Abstract
Logopenic primary progressive aphasia (lvPPA) is a progressive language disorder characterized by anomia, difficulty repeating complex sentences, and phonological errors. The majority, although not all, lvPPA patients have underlying Alzheimer’s disease. We aimed to determine whether clinical or neuroimaging features differ according to the deposition of Aβ on Pittsburgh-compound B PET in lvPPA. Clinical features, patterns of atrophy on MRI, hypometabolism on FDG-PET, and white matter tract degeneration were compared between six PiB-negative and 20 PiB-positive lvPPA patients. PiB-negative patients showed more asymmetric left-sided patterns of atrophy, hypometabolism and white matter tract degeneration, with greater left anteromedial temporal and medial prefrontal involvement, than PiB-positive patients. PiB-positive patients showed greater involvement of right temporoparietal and frontal lobes. There was very little evidence for clinical differences between the groups. Strikingly asymmetric neuroimaging findings with relatively preserved right hemisphere may provide clues that AD pathology is absent in lvPPA.
Keywords: logopenic, primary progressive aphasia, Pittsburgh Compound B, magnetic resonance imaging, FDG-PET, progranulin, beta-amyloid
1. Introduction
The logopenic variant of primary progressive aphasia (lvPPA) is a progressive language disorder in which patients have anomia, difficulty retrieving words and repeating complex sentences and phonological errors in their spoken speech (Gorno-Tempini et al., 2011). These patients have preserved single word comprehension, grammar and syntax, and typically do not have apraxia of speech or dysarthria. On neuroimaging, patients with lvPPA typically show abnormalities in the temporoparietal cortex, with greater involvement of the left hemisphere (Gorno-Tempini et al., 2004; Madhavan et al., 2013; Rogalski et al., 2011; Rohrer, Ridgway, et al., 2010; Teichmann et al., 2013). Pathological studies, and studies that have utilized beta-amyloid (Aβ) imaging or CSF biomarkers, have shown that the majority of patients with lvPPA have underlying Alzheimer’s disease (AD) (Leyton et al., 2011; M. Mesulam et al., 2008; Rabinovici et al., 2008; Teichmann et al., 2013). Hence, lvPPA is often considered an atypical clinical variant of AD (Whitwell et al., 2011). However, lvPPA patients have been reported that do not show Aβ deposition on imaging, suggesting a different underlying pathological etiology in these patients. It appears that in these instances lvPPA may arise from frontotemporal lobar degeneration (FTLD) pathology (Hu et al., 2010; M. Mesulam et al., 2008; M. M. Mesulam, Weintraub, et al., 2014), most commonly from FTLD characterized by the presence of the protein TDP-43, and may even be associated with FTLD-related genetic mutations, such as progranulin gene mutations (Hu et al., 2010; Josephs et al., 2014; Rohrer, Crutch, Warrington, & Warren, 2010). The proportion of lvPPA patients that do not have AD varies between 0 and 38% across studies (Chare et al., 2014; Hu et al., 2010; Leyton et al., 2011; M. Mesulam et al., 2008; Rabinovici et al., 2008; Teichmann et al., 2013).
It is unclear whether there are any clinical or neuroimaging differences between lvPPA patients that do or do not have underlying AD pathology, and hence whether it would be possible to determine which patients will not have AD. This will be critically important for patient care and prognosis, especially when treatments that can slow the AD neurodegenerative process become available. Predicting the underlying pathology would be particularly useful in non-tertiary care centers where amyloid imaging is not available. Previous studies utilizing autopsy-confirmed cohorts have suggested that neuroimaging can be useful to help predict underlying pathology, with specific signatures identified for AD and for FTLD across a number of clinical syndromes (Josephs et al., 2010; Josephs et al., 2008; Lee et al., 2011; Lehmann et al., 2010; Rohrer, Geser, et al., 2010; Whitwell, Jack, Boeve, et al., 2010; Whitwell, Jack, Parisi, et al., 2010; Whitwell et al., 2011). It is unknown, however, whether neuroimaging features differ according to pathology within the lvPPA syndrome.
The aim of this study was therefore to investigate whether there are any clinical or neuroimaging differences between lvPPA patients that do and do not have Aβ deposition on Pittsburgh Compound B (PiB) PET imaging, and to determine the degree to which these variables can differentiate the groups. The neuroimaging analysis included MRI, 18-F-fluorodeoxyglucose PET (FDG-PET) and diffusion tensor imaging (DTI), and we analyzed regions that have been particularly associated with AD pathology, FTLD pathology or the presence of progranulin mutations.
