Abstract
Although the role of IQ in the diagnosis of developmental dyslexia remains controversial, the dominant clinical and research approaches rely on a discrepancy definition requiring reading skill to be below the level expected by an individual’s IQ. Here, with functional magnetic resonance imaging (fMRI), we examined whether differences in brain activation during phonological processing that are characteristic of dyslexia are similar or dissimilar in children with poor reading ability who had either high (discrepant) or low (non-discrepant) IQ scores. In two independent samples of 131 children, using univariate and multivariate pattern analyses, poor readers with discrepant or non-discrepant IQ scores exhibited similar patterns of reduced brain activation in brain regions including left parieto-temporal and occipito-temporal regions. These results converge with behavioral evidence that poor readers have similar kinds of reading difficulties in relation to phonological processing regardless of IQ.
Keywords: academic achievement, aptitude measures, dyslexia, neuroimaging, reading
INTRODUCTION
Learning to read may be the single most critical skill that children acquire in early education because reading ability is necessary to access much of the educational curriculum. In the United States, approximately 5–10% of children in first through fifth grades are reported to have developmental dyslexia (Shaywitz, Escobar, Shaywitz, Fletcher, & Makugh, 1992). A critical issue, with important educational, clinical, and theoretical implications, is how to diagnose dyslexia. The notion of reading achievement being below expectation based on IQ has been central to the concept of dyslexia as a “specific” learning difficulty. Although the 2004 reauthorization of Individuals with Disabilities Education Act (IDEA) mandates that states can no longer require school districts to use IQ tests in identifying individuals with learning disabilities (Fletcher, Lyon, Fuchs, & Barnes, 2006), the majority of US schools and school psychologists still rely on discrepancy between reading achievement and IQ to define dyslexia (Machek & Nelson, 2007). The discrepancy standard posits that reading difficulties in the presence of intact general intellectual ability may arise from different causes, and require different forms of treatment, than reading difficulties accompanied by lower intellectual ability.
In contrast to the assumptions of discrepancy-based definitions, a number of behavioral studies indicate that an underlying phonological deficit is similar in poor readers regardless of whether poor readers are discrepant (low reading and higher IQ score) or non-discrepant (low reading and low IQ scores) (Fletcher, et al., 1994; O'Malley, Francis, Foorman, Fletcher, & Swank, 2002; Stanovich & Siegel, 1994; Stuebing, et al., 2002; Tunmer & Greaney, 2010). Further, both kinds of poor readers respond similarly to structured phonetically-based remedial reading instruction programs designed to ameliorate phonological deficits (Stuebing, et al., 2002; Vellutino, Scanlon, Small, & Fanuele, 2006). Moreover, longitudinal analyses indicate that there is a decoupling between reading and IQ in poor readers, and in particular, those who do not compensate for poor reading over time (Ferrer, Shaywitz, Holahan, Marchione, & Shaywitz, 2010). These findings suggest that the underlying brain basis of reading failure is similar in children with low reading scores whether or not those scores are discrepant from performance on IQ-type measures of broader intellectual ability (Stanovich, 2005).
Here we used functional magnetic resonance imaging (fMRI) to examine whether poor reading associated with impaired phonological processing of printed words involves similar or dissimilar brain processes in two independent samples of matched groups of children who were either discrepant (poor reading scores and normal IQ estimate) or non-discrepant (poor reading scores and low IQ estimates) poor readers and compared brain activation patterns to typically developing children. To the extent that a shared neurophysiological difference underlies impaired phonological processing of printed words in children with high or low IQ scores, we expected the two groups of poor readers to exhibit similar patterns of brain activation differences relative to typically developing readers. Alternatively, different patterns of brain activation in the two groups would suggest a different neurophysiological basis for impaired phonological processing that is related to IQ.
Studies of functional brain differences in dyslexia have frequently reported reduced left-hemisphere activations in a neural circuit implicated in reading and language, including inferior frontal, parieto-temporal, and occipito-temporal regions (Hoeft, et al., 2006; Hoeft, et al., 2007; Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008; Richlan, Kronbichler, & Wimmer, 2009). These studies overwhelmingly used a discrepancy criterion for inclusion of participants with dyslexia, and we therefore expected reduced activation in these brain regions in the poor readers with high IQs. It is unknown, however, as to whether poor readers with low IQs would also show a similar pattern of reduced activation in these brain regions.
METHODS
Participants
We utilized data collected at two sites, Carnegie Mellon University (CMU) and Stanford University (Table 1, Table S1). Fifty-seven participants age 8.5 to 12.6 years (mean = 10.3 ± 1.1) were drawn from a larger study (Torgesen, et al., 2006) at CMU of third- and fifth-grade typical and poor readers from public schools surrounding Pittsburgh in Allegheny County, Pennsylvania. Seventy-four typical and poor readers age 7.7 to 16.9 years old (mean = 13.4 ± 2.5) were recruited from the Stanford-San Francisco Bay Area. Participants were all native English speakers. Exclusion criteria for both studies were the diagnosis of a neurological or psychiatric disorder (e.g., sensory disorders, attention deficit hyperactivity disorder [ADHD]), the use of psychotropic medication, and/or the presence of contraindication to MRI (e.g., metal in their body). This study was approved by Institutional Review Boards at both sites. Written informed consent and assent were collected from parents and their children, respectively.
Table 1.
