION
The metabolic syndrome is a clustering of conditions that includes obesity, hypertension, dyslipidemia, and impaired glucose metabolism, and is associated with an increased risk of cardiovascular disease (Galassi, Reynolds, & He, 2006). There is agreement that over a quarter of the U.S. population has metabolic syndrome and its prevalence is rising (Ford, Giles, & Mokdad, 2004). It is believed that the increase is primarily due to lifestyle factors and the well documented rise of obesity (Mokdad et al., 2000).
There is also evidence linking the metabolic syndrome with cognitive decline and dementia, but not all studies have found an association (Muller et al., 2007; Raffaitin et al., 2009; Yaffe et al., 2004). In addition, the relative importance of individual components to cognition is less clear and has varied across studies (Komulainen et al., 2006; Vieira et al., 2011). While hypertension (Novak & Hajjar, 2010), diabetes (Luchsinger et al., 2007), obesity (Dahl et al., 2013), hypertriglyceridemia (Farr et al., 2008), and impaired glucose tolerance (Takahashi et al., 2011) have each been associated with cognitive impairment, ranging from mild cognitive changes to dementia, the relationship between each metabolic risk factor and cognition is complex. Also, generalizability has been limited due to differences in age, ethnicity, sex, and small sample sizes, as well as the inclusion of heterogeneous surrogate measures for primary components and exclusion of measures with established CVD pathophysiological relevance. In addition, many studies to date have focused on the impact of the metabolic syndrome on cognition using brief mental status tests to define cognitive impairment (Viscogliosi et al., 2012).
Quantitative modeling methods have been used to characterize the relationships among metabolic syndrome components (Pladevall et al., 2006). Structural equation modeling (SEM) is well-suited for this purpose and may be used to derive a comprehensive metabolic syndrome model. Shen et al. (2003) and Shen, Goldberg, Llabre, and Schneiderman (2006) used a hierarchical four-factor model in two studies that provided an empirical foundation for conceptualizing and measuring the metabolic syndrome. Similarly, a recent study using three preclinical adult cohorts concluded that a one-factor metabolic syndrome model adequately fit each data set (Stevenson, Wright, & Boydstun, 2012). Taken together, results from these studies suggest that metabolic syndrome represents related domains, with obesity and insulin resistance as integral components.
The role of the metabolic syndrome components in cognition is less well studied using these methods. The purpose of this study is to replicate the latent variable model of metabolic syndrome, test its stability over an average of 10 years in a population-based race/ethnically diverse sample, and estimate the extent to which metabolic syndrome, and its components, is associated with cognition.
METHOD
The Northern Manhattan Study (NOMAS) includes 3298 initially stroke-free participants identified using random digit dialing with dual-frame sampling to identify published and unpublished telephone numbers. Participants were eligible if never diagnosed with stroke, ≥ 40 years, and resident of Northern Manhattan ≥ 3 months in a household with a telephone. Participants were recruited for in-person assessments with an overall response rate of 68%. Data were collected between 1993 and 2001 by trained bilingual research assistants using standardized instruments, review of medical records, physical and neurological examinations by study physicians, and fasting blood samples for glucose and lipids. Study definitions for race-ethnicity, hypertension, diabetes, cardiac disease and other risk factors are reported in Sacco et al. (2001).
Selection of Subsample
Between 2003 and 2008, NOMAS participants were recruited into an magnetic resonance imaging (MRI) sub-study and those who were enrolled received a full neuropsychological assessment. Participants were recruited sequentially during annual follow-up of the sample using the following criteria: (1) remained clinically stroke free; (2) had no contraindications to MRI; (3) age >55; and (4) provided Institutional Review Board-approved informed consent.
Neuropsychological Evaluation
The NOMAS neuropsychological evaluation has been previously described (Siedlecki et al., 2009). In brief, English- and Spanish-speaking participants were given the same neuropsychological evaluation with the exception that the Wide Range Achievement (WRAT; Wilkinson, 1993) was only administered to English speakers and the Word Accentuation Test (WAT; Del Ser et al., 1997) was only administered to Spanish speakers. The cognitive domains assessed included: memory [immediate and delayed verbal memory (12-word list-learning )], visual/ motor (Grooved Pegboard, Color Trails) executive functioning (Odd-Man-Out, Color Trails 2, verbal and category fluency, digit ordering), and language [15-item Boston Naming, Peabody Picture Vocabulary Test/ Test de Vocabulario en Imagenes Peabody-Adaptacion Hispanoamericana, WRAT (English)/ WAT (Spanish)].