2. Material and Methods
2.1. Subjects
A total of 50 patients with lvPPA were consecutively recruited from the Department of Neurology, Mayo Clinic between October 1st 2010 and July 1st 2013. All patients underwent a detailed neurological and speech and language assessment as detailed below. Clinical diagnosis was rendered based solely on data from speech and language assessments without any reference to neurological or neuroimaging results. All patients presented with deficits in language, with language being the dominant symptom and the primary cause for problems in activities of daily living. The diagnosis of lvPPA was independently determined by two speech-language pathologists (JRD and EAS) by consensus. Criteria for the diagnosis of lvPPA were compatible with published consensus criteria (Gorno-Tempini et al., 2011), and included: 1) presence of aphasia, 2) impaired sentence repetition and comprehension, 3) presence of anomia with evidence of spared single word comprehension, 4) evidence of phonemic paraphasias, 5) normal rate of verbal expression or slowed verbal expression due to pauses for word retrieval without evidence of motoric slowing or apraxia of speech, and 6) absence of agrammatic/telegraphic verbal output. All patients showed patterns of left posterior perisylvian or parietal atrophy and hypometabolism characteristic of lvPPA. No patients showed the imaging patterns characteristic for the semantic and agrammatic variants of PPA, as defined in the consensus criteria (Gorno-Tempini et al., 2011).
All 50 patients qualitatively met published consensus criteria for lvPPA (Gorno-Tempini et al., 2011). All patients underwent PiB-PET scanning and patients were classified as PiB-positive or PiB-negative using a global SUVR ratio cut-point of 1.5 that was generated using an automated analysis pipeline previously described in detail (Jack et al., 2008). Of the 50 lvPPA patients, six were classified as PiB-negative (12%) and 44 were classified as PiB-positive (88%). The PiB-PET scans for the six PiB-negative patients are shown in Supplemental Figure 1, and a scatter-plot showing the global and regional SUVR values for each patient is shown in Supplemental Figure 2. For this study, we compared the six PiB-negative patients to all PiB-positive patients that had a similar disease duration of three years or less (n=20) to eliminate any potential biases that could have been caused by imbalances in disease duration.
2.2 Speech and language assessment
The speech and language battery was performed by one of two Speech-Language Pathologists (JRD or EAS). The battery included the Western Aphasia Battery (WAB), revised (Kertesz, 2007), Part 1, as a primary measure of global language ability. Specific subtest scores on the WAB were used to index information content, and fluency and grammatical adequacy and paraphasias, during narrative picture description; word and sentence repetition ability; and animal fluency. The 15-item Boston Naming Test (Lansing, Ivnik, Cullum, & Randolph, 1999) served as a sensitive measure of confrontation naming, the 22-item version of Part V of DeRenzi and Vignolo’s Token Test (DeRenzi & Vignolo, 1962) served as a challenging measure of verbal comprehension ability (Wertz, Keith, & Custer, 1971), and the Pyramids and Palm Trees test (Howard & Patterson, 1992) served as a measure of object knowledge. Action (verb) (Piatt, Fields, Paolo, Koller, & Troster, 1999) fluency was also assessed. Phonological errors were rated on a four-point scale (absent, mild, moderate-marked, severe) during consensus review of recorded conversation as well as spoken picture description and word and sentence repetition responses during the formal test battery. The presence or absence of motor speech abnormalities were determined by the two speech-language pathologists (JRD and EAS).
2.3. Cognitive assessment
All patients underwent detailed neuropsychological assessments (Josephs et al., 2012) including the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975), Trail Making Test (TMT) A (Spreen & Strauss, 1998), the Delis-Kaplan Executive Function System Card Sort (DKEFS) (Delis, Kaplan, & Kramer, 2001), the Wechsler Memory Scale III (Wechsler, 1987); and the Visual Object and Space Perception Battery (VOSP) (Warrington & James, 1991). Mayo Older American Normative Studies age and education-adjusted scaled scores (Ivnik et al., 1992) were used for all neuropsychological variables except for the DKEFS Card Sort and VOSP Cube Analysis. The MOANS and DKEFS Card Sort are constructed to have a mean of 10 and standard deviation of 3 in cognitively healthy participants.
