Abstract
Reactive Oxygen Species (ROS), produced during various electron transfer reactions in vivo are generally considered to be deleterious to cells1. In the mammalian haematopoietic system, haematopoietic stem cells (HSCs) contain low ROS levels, but unexpectedly, the common myeloid progenitors (CMPs), produce significantly elevated levels of ROS2. The functional significance of this difference in ROS level in the two progenitor types remains unresolved2,3. Here, we show that Drosophila multipotent haematopoietic progenitors which are largely akin to the mammalian myeloid progenitors4 display elevated levels of ROS under in vivo physiological conditions, which is downregulated upon differentiation. Scavenging the ROS from these haematopoietic progenitors using in vivo genetic tools, retards their differentiation into mature blood cells. Conversely, increasing the haematopoietic progenitor ROS beyond their basal level triggers precocious differentiation into all three mature blood cell types found in Drosophila, through a signaling pathway that involves JNK and FoxO activation as well as Polycomb downregulation. We conclude that the developmentally regulated, moderately high ROS level in the progenitor population sensitizes them to differentiation, and establishes a signaling role for ROS in the regulation of haematopoietic cell fate. Our results lead to a model that could be extended to reveal a probable signaling role for ROS in the differentiation of CMPs in mammalian haematopoietic development and oxidative stress response.
Keywords: Drosophila, blood, ROS, JNK, FoxO, Haematopoiesis
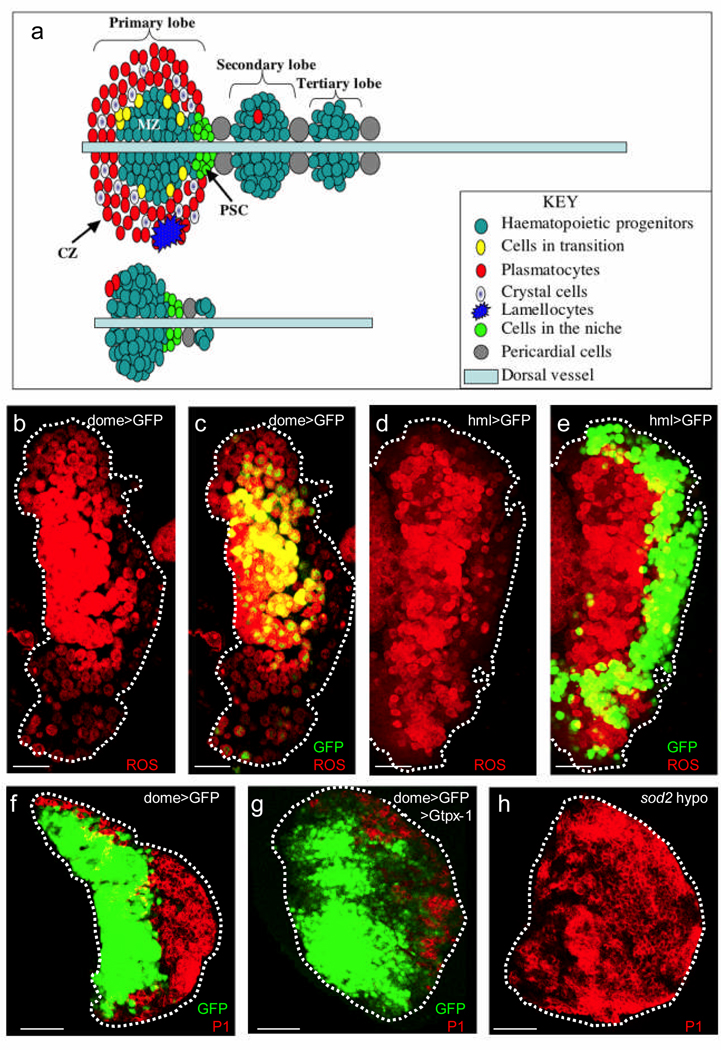
The Drosophila lymph gland is a specialized haematopoietic organ which produces three blood cell types – plasmatocytes, crystal cells and lamellocytes – with functions reminiscent of the vertebrate myeloid lineage5,6. During the first and early second larval instars, the lymph gland is comprised essentially of only the progenitor population (Fig. 1a, lower panel). However, by late third instar, multipotent stem-like progenitor cells become restricted to the medial region of the primary lymph gland lobe, in an area referred to as the Medullary Zone (MZ); while a peripheral zone, referred to as the Cortical Zone (CZ) contains differentiated blood cells. By late third instar, the progenitors within the MZ are essentially quiescent, while the mature, differentiated population in the CZ proliferates extensively5. The Posterior Signaling Center (PSC), is a group of about 30 cells (Fig. 1a, upper panel), that secretes multiple signaling molecules7–9 and serves as a stem cell niche regulating the balance between cells that maintain "stemness" and those that differentiate8,9.
