Abstract
In Parkinson’s Disease (PD), qualitative speech changes such as decreased variation in pitch and loudness are common, but quantitative vocal changes are not well documented. The variability of fundamental frequency (F0) in 32 individuals (23 male) with PD both ON and OFF levodopa medication was compared with 32 age-matched healthy controls (23 male). Participants read a single paragraph and estimates of fundamental frequency (F0) variability were determined for the entire reading passage as well as for the first and last sentences of the passage separately. F0 variability was significantly increased in controls relative to both PD groups and PD patients showed significantly higher F0 variability while ON medication relative to OFF. No significant effect of group was seen in the change in F0 variability from the beginning to the end of the reading passage. Female speakers were found to have higher F0 variability than males. F0 variability was both significantly reduced in PD relative to controls and significantly increased in patients with PD during use of dopaminergic medications. F0 variability changes over the course of reading a paragraph may not be indicative of PD but rather dependent on non-disease factors such as the linguistic characteristics of the text.
Introduction
Parkinson’s disease is a neurological disorder characterized by the progressive loss of dopaminergic neurons in specific brain areas and occurs most prevalently in older adults (Lang & Lozano, 1998). Characteristic symptoms include tremor, rigidity, dyskinesias, and postural gait changes (Lang & Lozano, 1998). Vocal symptoms are also common, with speech deficits occurring in 60–80% of patients (Canter, 1963; Darley, Aronson, & Brown, 1969; Mutch, Strudwick, Roy, & Downie, 1986). The Parkinsonian voice has been described qualitatively as breathy, rough, hoarse, tremulous, abnormally pitched, having reduced pitch range, and unusually quiet (Holmes, Oates, Phyland, & Hughes, 2000).
Fundamental frequency (F0), a result of the rate of vibration of vocal folds, is perceptually related to vocal pitch. Some studies have found higher mean F0 values in individuals with Parkinsonian voice (Goberman & Blomgren, 2008), particularly in males (Canter, 1963; Holmes et al., 2000; Metter & Hanson, 1986; Skodda, Gronheit, & Schlegel, 2011). However, average speaking fundamental frequencies show considerable inter-speaker and intra-speaker differences due to intentional prosodic changes (Atkinson, 1976). A reduction in these intentional prosodic pitch changes can be measured by examining F0 variability, which has been shown to contribute to overall speech intelligibility in both healthy and dysarthric speakers (Bunton, Kent, Kent, & Duffy, 2001; Laures & Weismer, 1999).
Acoustic characterization of F0 variability has been realized in a variety of ways, including the range (difference between the passage maximum and minimum) of F0 excursions, F0 standard deviation (F0SD), and the semitone standard deviation (STSD), which is normalized for mean speaker F0. Although F0 range has been studied in PD previously, measures of range are highly corruptible by single time-points and thus are less appropriate estimations of overall prosodic variation. In this study we focus on F0SD and STSD. STSD has been used previously (Ramig, Countryman, Thompson, & Horii, 1995) as an intervention outcome measure in PD; it provides an estimate of passage prosodic variation that is less likely to be confounded by the effects of mean F0 (and thus speaker sex) and is also robust to single instances of F0 deviations. Although F0SD is somewhat robust against corruption from a minority of datapoints, it may be affected by mean F0. It has however been studied in individuals with PD by several groups (Gamboa et al., 1997; Goberman, Coelho, & Robb, 2005; Holmes et al., 2000; Jimenez-Jimenez et al., 1997; Skodda et al., 2011). Here we include F0SD in order to make comparisons to previous literature.
F0 variability has thus far been investigated in PD with conflicting results. In read text ranging from a single sentence to a paragraph, some work has shown a decrease in F0SD with PD (Gamboa et al., 1997; Goberman et al., 2005; Jimenez-Jimenez et al., 1997; Skodda et al., 2011), while others found no effect of PD on F0SD in males but a decrease with PD in females (Holmes et al., 2000). A recent study by Skodda and colleagues found decreases in F0SD in a large group of PD patients independent from clinical manifestations of dysarthria while ON medication relative to age-matched controls (Skodda et al., 2011). F0SD was measured during a reading task of four complex sentences (Skodda et al., 2011). Skodda et al. (2011) also examined the difference in F0SD between the first and fourth sentences of their reading passage (ΔF0SD). Although both male and female participants with PD (ON medication) showed a negative average ΔF0SD (a decrease in F0 variability at the end of the reading passage relative to the beginning), both male and female control participants showed a positive average ΔF0SD (Skodda et al., 2011). In male participants with PD, neither F0SD nor ΔF0SD were significantly correlated with disease duration or the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS III). In female participants with PD, neither F0SD nor ΔF0SD were correlated with disease duration, but F0SD and UPDRS III scores were modestly correlated (R=−0.32, p=0.01), with decreases in F0SD associated with higher UPDRS scores (increased severity of PD symptoms; Skodda et al., 2011). Increased stiffness and rigidity of the larynx and vocal folds (Goberman & Coelho, 2002), reduced muscle activity in the larynx (Baker, Ramig, Luschei, & Smith, 1998), reduced stability in the larynx (Goberman & Coelho, 2002), and a lack of tension in the larynx needed to create sounds (Goberman & Blomgren, 2008) have been hypothesized to cause these F0 variability changes in PD. Although rigidity has commonly been hypothesized to underlie voicing changes in PD, more recent evidence suggests that additional factors such as sensorimotor deficits and internal cueing issues may contribute (Ramig, Fox, & Sapir, 2011).