2.4. Genetic testing
All patients underwent apolipoprotein E (APOE) genotype testing, as previously described (Josephs, Tsuboi, Cookson, Watt, & Dickson, 2004), and were tested for the presence of progranulin, microtubule associated protein tau (MAPT) and TARDBP gene mutations and the expanded GGGGCC hexanucleotide repeat in C9ORF72, as previously described (Baker et al., 2006; Dejesus-Hernandez et al., 2011; Hutton et al., 1998; Rutherford et al., 2008).
2.5. Image acquisition
All patients underwent MRI, 18-F fluorodeoxyglucose (FDG) and PiB PET scanning within two days of the clinical evaluations. The MRI imaging protocol was performed on a 3T GE scanner, and included a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence and a diffusion tensor imaging (DTI) sequence with 41 diffusion encoding steps and four non-diffusion (b0) weighted T2 images. All PET scans were acquired using a PET/CT scanner (GE Healthcare, Milwaukee, Wisconsin) operating in 3D mode. Detailed acquisition details have been previously published (Josephs et al., 2012).
2.6. MRI and FDG-PET analysis
Voxel-level and region-level analyses were utilized to assess both the MPRAGE and FDG-PET scans. The voxel-level analysis was performed using voxel-based morphometry (VBM) and SPM5 (Ashburner & Friston, 2000). All MPRAGE scans were normalized and segmented using customized priors and unified segmentation (Ashburner & Friston, 2005), followed by the hidden Markov random field clean-up step. All grey matter images were modulated and smoothed at 8mm full-width-at-half-maximum (FWHM). The FDG-PET images were co-registered to the patients’ MPRAGE using 6 degrees-of-freedom registration. All voxels in the FDG-PET image were divided by median uptake of the pons to form uptake ratio images. The FDG-PET uptake ratio images were then normalized to the customized template using the normalization parameters from the MPRAGE normalization. Two-sided t-tests were used to compare PiB-positive and PiB-negative lvPPA patients to each other, and to a healthy control cohort of 26 age and gender-matched subjects that had undergone identical imaging protocols and were PiB-negative. Comparisons between disease groups and controls were assessed corrected for multiple comparisons using the false discovery rate (FDR) at p<0.01, and direct comparisons between PiB-negative and PiB-positive groups were assessed uncorrected for multiple comparisons at p<0.001. Age and gender were included in all comparisons as covariates.
Atlas-based parcellation using the automated anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) was utilized in order to generate regional data. The AAL atlas was transformed into the MPRAGE native anatomical space for each subject and multiplied by a grey matter mask created using SPM5. Grey matter volumes and FDG-PET uptake ratios were calculated for 7 temporal, frontal, and parietal ROIs. Regional grey matter volumes were divided by total intracranial volume to correct for head size. Both grey matter volumes and FDG-PET uptake ratios were expressed as Z scores showing differences from the control cohort. Asymmetry scores were also calculated for all regions as follows: (left value-right value)/average of left and right values.
2.7. DTI analysis
Each of the 41 diffusion-weighted images was registered to the non-diffusion weighted b0 volumes using affine transformations. Images were brain-extracted using the FSL brain extraction tool (Smith, 2002) and fractional anisotropy (FA) and mean diffusivity (MD) maps were generated (Behrens et al., 2003). A whole-brain Voxel-Based Analysis (VBA) was performed on the FA and MD images. This analysis technique has been shown to be more sensitive for detecting true changes and has greater specificity in resisting false positives from misregistration than skeleton-based DTI analysis methods, such as tract-based spatial statistics (Schwarz et al., 2014). In brief, FA and MD images of all subjects were nonlinearly coregistered via an iterative, groupwise registration algorithm (Advanced Normalization Tools, ANTs (Avants et al., 2010)) and normalized to a 1mm isotropic Montreal Neurological Institute (MNI)152 standard space via the FMRIB58_FA template (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). To increase the validity of the Gaussian field assumptions used in voxel-wise parametric tests, the images were smoothed using a Gaussian kernel with 8mm FWMH. Group statistical comparisons were performed comparing PiB-negative lvPPA and PiB-positive lvPPA to each other and to the healthy control cohort, using two-sided t-tests in SPM5. Comparisons between disease groups and controls were assessed corrected for multiple comparisons using the false discovery rate (FDR) at p<0.01, and direct comparisons between PiB-negative and PiB-positive groups were assessed uncorrected for multiple comparisons at p<0.001. Age and gender were included in all comparisons as covariates. Voxels with mean FA across coregistered images <0.2 were masked out of the analysis to avoid CSF and grey matter. Regional FA and MD were calculated using the Johns Hopkins University atlas and expressed as Z scores. Asymmetry scores were calculated as follows: (left value-right value)/average of left and right values.