Figure 1. Reactive Oxygen Species Profile of third instar lymph glands.
(a) Schematic diagrams of late third instar (upper panel) and early second instar (lower panel) lymph glands. The second instar lymph gland consists mostly of the progenitor population, which by late third instar, becomes restricted to the central domain of the primary lobe, referred to as the Medullary Zone (MZ). At least three differentiated cell types can be distinguished: plasmatocytes, crystal cells and lamellocytes. Lamellocytes are rarely found in wild-type lymph glands as they are only induced upon infection. All three differentiated cell types are largely restricted to the Cortical Zone (CZ). The third instar lymph gland is comprised of several lobes; primary lobes are found in the most anterior region, and are followed posteriorly by two or more smaller lobes, referred to as secondary and tertiary lobes respectively.
(b) The progenitor population in the MZ show elevated ROS levels (red). The dotted outlines of lymph gland lobes in all panels are based on images acquired at high laser power.
(c) The expression of the MZ marker, dome-gal4, UAS-2xEYFP (green; genotype abbreviated on the panel as dome>GFP for clarity) overlaps with the ROS dye (red) in cells of the MZ (therefore yellow).
(d) As in panel (b), the progenitor population in the MZ show elevated ROS levels (red).
(e) hmlΔ-gal4, UAS-2xEGFP is restricted to cells in the CZ (green). Most of the cells that are marked by hmlΔ-gal4, UAS-2xEGFP are low in ROS (therefore green) when compared to cells in the MZ (red). A ring of hmlΔ-gal4, UAS-2xEGFP expressing cells can be seen along the edge of the MZ that are both GFP and ROS positive (therefore yellow). These appear to be cells in a state of transition between the stem-like and the differentiated cell fate.
(f) Unlike in previous panels, the red color here marks P1 expression in differentiated plasmatocytes in the CZ. By late third instar, the expression of dome-gal4, UAS-2xEYFP (green) is restricted to the MZ and the cells in the CZ (red) downregulate this marker.
(g) Overexpression of the antioxidant protein (GTPx-1) in the progenitor cell compartment (genotype: dome-gal4, UAS-2xEYFP; UAS-Gtpx1) results in a pronounced reduction in the number of cells that express the P1 marker (red). Some cells occupying the CZ region continue to express dome-gal4, UAS-2xEYFP while many others downregulate this marker without yet expressing the differentiation marker P1.
(h) In the hypomorphic (weak allele) sod2/sod2 homozygotes, in which the level of expression of a major ROS scavenger is reduced, P1 expression is expanded and can be found throughout the lymph gland (red), rather than being restricted to the CZ. This image is generated from the optical sections acquired from the central part of the gland.
Scale bars : 50µm.
Although several studies have identified factors that regulate the differentiation and maintenance of Drosophila blood cells and the stem-like progenitor population that generates them8–11, intrinsic factors within the stem-like progenitors are less explored, and forms the central theme of this investigation. We observed that by the third instar, the progenitor population in the normal wild-type lymph gland MZ contain significantly elevated ROS levels when compared to their neighboring differentiated progeny that express mature blood cell markers in the CZ (Fig. 1b–e). ROS is not elevated during the earlier larval instars but rises as the progenitor cells become quiescent and subside as they differentiate (Fig. 1b–e). This first suggested to us that the rise in ROS primes the relatively quiescent stem-like progenitor cells for differentiation. We reduced ROS by expressing antioxidant scavenger proteins GTPx-112 (Fig. 1f,g) or Catalase (Supplementary Fig. 1), specifically in the progenitor cell compartment using the GAL4/UAS system13, and found that suppressing elevated ROS levels in haematopoietic progenitors significantly retards their differentiation into plasmatocytes (Fig. 1f,g and supplementary Fig. 1). As a corollary, mutating the gene encoding the antioxidant scavenger protein Superoxide Dismutase (sod2)1 led to a significant increase in differentiated cells and decrease in progenitors (Fig. 1h).