The effects of dopamine agonists on F0 variability are still unclear. Goberman examined F0SD in a group of 9 individuals with PD both ON and OFF medication, finding a trend for increased F0SD while ON medication, but no statistically significant effect (Goberman et al., 2005). Skodda et al. examined both F0SD and ΔF0SD as a function of medication status in a subgroup of 20 participants with PD. Their work also found that medication state did not significantly affect F0SD or ΔF0SD. However, they noted a trend in which male patients (N=7) showed somewhat increased ΔF0SD ON medication (−0.39 Hz) relative to more negative OFF medication ΔF0SD (−2.17 Hz) (Skodda et al., 2011). Skodda et al. hypothesized that this decrease in variability over the reading passage in the OFF medication state is a result of general “motor instability.” They suggested that dopaminergic stimulation may stabilize otherwise declining F0SD and called for study with a larger sample of participants.
Current understanding of F0 changes and variability remains incomplete. Most vocal symptoms are qualitative and what defines Parkinsonian speech is often subjective and unclear. In addition, early biomarkers are neither well developed nor understood. A case study by Harel et al found visible changes in F0 5 years before PD diagnosis (Harel, Cannizzaro, Cohen, Reilly, & Snyder, 2004) suggesting that if quantified and developed, F0 changes could detect PD much earlier and more accurately. F0 changes may also assist in developing quantitative measures of disease progression since few exist. Many studies have conflicting results or small participant populations, indicating the need for further research.
The purpose of the current study is to examine F0 variation in PD patients independent from the clinical manifestation of dysarthria while both ON and OFF medication. We compare the voices of participants with PD to those of healthy age-matched controls. We hypothesize that individuals with PD have reduced F0 variation compared to controls and that individuals with PD will have increased F0 variability while ON medications relative to their OFF medication states.
Methods
Participants
32 participants with PD (9 females and 23 males) and 32 controls (9 females and 23 males) completed the study with informed consent. No participants reported any other neurological, speech, or language disorders, with the exception of some (~10%) reported some minor age-related hearing loss. The mean age of female control participants was 67 years (STD 7.4; range 58 – 81) and the mean age of male control participants was 66 years (STD 6.7, range 56 – 79).
Participants with PD had been previously diagnosed with idiopathic Parkinson’s Disease and were currently under the care of a movement disorders specialist. The mean age of female participants with PD was 66 years (STD = 10.1, range = 49 – 79) and they had a mean disease duration of 8.61 years (SD = 7.3, range = 1 – 20). The mean age of male participants with PD was 69 years (STD 8.8; range 51 – 89) and they had a mean disease duration of 5.283 years (SD = 3.655, range =0.5–16). Participants with PD presented with a range of disease severities. The majority considered speech symptoms to be nonexistent (UPDRS subsection 2.1 = 0: N = 14, 44%) or slight (UPDRS subsection 2.1 = 1: N = 8, 25%), whereas the rest reported mild (UPDRS subsection 2.1 = 3: N = 4, 13%) or moderate (UPDRS subsection 2.1 = 4: N = 6, 19%) speech impairment. No participant with PD reported severe speech impairment (score of 4). Participant report of PD-related speech impairment was relatively similar in males and females. The mean UPDRS subsection 2.1 score was 0.78 for women and 1.17 for men, both of which are near the “slight” impairment stage.
Procedures
All participants with PD were typically on levodopa and/or carbidopa medication and underwent a medication challenge as part of this study; thus each participant with PD was tested first OFF and then ON medication. Individuals with PD were instructed to forgo morning medication so that they last took medication the night before, at least 8 hours prior to testing. After completion of OFF testing, each participant took his or her medication. ON testing occurred after the participant felt the medication take effect (usually within 45 minutes), consistent with the known pharmacokinetics of levodopa and carbidopa (Robertson et al., 1989). Control participants did not undergo a medication challenge, so vocal data were collected only at a single time point.