2.8. Statistical analysis
Statistical analyses were performed utilizing JMP computer software (JMP Software, version 9.0.0; SAS Institute Inc., Cary, NC) with significance assessed at p≤0.05. Chi-Square tests were used to compare categorical data across groups and non-parametric Wilcoxon Rank Sum tests were used to compare continuous data. Receiver operating characteristic (ROC) curve analysis was performed in order to calculate area under the ROC curve (AUROC) values, and optimum sensitivity, specificity and positive predictive value (PPV) to predict the lvPPA-negative group. ROC statistics were calculated for all clinical variables, regional MRI, FDG and DTI values, and asymmetry scores that showed significant differences between PiB-negative and PiB-positive lvPPA (Table 1 and Supplemental Table 1). For each variable, sensitivity, specificity and PPV were reported at the optimum cut-point which provided the greatest sensitivity – (1-specificity).
Table 1.
Demographic and clinical data of the PiB-negative and PiB-positive lvPPA patients
| PiB-negative | PiB-positive | P value | |
|---|---|---|---|
| N | 6 | 20 | NA |
| Global PiB SUVR | 1.2 (1.2–1.3) | 2 (1.9–2.2) | <0.001 |
| Gender (% female) | 2 (33%) | 8 (40%) | 0.77 |
| Education, years | 17.5 (14.8–18) | 14.5 (14–16.5) | 0.30 |
| Age at onset, years | 63 (57.3–65.8) | 62 (54–70) | 0.90 |
| Age at examination, years | 66 (59.5–68) | 63 (56–73) | 0.90 |
| Disease Duration, years | 2 (2–2.8) | 2 (1.9–3) | 0.80 |
| APOE e4 (%) | 2 (33%) | 11 (55%) | 0.34 |
| MMSE (/30) | 26.5 (25.3–27.8) | 25 (23.8–26.3) | 0.34 |
| WAB aphasia quotient (/100) | 82.3 (75.6–88.7) | 85.5 (81.3–88.3) | 0.58 |
| WAB information content (/10) | 8 (8–8) | 9 (7.8–10) | 0.71 |
| WAB repetition (/10) | 7.5 (6.8–8.1) | 8.4 (7.2–8.7) | 0.30 |
| WAB fluency (/10) | 8 (6–9) | 8 (8–9) | 0.23 |
| Boston Naming Test (/15) | 11 (4.3–11.8) | 8 (5.8–10.3) | 0.50 |
| Phonological error severity (0–3) | 1 (1–1.8) | 1 (1–1.3) | 0.85 |
| Pyramids and Palm Trees (/52) | 47 (45.3–50.3) | 47 (44–49) | 0.65 |
| Token Test (/22) | 9 (7–13) | 12 (8–16) | 0.43 |
| Action fluency | 6 (4.3–8.5) | 10.5 (5.8–14) | 0.05 |
| TMT A MOANSa | 9 (6.8–9.8) | 6 (3–9.3) | 0.23 |
| DKEFS card sorta | 5 (1.8–6) | 7 (3.5–9.0) | 0.14 |
| WMS-III VR % retention SSa | 9.5 (8–12.5) | 8 (6–10.5) | 0.32 |
| VOSP cube analysis (/10) | 10 (9.3–10) | 7.5 (1.5–9) | 0.05 |
Data shown as median (inter-quartile range). APOE= apolipoprotein E; DKEFS = Delis-Kaplan Executive Function System; MOANS = Mayo Older American Normative Studies; MMSE = Mini-Mental State Examination; TMT = Trail Making Test; VOSP = Visual Object and Space Perception Battery; WAB = Western Aphasia Battery; WMS-III VR % retention SS = Wechsler memory scale visual reproduction, percent retention, scaled score. P values were calculated using Wilcoxon Rank Sum tests for continuous data and Chi-squared tests for categorical data.