Demographic Information
| Typical Readers |
Discrepant Poor Readers |
Non- Discrepant Poor Readers |
||
|---|---|---|---|---|
| CMU Group | N | 26 | 16 | 15 |
| Age | 10.0 (1.0)a | 10.3 (1.0)a,b | 10.9 (1.1)b | |
| PPVT[SS] | 114.2 (10.6) | 103.8 (10.5) | 82.6 (5.2) | |
| WID[SS] | 109.6 (12.3) | 81.7 (9.5)a | 84.3 (5.5)a | |
| Discrepancy (PPVT-WID[SS]) | 4.6 (10.3)a | 22.1 (17.6) | −1.7 (7.1)a | |
| WA[SS] | 114.6 (13.7) | 88.6 (9.4)a | 89.1 (8.6)a | |
| PC[SS] | 112.8 (10.3) | 87.8 (14.3)a | 87.2 (11.3)a | |
| Task performance (correct %) | 95.2 (6.7) | 71.9 (18.2)a | 73.7 (19.9)a | |
| Stanford Group | N | 36 | 18 | 20 |
| Age | 12.7 (3.0) | 14.1 (1.8) | 14.0 (1.6) | |
| PPVT[SS] | 116.4 (13.8) | 99.2 (7.9) | 80.2 (8.4) | |
| WID[SS] | 112.1 (11.3) | 82.5 (6.5)a | 79.8 (7.7)a | |
| Discrepancy (PPVT-WID[SS]) | 4.3 (14.2)a | 16.7 (8.2) | 0.5 (9.2)a | |
| WA[SS] | 109.9 (10.8) | 87.3 (6.3)a | 89.0 (9.2)a | |
| PC[SS] | 113.9 (8.5) | 90.1 (9.8) | 79.5 (8.5) | |
| Task performance (correct %) | 94.9 (6.8) | 81.7 (13.1)a | 77.5 (11.6)a |
PPVT: Peabody Picture Vocabulary Test, WID: WRMT Word Identification subtest, WA: WRMT Word Attack subtest, PC: WRMT Passage Comprehension subtest, SS: standard score Numbers in brackets indicate SD of the mean, Superscripts: same superscripts indicate no significant difference.
Group Assignment
Participants were assigned to one of three groups based on the performance of Word Identification (Woodcock Reading Mastery Test Revised Normative Update Word Identification subtest [WRMT-R/NU WID]), a single-word reading measure, and Peabody Picture Vocabulary Test (3rd edition; PPVT) to assess estimated IQ. These measures have been used in several previous studies to assess reading ability and IQ (Hoeft, et al., 2006; Hurford, Schauf, Bunce, Blaich, & Moore, 1994) and in behavioral studies examining the discrepancy and low-achievement models (Stuebing, et al., 2002). PPVT is highly (0.90) correlated with full-scale IQ scores from other measures (Dunn & Dunn, 1997). Children were classified as having low reading achievement if they scored equal to or less than 25th percentile (SS≤90) on the WID (Fletcher, et al., 1994; O'Malley, et al., 2002; Stanovich & Siegel, 1994). Children were classified as having low IQ if they had an estimated IQ of equal to or less than 25th percentile (SS≤90). Using these criteria, three groups were identified: (1) Typical readers (N=26 and N=36 for the CMU and Stanford groups, respectively) with typical reading ability and IQ; (2) Poor readers with typical IQ (discrepant poor readers, N=16 and N=18, respectively); and (3) Poor readers with low IQ (non-discrepant poor readers, N=15 and N=20, respectively). There were no significant differences in demographics among the three groups except that the typical readers were significantly younger (less than one year) than the non-discrepant poor readers in the CMU group only (p=0.04, Table 1, Table S1). Socio-economic status (SES) measured by parental education was also not significantly different between the two groups (CMU data: p=0.16, Stanford data: p=0.31).
fMRI Task Design
A block-design word-rhyme task, with alternating rhyme and rest conditions, was used in the scanner to assess brain activation associated with awareness of the phonology of printed words (Hoeft, et al., 2006; Hoeft, et al., 2007). During the rhyme condition, participants judged whether or not two visually presented words rhymed (e.g., bait, gate) or not (e.g., price, miss), and indicated each response with a right- or left-handed button press, respectively. Word pairs were selected so that the visual appearance of the last letters of the two words could not be used to determine whether they rhymed. Stimuli were balanced for frequency of occurrence, number of letters, and syllables between rhyme and non-rhyme trials and across blocks (Zeno, Ivens, Millard, & Duvvuri, 1995) (full list of stimuli in (Hoeft, et al., 2006) supplemental material). Each 6s trial consisted of a 4s presentation of two words followed by a 2s fixation cross. Each task block consisted of a 2s cue period followed by five trials (32s total). During rest blocks, participants saw a fixation cross on the screen for either 16s (CMU group) or 15s (Stanford group). The entire scan was 234s (CMU group) or 223s (Stanford group) long, including two practice trials at the beginning, and consisted of four rhyme blocks and five rest blocks. Image acquisition and preprocessing methods are detailed in Supplementary Appendix.
