Abstract
Background and Purpose
The chronological development and natural history of cerebral aneurysms (CA) remains incompletely understood. We used 14C birth dating of a main constituent of CAs, i.e. collagen type I, as an indicator for biosynthesis and turnover of collagen in CAs in relation to human cerebral arteries to further investigate this.
Methods
Forty-six ruptured and unruptured CA samples from 43 patients as well 10 cadaveric human cerebral arteries were obtained. The age of collagen, extracted and purified from excised CAs, was estimated using 14C birth dating and correlated with CA and patient characteristics, including the history of risk factors associated with atherosclerosis and potentially aneurysm growth and rupture.
Results
Nearly all CA samples contained collagen type I which was less than 5 years old, irrespective of patient age, aneurysm size, morphology, or rupture status. However, CAs from patients with a history of risk factors (smoking or hypertension), contained significantly younger collagen than CAs from patients with no risk factors (mean 1.6±1.2 years versus 3.9±3.3 years, respectively, p= 0.012). CAs and cerebral arteries did not share one dominant structural protein, such as collagen type I, which would allow comparison of their collagen turnover.
Conclusions
The abundant amount of relatively young collagen type I in CAs suggests that there is on-going collagen remodeling in aneurysms, which is significantly more rapid in patients with risk factors. These findings challenge the concept that cerebral aneurysms are present for decades and that they undergo only sporadic episodes of structural change.
Keywords: Cerebral aneurysms, risk factors, radiocarbon birth dating, natural history
Introduction
The prevalence of unruptured cerebral saccular aneurysms (CAs) in the general population is two to three percent. 1 CAs can remain clinically silent, present with symptoms of mass effect or they can rupture, causing subarachnoid hemorrhage (SAH) or hemorrhage into other brain compartments. The high case fatality rate of SAH of up to 35% has stimulated interest in understanding the formation and natural history of these lesions, in order to define standards for screening or prophylactic treatment and to identify those CAs that are at increased risk of rupture.2, 3 One hypothesis suggests that CAs form and then grow at a constant rate.4 Alternatively, CA growth has been noted to alternate stochastically between periods of stability and instability or growth, during which they are prone to rupture.5 Nevertheless, no compelling biological evidence exists to support either theory, as most data are derived from mathematical modeling studies. One possibility to better investigate the chronological development of CAs is radiocarbon birth dating of collagen type I, which is the most dominant molecular constituent of CAs.
Radiocarbon birth dating takes advantage of the sharp increase and subsequent slow attenuation of atmospheric 14CO2 concentrations that occurred after above ground nuclear bomb tests between 1955 and 1963. After the implementation of the Limited Test Ban Treaty, there was no additional significant elevation of atmospheric 14CO2, resulting in a nearly exponential decrease of atmospheric 14CO2 levels, not due to 14C radioactive decay (half-life = 5730 y) but instead due to 14CO2 diffusion from the atmosphere and equilibration with the oceans and biosphere.6, 7 Therefore, the consumption of plants and of animals that live of plants results in F14C levels in the human body parallel to those in the atmosphere. 8 14C is integrated into any human biomolecule or genomic deoxyribonucleic acid (DNA), reflecting the F14C level in the atmosphere at the time the biomolecule were synthesized. 9 While F14C content can be measured in DNA to birth date human cells and birth dating of proteins can be used to estimate development and turnover of pathological structures.9, 10 We previously reported on the feasibility of this method for CAs, provided that the extracted CA collagen was pure and not mixed with other proteins.11 However, the small sample size and the lack of human artery control tissues did not permit more definitive conclusions based on this preliminary data. Here, we investigated the age of CA collagen as an indicator for collagen turnover in a distinctly larger patient cohort to identify determinants for aneurysm development and the potential relation with collagen turnover in human cerebral arteries.
Materials and Methods
For details on CA and cerebral artery sample processing as well as birth dating of CA collagen, please see http://stroke.ahajournals.org.