Analysis Plan
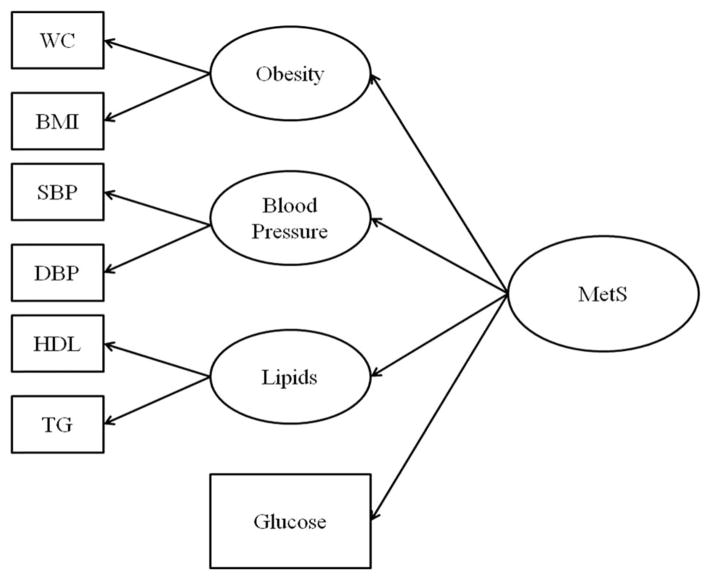
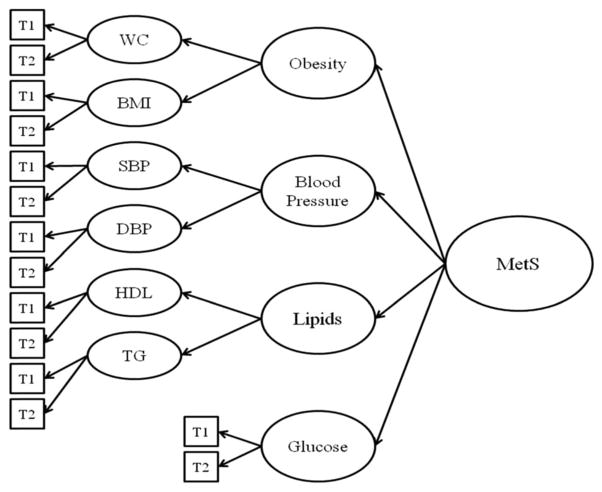
We first modeled the metabolic syndrome as a second order latent variable similar in structure to Shen et al. (2003, 2006) using structural equation modeling where all indicators were treated as continuous variables (Figure 1). The second order latent variable was measured by four first order constructs, the components of the metabolic syndrome: obesity, blood pressure, lipids, and glucose metabolism. Obesity was measured by waist circumference and body mass index (BMI). Systolic (SBP) and diastolic blood pressure (DBP) were the two blood pressure indicators. Lipids were assessed with measures of HDL and triglycerides. Fasting blood glucose was the single indicator of glucose metabolism. This model was tested at two points in time: baseline and at the MRI visit, an average of 10 years later (ranging from 8 to 13 years post baseline assessment. Model fit was determined using the Comparative Fit Index (CFI), Root Mean Squared Error of Approximation (SRMA), and Standardized Root Mean Squared Residual (SRMR). We then combined the two time-specific measures into an overall model that was tested for fit and allowed the assessment of reliability over time (Figure 2).
Fig. 1.
Model of MetS. Note. WC = Waist Circumference, BMI = Body Mass Index, SBP = systolic Blood Pressure, DBP = Diastolic Blood Pressure, HDL = High Lipid Lipoprotein, TG = triglycerides.
Fig. 2.
Model of MetS for both time points. Note. WC = Waist Circumference, BMI = Body Mass Index, SBP = systolic Blood Pressure, DBP = Diastolic Blood Pressure, HDL = High Lipid Lipoprotein, TG = triglycerides.
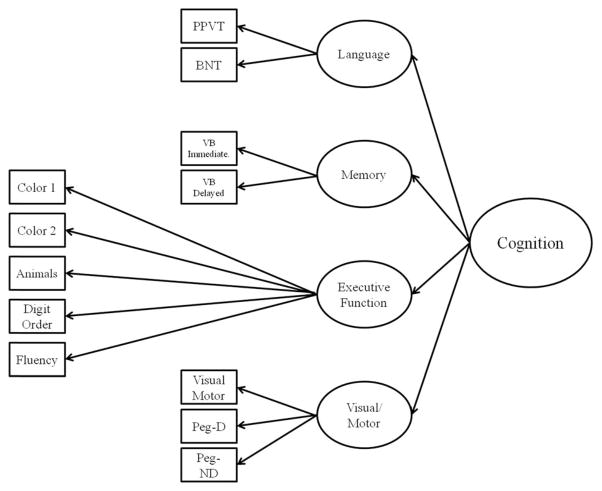
Using similar methodology, we modeled the cognitive measures with a second order latent variable model with the first order represented by the cognitive domains of language, memory, executive function, and visual/motor. Both second order factor models were specified together to assess the ability of metabolic syndrome to predict cognitive performance, controlling for measurement error and domain specific variance (Figure 3).
Fig. 3.
Cognitive Model. Note. PPVT = Peabody Picture Vocabulary Test, BNT = Boston Naming Test, VB = Verbal Memory, Pgeg = Grooved Pegboard.
Finally, we used the components of the metabolic syndrome as predictors of the different cognitive domains to determine the unique contribution of each to the individual cognitive domains. This model was first tested without covariates, and then controlling for age, sex, race/ethnicity, education, smoking, reported alcohol consumption, and use of blood pressure, cholesterol, and diabetes medications.