During testing, all participants read the first paragraph of “The Rainbow Passage” (Fairbanks 1960) in order to produce continuous speech samples. This read text was used rather than spontaneous speech in order to control for content, which could induce substantial inter- and intra-subject variability (Fitch, 1990). Speech samples were recorded in quiet using a portable digital audio recorder (Olympus Linear PCM recorder, LS-10) at 44.1 kHz, 16 bit, with a headset microphone (Shure WH20) placed at a 45 degree angle and 10 cm from the lips. Since sound pressure level was not a primary variable of interest, the microphone was not calibrated using a sound pressure level meter. However, each participant with PD was recorded with identical microphone distance and recorder amplification settings during both ON and OFF medication recordings. All acoustic recordings obtained were of high quality with good signal-to-noise ratios. Participants were directed to speak in comfortable, normal, conversational voices. All participants were able to read the provided text without difficulty.
During both the ON and OFF medication states, a licensed physical therapist and clinical researcher (author S.P.) administered and scored the UPDRS (Unified Parkinson’s Disease Ratings Scale; Sections I, II, and III) for participants with PD.
Data Analysis
Speech samples were analyzed using Praat software (Boersma, 2011). Analysis was performed by a total of three trained researchers (see acknowledgements) who were blinded to the study goals. One individual completed analysis for all 96 samples (32 individuals with PD while ON and OFF, 32 control participants). Two additional individuals each completed analysis for roughly 50% of the data such that each sample was analyzed by two independent researchers. For further analysis, data were averaged over the two independent researchers to result in a single estimate of F0SD and ΔF0SD for each speaker.
F0SD was measured using corresponding functions in Praat (Boersma, 2011) over the entire first paragraph of “The Rainbow Passage.” Using the average F0SD value and the mean F0 over the relevant passage, the corresponding STSD value was calculated as in equation 1.
| (1) |
ΔF0SD was calculated as the difference between the F0SD for the last sentence of the first paragraph (“When a man looks for something beyond his reach, his friends say he is looking for the pot of gold at the end of the rainbow”) and the first sentence of the paragraph (“The rainbow is a division of white light into many beautiful colors”). The corresponding ΔSTSD was calculated by converting the F0SD for each sentence using equation 1 and then subtracting the value from the first sentence from the value from the last sentence.
The Praat settings were adjusted manually for each voice and starting values were based on whether the sample recording was of a male or a female. The initial fundamental frequency range setting for females was set at 80–500 Hz whereas for males it was set at 60–400 Hz. The accuracy of Praat’s fundamental frequency estimates was ascertained by visually inspecting each pitch contour while simultaneously listening to the relevant segment of the sound file. Any inconsistencies due to glottalization or incorrect detection of voicing were removed from the sample manually.
Reliability Measures
Inter-rater reliability between the two independent judges of each sample was calculated using Pearson’s correlation coefficient, yielding inter-rater reliabilities of 0.97 and 0.96 for F0SD and ΔF0SD between raters 1 and 2 respectively, and 0.98 and 0.82 between raters 1 and 3 respectively. Rater 1 (the single investigator who analyzed all samples) re-analyzed roughly 10% of samples independently 3 months after the original analysis yielding intra-rater reliability of 0.99 for both F0SD and ΔF0SD. Given the correspondence of STSD and ΔSTSD to F0SD and ΔF0SD, separate reliability measures were not computed.
Statistical Analysis
All statistical analyses were completed using Minitab Statistical Software (Minitab Inc., State College, PA). Two-way analysis of variance (ANOVA) was performed on F0SD, ΔF0SD, STSD and ΔSTSD as a function of sex (male or female), group (PDOFF, PDON, and CTRL), and the interaction of sex × group. Post hoc Tukey simultaneous tests and paired Student’s t-tests were used when appropriate. An alpha level of 0.05 was adopted for significance testing. Pearson product moment correlations were used to assess relationships among measures.
Results
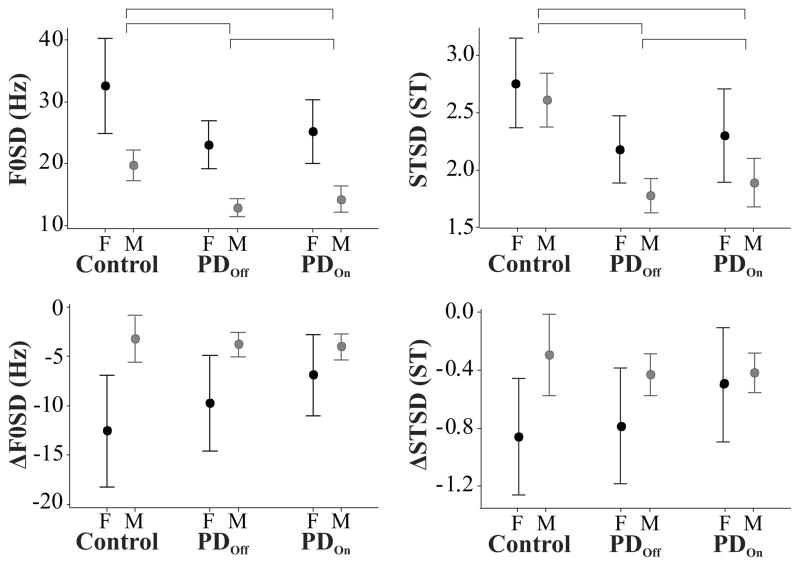
The mean female UPDRS total score was 52.1 (SD=35.8, range = 9 – 97) and the mean male UPDRS score was 40.9 (SD =19.8, range = 9 – 91). Disease duration and OFF medication UPDRS scores were statistically significantly correlated (p = 0.003) with R = 0.50. Figure 1 shows F0SD, STSD, ΔF0SD, and ΔSTSD as a function of sex and group.