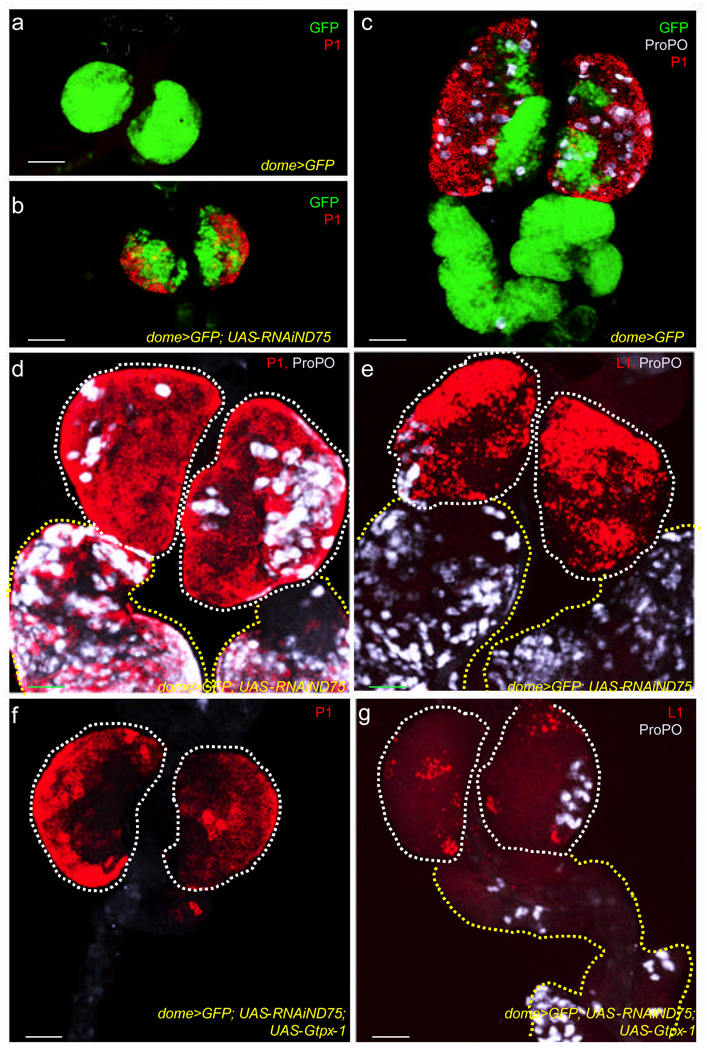
ROS levels in cells can be increased by the genetic disruption of complex I proteins of the mitochondrial electron transport chain14,15, such as ND75 and ND42 (Supplementary Fig. 2). Unlike in wild type, where early second instar lymph glands are exclusively comprised of undifferentiated cells (Fig. 2a), mitochondrial complex I depletion triggers premature differentiation of the progenitor population (Fig. 2b). This defect is even more evident in the third instar (Compare Fig. 2c and 2d), where a complete depletion of the progenitors is seen as primary lobes are populated with differentiated plasmatocytes and crystal cells. The third differentiated cell type, lamellocyte, defined by the expression of the antigen L1, is rarely observed in the wild-type lymph gland (Supplementary Fig. 3) but is abundantly seen in the mutant (Fig. 2e). Finally, the secondary and tertiary lobes, largely undifferentiated in wild type, also embark on a robust program of differentiation upon complex I depletion (Fig. 2d, e and Supplementary Fig. 4). Importantly, the phenotype resulting from ND75 disruption can be suppressed by the co-expression of the ROS scavenger protein GTPx-1 (Fig. 2f, g; compare with Fig. 2d, e) providing a causal link between increased ROS and the premature differentiation phenotype. Combining these results with those in figure 1, we conclude that the normally elevated ROS levels in the stem-like progenitors serves as an intrinsic factor that sensitizes them to differentiation into all three mature cell types. Any additional increase or decrease in the level of ROS away from the wild-type level enhances or suppresses differentiation respectively.
Figure 2. Increased ROS production triggers precocious differentiation of the multipotent progenitors.
In all panels, the progenitor population expresses the MZ marker, dome-gal4, UAS-2xEYFP (green). In panels (d–g), the green channel has been omitted for clarity. The two genotypes used in these panels are control lymph glands (dome-gal4, UAS-2xEYFP), abbreviated as wild-type (WT), and experimental lymph glands which express a RNAi construct to ND75 (dome-gal4, UAS-2xEYFP; UAS-RNAiND75), abbreviated as ND75RNAi. Scale bars :50µm.