Ethics Committee Approval
The research ethics committees of the Medical Faculty of Heinrich-Heine University, Düsseldorf, Germany (ID # 3365), St. Michael's Hospital, Toronto, Canada (ID # 09-309), and Lawrence Livermore National Laboratory, Livermore, California (ID # 10-108) approved this study.
Sample and data collection
Tissue from ruptured and unruptured CAs was collected from patients undergoing surgical repair of their CA. Patients were included if: a) the CA diameter was five or larger than five mm b) scheduled to undergo neurosurgical clipping, c) the operating neurosurgeon considered it medically indicated or safe to resect a portion of the CA after clipping, and d) informed consent was obtained for scientific data analysis. Patients were excluded if they: a) were treated for an CA smaller than five mm, and b) when CA sampling was not safe or technically feasible. For further analysis, data on potential risk factors for CA formation/rupture (i.e. hypertension, cigarette smoking or cocaine consumption) were recorded. CA location, irregularity, diameter and other size measurements were assessed by neuroradiologists blinded to further analyses. Following surgical removal, CA domes were placed in sterile containers and frozen at -80°C until subjected to collagen purification. In three larger CAs, two separate samples were retrieved to confirm 14C intercepts within different parts of these CAs for internal validation. Ten samples from human cerebral as well as two samples from extra-cerebral arteries from five cadavers and five collagen samples of known age from newborn mouse tendons served as controls. For the cadaveric cerebral arteries, five samples were obtained from the proximal and intradural portion of the internal carotid artery and five samples from the distal middle cerebral artery.
Definitions
CA size was defined as the greatest CA diameter, measured using three-dimensional reconstruction of the catheter angiograms. CA irregularity was defined as multiple lobes, presence of daughter-sac or significant irregularity of the CA wall on the three-dimensional angiographic reconstruction images. Aspect ratio was defined as the ratio of CA neck width and CA largest diameter.12 The presence of risk factors potentially associated with an increased risk of CA rupture were assessed during the first clinical presentation or following admission due to SAH. 13 Hypertension, irrespective of whether it was treated or untreated, was defined as systolic blood pressures greater than 140 mmHg and/or diastolic blood pressure greater than 90 mmHg on admission or a prior diagnosis of hypertension. Current cigarette smoking was defined as a risk factor for adults who had smoked at least 100 cigarettes in their lifetime and smoked cigarettes every day (daily) or some days (non-daily) at the time of clinical presentation. Cocaine use was considered as a potential risk factor if used within 1 year of clinical presentation.
Statistical Analysis
Pearson's product-moment was used to correlate continuous variables, such as patient age and morphological CA measurements with CA F14C levels and estimated collagen age. Spearman's correlation was used to analyze correlations between dichotomized variables (CA rupture status, irregularity or presence of risk factors) and continuous variables (estimated collagen age and F14C levels). Differences between groups were compared by the non-parametric Mann-Whitney test to account for small group sizes. Correlations and differences were always analyzed for both F14C levels and estimated CA collagen age to avoid over-interpretation of single results due to sample size. For statistical analysis risk factors were classified and defined as any risk factor versus no risk factor. The odds ratio (OR) for patients to harbor a CA with an estimated collagen age of one year or less was calculated for patients with risk factors compared to those without. Significance was accepted at a level of p ≤ 0.05. All statistical analysis was performed using SPSS 15.0.1 (Ulead Technologies, Chicago, Illinois).