The MOANS, WMS-III VR % SS and DKEFS Card Sort are constructed to have a mean of 10 and standard deviation of 3 in cognitively healthy participants
3. Results
3.1. Clinical findings
No differences were observed between PiB-positive and PiB-negative lvPPA patients in age, gender or proportion of APOE e4 carriers (Table 1). The PiB-negative lvPPA group performed worse on action fluency and better on VOSP Cube Analysis than the PiB-positive lvPPA group (Table 1). Of the six PiB-negative patients, three (50%) were found to carry a pathogenic progranulin gene mutation. We identified the following mutations: 1) c.912G>A, p.Trp304X, 2) c.139delG, p.D47TfsX7, and 3) c.709-2A>G, p.Ala237fs. A family history of dementia was present in the three PiB-negative progranulin patients but absent in the remaining three PiB-negative patients.
3.2. MRI and FDG findings
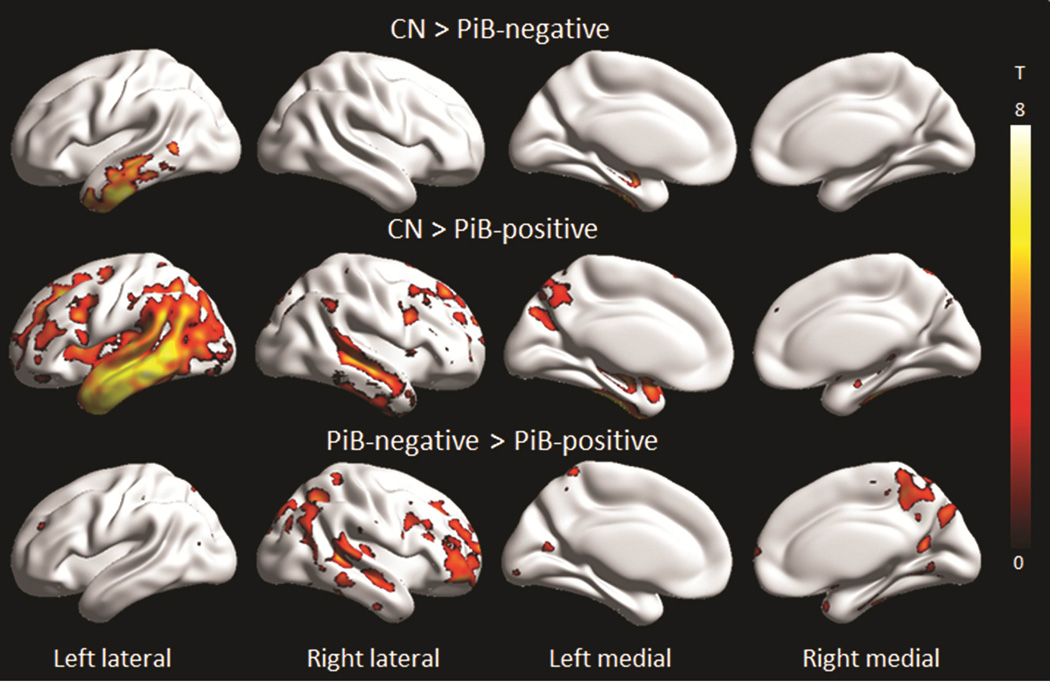
The PiB-positive lvPPA group showed grey matter loss predominantly in the left temporoparietal cortex, with additional involvement of the left frontal and occipital lobes and right temporal, parietal and frontal lobes compared to controls (Figure 1 and 2). In contrast, the PiB-negative lvPPA group showed grey matter loss restricted to left temporal lobe (Figure 1 and 2). On direct comparison, the PiB-positive lvPPA group showed greater loss throughout right lateral temporal, parietal and frontal lobe, precuneus and striatum, compared to the PiB-negative lvPPA group (Figure 1 and 2). No regions showed greater loss in the PiB-negative lvPPA group compared to the PiB-positive lvPPA group.
Figure 1. Voxel-level maps showing MRI grey matter atrophy.