(a) P1 is not expressed (note absence of red) in early second instar WT lymph glands.
(b) P1 expression (red) is robustly induced in early second instar ND75RNAi lymph glands.
(c–e) Disruption of ND75 triggers precocious differentiation.
(c) In WT third instar lymph glands, plasmatocytes marked with P1 (red) and crystal cells with ProPO (gray) are restricted to the CZ. These differentiated cell types are rarely if ever found in secondary and tertiary lobes.
(d) In third instar ND75RNAi lymph glands, there is a dramatic increase in P1 (red) and ProPO (gray) expressing cells, throughout the primary, as well as in the secondary and tertiary lobes (tertiary lobes are shown in Supplementary Fig. 4).
(e) Lamellocytes, marked by L1 (red) are prominently seen in third instar ND75RNAi lymph glands. Crystal cells are shown in gray. Lamellocytes are rarely found in secondary lobes.
(f, g) Scavenging ROS suppresses differentiation associated with ND75 disruption. Overexpression of Gtpx-1 in ND75RNAi lymph glands (in f and g) potently suppresses differentiation into all three lineages as there is a decrease in P1 (red in f), ProPO (gray in g) and L1 (red in g) expression. Compare (f, g) with (d, e). Controls for titration of GAL4 are shown in Supplementary Figure 6.
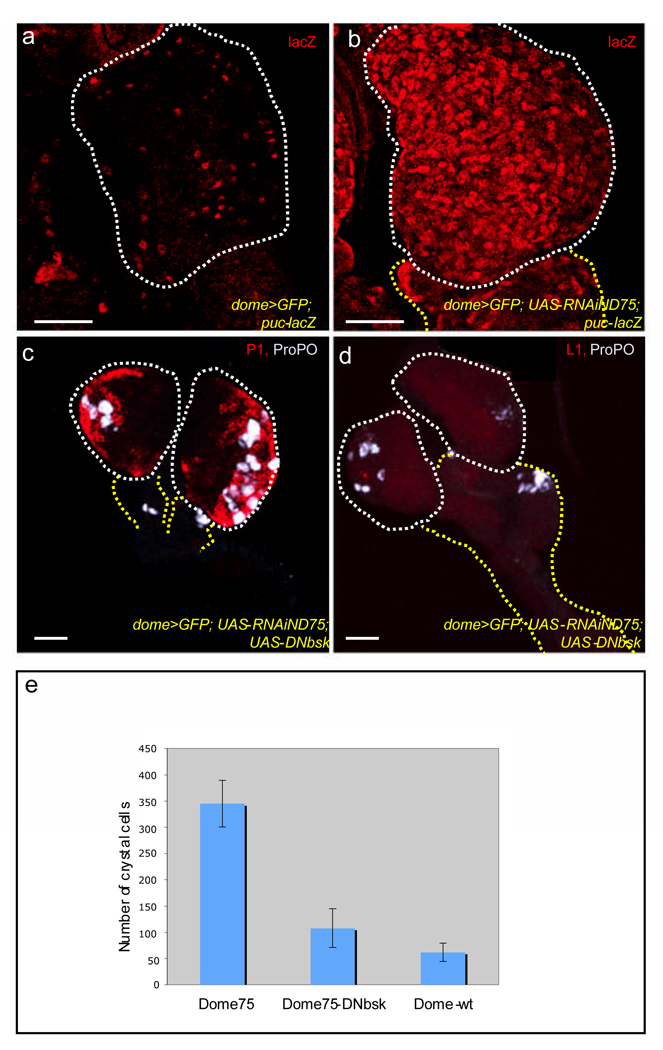
In unrelated systems, elevated ROS levels have been demonstrated to activate the JNK signal transduction pathway1,16,17. Consequently, we tested whether the mechanism by which the progenitors in the MZ differentiate when ROS levels increase could involve this pathway. puckered (puc), is a downstream target of JNK signaling and its expression has been used extensively to monitor JNK activity18. Although puc transcripts are detectable by RT-PCR (Supplementary Fig. 5), the puc-lacZ reporter is very weakly expressed in wild-type (Fig. 3a). Upon disruption of ND75, however, a robust transcriptional upregulation of puc-lacZ expression can be seen (Fig. 3b), indicating that JNK signaling is induced in these cells in response to high ROS levels. The precocious progenitor cell differentiation caused by mitochondrial disruption is suppressed upon expressing a dominant negative version of Basket (Bsk), the sole Drosophila homologue of JNK (Fig. 3c, d; compare with Fig. 2d, e; also see Supplementary Fig. 5). This suppression was associated with a decrease in the level of expression (Supplementary Fig. 5) of the stress response gene encoding Phosphoenol pyruvate carboxykinase (PEPCK)19, and quantitatively, a 68% suppression of the ND75 crystal cell phenotype was observed when JNK function was removed as well (Fig 3e). Although disrupting JNK signaling suppressed differentiation, ROS levels remain elevated in the mutant cells (Supplementary Fig. 2f) as would be expected from JNK functioning downstream of ROS.