fMRI Univariate Analyses
We perfomed univariate modeling of fMRI data to compare regional brain activation among the three groups. Six regions of interest (ROIs) based on previous neuroimaging reports of dyslexia (Brunswick, McCrory, Price, Frith, & Frith, 1999; Cao, Bitan, & Booth, 2008; Hoeft, et al., 2007; Kronbichler, et al., 2006; Maisog, et al., 2008; McCrory, Mechelli, Frith, & Price, 2005; Paulesu, et al., 2001; Richlan, et al., 2009; Rumsey, et al., 1997; Schulz, et al., 2008; Shaywitz, et al., 1998) were combined to form one mask comprised of bilateral inferior frontal (pars triangularis, pars opercularis), parieto-temporal (inferior parietal lobule, IPL), and occipito-temporal (fusiform gyrus, FG) regions,using the Automated Talairach Atlas Label (AAL) (http://www.cyceron.fr/freeware/) in the WFU PickAtlas toolbox (http://fmri.wfubmc.edu/software/PickAtlas). Conjunction analyses were performed (conjunction null method as in (Nichols, Brett, Andersson, Wager, & Poline, 2005)) with a random effects model (Friston, Holmes, Price, Buchel, & Worsley, 1999) using the rhyme vs. rest contrast images to identify brain regions that showed significantly greater activation for typical readers compared to both discrepant and non-discrepant poor readers. A voxel-wise statistical threshold of p=0.05 false-discovery-rate (FDR) post small volume correction (SVC) was applied. Because there was a significant difference in age between typical readers and non-discrepant poor readers in the CMU dataset (Table 1, Table S1), we also repeated the analyses regressing out age
fMRI Multi-Voxel Pattern Analyses (MVPA)
Multi-voxel pattern analysis (MVPA) was used to examine all voxels with pattern classification algorithms aimed at identifying naturally occurring groupings of children. We performed leave-one-out linear support vector machine (SVM) analyses (regularization parameter C=1) using in-house Matlab-based (Mathworks, Natick MA) tools which adopts LIBSVM (http://www.csie.ntu.edu.tw/~cjlin/libsvm/), and has been used successfully in several prior studies (Gothelf, et al., 2011; Hoeft, et al., 2008; Hoeft, McCandliss, et al., 2011; Hoeft, Walter, et al., 2011; Marzelli, Hoeft, Hong, & Reiss, 2011). First, we constructed a class vector constituting either ‘+1’s and ‘−1’s assigning each of the child from the three groups to either class label depending on the analysis. Next, we converted contrast images (rhyme > rest) into a S-by-N matrix where S is the number of subjects and N is the number of features/voxels (2×2×2mm) and normalized the matrix in both directions so that mean=0 and SD=1. Numbers of features were reduced by using a grey matter mask to include only grey matter voxels, by performing principal component analyses (PCA) and transforming the matrix to principal components, and by recursive feature elimination (RFE) iteratively, removing 30% of worst-discriminating features at a time until performance started deteriorating (De Martino, et al., 2008). Classification accuracy (accuracy of children with a label of “+1” actually classified as “+1” and children with a label of “−1” actually classified as “−1” relative to the total group), sensitivity (the proportion of actual children labeled as “+1” which are correctly identified as “+1), specificity (the same for “−1”) and positive predictive value were calculated for each classification. Further, distance measure derived from the classifier (e.g. a large positive distance measure of a child who had a label of “+1” indicates that the brain-based classifier was highly confident that the child was in the group “+1”) was derived and plotted for each participant and classifier to examine “brain-based representation” of all participants.
All procedures were performed by keeping training data to construct the classifier and test data independent using leave-one-out cross-validation to avoid overfitting and allow generalization of the models (classifier). Significance was determined using permutation analysis by randomly reassigning class labels 2000 times (p<0.05). Brain maps were constructed by transforming features (principal components, PCs) that remained during RFE back into voxel-space. Thresholding was done using permutation analyses (p=0.05) for visualization purposes to show brain regions that carried greater positive and negative weights.
Using this approach, we performed a series of classification analyses to examine the similarity between the two groups of poor readers and differences between these poor readers and typical readers. First, we performed SVM analyses that paralleled univariate analyses. We examined in each sample, Stanford and CMU, whether differences in brain activation patterns could discriminate between typical readers and the combined group of poor readers (discrepant and non-discrepant), and between the two groups of poor readers.
Second, we performed an additional MVPA analysis to address the possibility that failure to find significant differences between the two groups of poor readers would reflect only null or negative findings. We performed pattern classification between one group of poor readers (e.g. discrepant poor readers) and typical readers and applied that classifier to the other group of poor readers (e.g. non-discrepant poor readers). If the classifier developed to discriminate typical readers from one group of poor readers also significantly classify the other group of poor readers as poor readers, then this would constitute positive evidence that the two groups of poor readers are described by a common pattern of brain activation. In order to do this, leave-one-out SVM was first performed to classify discrepant poor readers vs. typical readers with these data as training data (leaving one child from either group out at a time, hence producing N classifiers where N is the number of participants). Then data from non-discrepant poor readers were used as test-data for each classifier to examine how likely each non-discrepant poor reader was likely to classified as discrepant poor reader. Similarly, we examined when the classifier derived from non-discrepant poor readers vs. typical readers is applied to discrepant poor readers, how likely discrepant poor readers are more likely to be classified as non-discrepant poor readers rather than typical readers. These analyses were performed for both samples.
RESULTS
Behavioral Analysis
Typical readers, compared to the two groups of poor readers, showed significantly higher reading-related scores and more accurate performance on the rhyme-judgment task, but there were no significant differences between the two groups of poor readers on these measures (Table 1, Table S1). IQ scores in the typical readers were significantly higher than in the discrepant poor readers, who had significantly higher IQ scores than the non-discrepant poor readers. The discrepant poor readers showed a significant discrepancy between reading ability and IQ scores, whereas the non-discrepant poor readers did not.