RESULTS
Descriptive Statistics for all Measures
There were 1290 participants with data on metabolic syndrome variables and cognitive assessments available. Table 1 shows the characteristics of the study sample. There were more women than men. Mean age was 64 years at baseline and 71 at enrollment in the substudy. The sample was ethnically diverse, with a majority of Hispanic participants. Nearly half of the sample were moderate to heavy drinkers, nonsmokers, and had less than a high school education. Over half of the sample was taking blood pressure medication at baseline, but only 16% and 12% were on cholesterol and diabetes medication, respectively.
Table 1.
Sample characteristics and sample means for cardiometabolic risk factors and cognitive variables
| Baseline | 2nd Assessment (N = 1290) | |
|---|---|---|
|
| ||
| 1993–2001 | 2003–2008 | |
| Sample characteristics (%) | ||
| Age (M = 64, SD = 8.4) | ||
| Gender | ||
| Female | 60.8 | – |
| Male | 92.2 | – |
| Race/ethnicity | ||
| Non-Hispanic White | 14.8 | – |
| Non-Hispanic Black | 17.3 | – |
| Hispanic | 65.6 | – |
| Other | 2.3 | – |
| Alcohol consumption | ||
| Non-drinker | 20.5 | – |
| Past-drinker | 20.0 | – |
| Light-drinker | 12.6 | – |
| Moderate-heavy drinker | 47.0 | – |
| Smoking | ||
| Never | 47.5 | – |
| Former | 36.0 | – |
| Current | 16.0 | – |
| Medication | ||
| Blood pressure | 53.0 | – |
| Cholesterol | 16.0 | – |
| Diabetes | 12.0 | – |
| Education | ||
| <8th grade | 40.6 | – |
| Not HS graduate | 13.6 | – |
| HS graduate | 15.5 | – |
| Some college | 14.1 | – |
| College graduate | 12.0 | – |
| Cardiometabolic risk factors M(SD) | ||
| SBP (mmHg) | 141.0 (19.8) | 136.0 (14.5) |
| DBP (mmHg) | 84.0 (10.6) | 78.0 (9.7) |
| HDL cholesterol (mg/dl) | 46.0 (13.9) | 53.0 (17.0) |
| Triglycerides (mg/dl) | 136.0 (81.8) | 127.0 (78.1) |
| BMI (kg/m2) | 28.0 (4.8) | 28.0 (5.0) |
| Waist circumference (in) | 36.6 (5.1) | 38.0 (4.9) |
| Blood glucose (mg/dL) | 102.0 (41.2) | 101.0 (33.9) |
| Cognitive domains M(SD) | ||
| Language | ||
| PPVT | – | 126.0 (43.8) |
| BNT | – | 13.0 (1.8) |
| Memory | ||
| Immediate Verbal Memory | – | 29.0 (7.6) |
| Delayed Verbal Memory | – | 6 (2.6) |
| Executive Function | ||
| Color Trails 2 | – | 178 (71.9) |
| Animals | – | 16 (5.3) |
| Digit Ordering | – | 4 (2.2) |
| Odd Man Out (Items 2 + 4) | – | 12 (4.9) |
| Fluency | – | 28 (12.5) |
| Visual/Motor | ||
| Color Trails 1 | – | 86 (45.9) |
| Grooved Pegboard-Dominant | – | 109 (27.3) |
| Grooved Pegboard-Non-Dominant | – | 114 (25.7) |
Note. SBP = systolic blood pressure; DBP = diastolic blood pressure; BMI = body mass index; PPVT = Peabody Picture Vocabulary Test; BNT = Boston Naming Test.
Table 1 displays sample characteristics as well as the means and standard deviations for the metabolic syndrome components at baseline and at the second assessment and the means and standard deviations for the cognitive domains. In general, this urban sample would be considered overweight, but not obese, with elevated blood pressure, and borderline glucose and lipid levels.
Latent Variable Models
Results of the standardized factor loadings for the first order factors within the second order factor model of metabolic syndrome at each time point are not shown but were all above 0.4. Both models fit the data, and all factor loadings were statistically significant (p <.05). The fit of the model at baseline was confirmed by CFI = .97, RMSEA = .054, and SRMR = .032. Model fit during the MRI visit was confirmed with CFI = .97, RMSEA = .058, and SRMR = .029. The χ2 test is not reported because its sensitivity in our large sample resulted in a significant value for all tests. The model replicated well over the two assessments and factor loadings were consistent over time. With respect to the second order factor, obesity (.60) and lipids (.55) loaded more strongly than did blood pressure (.32) and glucose (.30). Not all indicators were stable over time. The stability reliability of the indicators of obesity (r = .82 and .64 for BMI and waist, respectively) and lipids (.78 and .60 for HDL and triglycerides, respectively) tended to be more stable than measures of blood pressure (.38 and.37 for SBP and DBP, respectively) and blood glucose (.51). This could be partially due to changes in medication use over time.
To capture the stable components of the metabolic syndrome, we specified a third order factor model incorporating the measurements from both assessments. When the indicators were combined into a single model with the indicators at the two different times loading on stable factors for each measure, the specific measure factors loading on the metabolic syndrome components, and the components loading on a third order metabolic syndrome factor, the model fit the data, as evidenced by CFI = .97, RMSEA = .048, and SRMR = .032. All first order factor loadings exceeded 0.40 and were consistent between the baseline and second assessment with the exception of DBP where the 2nd assessment loading was significantly lower. These first order factors represent the shared variance between the two indicators over time and therefore are stable and free from time-based fluctuations. The loadings for the second and third order factors, presented in Tables 2 and 3, are comparable to those for the model at each time point, but are stronger because they are based on the reliable variance.