Figure 1.
Effects of group (Control, PD OFF medication, PD ON medication) and sex (F: female, M: male) on the standard deviation of the fundamental frequency (F0SD; left upper panel), the semitone standard deviation (STSD; right upper panel), the difference in F0SD between the last and first sentences of the reading passage (ΔF0SD; left lower panel), and the difference in STSD between the last and first sentences of the reading passage (ΔSTSD; right lower panel). Error bars indicate 95% confidence intervals around the mean. Horizontal brackets indicate statistically significant (p < 0.05) differences between groups. All four measures showed a statistically significant effect of sex.
The results of the ANOVA on F0SD are shown in Table 1. Statistically significant effects of sex (male or female) and group (PDOFF, PDON, and CTRL) were found, but no significant interaction of sex × group. Post hoc Tukey simultaneous tests indicated that females had statistically significantly higher F0SD than males (T = −8.9, padj < 0.001) and that control speakers had significantly higher F0SD than individuals with PD, both ON (T = −4.1, padj < 0.001) and OFF (T = −5.2, padj < 0.001) medication. A post hoc paired two-sided Student’s t-test found that individuals with PD had statistically significantly increased F0SD while ON medication relative to OFF medication (T = −3.1, p = 0.004).
Table 1.
Results of ANOVA on passage F0SD
| Factor | DF | F | ηp2 | p |
|---|---|---|---|---|
| Sex | 1 | 78.8 | 0.47 | <0.001 |
| Group (PDOFF, PDON, CTRL) | 2 | 15.1 | 0.26 | <0.001 |
| Sex × Group | 2 | 0.36 | 0.01 | 0.700 |
The results of the ANOVA on STSD are shown in Table 2 and largely mirror those found for F0SD. ANOVA showed statistically significant effects of sex (male or female) and group (PDOFF, PDON, and CTRL), but no significant interaction of sex × group. Post hoc Tukey simultaneous tests indicated that females had statistically significantly higher F0SD than males (T = −3.0, padj = 0.003) and that control speakers had significantly higher F0SD than individuals with PD, both ON (T = −4.5, padj < 0.001) and OFF (T = −5.4, padj < 0.001) medication. A pos thoc paired two-sided Student’s t-test found that individuals with PD had statistically significantly increased F0SD while ON medication relative to OFF medication (T = −2.2, p = 0.03).
Table 2.
Results of ANOVA on passage STSD
| Factor | DF | F | ηp2 | p |
|---|---|---|---|---|
| Sex | 1 | 9.0 | 0.09 | 0.003 |
| Group (PDOFF, PDON, CTRL) | 2 | 16.7 | 0.35 | <0.001 |
| Sex × Group | 2 | 0.7 | 0.01 | 0.517 |
The results of the ANOVA on ΔF0SD are shown in Table 3 and showed statistically significant effects of sex (male or female), but not of group (PDOFF, PDON, and CTRL). In addition the interaction of sex × group approached our predetermined alpha level, but was not significant (p = 0.052). A post hoc Tukey simultaneous test indicated that females had statistically significantly lower ΔF0SD (greater decline in F0SD over the course of the passage) than males (T = 5.6, padj < 0.001).
Table 3.
Results of ANOVA on passage ΔF0SD
| Factor | DF | F | ηp2 | p |
|---|---|---|---|---|
| Sex | 1 | 31.6 | 0.26 | <0.001 |
| Group (PDOFF, PDON, CTRL) | 2 | 1.8 | 0.01 | 0.178 |
| Sex × Group | 2 | 3.0 | 0.06 | 0.052 |
The results of the ANOVA on ΔSTSD are shown in Table 4 and again mirrored ΔF0SD results. ΔSTSD showed statistically significant effects of sex (male or female), but not of group (PDOFF, PDON, and CTRL). In addition the interaction of sex × group was not significant. A post hoc Tukey simultaneous test indicated that females had statistically significantly lower ΔF0SD than males (T = 3.1, padj = 0.003).