fMRI Univariate Analyses
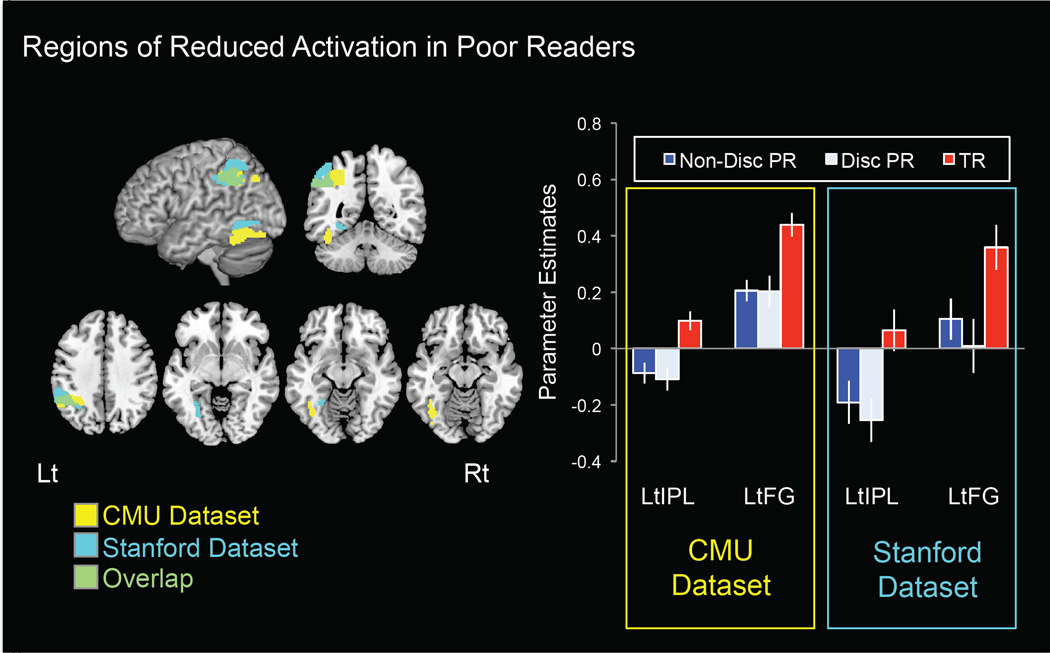
In the CMU group, both discrepant and non-discrepant poor readers exhibited significantly reduced activations relative to typical readers in left IPL (Talairach coordinates TAL[X: −32, Y: −47, Z: 41], Z-val=4.05, p=0.02 corrected) and left FG (TAL[−44, −53, −14], Z-val=4.05, p=0.02 corrected) (Figure 1). The two groups of poor readers (discrepant and non-discrepant) did not exhibit significant differences in activation from one another. Covarying for age in the CMU group did not change the results (Figure S1).
Figure 1.
Brain activation differences (rhyme > rest) between typical reading children (TR) and IQ-discrepant (Disc PR) and non-discrepant poor readers (Non-Disc PR). Typical readers exhibited significantly greater activation than both groups of poor readers in left inferior parietal lobule (LtIPL) and fusiform gyrus (LtFG) in both the CMU and Stanford groups (left panel). Mean parameter estimates from those brain regions shown for each group (right panel). Error bars represent standard error of the mean.
In the Stanford group, both groups of poor readers also exhibited significantly reduced activations relative to typical readers in left IPL (TAL[X: −55, Y: −42, Z: 48], Z-val= 3.82 p=0.046 corrected) and left FG (TAL[−26, −70, 0], Z-val=3.47, p=0.046 corrected) (Figure 1), and, again, the two groups of poor readers did not significantly differ from one another.
fMRI Multi-Voxel Pattern Analyses (MVPA)
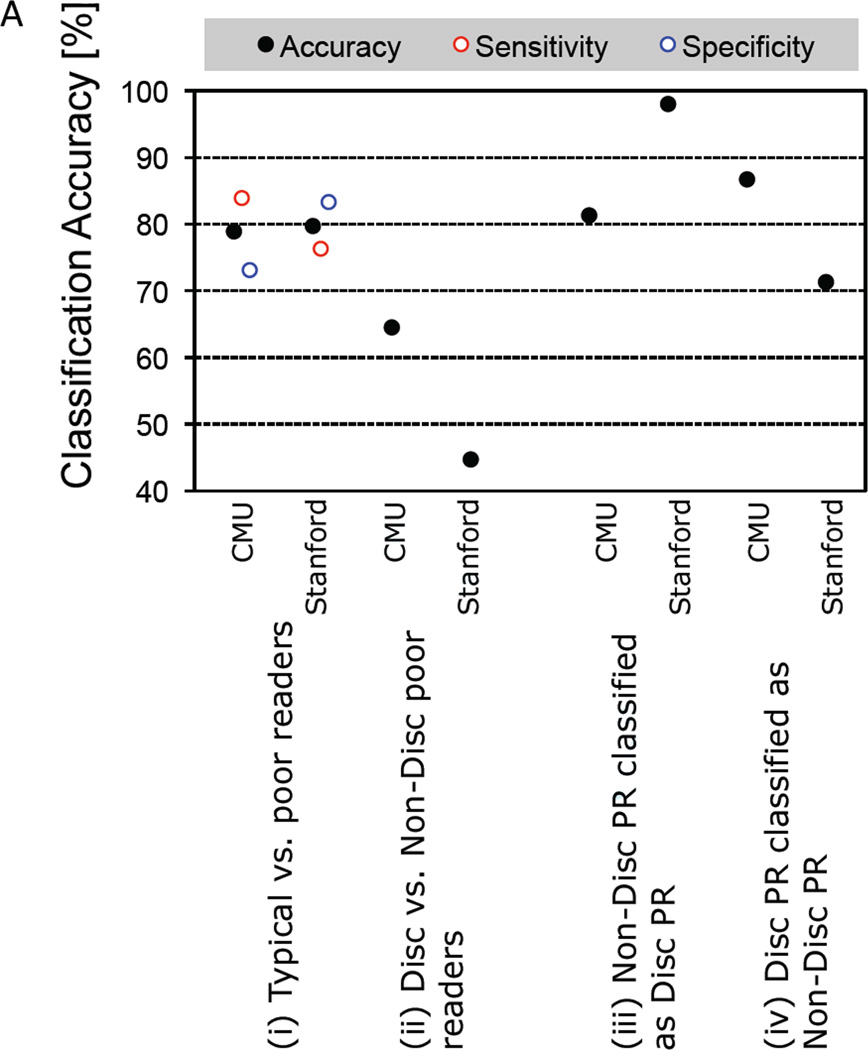
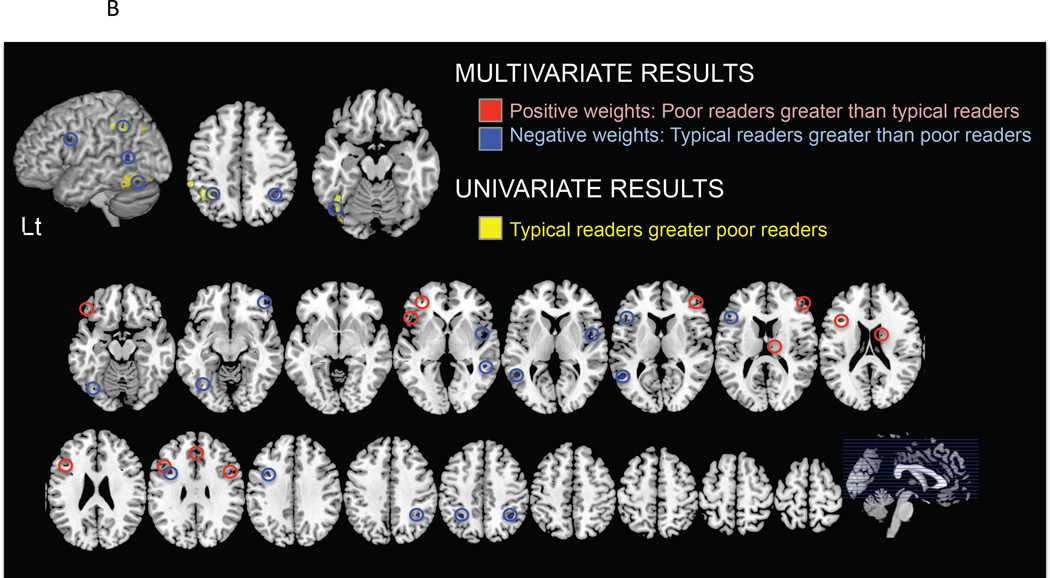