Table 2.
Standardized factor loadings for second order cardiometabolic syndrome factor model at two time points
| Indicator | Baseline | 2nd Assessment | |
|---|---|---|---|
|
| |||
| 1993–2001 | 2003–2008 | ||
| First order factor | |||
| Obesity | |||
| BMI | 0.75 (.04) | 0.77 (.03) | |
| Waist Circumference | 0.82 (.04) | 0.96 (.04) | |
| Blood pressure | |||
| Systolic BP | 0.70 (.07) | 0.61(.08) | |
| Diastolic BP | 0.82 (.07) | 0.84 (.10) | |
| Lipids | |||
| Triglycerides | 0.54 (.06) | 0.50 (.05) | |
| HDL Cholesterol | −0.76 (.07) | −0.86 (.08) | |
| Glucose | |||
| Fasting blood glucose | 1.00 | 1.00 | |
| Second order factor | |||
| Metabolic syndrome | |||
| Obesity | 0.60 (.08) | 0.68 (.09) | |
| Lipids | 0.55 (.09) | 0.47 (.08) | |
| Blood Pressure | 0.32 (.06) | 0.24 (.05) | |
| Glucose | 0.30 (.06) | 0.25 (.05) | |
Note. BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein.
Table 3.
Standardized factor loadings for third order cardiometa-bolic factor model
| Baseline | 2nd Assessment | |
|---|---|---|
|
| ||
| 1993–2001 | 2003–2008 | |
| First order factor | ||
| BMI (F1) | 0.91 (.03) | 0.90 (.03) |
| Waist circumference (F2) | 0.72 (.03) | 0.88 (.03) |
| Systolic BP (F1) | 0.64 (.08) | 0.60 (.07) |
| Diastolic BP (F2) | 0.74 (.08) | 0.50 (.06) |
| Triglycerides (F1) | 0.77 (.03) | 0.78 (.03) |
| HDL Cholesterol (F2) | 0.88 (.02) | 0.90 (.02) |
| Glucose | 0.69 (.06) | 0.74 (.06) |
| Second order factor | F1 | F2 |
| Obesity | 0.83 (.02) | 1.00 (–) |
| Blood pressure | 0.69 (.07) | 0.84 (.08) |
| Lipids | 0.59 (.05) | −0.95 (.08) |
| Third order factor | ||
| Obesity | 0.73 (.07) | |
| Lipids | 0.47 (.07) | |
| Blood pressure | 0.41 (.06) | |
| Glucose | 0.36 (.06) | |
Note. BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein.
The third order model had each variable with indicators measured at time 1 and time 2. Variable pairs were combined into the second order factors (F1–F2) and finally obesity, blood pressure, lipids, glucose combined into the third order factor.
The second order factor model of cognitive performance also fit the data as evidenced by CFI = .95, RMSEA = .078, and SRMR = .051. The standardized loadings are displayed in Table 4 and were all statistically significant. All standardized loadings exceeded 0.4 for both the first and second order factors. The second order factor was strongly defined by the executive function, language, and visuomotor factors. When the two models were combined, the correlation between metabolic syndrome and cognition was estimated at −0.31. This coefficient represents the association between these two constructs free from measurement error and variance specific to any one component.
Table 4.
Standardized loadings for second order factor model of cognitive performance
| First order factor | Indicator | 2nd Assessment (2003–2008) |
|---|---|---|
| Language | ||
| PPVT | 0.81(.02) | |
| BNT | 0.60 (.02) | |
| Memory | ||
| Immediate Verbal Memory | 0.92 (.02) | |
| Delayed Verbal Memory | 0.82 (.02) | |
| Executive Function | ||
| Color Trails 1 | −0.69 (.02) | |
| Color Trails 2 | −0.79 (.01) | |
| Animals | 0.63 (.02) | |
| Digit Ordering | 0.67 (.02) | |
| Odd Man Out (Items 2 + 4) | 0.71 (.02) | |
| Fluency | 0.76 (.02) | |
| Visual/Motor | ||
| Color Trail 1 | 0.62 (.02) | |
| Grooved Pegboard-Dominant | −0.62 (.02) | |
| Grooved Pegboard-Non-dominant | −0.56 (.03) | |
| Second Order Factor | ||
| Cognitive | ||
| Language | 0.90 (.02) | |
| Memory | 0.61 (.02) | |
| Executive Function | 1.09 (.01) | |
| Visual/Motor | 0.87 (.03) | |
Note. PPVT = Peabody Picture Vocabulary Test; BNT = Boston Naming Test.