Table 4.
Results of ANOVA on passage ΔSTSD
| Factor | DF | F | ηp2 | p |
|---|---|---|---|---|
| Sex | 1 | 9.5 | 0.10 | 0.003 |
| Group (PDOFF, PDON, CTRL) | 2 | 0.7 | 0.01 | 0.498 |
| Sex × Group | 2 | 1.7 | 0.04 | 0.192 |
When patients were OFF medication, the Pearson’s correlations between time after diagnosis and F0SD and ΔF0SD were r = 0.30 and r = 0.10, respectively. Neither were statistically significant (p < 0.05). The Pearson’s correlations between time after diagnosis and STSD and ΔSTSD were r = 0.12 and r = 0.12, respectively, neither of which were statistically significant (p < 0.05). Due to the significant effects of sex on the acoustic measures, correlations were also examined as a function of sex. Again, no significant correlations (p > 0.05) were found.
Discussion
Overall F0 variability as measured by both STSD and F0SD gave rise to in the same results, as did measures of the changes in F0 variability over the course of the reading passage (ΔF0SD and ΔSTSD). F0 variability was significantly increased in control speakers relative to individuals with PD. Further, while ON medication individuals with PD showed statistically significantly increased F0 variability relative to while OFF medication. F0 variability as well as the decline in F0 variability over the reading passage were higher in females than males.
Effects of PD and sex on F0 variability (F0SD and STSD)
PD disease status had a significant effect on both F0SD and STSD, with control speakers showing significantly higher F0 variability than individuals with PD, both ON and OFF medication. These findings confirm previous studies that found higher values of F0SD in control participants relative to participants with PD (Jimenez-Jimenez et al., 1997; Skodda et al., 2011).
Within the participants with PD, medication status also proved significant. Individuals showed statistically significantly increased F0SD and STSD while ON medication relative to OFF medication. These findings are at odds with previous studies that found no significant effect of medication status (Goberman et al., 2005; Skodda et al., 2011). However, these previous medication challenge studies were more limited in statistical power, which may explain this difference.
We found that female participants had statistically significantly higher F0 variability than males, even when estimated using the normalized STSD measure. Although the mean disease duration differed slightly between our female and male participants with PD, this difference is not likely to be the source of the effects of sex on F0SD. The mean disease duration was higher in our female participants than in the males, suggesting that female patients with PD might be expected to show decreased F0 variability. In fact females with PD and control female participants showed increased F0 variability relative to male participants. Skodda et al. also reported a significant effect of sex on F0SD, with higher F0SD values reported for females relative to males (Skodda et al., 2011).
Skodda et al. chose to analyze male and female data separately due to finding a significant effect of sex on acoustic parameters. Although we too found a significant effect of sex on both F0SD and STSD, our statistical analysis did not uncover significant interactions between group and sex for either measure. Although the male participants in our study do seem to show systematically lower F0 variability than the females, the trends within groups are the same regardless of sex: individuals with PD show decreases in F0 variability relative to controls and medication increases F0 variability in individuals with PD.
One explanation for lowered F0 variability in individuals with PD could be the coincidence of depression. Depression is common in individuals with PD (Slaughter, Slaughter, Nichols, Holmes, & Martens, 2001), and has been further associated with decreases in F0 variability (e.g., Mundt, Snyder, Cannizzaro, Chappie, & Geralts, 2007). However, we did not find strong evidence for depression in our sample of individuals with PD. Of the 32 participants with PD, N = 28 (88%) reported a score of 0 to UPDRS subsection 1.3, indicating no depressed mood. Two participants reported a slight (UPDRS subsection 1.3 = 1) depressed mood and two participants reported a mild depressed mood (UPDRS subsection 1.3 = 2).
Effects of PD and sex on F0 variability decline (ΔF0SD and ΔSTSD)
Our results indicate that females had statistically significantly lower ΔF0SD and ΔSTSD over the course of reading the paragraph than males, corresponding to an increase in F0 variability decline over the reading passage. The work of Skodda et al. did not report an effect of sex on ΔF0SD, but did note a trend in which seven male PD patients showed increased ΔF0SD while ON medication relative to OFF medication (Skodda et al., 2011). They suggested that a larger number could elucidate this finding (Skodda et al., 2011); however, we did not find a significant effect of group on ΔF0SD or ΔSTSD.