Results
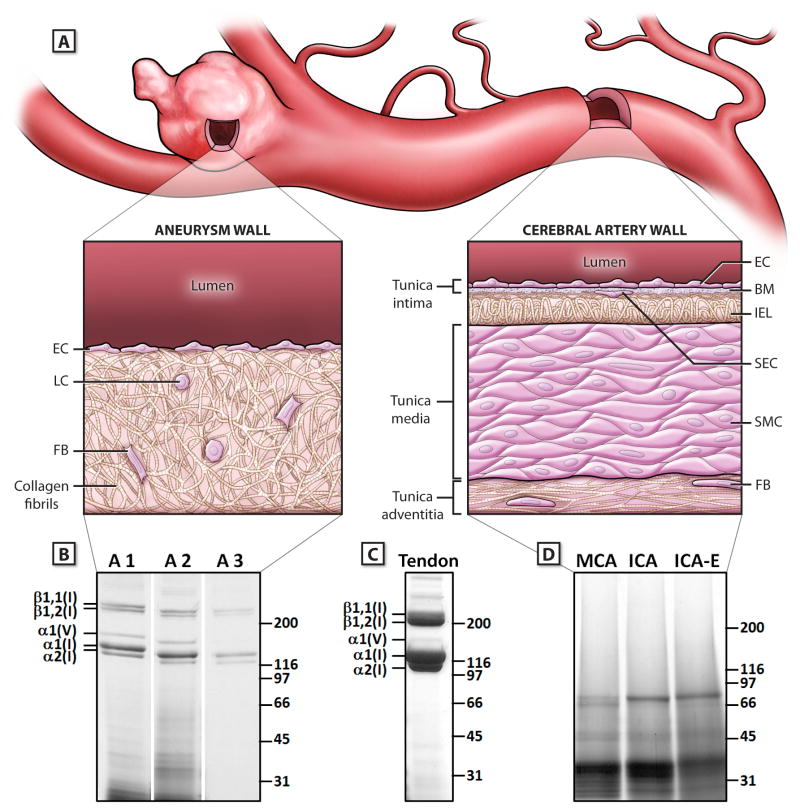
Between 03/2009 and 04/2013 a total of 293 CAs were surgically repaired at the first author's institution. During this period 53 CA samples from 50 patients met the inclusion criteria and were collected. None of the CAs was known to have existed for a long time, i.e. more than 2 months before aneurysm repair. Ultimately, samples from 36 ruptured and 10 unruptured CAs from 43 patients yielded sufficient amounts of collagen for further AMS analysis (Table I, please see http://stroke.ahajournals.org). Mean patient age in this cohort was 55.1 ± 11.5 years. Gel electrophoresis confirmed the high purity of the collagen from CA samples, with collagen type I and lesser amounts of collagen type V being the only detectable components (Figure 1). Despite some variability in the amount of purified collagen after pepsin digestion there was no association between the yield of collagen from a CA and its F14C measurements. Importantly, CAs and cerebral or extracerebral arteries did not share a dominant structural protein which could be used to compare collagen age and turnover in normal arteries and CAs: Gel electrophoresis of the pepsin digested human cerebral arteries revealed no substantial enrichment of collagen types I or V, but several unidentified proteins, or fragments thereof, which were stable against further digestion with pepsin (Figure 1). Due to their low amounts of collagen type I, the normal cerebral arteries were not amenable to birth dating of collagen type I. The five control collagen samples from mouse tendons confirmed the validity of the method since all of these samples gave F14C values for collagen corresponding to the F14C values of the mouse chow fed to the animals (data not shown).
Figure 1.
Differences in the structural and molecular composition of cerebral aneurysms and cerebral arteries. A) Schematic illustration of the structural composition of a cerebral aneurysm and the parent cerebral artery. The aneurysm wall contains collagen type I fibrils as the dominant structural component, with loss of the typical arterial wall features, such as the internal elastic lamina (IEL) and the tunica layers. The cerebral artery contains different organized layers of cellular and extracellular matrix components, without a meaningful proportion of collagen type I. B) Electrophoretic mobility of pepsin digested aneurysm samples analysed on Coomassie brilliant blue-stained SDS-PAGE gels (4.5% to 15%, reducing conditions).: Three representative aneurysm samples (A1-A3) are shown. The predominant bands represent α bands of collagen type I (α1[I],α2[I]) and type V (α1[V], α2[V]), as well as β components of collagen type I (β1, 1[I], β1, 2[I]). C: Collagen type I amount and purity in aneurysm and artery samples was compared to pepsin digested collagens from mouse tail tendons (T). D: Cadaveric cerebral arteries were analyzed under the same digestion and separation conditions as aneurysms. One representative set is shown without any bands for collagen type I and V. Abbreviations: BM indicates basal membrane; EC, endothelial cells; FB, fibroblasts; ICA, Internal Carotid Artery; ICA-E, extracranial Internal Carotid Artery; IEL, internal elastic lamina; LC, lymphocytes; MCA, Middle Cerebral Artery; SEC, subendothelial cells; SMC, smooth muscle cells.