Figure 3. Disrupting JNK signaling suppresses the ROS-dependent differentiation Phenotype.
The progenitor population also expresses the MZ marker, dome-gal4, UAS-2xEYFP (green), in panels (a–d), but this has been omitted for clarity. Lymph glands that express a RNAi construct to ND75 (dome-gal4, UAS-2xEYFP; UAS-RNAiND75), are abbreviated as ND75RNAi. Scale bars: 50µm.
(a, b) JNK signaling is activated upon ROS increase. puc-lacZ expression (red) in WT lymph glands (a) and ND75RNAi lymph glands (b). puc-lacZ, which is a transcriptional reporter of JNK signaling is dramatically elevated in ND75RNAi cells.
(c, d) JNK signaling is required for triggering differentiation associated with ND75 disruption.
Expressing a dominant negative construct of JNK in the precursor population ameliorates the effect of complex I disruption as the number of plasmatocytes (red in c) crystal cells (gray in c and d) and lamellocytes (red in d) are reduced virtually to WT levels. Compare (3c, d) with (2d, e)
(e) Suppression of the number of crystal cells formed in ND75RNAiUAS-DNbsk lymph glands relative to ND75RNAi lymph glands. Error bars are s.e.m and n = 10.
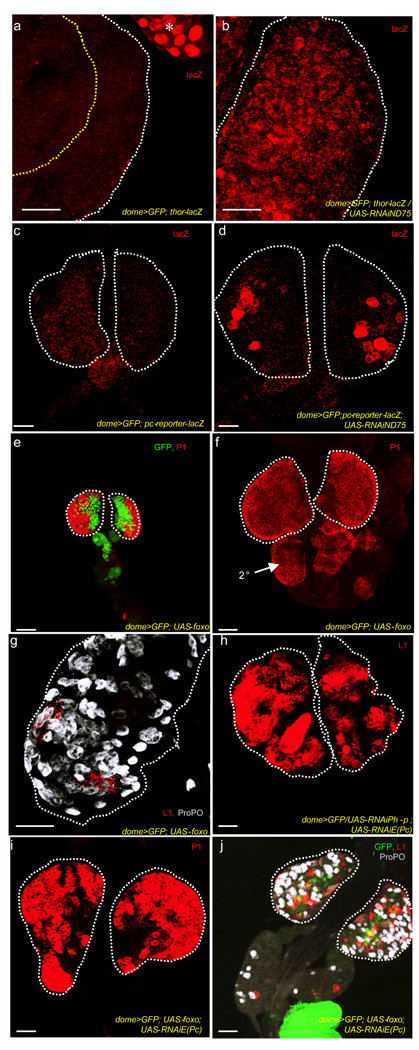
In multiple systems and organisms, JNK function can be mediated by activation of FoxO as well as through repression of Polycomb activity17,20,21. FoxO activation can be monitored via expression of its downstream target thor, using thor-lacZ as a transcriptional read-out22–24. thor-lacZ is undetectable in wild type lymph glands (Fig. 4a) although thor transcripts are detectable by RT-PCR (Supplementary Fig. 5), but the reporter is robustly induced when complex I is disrupted (Fig. 4b), suggesting that the complex I loss mediated increase in ROS activates FoxO. To monitor Polycomb derepression, we used a Polycomb reporter, which expresses lacZ when Polycomb proteins are downregulated. Although undetectable in wild-type lymph glands (Fig. 4c), disrupting ND75 leads to lacZ expression (Fig. 4d), suggesting that Polycomb activity is downregulated by the altered ROS and resulting JNK activation. Direct FoxO overexpression causes a remarkable advancement in differentiation to a time as early as the second instar (Fig. 4e), never seen in wild type (Fig. 2a). By early third instar, the entire primary and secondary lobes stained for plasmatocyte (Fig. 4f) and crystal cell (Fig. 4g) markers when FoxO is expressed in the progenitor population. Unlike with ROS increase, we did not find a significant increase in lamellocytes upon FoxO overexpression. However, downregulating the expression of two polycomb proteins, Polyhomeotic Proximal (Php-x) and Enhancer of Polycomb (E(Pc)) that function downstream of JNK21 dramatically increased lamellocyte number (Fig. 4h) without affecting plasmatocytes and crystal cells (not shown). When FoxO and an RNAi against E(Pc) are expressed together in the progenitor cell population, differentiation to all three cell types is evident (Fig. 4i, j). We conclude that FoxO activation and Polycomb downregulation act combinatorially downstream of JNK to trigger the full differentiation phenotype – an increase in plasmatocytes and crystal cells due to FoxO activation, and an increase in lamellocytes primarily due to Polycomb downregulation.