Predicting Cognition from Metabolic Factors
Standardized regression coefficients associated with regressing the four cognitive domains on sociodemographic and lifestyle factors and the metabolic syndrome components are shown in Table 5. First, considering the non-metabolic syndrome variables, older age was significantly associated with worse memory, executive function, and visual/motor skills, but was not related to language. Women had better memory but did less well in language than men. Relative to whites, blacks and Hispanics did worse on language, memory, and executive function. More years of formal schooling was significantly associated with better performance across all domains. Participants who reported being current smokers performed worse in memory and visual/ motor skills. Consumption of moderate alcohol was associated with better performance on all four domains except for visuomotor, relative to all others.
Table 5.
Standardized path coefficients (standard errors) for covariates and metabolic factors, not controlling for covariates
| Predictor | Outcomes
|
|||
|---|---|---|---|---|
| Language | Memory | Ex. Function | Visual/Motor | |
| Covariates | ||||
| Age | .01(.02) | −.32(.03) * | −.20(.03) * | −.42(.04) * |
| Gender | .05(.02)* | −.23(.03) * | −.02(.03) | .08(.04) * |
| Race/ethnicity | ||||
| Hispanic | −.57(.03)* | −.11(.05) * | −.26(.04)* | .03(.06) |
| Non-Hispanic Black | −.05(.02)* | −.11(.04)* | −.12(.04)* | −.01(.05) |
| Other | −.03(.02)* | −.07(.03)* | −.04(.03)* | .00(.04) |
| Education | .33(.02)* | .36(.04)* | .55(.03)* | .45(.04)* |
| Past smoker | .02(.02) | −.01(.03) | −.04(.03) | −.03(.04) |
| Current smoker | −.00(.02) | −.07(.03)* | .02(.03) | −.11(.04)* |
| Alcohol consumption | .07(.02)* | .08(.03)* | −.10(.03)* | .09(.04)* |
| BP meds | −.01(.02)* | .02(.04)* | .02(.04) | −.03(.05) |
| Cholesterol meds | −.01(.01) | .02(.03)* | .00(.03) | .02(.04) |
| Diabetes meds | −.01(.03) | −.10(.05)* | .04(.05) | .01(.06) |
| Cardiometabolic factors (CFs) | ||||
| Obesity | .03(.02) | −.02(.04) | −.03(.03) | −.15(.04)* |
| Blood pressure | −.06(.03)* | −.05(.05) | .09(.05) | −.14(.07)* |
| Lipids | .04(.02)* | .00(.03) | .02(.03) | −.01(.04) |
| Glucose | .01(.03) | .09(.06) | .04(.05) | −.17(.07)* |
| CFs without covariates | ||||
| Obesity | .12(.05)* | .02(.04) | −.06(.04) | −.09(.05) |
| Blood pressure | −.24(.62)* | −.20(.04)* | .33(.06)* | −.40(.06)* |
| Lipids | −.30(.04)* | −.11(.03)* | .12(.04)* | −.02(.04)* |
| Glucose | −.09(.05) | −.00(.04) | .15(.04)* | −.21(.05)* |
Note.
p <0.05.
Reference groups: meds = no; race/ethnicity = white/anglo; smoking = no smoking; gender = female.
Table 5 shows the path coefficients for the cardiometabolic factors with and without covariates. In the unadjusted model, all four factors were significant predictors of performance on tests in the language domain; lipids and blood pressure were significant predictors of memory; lipids, blood pressure, and fasting glucose were significant predictors of executive function; and blood pressure and fasting glucose were significant predictors of visual/motor skills. Blood pressure was the one component that predicted all four cognitive domains. Once all covariates were controlled for, path coefficients were attenuated but blood pressure remained a significant unique predictor of all cognitive domains except memory. In fact, no factor uniquely predicted the memory domain, once covariates were introduced. With respect to visual/motor skills, in addition to blood pressure, obesity, and glucose remained significant predictors. Lipids remained a significant predictor of language.
DISCUSSION
This study replicates previous work by Shen et al. (2003, 2006) demonstrating that the metabolic syndrome can be modeled using a hierarchical factor structure composed of obesity, blood pressure, lipid levels, and insulin sensitivity, and extends their findings to a larger, race/ethnically diverse sample. The advantage of SEM is that it allows for the creation of latent variables controlling for measurement error and using the full range of values of the continuous variables. This is the first prospective study to successfully model MetS at two points and to confirm that the individual components were stable over an average of 10 years of follow-up. We were also able to model cognition as a four-factor hierarchical model, composed of language, executive function, memory and psychomotor skills. This study illustrates the advantage of structural equation modeling to better understand the clustering of metabolic syndrome, the interrelationships between cognitive domains, and the link between cognition and metabolic risk factors. Furthermore, working with continuous variables maximizes power and does not assume the relationship between predictor and criterion is flat within category (Royston, Altman, & Sauerbrei, 2006).
Although high levels of blood pressure (Novak & Hajjar, 2010; Waldstein & Katzel, 2001), obesity (Dahl et al., 2013; Gunstad et al., 2007), dyslipidemia (Muldoon et al., 2000), and impaired glucose metabolism (McCrimmon, Ryan, & Frier, 2012) have all been shown to be adversely associated with cognitive function, few studies have examined the relative importance of these factors using structural equation modeling. When the individual components of metabolic syndrome were used as simultaneous predictors of the cognitive domains, after adjusting for demographic, behavioral, and medication variables, only blood pressure remained a significant predictor of three domains. We found that lipid levels predicted language performance and no factor predicted the memory domain. Obesity and glucose were predictors of visual/motor performance, a finding consistent with recent research showing higher BMI is linked to reduced visuomotor speed even among those in exceptional cardiovascular shape (Fedor & Gunsted, 2013) and that impaired glucose is associated with reduced dexterity (Pfützner et al., 2012).