Birth dating of Collagen from Cerebral Aneurysms
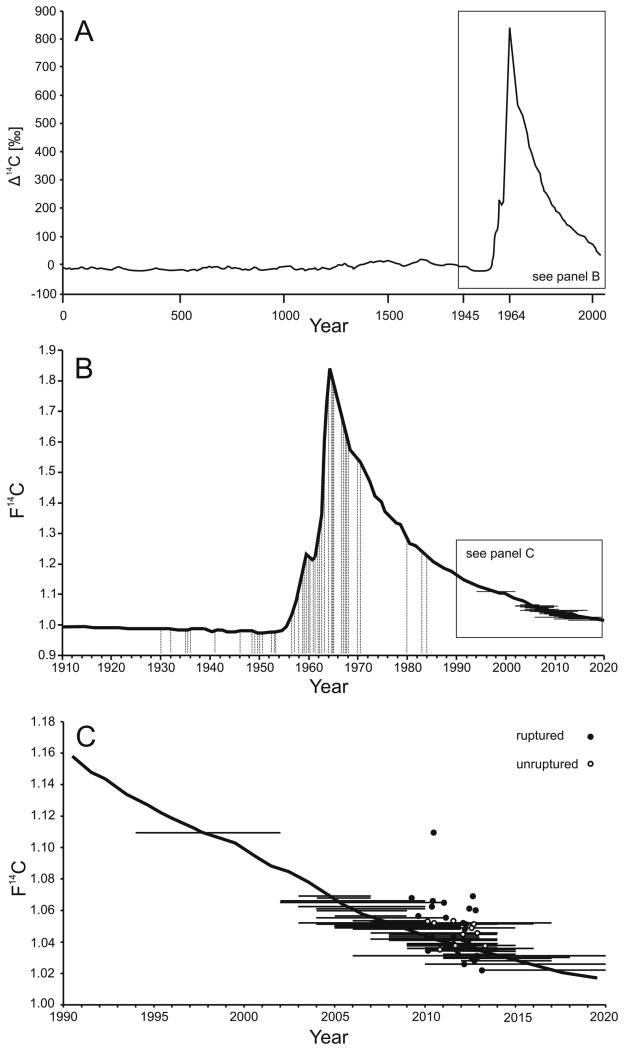
There was a distinct dissociation between the patients' years of birth and age intercepts of their corresponding CAs (Figure 2, Table I please see http://stroke.ahajournals.org): Except for three samples (samples 8, 30, 33), all CA collagen samples were five years old or less (overall median age estimate: 1.5 years, IQR 2.5 years). There was no difference in the estimated age (p = 0.946) or F14C levels (p = 0.739) of collagen from ruptured and unruptured CAs (Figure 3). However, CAs from patients with a history of potential risk factors for CA formation and rupture (hypertension, cigarette smoking or cocaine use) contained significantly younger collagen. Mean collagen age was 1.6 ± 1.2 years for patients with risk factors compared to 3.9 ± 3.3 years for patients without risk factors (p = 0.012) (Figure 3). This difference was also reflected in the actual F14C levels (1.043 ± 0.0106 for patients with risk factors versus 1.0563 ± 0.0207, without risk factors, p = 0.027). Patients with a history of risk factors were more likely [OR: 4.29 (95% CI: 0.98-18.72, p=0.053)] to have CA collagen younger than one year, as compared to patients without risk factors. The modest inverse correlation between patient age and estimated collagen age (p = 0.022) was not confirmed following the correlation with actual F14C levels (p=0.115) (Figure 3). There was no association between estimated CA collagen age and CA characteristics, with non-significant correlations of estimated CA collagen age and largest CA diameter (r =-0.005, p = 0.974), CA irregularity (r=-0.08, p=0.598) and aspect ratio (r=-0.005, p=0.971).