Figure 4. FoxO activation and Polycomb downregulation phenocopy aspects of the ROS induced differentiation.
In all panels, the progenitor cells express the MZ marker, dome-gal4, UAS-2xEYFP (green), omitted in some panels for clarity. Lymph glands from dome-gal4, UAS-2xEYFP larvae were used as wild-type controls (abbreviated WT). Lymph glands which express a RNAi construct to ND75 in the progenitor cells (dome-gal4, UAS-2xEYFP; UAS-RNAiND75), are abbreviated as ND75RNAi. Scale bars :40µm.
(a, b) Disruption of ND75 leads to induction of the FoxO reporter, thor-lacZ. WT lymph glands (a) do not express thor-lacZ (absence of red). The asterisk in a, points to thor-lacZ expression in the ring gland, adjacent to the lymph gland which serves as an internal control. thor-lacZ expression is significantly induced in ND75RNAi lymph glands (b).
(c, d) Disruption of ND75 leads to expression of the polycomb reporter. The polycomb reporter (red) is not expressed in WT lymph glands (c), but is induced in ND75RNAi lymph glands (d).
(e–g) FoxO overexpression causes an increase in plasmatocytes and crystal cells, but has virtually no effect on lamellocytes.
(e) Overexpression of FoxO in the progenitor cells (dome-gal4, UAS-2xEYFP; UAS-foxo) causes their premature differentiation into plasmatocytes as shown for earlier than normal P1 staining (red) in a second instar lymph gland. Compare with Figure 2a.
(f) Progenitor cells expressing FoxO in the MZ of the third instar lymph gland also initiate extensive differentiation into plasmatocytes (red). In addition, there is ectopic differentiation in the secondary lobes (arrow, 2°).
(g) FoxO expression in the MZ results in an increase in the number of crystal cells (gray). However, only a few isolated L1-positive cells (red) are evident even in late third instar lymph glands. This image is acquired at twice the magnification of the other panels to highlight the few lamellocytes (red).
(h) RNAi-mediated downregulation of the expression of two polycomb proteins, Enhancer of polycomb, E(Pc) and polyhomeotic proximal (Ph-p), leads to a robust increase in lamellocytes, that stain for L1 (red).
(i, j) When FoxO and the RNAi construct to E(Pc) are expressed together in the MZ progenitors there is an increase in all three mature cell markers.
Co-expression of FoxO and an RNAi construct to E(Pc) trigger the full differentiation phenotype associated with complex I disruption as there is an increase in the number of plasmatocytes (red in i), crystal cells (gray in j) and lamellocytes (red in j).