In fact, our data differed from that of Skodda et al. in several ways. In the large group of patients studied, Skodda reported ΔF0SD for individuals with PD (ON medication) of −1.20 Hz (males) and −3.15 Hz (females) and control speakers of 0.67 Hz (males) and 1.26 Hz (females), suggesting a decline of 1 – 3 Hz in variability in individuals with PD over the passage and a small (roughly 1 Hz) overall increase in variability in control speakers over the course of reading (Skodda et al., 2011). Our data in controls and participants with PD ON and OFF medication show ΔF0SD values ranging from roughly −13 Hz to −3 Hz. Thus, unlike Skodda et al., we see a much larger overall decline in variability in all of our participants. Further, although we did not find a significant effect of group on ΔF0SD, the trend in our data is for control participants to show a greater decline in variability (a mean decrease of 5.8 Hz in control participants versus a mean decrease of 5.4 Hz in individuals with PD while OFF medication). Combining the male and female ΔF0SD data reported in Skodda et al., they report statistics in individuals with PD (ON medication) and controls consistent with an effect size of d = 1.05 for ΔF0SD. Power calculations show that if our speakers showed an effect of this size, we could detect it with power of 98% (alpha = 0.05).
Likewise, Skodda et al. (2011) reported that males increased from ΔF0SD values of −2.2 Hz to −0.4 Hz with medication and that females increased from −1.9 Hz to −1.7 Hz. Combining the male and female ΔF0SD data from the medication challenge experiment in Skodda et al. (2011) results in an effect size of d = 0.20 for ON versus OFF medication in individuals with PD, detection of which at 80% power would require 379 individuals with PD to undergo medication challenge. Overall, we cannot confirm the hypothesis set forth by Skodda et al. that PD medications stabilize F0SD over the course of speech production given an effect size of this small magnitude. We can assert that there is no evidence in our data to support their hypothesis.
One difference between the methodology employed here and that employed by Skodda is the reading text used to elicit speech production. Skodda et al. used a series of 4 complex sentences in German whereas we used a short paragraph with variable sentence structure in English. Previous work has shown that German and English speakers display different fundamental frequency contours, a difference which could contribute to our findings here (Grover, 1987). Overall, the large differences in our absolute values of ΔF0SD suggest that the reading text and linguistic context may strongly influence this measure and that further study in this area is necessary before ΔF0SD can be clinically useful.
Relationship between disease duration and F0 variability
Correlations between disease duration and all four acoustic measures while OFF medication were not statistically significant. These findings confirm those reported by previous work indicating that measures of fundamental frequency variability do not correlate well with disease duration (Gamboa et al., 1997; Holmes et al., 2000; Skodda et al., 2011). Given that F0 variability is thought to be decreased in PD, we hypothesized that individuals with greater disease durations would have lower F0SD and STSD than newly diagnosed individuals, resulting in a negative correlation. In fact, no trends were seen in these data and none of the four measures seem to show promise as indicators of disease duration.
Potential neural mechanisms of F0 variability
The source of F0 variability changes in PD is not yet fully understood. Electromyographic studies have shown both reduced (Baker et al., 1998) as well as elevated (Gallena, Smith, Zeffiro, & Ludlow, 2001) laryngeal muscle activity. In this study we have found that F0 variability is reduced in individuals with PD and that use of medication increases F0 variability in individuals with PD. Damage to neural systems in PD may be responsible for this change in F0 modulation. However, we did not find evidence for a relationship between disease duration and F0 variability. Neurological findings indicate that PD progression may be marked by the progressive involvement of differing brain structure, beginning in brainstem structures during the presymptomatic phase and later moving to higher level dopaminergic systems that are associated with the common motor symptoms (Braak, Ghebremedhin, Rub, Bratzke, & Del Tredici, 2004).
Our finding of a significant medication effect on F0 variability suggests that damage to dopaminergic systems may be the cause of impaired F0SD. However, we did not find a relationship between PD progression and measures of F0 variability, which may suggest that lower-level systems implicated in all patients studied could also contribute to decreased F0 variability. Future prospective studies in individuals during the presymptomatic phase of PD (prior to diagnosis) could help elucidate the sources of F0 variability changes.
One potential interpretation of the effects of medication seen here is that decreased lung rigidity and the subsequent increases in sound pressure levels and variations are responsible for increases in F0 variability. Previous work has shown that therapy targeting increases in sound pressure level (loudness) also causes concurrent increases in F0 variability estimated using STSD (Dromey, Ramig, & Johnson, 1995). However previous work examining the effects of medication on sound pressure level in PD have shown equivocal results (Goberman, Coelho, & Robb, 2002; Jiang, Lin, Wang, & Hanson, 1999). The current data were not collected using a microphone calibrated by a sound pressure level meter. However, each participant with PD was recorded with identical microphone distance and recorder amplification settings during both ON and OFF medication recordings. In order to comment on the potential role of changes in sound pressure loudness on the current findings, we performed a post hoc analysis. A paired, two-sided Student’s t-test was performed on the ON and OFF data from individuals with PD. No statistically significant difference was noted (T = 1.4, p = 0.17), and the mean change was 0.54 dB SPL.Although our post hoc analysis suggests that changes in intensity are not related to the changes seen in F0 variability, recordings using a microphone calibrated with a sound pressure level meter would be necessary to confirm this. Thus, it is possible that the effects of medication are related to changes in sound pressure level in this population; however even if both variables were assessed a concurrent change would not necessarily imply causality. Because these two variables are physically correlated, it is not possible in natural contexts to disentangle them. Future work using real-time manipulations of prosodic variations (Patel, Niziolek, Reilly, & Guenther, 2011) in PD could answer this question.