Figure 2.
Birth dating of collagen in CAs. A) Atmospheric 14CO2 levels are illustrated for the past 2000 years. These have been relatively stable except for a large increase between 1955-1963, due to atmospheric nuclear testing; the resulting spike of atmospheric 14CO2 is called the bomb pulse. The Δ14C nomenclature corrects for radioactive decay and shows the historical 14CO2 production rate.14 Note the change in temporal resolution after 1945 for illustration purposes. B) The birth dates of patients' (vertical lines) and CA age intercepts (horizontal lines) are projected onto the F14C bomb pulse curve, illustrating the dissociation between the patient age and the estimated CA age. C) CA age intercepts (horizontal lines) in relation to time of sample acquisition for ruptured (filled circles) and unruptured (open circles) CAs.
Figure 3.
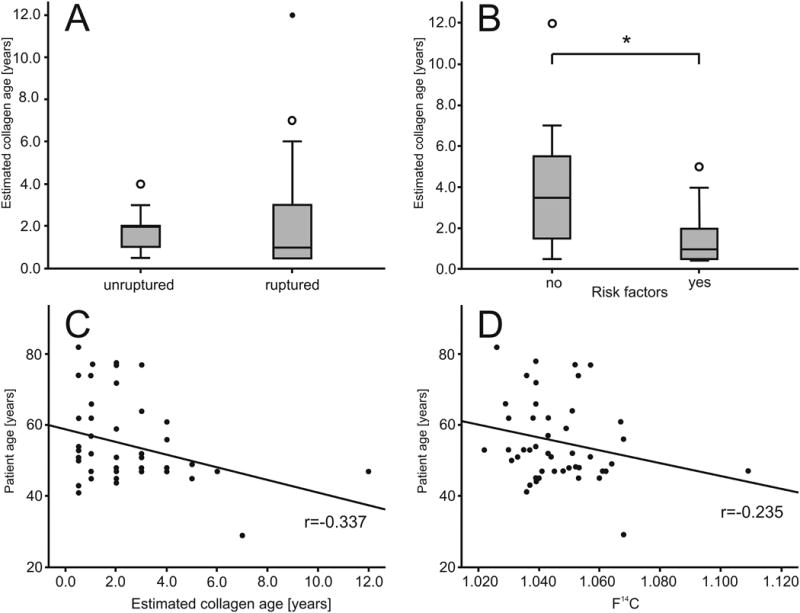
Determinants for CA collagen age. A) Estimated collagen age for patients with ruptured and unruptured CAs. Open circles denote outliers, filled circles denote extreme outliers in panel A and B. B) Estimated CA collagen age for patients with and without a history of risk factors for aneurysm formation and rupture. Open circles denote outliers, filled circles denote extreme outliers in panel A and B. C, D) Correlation of patient age with estimated CA collagen age or actual F14C levels in CA collagen, respectively. Correlations remained significant after exclusion of the extreme outlier.
Discussion
We used 14C birth dating of collagen extracted from CAs, as a measure of collagen age and turnover to demonstrate that CAs invariably contain recently formed collagen type I and/or that they are dynamic structures containing collagen that is typically less than five years old. The age of collagen in CAs is independent of CA clinical presentation (ruptured versus incidental) and, moreover, of factors which may be associated with CA formation and/or rupture, i.e. patient age, aneurysm size and morphology. Importantly, we could not compare the age and turnover of collagen type I in CAs to that in cerebral arteries since CAs and normal arteries of the circle of Willis did not share a representative and exclusive structural protein, such as collagen type I.