Our finding that none of the metabolic components uniquely predicted memory performance may seem at odds with some studies showing a strong link between metabolic syndrome and dementia (Birdsill et al., 2013). In our model, all components were specified; therefore, the coefficients reflect the unique contribution of the individual components rather than their shared contribution. It is possible that the extent to which metabolic syndrome is associated with reduced memory performance may have been masked given that all components were simultaneously considered. An alternative explanation is that the prevalence of both mild cognitive impairment and dementia was low in this sample. NOMAS participants in the current study had an average age of 71 at the time of the neuropsychological assessment, an age group at relatively low risk of cognitive disorders. In addition, elevated blood pressure is associated with white matter disease in NOMAS and these lesions, along with subclinical infarcts, have been associated with worse cognitive flexibility and psychomotor speed. These domains may be more susceptible to damage to frontal subcortical systems caused by exposure to metabolic syndrome and its components (Wright et al., 2008). It is also likely that subtle memory dysfunction is somewhat more dependent than the other domains on underlying neurodegenerative processes (i.e., Alzheimer pathology), and these may be less relevant to the metabolic syndrome to the extent that these risk factors cause vascular damage. While metabolic syndrome has been associated with dementia in prior studies, the relative effects among those with cerebrovascular disease in these studies has usually not been examined and not all studies have found an association between metabolic syndrome and Alzheimer disease (Raffaitin et al., 2009). Also, free recall of a word list may not be as sensitive to subtle memory deficits compared with some other indices, such as those that include an interference or distractor list.
Blood pressure emerged as the most significant metabolic syndrome variable uniquely predicting cognition in this cohort. An inverse relationship between blood pressure and cognitive dysfunction is well documented by cross-sectional and longitudinal studies carried out in the United States and other countries (Gifford et al., 2013). Specifically, Kilander, Nyman, Bobaerg, Hansson, and Lithell (1998) reported that high DBP at age 50 years predicted cognitive performance on the Mini Mental State Exam and trail-making test at age 70. In the Honolulu Asia Aging Study, each 10 mmHg increase in SBP was associated with a 7% increased risk for some degree of cognitive dysfunction and a 9% risk for poor cognitive function. The same investigators reported that elevated BP in the middle years predicted later dementia in men who had never been treated for hypertension (Launer et al., 2000). Low and high baseline diastolic and mean arterial pressure, but not systolic blood pressure or pulse pressure, in midlife was linked to cognitive impairment in a multiethnic sample followed up 20 years later (Taylor et al., 2013). In middle aged women, the combination of type 2 diabetes and hypertension is associated with greater cognitive impairment compared to normotensive diabetic patients (Petrova, Prokopenko, Pronina, & Mozheyko, 2010). Several other studies examining the link between metabolic syndrome and cognition using multivariable linear regression have been conducted, but the relative importance of specific factors has differed across studies. In the Longitudinal Aging Study Amsterdam (Dik et al., 2007), hyperglycemia was a key predictor, while HDL-C was found to be the most important predictor in the Finnish study noted above (Komulainen et al., 2006), and in the Dijon 3C study, high triglycerides were the most important predictor of vascular dementia (Raffaitin et al., 2009). Most recently, Yaffe et al. (2014) demonstrated in a prospective study of 3381 adults that higher SBP and DBP and fasting glucose assessed in young adulthood was significantly associated with poorer performance on multiple neuropsychological measures 25 years later. Sabayan et al. (2013) showed that variability in SBP, independent of average blood pressure, assessed on approximately every 3 months was associated with poorer attention, slower processing speed and impaired pictorial recall.
The mechanism by which hypertension may lead to cognitive decline is controversial and both direct and indirect pathways have been proposed. We have previously found that elevated blood pressure, and increases in blood pressure from baseline to the time of MRI, are associated with a greater burden of white matter lesions, suggesting a role for small vessel disease (Marcus et al., 2011). Hypertension has also been associated with reduced brain volume (Nagai, Hoshide, Ishikawa, Shimada, & Kario, 2008; Narayan et al., 2011). Strassburger et al. (1997) found that hypertensive individuals ranging in age between 56 and 84 years performed worse than their age-matched counterparts on language and memory measures and had reduced volume in the thalamic nuclei and more CSF in temporal and cerebellar regions. In the Honolulu Asia Aging Study, SBP >160 mmHg and DBP >95 mmHg in the middle years was associated with an increased number of neurofibrillary tangles, and increased SBP was also associated with reduced brain weight and poorer cognitive function. Thus elevated blood pressure could contribute to worse cognitive performance through both vascular damage and neurodegeneration.