Another related possible issue is that our ON and OFF medication conditions are confounded by an order effect. As with a variety of previous studies examining medication effects on speech production (De Letter, Santens, De Bodt, Boon, & Van Borsel, 2006; Jiang et al., 1999; Skodda et al., 2011), ON medication testing always occurred after OFF testing due to the pragmatic difficulties surrounding multi-day recordings in this population. Although previous work has shown that learning can demonstrate an effect on speech variables such as maximal vowel tasks, no such effect has been shown on F0 variability in reading (King, Ramig, Lemke, & Horii, 1994)
Conclusions
We found significant group (Control, PDON, PDOFF) and sex effects on F0SD and STSD. Control speakers showed significantly higher F0SD and STSD than individuals with PD, both ON and OFF medication. While ON medication, individuals with PD showed statistically significantly increased F0SD and STSD relative to while OFF medication. Females showed significantly higher F0SD and STSD and significantly lower ΔF0SD and ΔSTSD relative to males. No effect of group was found for ΔF0SD or ΔSTSD. No significant correlations were found between PD disease duration and any acoustic measure. This work suggests that while F0 variability (F0SD and STSD) may have promise as a reliable objective indicator of PD, that further study is necessary before utilizing measures of the changes in F0 variability across utterances (ΔF0SD and ΔSTSD).
Acknowledgments
The authors would like to thank Margaux Canevari and Howard Terry of Boston University for their assistance with acoustic analysis. This study was funded in part by grant 5T32HD007424 from the National Institute of Child Health and Human Development (NICHD), through the National Center for Medical Rehabilitation Research (NCMRR), the University of Washington – Advanced Rehabilitation Research Training (NIDRR training grant number H133P080008), and internal funds from Boston University.
References
- Atkinson JE. Inter- and intraspeaker variability in fundamental voice frequency. J Acoust Soc Am. 1976;60(2):440–446. doi: 10.1121/1.381101. [DOI] [PubMed] [Google Scholar]
- Baker KK, Ramig LO, Luschei ES, Smith ME. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology. 1998;51(6):1592–1598. doi: 10.1212/wnl.51.6.1592. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat: Doing phonetics by computer (Version 5.0.20) [Computer software] 2011 Retrieved from http://www.praat.org.
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Bunton K, Kent RD, Kent JF, Duffy JR. The effects of flattening fundamental frequency contours on sentence intelligibility in speakers with dysarthria. Clinical Linguistics & Phonetics. 2001;15(3):181–193. [Google Scholar]
- Canter GJ. Speech Characteristics of Patients with Parkinson’s Disease: I. Intensity, Pitch, and Duration. J Speech Hear Disord. 1963;28:221–229. doi: 10.1044/jshd.2803.221. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. 1969;12(2):246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, De Bodt M, Boon P, Van Borsel J. Levodopa-induced alterations in speech rate in advanced Parkinson’s disease. Acta Neurol Belg. 2006;106(1):19–22. [PubMed] [Google Scholar]
- Dromey C, Ramig LO, Johnson AB. Phonatory and articulatory changes associated with increased vocal intensity in Parkinson disease: a case study. J Speech Hear Res. 1995;38(4):751–764. doi: 10.1044/jshr.3804.751. [DOI] [PubMed] [Google Scholar]
- Fitch JL. Consistency of fundamental frequency and perturbation in repeated phonations of sustained vowels, reading, and connected speech. J Speech Hear Disord. 1990;55(2):360–363. doi: 10.1044/jshd.5502.360. [DOI] [PubMed] [Google Scholar]
- Gallena S, Smith PJ, Zeffiro T, Ludlow CL. Effects of levodopa on laryngeal muscle activity for voice onset and offset in Parkinson disease. J Speech Lang Hear Res. 2001;44(6):1284–1299. doi: 10.1044/1092-4388(2001/100). [DOI] [PubMed] [Google Scholar]
- Gamboa J, Jimenez-Jimenez FJ, Nieto A, Montojo J, Orti-Pareja M, Molina JA, Cobeta I. Acoustic voice analysis in patients with Parkinson’s disease treated with dopaminergic drugs. J Voice. 1997;11(3):314–320. doi: 10.1016/s0892-1997(97)80010-0. [DOI] [PubMed] [Google Scholar]
- Goberman A, Coelho C, Robb M. Phonatory characteristics of parkinsonian speech before and after morning medication: the ON and OFF states. J Commun Disord. 2002;35(3):217–239. doi: 10.1016/s0021-9924(01)00072-7. [DOI] [PubMed] [Google Scholar]