In the CMU group, typical and poor reader (discrepant and non-discrepant combined) were discriminated significantly from one another with an accuracy of 78.9% (sensitivity 83.9%, specificity 73.1%; p<0.001; Table 2, Figure 2A(i)). Brain regions that contributed to the classification of typical and poor readers included left IPL, FG, IFG, caudate, insula, and middle temporal gyrus (Figure 2B). Discrimination between the two groups of poor readers was not reliably above chance (accuracy: 64.5%, p=0.16; Table 2, Figure 2A(ii)). Analysis of the Stanford group yielded similar results with a discrimination accuracy of 79.7% (sensitivity 76.3%, specificity 83.3%; p<0.001; Table 2, Figure 2A(i)) between typical and poor readers, and 44.7% (p>0.1) between the two groups of poor readers (Table 2, Figure 2A(ii)).
Table 2.
Multivariate Pattern Classification Results
| Classiciation | Data-Set | Classification Accuracy (%) |
|
|---|---|---|---|
| (i) | Typical vs. Poor readers | CMU | 78.90 |
| Stanford | 79.70 | ||
| (ii) | Discrepent vs. Non-discrepent poor readers | CMU | 64.50 |
| Stanford | 44.70 | ||
| (iii) | Non-discrepent poor readers classified as Discrepent poor readers | CMU | 81.30 |
| Stanford | 98.00 | ||
| (iv) | Discrepent poor readers classified as Non-discrepent poor readers | CMU | 86.70 |
| Stanford | 71.30 |
Figure 2. Multi-voxel pattern classification analysis (MVPA) results.
A. Classification accuracy from MVPA of both the CMU and Stanford groups. Asterisks at the top indicate whether the accuracy was significantly better than chance. ***: p < 0.001, n.s.: p > 0.1. IQ-discrepant poor readers: Disc, non-discrepant poor readers: Non-Disc.
B. Brain activation differences between typical readers and both IQ-discrepant and non-discrepant poor readers for the CMU group. LtIPL and LtFG show overlap with univariate and multivariate results (top row). MVPA results thresholded as p=0.05 (2000 permutations) for display purposes.