It is interesting to note that obesity was the strongest correlate of metabolic syndrome but blood pressure was the strongest correlate of cognitive performance. It is not surprising that obesity was the strongest correlate of metabolic syndrome as it has been found to be the strongest predictor of incident metabolic syndrome in a large prospective study (Palaniappan et al., 2004). In addition, obesity causes worsening of other risk factors within the syndrome, including insulin resistance and high blood pressure. Thus, obesity itself may be less important as a direct determinant of cognitive performance than as a contributor to elevated blood pressure, that in turn leads to ischemic damage and subclinical infarction or increases Alzheimer pathology.
A major strength of this study is the well-characterized sample of the fastest growing minority demographic in the United States. Another strength is the use of structural equation modeling, an approach that uses continuous scale for measures of components of the metabolic syndrome, as opposed to imposing categorical classifications or arbitrary thresholds to define metabolic syndrome. There are also limitations to this study. One is a survival effect, since only NOMAS participants remaining alive, stroke-free and older than 55 years were eligible to be included. In addition, participants with contraindications to MRI were not eligible. While this could lead to a healthy cohort effect, it would have tended to bias our results toward the null and minimized any vascular problems or cognitive deficits in the sample, making it less likely to find an association between cardiometabolic factors and cognition. While we used our measurements of metabolic syndrome determinants at two visits an average of 10 years apart to improve reliability, we did not model incident metabolic syndrome. In addition, the cognitive measures came from a single time point and we are thus unable to determine the effect of metabolic syndrome and its components on cognitive decline. This was also true of the covariates, where they were assessed systematically at baseline but after that, they were measured during an annual telephone follow-up not at the time of the MRI. The second time point is nearly complete which will allow for a prospective analysis of our data. This will also permit a more careful examination of alcohol consumption, a covariate that has been shown to be either positively or negatively linked to cognition depending on the amount consumed. In the current study, most NOMAS participants were either non-drinkers or drank light to moderate amounts of alcohol (moderate was defined as up to two drinks daily). We have very few heavy drinkers (2%), which was why the moderate and heavy drinkers were combined. Several other studies using regression have found specific markers of inflammation to be effect modifiers of the association between metabolic syndrome and cognitive problems, including among Latinos (Yaffe et al., 2007). We did not examine inflammation in the current study, as it would have reduced our sample size. However, further studies are planned to examine this question in NOMAS.
These findings add to the growing body of research showing a strong relationship between metabolic syndrome and neuropsychological test performance. In this race/ethnically diverse urban U.S. community, a four-factor model of MetS that includes blood pressure, fasting glucose and lipid levels, and obesity provided the best structural representation. Furthermore, we have demonstrated that these components remain stable and reliable over time. The finding that blood pressure emerged as the strongest unique correlate of neuro-cognitive performance suggests that further studies should focus on understanding the hemodynamic changes underlying vascular aging, arterial damage and neurodegenerative changes associated with age related cognitive decline.
Acknowledgments
We acknowledge Maria Agustina Rossetti for her help in manuscript preparation. All authors report no disclosures. Study Funding: Supported by NINDS Grant # R37 NS029993.
References
- Birdsill AC, Carlsson CM, Willette AA, Okonkwo OC, Johnson SC, Xu G, Bendlin BB. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity. 2013;21(7):1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl AK, Hassing LB, Fransson EI, Gatz M, Reynolds CA, Pedersen NL. Body mass index across midlife and cognitive change in late life. International Journal of Obesity. 2013;37(2):296–302. doi: 10.1038/ijo.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Ser T, Gonzalez-Montalvo JI, Martinez-Espinosa S, Delgado-Villapalos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain and Cognition. 1997;33:343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Comijs HC, Deeg DJ, Kok A, Yaffe K, Penninx BW. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30(10):2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Morley JE. Obesity and hypertriglyceridemia produce cognitive Impairment. Endocrinology. 2008;149(5):2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor A, Gunstad J. Higher BMI is associated with reduced cognitive performance in division I athletes. Obesity Facts. 2013;6(2):185–192. doi: 10.1159/000351138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care. 2004;27(10):2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. The American Journal of Medicine. 2006;119(10):812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Gifford KA, Badaracco M, Liu D, Tripodis Y, Gentile A, Lu Z, Jefferson AL. Blood pressure and cognition among older adults: A meta-analysis. Archives of Clinical Neuropsychology. 2013;28(7):649–664. doi: 10.1093/arclin/act046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment a 20-year follow-up of 999 men. Hypertension. 1998;31(3):780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Helkala EL, Haapala I, Rauramaa R. Metabolic syndrome and cognitive function: A population-based follow-up study in elderly women. Dementia and Geriatric Cognitive Disorders. 2006;23(1):29–34. doi: 10.1159/000096636. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: The Honolulu-Asia aging study. Neurobiology of Aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Archives of Neurology. 2007;64(4):570. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- Marcus J, Gardener H, Rundek T, Elkind MS, Sacco RL, DeCarli C, Wright CB. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume The Northern Manhattan Study. Stroke. 2011;42(9):2639–2641. doi: 10.1161/STROKEAHA.111.617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. The Lancet. 2012;379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The continuing epidemic of obesity in the United States. The Journal of the American Medical Association. 2000;284(13):1650–1651. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB. Effects of lovastatin on cognitive function and psychological well-being. The American Journal of Medicine. 2000;108(7):538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dementia and Geriatric Cognitive Disorders. 2007;24(3):185–192. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. Journal of Hypertension. 2008;26(8):1636–1641. doi: 10.1097/HJH.0b013e3283018333. [DOI] [PubMed] [Google Scholar]