Previous observational data on a selected cohort of patients with unruptured CAs suggested that CA location in the posterior circulation and increased CA size are significantly associated with risk of rupture and, moreover, that small, unruptured CAs have a very low risk of rupture.15 Higher rates of rupture, but still highly correlated with CA size were reported in another series from Japan.16 The discrepancy between the low risk of rupture of small, incidentally detected CAs in previous observational studies and the high proportion of small CAs in patients with SAH has been difficult to reconcile. The prevailing concept remains that there are different populations of small CAs, including those that grow and reach a stable size, those that continue to enlarge and either reach a stable larger size or rupture over the long-term, and that those that form, enlarge, and then rupture over a relatively short period of time. Aside from our preliminary birth dating study in a small patient cohort, data to support or challenge this concept is mainly derived from animal studies or mathematical models and observational studies of human CAs. 15, 17-21 Additionally, the existing knowledge on collagen turnover in arteries is mainly derived from animal models or extracranial vessels: In general, increased overall collagen turnover in extracranial arteries has been reported under conditions inducing arterial remodeling but studies have not differentiated turnover of specific collagen types and have rarely examined human cerebral arteries.22-25 Thus, biological data on collagen age and turnover in larger human CA cohorts in relation to cerebral arteries is lacking.
In line with our preliminary report, the most likely explanation for our current findings is that there is ongoing collagen type I biosynthesis in CAs due to dynamic remodeling, comparable to a continuous attempt for fibrotic tissue repair to a previous event causing vessel wall instability and the aneurysm formation. 11, 26-28 Interestingly, this remodeling seems to be significantly accelerated in patients with risk factors associated with atherosclerosis and potentially aneurysm growth and rupture (hypertension, cigarette smoking or cocaine use), possibly due to increased hemodynamic stress. 13, 18, 29 This is consistent with serial imaging data showing that some unruptured CAs do undergo growth, including selected smaller aneurysms and a substantial proportion of larger aneurysms especially in patients who smoke. 30, 31 Some unruptured CAs may grow for short times punctuated by long periods of non-growth, suggesting that collagen remodeling is ongoing and dynamic. 5, 31, 32 Thus, variable, non-linear growth rates of some CAs may also better explain why ruptured CAs are usually small and would not be predicted to rupture if found incidentally.5, 15, 18 Ultimately, our findings also underline that the extracellular matrix and the biomechanical properties of CAs and cerebral arteries are fundamentally different: While collagen type I is a main constituent of the CA wall, mechanical stability in cerebral arteries is maintained by a complex network of elastin, collagen types III, IV and VI and smooth muscle cells, but without a significant contribution of collagen I. Additionally, cerebral arteries do not comprise a large tunica adventitia as a fibrous tissue component which explains the relative absence of collagen type I.22, 25
There are some limitations to our findings. Due to the cross-sectional nature of our study and the lack of CA samples which have been followed for many years, we cannot draw definite causal inferences regarding the actual chronological onset of aneurysm formation or a potential association with a heightened risk of CA rupture. Further, it cannot be ascertained whether the age of collagen is different in the neck or in the remaining CA tissue. Additionally, we can only estimate relative collagen turnover by using radiocarbon birth dating as an indicator, since the total collagen content in human CA samples cannot be monitored over time.33 However, since collagen I is the main constituent of CAs and since we generally excised and analyzed more than 2/3 of the aneurysm dome, the birth dating measurements are likely to reflect the majority of the aneurysmal mass. Further, the measurement precision in radiocarbon birth dating corresponds to a chronological uncertainty of 1 to 3 years and current technology does not allow for better temporal resolution in samples. Lastly, the extractability of collagens from different CAs was variable. However, while the extractable, thus analyzable collagen I fraction varied from 11-100% (mean 41 ± 26%) of the total collagen content in the CA, F14C levels throughout all samples were consistent.