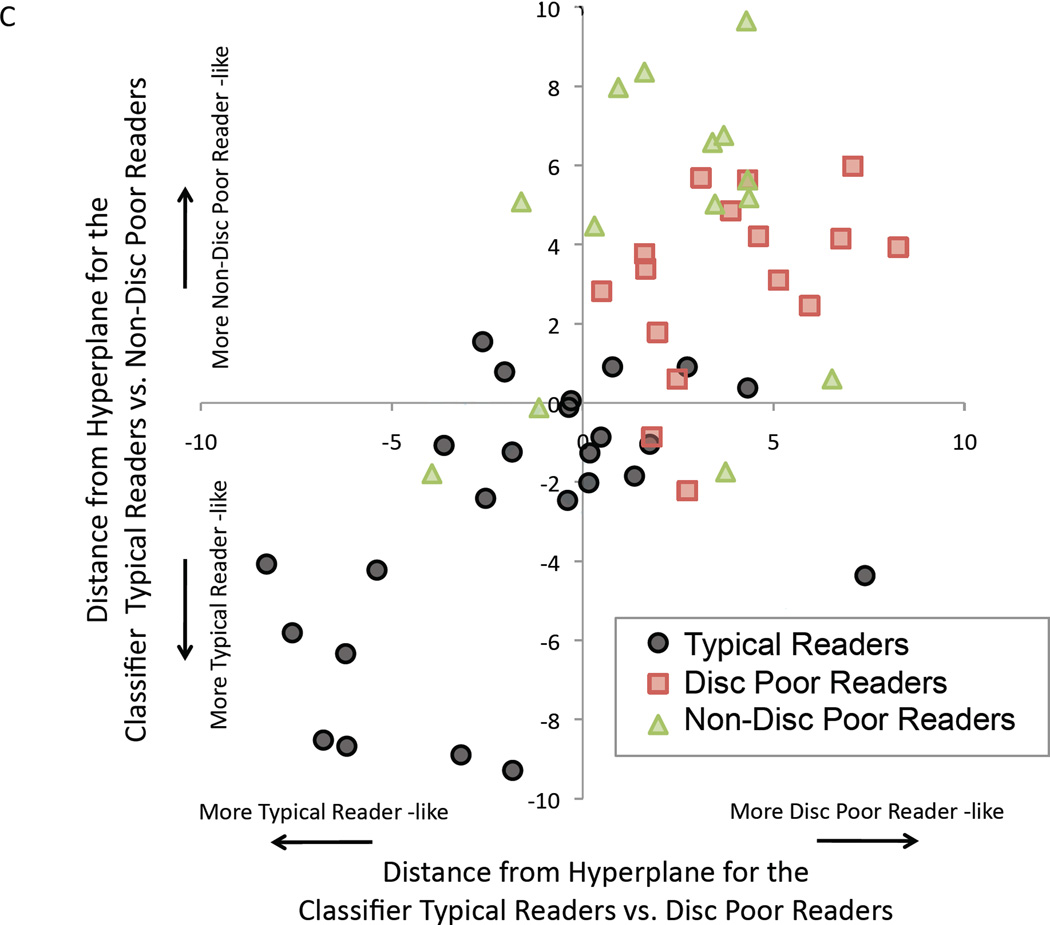
C. Distance from hyperplanes for each child is plotted for the CMU group. X-axis is the distance from the hyperplane (i.e., distance from plane drawn in multidimensional space) of classifier typical readers vs. IQ-discrepant (Disc) poor readers. Non-discrepant (Non-Disc) poor readers children are plotted by applying the classifier from typical readers vs. Disc poor readers to the Non-Disc poor readers. Y-axis is the distance from the hyperplane of classifier typical readers vs. Non-Disc poor readers. Disc poor readers are plotted by applying the classifier from typical readers vs. Non-Disc poor readers to the Disc poor readers.
To show a positive similarity between the two groups of poor readers, we applied the classification model that discriminated between typical readers and one group of poor readers to classify the other group of poor readers (Table 2, Figure 2A). In the CMU group, the classification model that discriminated typical readers from discrepant poor readers significantly classified non-discrepant poor readers as discrepant poor readers with 81.3% accuracy (p<0.001, permutation based; Table 2, Figure 2A(iii)). The classification model that discriminated typical readers from non-discrepant poor readers also significantly classified discrepant poor readers as non-discrepant poor readers with 86.7% accuracy (p<0.001; Table 2, Figure 2A(iv)). Identical analyses in the Stanford data showed that discrepant poor readers were classified as non-discrepant poor readers with 98.0% accuracy (Table 2, Figure 2A(iii)), and non-discrepant poor readers were classified as discrepant poor readers with 71.8% accuracy (both p’s<0.001; Table 2, Figure 2A(iv)). Distance from the hyperplane for each subject for each classifier is plotted in Figure 2C to indicate similarity between the two groups of poor readers. A hyperplane is a multidimensional plane that optimally separates two groups. Distance from the hyperplane indicates how much that particular individual (vector) is like the group on the same side of the hyperplane. Typical readers with positive values and Disc and Non-Disc poor readers children with negative values are those that were misclassified.
DISCUSSION
Overall, atypical brain function for the phonological processing of printed words was highly similar in two carefully matched groups of poor readers who had IQ estimates that were either discrepant (higher IQ) or non-discrepant (equivalent IQ) with their poor reading scores. Although typical univariate analyses and pattern classification methods of brain activation could reliably distinguish patterns of brain activation between good and poor readers, there were no reliable functional brain differences between the two types of poor readers. The shared reductions of activation occurred in left-hemisphere brain regions that often exhibit reduced activation in dyslexia, the left FG, which is thought to be important for specialized visual analysis of print, and the left IPL, which may be important for relating print to sound. Altogether, multiple independent analyses of brain activations in two independent samples converge on the conclusion that the brain basis of a weakness in phonological awareness, which is thought to be the leading cause of dyslexia, is similar in poor readers irrespective of their IQ scores.
There was great similarity in patterns of brain activation between poor readers who were discrepant or non-discrepant for IQ, but a concern about such similarity is that it is based on the absence of statistical differences between the groups of poor readers. Several findings, however, mitigate this concern. First, both poor reading groups in both samples showed reliable differences relative to typical readers with reduced activations in left IPL and FG regions, so the study had suitable and replicable statistical power to reveal brain activation differences. Second, both groups in both samples could reliably be discriminated from typical readers in MVPA analyses. None of these analyses, however, could differentiate reliably the two groups of poor readers. Third, and perhaps most compelling, is the positive evidence in both samples that the statistical models from the MVPA analyses that reliably discriminated one group of poor readers from typical readers classified the other group of poor readers as poor readers rather than typical readers.