- Narayan SK, Firbank MJ, Saxby BK, Stansby G, Hansrani M, O’Brien JT, Ford GA. Elevated plasma homocysteine is associated with increased brain atrophy rates in older subjects with mild hypertension. Dementia and Geriatric Cognitive Disorders. 2011;31(5):341–348. doi: 10.1159/000328118. [DOI] [PubMed] [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nature Reviews Cardiology. 2010;7(12):686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan L, Carnethon MR, Wang Y, Hanley AJ, Fortmann SP, Haffner SM, Wagenknecht L. Predictors of the Incident Metabolic Syndrome in Adults: The Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27(3):788–793. doi: 10.2337/diacare.27.3.788. [DOI] [PubMed] [Google Scholar]
- Petrova M, Prokopenko S, Pronina E, Mozheyko E. Diabetes type 2, hypertension and cognitive dysfunction in middle age women. Journal of the Neurological Sciences. 2010;299(1):39–41. doi: 10.1016/j.jns.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Pladevall M, Singal B, Williams LK, Brotons C, Guyer H, Sadurni J, Haffner S. A single factor underlies the metabolic syndrome A confirmatory factor analysis. Diabetes Care. 2006;29(1):113–122. doi: 10.2337/diacare.29.1.113. [DOI] [PubMed] [Google Scholar]
- Pfützner A, Musholt PB, Schipper C, Niemeyer M, Qvist M, Schorsch A, Forst T. Self-assessment and objective determination of dexterity in patients with type 1 or type 2 diabetes mellitus. Current Medical Research & Opinion. 2012;28(1):15–21. doi: 10.1185/03007995.2011.638911. [DOI] [PubMed] [Google Scholar]
- Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, Barberger-Gateau P. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: The Three-City Study. Diabetes Care. 2009;32(1):169–174. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: A bad idea. Statistics in Medicine. 2006;25(1):127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, Mooijaart SP. Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. British Medical Journal Open. 2013;347:f4600. doi: 10.1136/bmj.f4600. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin IF, Berglund L. High-density lipoprotein cholesterol and ischemic stroke in the elderly. The Journal of the American Medical Association. 2001;285(21):2729–2735. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- Shen BJ, Todaro JF, Niaura R, McCaffery JM, Zhang J, Spiro A, III, Ward KD. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. American Journal of Epidemiology. 2003;157(8):701–711. doi: 10.1093/aje/kwg045. [DOI] [PubMed] [Google Scholar]
- Shen BJ, Goldberg RB, Llabre MM, Schneiderman N. Is the factor structure of the metabolic syndrome comparable between men and women and across three ethnic groups: The Miami Community Health Study. Annals of Epidemiology. 2006;16(2):131–137. doi: 10.1016/j.annepidem.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MS, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: The Northern Manhattan Study. Journal of the International Neuropsychological Society. 2009;15(4):558. doi: 10.1017/S1355617709090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JE, Wright BR, Boydstun AS. The metabolic syndrome and coronary artery disease: A structural equation modeling approach suggestive of a common underlying pathophysiology. Metabolism. 2012;61(11):1582–1588. doi: 10.1016/j.metabol.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28(7):1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Iseki C, Wada M, Momma T, Ueki M, Kawanami T, Kato T. Impaired glucose metabolism slows executive function independent of cerebral ischemic lesions in Japanese elderly: The Takahata study. Internal Medicine. 2011;50(16):1671–1678. doi: 10.2169/internalmedicine.50.4871. [DOI] [PubMed] [Google Scholar]
- Taylor C, Tillin T, Chaturvedi N, Dewey M, Ferri CP, Hughes A, Stewart R. Midlife hypertensive status and cognitive function 20 years later: The Southall and Brent Revisited Study. Journal of the American Geriatrics Society. 2013;61(9):1489–1498. doi: 10.1111/jgs.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira JR, Elkind MS, Moon YP, Rundek T, Boden-Albala B, Paik MC, Wright CB. The metabolic syndrome and cognitive performance: The Northern Manhattan Study. Neuroepidemiology. 2011;37(3–4):153–159. doi: 10.1159/000332208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscogliosi G, Andreozzi P, Chiriac IM, Cipriani E, Servello A, Ettorre E, Marigliano V. Screening cognition in the elderly with metabolic syndrome. Metabolic Syndrome and Related Disorders. 2012;10(5):358–362. doi: 10.1089/met.2012.0043. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Katzel LI. Hypertension and cognitive function. In: Wldstein I SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 5–36. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test 3–Administration Manual. Wilimington, DE: Jastak Associates; 1993. [Google Scholar]
- Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, Stern Y. White matter hyperintensities and subclinical infarction associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39(3):800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. The Journal of the American Medical Association. 2004;292(18):2237. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: Findings from the Sacramento Area Latino Study of Aging study. Journal of the American Geriatrics Society. 2007;55(5):758–762. doi: 10.1111/j.1532-5415.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Pletcher MJ, Hoang T, Launer L, Whitmer R, Sidney S. Early adult to mid-life cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]