Results are shown on three dimensional surface renderings after correction for multiple comparisons using the false discovery rate at p<0.01. Direct comparisons between the PiB-positive and PiB-negative lvPPA groups were performed uncorrected at p<0.001. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
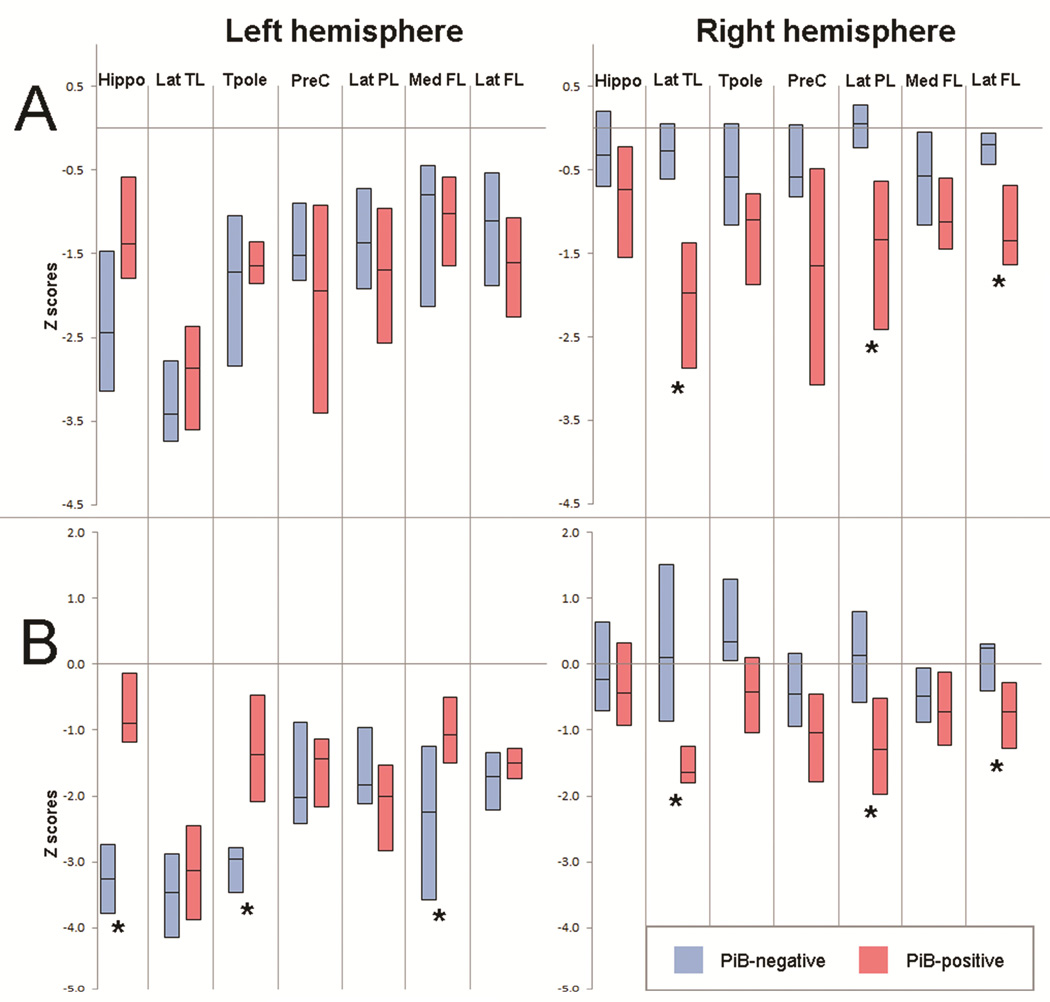
Figure 2. Box-plots of regional MRI grey matter volumes (A) and FDG-PET uptake ratios (B) expressed as Z scores.