In summary, the abundance of young age of collagen in CAs indicates that structural remodeling of collagen in CAs is an ongoing and dynamic process. This process seems to be more rapid in the presence of risk factors such as hypertension and cigarette smoking. Because of fundamentally different biochemical composition of CAs and cerebral arteries, these data cannot be directly compared or extrapolated to the normal cerebral vasculature. However, our data suggest that collagen in CAs, irrespectively of their size or rupture status, has developed rather recently in the patient's life. Ultimately and, in line with more recent data on serial aneurysm imaging, our data imply that patients with conservatively managed, incidental CAs and concomitant risk factors should be advised for strict control and effective modification of the risk factors.
Supplementary Material
Acknowledgments
None
Funding Source: NE and RLM received grant support from the Physicians Services Incorporated Foundation RLM received grant support from the Brain Aneurysm Foundation, Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario. This work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. BAB received support from NIGMS 8P41GM103483.
Footnotes
Disclosures: The authors report no relevant conflict of interest.
References
- 1.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain mri in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 3.Etminan N, Beseoglu K, Barrow DL, Bederson J, Brown RD, Jr, Connolly ES, Jr, et al. Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: Proposal of an international research group. Stroke. 2014 doi: 10.1161/STROKEAHA.114.004519. published online March 25th 2014. [DOI] [PubMed] [Google Scholar]
- 4.Chang HS. Simulation of the natural history of cerebral aneurysms based on data from the international study of unruptured intracranial aneurysms. J Neurosurg. 2006;104:188–194. doi: 10.3171/jns.2006.104.2.188. [DOI] [PubMed] [Google Scholar]
- 5.Koffijberg H, Buskens E, Algra A, Wermer MJ, Rinkel GJ. Growth rates of intracranial aneurysms: Exploring constancy. J Neurosurg. 2008;109:176–185. doi: 10.3171/JNS/2008/109/8/0176. [DOI] [PubMed] [Google Scholar]
- 6.Hua Q, Barbetti M. Review of tropospheric bomb c-14 data for carbon cycle modeling and age calibration purposes. Radiocarbon. 2004;46:1273–1298. [Google Scholar]
- 7.Levin I, Naegler T, Kromer B, Diehl M, Francey RJ, Gomez-Pelaez AJ, et al. Observations and modelling of the global distribution and long-term trend of atmospheric 14co2. Tellus. 2010:26–46. [Google Scholar]
- 8.Harkness DD. Further investigations of the transfer of bomb 14 c to man. Nature. 1972;240:302–303. doi: 10.1038/240302a0. [DOI] [PubMed] [Google Scholar]
- 9.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Lovell MA, Robertson JD, Buchholz BA, Xie C, Markesbery WR. Use of bomb pulse carbon-14 to age senile plaques and neurofibrillary tangles in alzheimer's disease. Neurobiology of aging. 2002;23:179–186. doi: 10.1016/s0197-4580(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 11.Etminan N, Dreier R, Buchholz BA, Bruckner P, Steiger HJ, Hanggi D, et al. Exploring the age of intracranial aneurysms using carbon birth dating: Preliminary results. Stroke. 2013;44:799–802. doi: 10.1161/STROKEAHA.112.673806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, et al. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: A possible index for surgical treatment of intracranial aneurysms. Neurosurgery. 1999;45:119–129. doi: 10.1097/00006123-199907000-00028. discussion 129-130. [DOI] [PubMed] [Google Scholar]
- 13.Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: A long-term follow-up study. Stroke. 2001;32:485–491. doi: 10.1161/01.str.32.2.485. [DOI] [PubMed] [Google Scholar]
- 14.Stuiver M, Polach HA. Reporting of c-14 data - discussion. Radiocarbon. 1977;19:355–363. [Google Scholar]