The present findings are consistent with substantial and convergent evidence, including a prior neuroimaging study (Temple, et al., 2001), suggesting that the relationship between IQ and the phonological awareness deficit underlying dyslexia may be quite weak (O'Malley, et al., 2002; Stuebing, et al., 2002; Tunmer & Greaney, 2010). Thus, the validity of the discrepancy definition of dyslexia is called into question, despite several lines of reasoning that have previously favored that definition. From a research perspective, behavioral difficulties are often most easily analyzed in the context of strong dissociations in which a single disability is isolated among many spared abilities such that the disability does not seem secondary to other deficits. From a clinical or educational perspective, remediation seems most targeted and effective when it addresses an isolated disability. Further, in unselected populations, there is a correlation between IQ and reading ability (in the 0.3 to 0.6 range) (Hulme & Snowling, 2009), suggesting some link between the broad cognitive abilities assayed by IQ-type measures and reading ability, although it appears that dyslexia may break this link (Ferrer, et al., 2010). Finally, it seems likely that children or adults with broad and severe cognitive deficits, well below those of the non-discrepant children in the present study, would fail to read as a secondary consequence of their cognitive disabilities, and therefore would not benefit from interventions focused on reading skills per se. In sum, there is a complexity of factors that have encouraged the scientific and educational communities to rely on the apparent straightforwardness of the discrepancy criterion.
The discrepancy criterion, however, seems to lack validity and reliability in predicting the course of reading failure, the response to remedial intervention (Stuebing, et al., 2002; Vellutino, et al., 2006), or the brain dysfunctions that underlie dyslexia. Although the discrepancy criterion may be intuitively appealing, its strict application would deprive non-discrepant children of the educational interventions that could promote their advancement in reading ability. Further, the exclusion of non-discrepant children from research studies examining the genetic, neural, and psychological bases of dyslexia would slow progress in truly understanding dyslexia by arbitrarily excluding many children from such research studies. Expanding the definition of dyslexia to include children with non-discrepant IQ scores increases the dissociation of reading from broad cognitive abilities by suggesting that the impairment in phonological awareness leading to dyslexia can occur across a broad range of IQ abilities.
There are several limitations to this study. First, IQ was estimated from the Peabody Picture Vocabulary Test, which does not require reading, is a strong indicator of general verbal ability, correlates highly with full-scale IQ (0.90) (Dunn & Dunn, 1997), and has been used in many studies investigating the effect of IQ in reading outcome (Stuebing, et al., 2002). Previous studies have demonstrated that the choice of IQ measure (e.g., verbal vs. nonverbal) has not improved the validity of the IQ-discrepancy model (Fletcher, et al., 1994; O'Malley, et al., 2002; Stanovich & Siegel, 1994; Stuebing, et al., 2002). Verbal IQ tests, such as vocabulary, tend to show the most resistance to effects of neurobiologic alterations (Brown, et al., 2011), but they are influenced by differences in environmental enrichment and opportunity (Stern, 2009). The groups in the present study were matched for SES, (indeed, the control participants in the CMU group were classmates of the poor readers), so it is unlikely that SES differences accounted for any findings. Alternate IQ tests, however, might yield different results. Second, the functional brain differences were found by comparison of a task that demanded phonological awareness of the sounds of printed words relative to a rest condition, and other functional contrasts may reveal additional differences between poor readers. For example, the fMRI task used here does not distinguish between the initial underlying cause and the additional consequence of reduced reading experience in poor readers. Although the poor reader groups were not different in terms of brain activation in reading-related regions, they might show differences using other imaging techniques such as volumetric or diffusion tensor imaging studies that may reflect the effect of IQ. Additionally, although the entire sample utilized in this study was quite large, sample sizes in each reading group may have been insufficient to detect effects. Continued studies using larger groups, more comprehensive cognitive-behavioral assessment, and complementary neuroimaging methods are required.
In summary, neurobehavioral disorders have so far proven remarkably elusive in their precise psychological characterization, perhaps due to the diversity of difficulties that occur within current diagnostic categories. It has been a hope that biological measures, such as genetics and neuroimaging, would provide new insights into these disorders that would, in turn, help restructure diagnostic categorizations into more precise and validated taxonomies. Here, convergent psychological, educational, and now neurobiological evidence suggests that the long-standing and widely applied diagnosis of dyslexia by IQ discrepancy is not supported. The evidence indicates that any child with a reading difficulty, regardless of his or her general level of cognitive abilities (IQ), should be encouraged to seek reading intervention.
Supplementary Material
Acknowledgments
This work was supported by grants from the William and Flora Hewlett Foundation and the Richard King Mellon Foundation and the Ellison Medical Foundation to JG, and NICHD K23HD054720, Lucile Packard Foundation for Children’s Health, Spectrum Child Health & Clinical and Translational Science Award, the Dyslexia Foundation, the National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award to FH.
Footnotes
Disclosures: The authors have no conflicts of interest related to this work.
Portions of this work were previously included in an oral presentation at the Society for Scientific Study of Reading Annual Meeting and a poster presentation at the Association for Psychological Science Annual Meeting in June 2009.