- Goberman AM, Blomgren M. Fundamental frequency change during offset and onset of voicing in individuals with Parkinson disease. J Voice. 2008;22(2):178–191. doi: 10.1016/j.jvoice.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Goberman AM, Coelho C. Acoustic analysis of parkinsonian speech I: speech characteristics and L-Dopa therapy. Neuro Rehabilitation. 2002;17(3):237–246. [PubMed] [Google Scholar]
- Goberman AM, Coelho CA, Robb MP. Prosodic characteristics of Parkinsonian speech: The effect of levodopa-based medication. Journal of Medical Speech-Language Pathology. 2005;13(1):51–68. [Google Scholar]
- Grover Intonation in English, French, and German: Perception and Production. Language and Speech. 1987;30(3):277–295. [Google Scholar]
- Harel BT, Cannizzaro MS, Cohen H, Reilly N, Snyder PJ. Acoustic characteristics of Parkinsonian speech: a potential biomarker of early disease progression and treatment. Journal of Neurolinguistics. 2004;17(6):439–453. [Google Scholar]
- Holmes RJ, Oates JM, Phyland DJ, Hughes AJ. Voice characteristics in the progression of Parkinson’s disease. Int J Lang Commun Disord. 2000;35(3):407–418. doi: 10.1080/136828200410654. [DOI] [PubMed] [Google Scholar]
- Jiang J, Lin E, Wang J, Hanson DG. Glottographic measures before and after levodopa treatment in Parkinson’s disease. Laryngoscope. 1999;109(8):1287–1294. doi: 10.1097/00005537-199908000-00019. [DOI] [PubMed] [Google Scholar]
- Jimenez-Jimenez FJ, Gamboa J, Nieto A, Guerrero J, Orti-Pareja M, Molina JA, Cobeta I. Acoustic voice analysis in untreated patients with Parkinson’s disease. Parkinsonism Relat Disord. 1997;3(2):111–116. doi: 10.1016/s1353-8020(97)00007-2. [DOI] [PubMed] [Google Scholar]
- King JB, Ramig LO, Lemke JH, Horii Y. Parkinson’s Disease: Longitudinal Changes in Acoustic Parameters of Phonation. Journal of Medical Speech-Language Pathology. 1994;2(1):29–42. [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Laures JS, Weismer G. The effects of a flattened fundamental frequency on intelligibility at the sentence level. Journal of Speech Language and Hearing Research. 1999;42(5):1148–1156. doi: 10.1044/jslhr.4205.1148. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Hanson WR. Clinical and acoustical variability in hypokinetic dysarthria. J Commun Disord. 1986;19(5):347–366. doi: 10.1016/0021-9924(86)90026-2. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Snyder PJ, Cannizzaro MS, Chappie K, Geralts DS. Voice acoustic measures of depression severity and treatment response collected via interactive voice response (IVR) technology. J Neurolinguistics. 2007;20(1):50–64. doi: 10.1016/j.jneuroling.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch WJ, Strudwick A, Roy SK, Downie AW. Parkinson’s disease: disability, review, and management. Br Med J (Clin Res Ed) 1986;293(6548):675–677. doi: 10.1136/bmj.293.6548.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Niziolek C, Reilly K, Guenther FH. Prosodic adaptations to pitch perturbation in running speech. J Speech Lang Hear Res. 2011;54(4):1051–1059. doi: 10.1044/1092-4388(2010/10-0162). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Countryman S, Thompson LL, Horii Y. Comparison of two forms of intensive speech treatment for Parkinson disease. J Speech Hear Res. 1995;38(6):1232–1251. doi: 10.1044/jshr.3806.1232. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Fox C, Sapir S. Speech and Voice Disorders in Parkinson’s Disease. In: Olanow CW, Stocchi F, Lang AE, editors. Parkinson’s Disease: Non-Motor and Non-Dopaminergic Features. Oxford, UK: Blackwell Publishing Ltd; 2011. pp. 346–360. [Google Scholar]
- Robertson DR, Wood ND, Everest H, Monks K, Waller DG, Renwick AG, George CF. The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br J Clin Pharmacol. 1989;28(1):61–69. doi: 10.1111/j.1365-2125.1989.tb03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda S, Gronheit W, Schlegel U. Intonation and speech rate in Parkinson’s disease: general and dynamic aspects and responsiveness to levodopa admission. J Voice. 2011;25(4):e199–205. doi: 10.1016/j.jvoice.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Slaughter JR, Slaughter KA, Nichols D, Holmes SE, Martens MP. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13(2):187–196. doi: 10.1176/jnp.13.2.187. [DOI] [PubMed] [Google Scholar]