- 15.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 16.Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al. The natural course of unruptured cerebral aneurysms in a japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 17.Chatziprodromou I, Tricoli A, Poulikakos D, Ventikos Y. Haemodynamics and wall remodelling of a growing cerebral aneurysm: A computational model. Journal of biomechanics. 2007;40:412–426. doi: 10.1016/j.jbiomech.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Etminan N, Beseoglu K, Steiger HJ, Hanggi D. The impact of hypertension and nicotine on the size of ruptured intracranial aneurysms. Journal of neurology, neurosurgery, and psychiatry. 2011;82:4–7. doi: 10.1136/jnnp.2009.199661. [DOI] [PubMed] [Google Scholar]
- 19.Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. Stroke. 2013;44:2414–2421. doi: 10.1161/STROKEAHA.113.001838. [DOI] [PubMed] [Google Scholar]
- 20.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sluijter JP, Smeets MB, Velema E, Pasterkamp G, de Kleijn DP. Increased collagen turnover is only partly associated with collagen fiber deposition in the arterial response to injury. Cardiovascular research. 2004;61:186–195. doi: 10.1016/j.cardiores.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Mimata C, Kitaoka M, Nagahiro S, Iyama K, Hori H, Yoshioka H, et al. Differential distribution and expressions of collagens in the cerebral aneurysmal wall. Acta neuropathologica. 1997;94:197–206. doi: 10.1007/s004010050694. [DOI] [PubMed] [Google Scholar]
- 23.Nissen R, Cardinale GJ, Udenfriend S. Increased turnover of arterial collagen in hypertensive rats. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:451–453. doi: 10.1073/pnas.75.1.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ooshima A, Fuller G, Cardinale G, Spector S, Udenfriend S. Collagen biosynthesis in blood vessels of brain and other tissues of the hypertensive rat. Science. 1975;190:898–900. doi: 10.1126/science.171771. [DOI] [PubMed] [Google Scholar]
- 25.Rowe AJ, Finlay HM, Canham PB. Collagen biomechanics in cerebral arteries and bifurcations assessed by polarizing microscopy. Journal of vascular research. 2003;40:406–415. doi: 10.1159/000072831. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey JD. Remodeling of a collagenous tissue at fixed lengths. Journal of biomechanical engineering. 1999;121:591–597. doi: 10.1115/1.2800858. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S, Tsubokawa T, Yoshida K, Hirasawa T, Nakano M. Appearance of collagen fibers in the cerebral vascular wall following subarachnoid hemorrhage. Neurologia medico-chirurgica. 1992;32:877–882. doi: 10.2176/nmc.32.877. [DOI] [PubMed] [Google Scholar]
- 28.Verzijl N, DeGroot J, Bank RA, Bayliss MT, Bijlsma JW, Lafeber FP, et al. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: The use of pentosidine levels as a quantitative measure of protein turnover. Matrix biology : journal of the International Society for Matrix Biology. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 29.Chatziprodromou I, Poulikakos D, Ventikos Y. On the influence of variation in haemodynamic conditions on the generation and growth of cerebral aneurysms and atherogenesis: A computational model. Journal of biomechanics. 2007;40:3626–3640. doi: 10.1016/j.jbiomech.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Burns JD, Huston J, 3rd, Layton KF, Piepgras DG, Brown RD., Jr Intracranial aneurysm enlargement on serial magnetic resonance angiography: Frequency and risk factors. Stroke. 2009;40:406–411. doi: 10.1161/STROKEAHA.108.519165. [DOI] [PubMed] [Google Scholar]
- 31.Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J, et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at ct angiography: Growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013;269:258–265. doi: 10.1148/radiol.13121188. [DOI] [PubMed] [Google Scholar]
- 32.Balakhovsky K, Jabareen M, Volokh KY. Modeling rupture of growing aneurysms. Journal of biomechanics. 2014;47:653–658. doi: 10.1016/j.jbiomech.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 33.Sluijter JP, Smeets MB, Velema E, Pasterkamp G, de Kleijn DP. Increase in collagen turnover but not in collagen fiber content is associated with flow-induced arterial remodeling. Journal of vascular research. 2004;41:546–555. doi: 10.1159/000081972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.