REFERENCES
- Brown LJ, Ferner HS, Robertson J, Mills NL, Pessotto R, Deary IJ, et al. Differential effects of delirium on fluid and crystallized cognitive abilities. [Research Support, Non-U.S. Gov't] Archives of gerontology and geriatrics. 2011;52(2):153–158. doi: 10.1016/j.archger.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Booth JR. Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 2008;107(2):91–101. doi: 10.1016/j.bandl.2007.12.009. doi: S0093-934X(07)00304-5 [pii]10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino F, Valente G, Staeren N, Ashburner J, Goebel R, Formisano E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. Neuroimage. 2008;43:44–58. doi: 10.1016/j.neuroimage.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 3rd ed. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Ferrer E, Shaywitz BA, Holahan JM, Marchione K, Shaywitz SE. Uncoupling of reading and IQ over time: empirical evidence for a definition of dyslexia. Psychol Sci. 2010;21(1):93–101. doi: 10.1177/0956797609354084. doi: 0956797609354084 [pii]10.1177/0956797609354084. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Fuchs LS, Barnes M. Learning disabilities: from identification to intervention. New York: Guilford Press; 2006. [Google Scholar]
- Fletcher JM, Shaywitz SE, Shankweiler DP, Katz L, Liberman IY, Stuebing KK, et al. Cognitive profiles of reading disability: Comparisons of discrepancy and low achievement definitions. Journal of Educational Psychology. 1994;86:6–23. [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P, et al. Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J Psychiatr Res. 2011;45(3):322–331. doi: 10.1016/j.jpsychires.2010.07.008. doi: S0022-3956(10)00220-7 [pii]10.1016/j.jpsychires.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26(42):10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. doi: 26/42/10700 [pii]10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Archives of General Psychiatry. 2008;65(9):1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci U S A. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. doi: 1008950108 [pii]10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci U S A. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. doi: 0609399104 [pii]10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, et al. Neuroanatomical differences in toddler boys with fragile x syndrome and idiopathic autism. Arch Gen Psychiatry. 2011;68(3):295–305. doi: 10.1001/archgenpsychiatry.2010.153. doi: archgenpsychiatry.2010.153 [pii]10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C, Snowling MJ. Developmental disorders of language learning and cognition. West Sussex: Wiley-Blackwell; 2009. [Google Scholar]
- Hurford DP, Schauf JD, Bunce L, Blaich T, Moore K. Early identification of children at risk for reading disabilities. J Learn Disabil. 1994;27(6):371–382. doi: 10.1177/002221949402700604. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2(9):635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 2006;44(10):1822–1832. doi: 10.1016/j.neuropsychologia.2006.03.010. doi: S0028-3932(06)00077-7 [pii]10.1016/j.neuropsychologia.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Machek GR, Nelson JM. How Should Reading Disabilities Be Operationalized? A Survey of Practicing School Psychologists. Learning Disabilities Research & Practice. 2007;22(2):147–157. [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. doi: NYAS1145024 [pii]10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Marzelli MJ, Hoeft F, Hong DS, Reiss AL. Neuroanatomical spatial patterns in Turner syndrome. NeuroImage. 2011;55(2):439–447. doi: 10.1016/j.neuroimage.2010.12.054. doi: S1053-8119(10)01654-X [pii]10.1016/j.neuroimage.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, Mechelli A, Frith U, Price CJ. More than words: a common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128(Pt 2):261–267. doi: 10.1093/brain/awh340. doi: awh340 [pii]10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. doi: S1053-8119(04)00750-5 [pii]10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O'Malley KJ, Francis DJ, Foorman BR, Fletcher JM, Swank PR. Growth in precursor and reading-related skills: do low-achieving and IQ-discrepant readers develop differently? Learning Disabilities: Research & Practice. 2002;17(1):19–34. [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, et al. Dyslexia: cultural diversity and biological unity. Science. 2001;291(5511):2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Arch Neurol. 1997;54(5):562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, et al. Impaired semantic processing during sentence reading in children with dyslexia: combined fMRI and ERP evidence. NeuroImage. 2008;41(1):153–168. doi: 10.1016/j.neuroimage.2008.02.012. doi: S1053-8119(08)00133-X [pii]10.1016/j.neuroimage.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Escobar MD, Shaywitz BA, Fletcher JM, Makugh R. Evidence that dyslexia may represent the lower tail of a normal distribution of reading ability. New England Journal of Medicine. 1992;326:145–150. doi: 10.1056/NEJM199201163260301. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A. 1998;95(5):2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanovich KE. The future of a mistake: Will discrepancy measurement continue to make the learning disabilities field a pseudoscience? Learn Disabil Q. 2005;28(2):103–106. [Google Scholar]
- Stanovich KE, Siegel LS. Phenotypic performance profile of children with reading disabilities: a regression-based tests of the phonological-core variable-difference model. Journal of Educational Psychology. 1994;86(1):24–53. [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. doi: S0028-3932(09)00123-7 [pii]10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebing KK, Fletcher JM, LeDoux JM, Lyon GR, Shaywitz SE, Shaywitz BA. Validity of IQ-discrepancy classifications of reading disabilties: A meta-analysis. American Educational Research Journal. 2002;39(2):469–518. [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, et al. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuroreport. 2001;12(2):299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Torgesen J, Myers D, Schirm A, Stuart E, Vartivarian SWM. Closing the Reading Gap: First Year Findings from a Randomized Trial of Four Reading Interventions for Striving Readers. Volume II. [Retrieved March 30, 2009];National Assessment of Title I: Interim Report to Congress. 2009 2006, from http://www2.ed.gov/rschstat/eval/disadv/title1interimreport/index.html.
- Tunmer W, Greaney K. Defining dyslexia. J Learn Disabil. 2010;43(3):229–243. doi: 10.1177/0022219409345009. doi: 0022219409345009 [pii]10.1177/0022219409345009. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Scanlon DM, Small S, Fanuele DP. Response to intervention as a vehicle for distinguishing between children with and without reading disabilities: Evidence for the role of kindergarten and first-grade interventions. J Learn Disabil. 2006;39(2):157–169. doi: 10.1177/00222194060390020401. [DOI] [PubMed] [Google Scholar]
- Zeno SM, Ivens SH, Millard RT, Duvvuri R. The educator's word frequency guide. New York: Touchstone Applied Science Associates, Inc.; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.