The analysis of ROS in the wild-type lymph gland highlights a previously unappreciated role for ROS as an intrinsic factor that regulates differentiation of multipotent haematopoietic progenitors in Drosophila. Any further increase in ROS beyond the developmentally regulated levels, due to oxidative stress, will cause the progenitors to differentiate into one of three myeloid cell types. Tothova et al.2 reported that the ROS levels in mammalian HSCs is low but that in the CMPs is relatively high. The Drosophila haematopietic progenitors give rise entirely to a myeloid lineage and therefore, are functionally more similar to CMPs than they are to HSCs. It is therefore a remarkable example of conservation to find that they too have high ROS levels. The genetic analysis makes it clear that the high ROS in Drosophila haematopoietic progenitors primes them towards differentiation. It will be interesting to determine, if such a mechanism operates in mammalian CMPs. In mice, as in flies, a function of FoxO is to activate antioxidant scavenger proteins. Consequently, deletion of FoxO elevates ROS levels in the mouse HSC and drives myeloid differentiation2. However, even in the mouse haematopoietic system, FoxO function is dose and context dependent, as ROS levels in CMPs are independent of FoxO2. Thus, while the basic logic of elevated ROS in myeloid progenitors is conserved between flies and mice, the exact function of FoxO in this context may have diverged.
Our past work14 and those of others1,25,26 has hinted that ROS can function as signaling molecules at physiologically moderate levels. This work supports and further extends this notion. While excessive ROS is damaging to cells, developmentally-regulated ROS production, can be beneficial. The finding that ROS levels are moderately high in normal Drosophila haematopoietic progenitors and mammalian CMPs raises the possibility that wanton overdose of antioxidant products may infact inhibit formation of cells participating in innate immune response.
Methods Summary
Lymph glands were stained as previously described5,8 using the following antibodies: mouse anti-P1 and L1 (Ando, I.), rat anti-ProPO (Müller, H.), rabbit anti-βgal (Cappell) and mouse anti -βgal (Promega). Cy3, Cy5 and FITC conjugated secondary antibodies were from Jackson Laboratory. ROS staining was conducted as previously described14,15. Images were captured using a BioRad Radiance 2000 confocal microscope with LaserSharp 2000 acquisition software.
Supplementary Material
Acknowledgments
We thank I. Ando and H Muller for antibodies; and E. Hafen, A. Martinez-Arias, F. Missirlis, S. Noselli, R. Paro, S Sinenko, the National Institute of Genetics Fly Stock Center (Japan) and the Bloomington Stock Center for fly stocks. We acknowledge Meghana Kulkarni and Chrysoula Pitsouli of the Perrimon lab, for technical assistance. Due to space limitations, we apologize to our colleagues whose work is not referenced. This study was supported by US National Institutes of Health grant R01HL067395 to U.B and a T32 institutional postdoctoral fellowship T32-HL069766 to E.O.A.
Footnotes
Full methods accompany this paper.
Supplementary information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions UB supervised the project. EOA conceived, designed and performed all experiments. EOA and UB discussed results and wrote the manuscript.
Reprints and permissions information is available at www.nature.com/reprints
REFERENCES
- 1.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Coffer PJ, Burgering BM. Stressed marrow: FoxOs stem tumour growth. Nat Cell Biol. 2007;9:251–253. doi: 10.1038/ncb0307-251. [DOI] [PubMed] [Google Scholar]
- 4.Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 5.Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 6.Wood W, Jacinto A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat Rev Mol Cell Biol. 2007;8:542–551. doi: 10.1038/nrm2202. [DOI] [PubMed] [Google Scholar]
- 7.Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krzemien J, et al. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- 10.Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 11.Bruckner K, et al. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Missirlis F, et al. A putative glutathione peroxidase of Drosophila encodes a thioredoxin peroxidase that provides resistance against oxidative stress but fails to complement a lack of catalase activity. Biol Chem. 2003;384:463–472. doi: 10.1515/BC.2003.052. [DOI] [PubMed] [Google Scholar]
- 13.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 14.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 15.Owusu-Ansah E, Yavari A, Banerjee U. A protocol for in vivo detection of Reactive Oxygen Species. Nature Protocol Network. (published online 3 February 2008) [Google Scholar]
- 16.Gotoh Y, Cooper JA. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 17.Essers MA, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. Embo J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Blanco E, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey KF, et al. FOXO-regulated transcription restricts overgrowth of Tsc mutant organs. J Cell Biol. 2008;180:691–696. doi: 10.1083/jcb.200710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Lee N, Maurange C, Ringrose L, Paro R. Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature. 2005;438:234–237. doi: 10.1038/nature04120. [DOI] [PubMed] [Google Scholar]
- 22.Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teleman AA, Hietakangas V, Sayadian AC, Cohen SM. Nutritional control of protein biosynthetic capacity by insulin via Myc in Drosophila. Cell Metab. 2008;7:21–32. doi: 10.1016/j.cmet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Junger MA, et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda S, et al. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 26.Foreman J, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.