Hippo=hippocampus; Inf TL=inferior temporal lobe; Mid TL=middle temporal lobe; Sup TL=superior temporal lobe; Tpole=temporal pole; PreC=precuneus; Inf PL=inferior parietal lobe; Sup PL=superior parietal lobe; Med FL=medial frontal lobe; Lat FL=lateral frontal lobe; OL=occipital lobe. * Significant difference between PiB-negative and PiB-positive groups
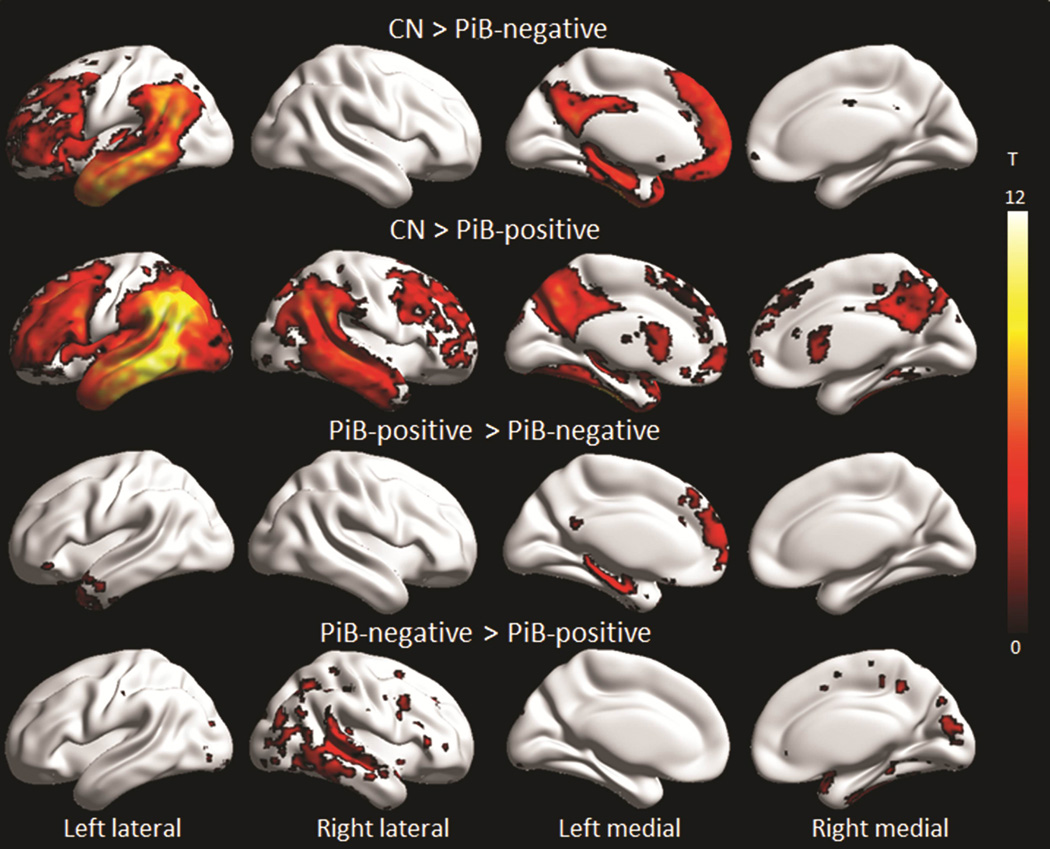
Both groups showed reduced FDG metabolism in temporal, parietal and frontal lobes, although findings were limited to the left hemisphere in the PiB-negative lvPPA group and were more bilateral in the PiB-positive lvPPA group (Figure 2 and 3). On direct comparison, the PiB-positive lvPPA group showed greater hypometabolism throughout right lateral temporal, parietal and frontal lobe, and right precuneus and putamen, compared to the PiB-negative lvPPA group (Figure 2 and 3). Conversely, the PiB-negative lvPPA group showed greater hypometabolism in left temporal pole, hippocampus, inferior temporal gyrus, fusiform gyrus, caudate nucleus, posterior cingulate and medial prefrontal cortex, compared to the PiB-positive lvPPA group (Figure 2 and 3).
Figure 3. Voxel-level maps showing FDG-PET hypometabolism.
Results are shown on three dimensional surface renderings after correction for multiple comparisons using the false discovery rate at p<0.01. Direct comparisons between the PiB-positive and PiB-negative bvFTD groups were performed uncorrected at p<0.001. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
The PiB-negative lvPPA group showed significantly greater asymmetry scores in lateral temporal lobe, hippocampus, and temporal pole on both MRI and FDG, and in precuneus and medial frontal lobe on FDG, compared to the PiB-positive lvPPA group (Supplemental Table 1). Asymmetry scores did not differ between PiB-negative patients with and without progranulin mutations (e.g. the median lateral temporal MRI asymmetry score was −0.41 in PiB-negative progranulin patients and −0.32 in PiB-negative patients without progranulin mutations, compared to −0.16 in the PiB-positive patients).
3.3. DTI findings
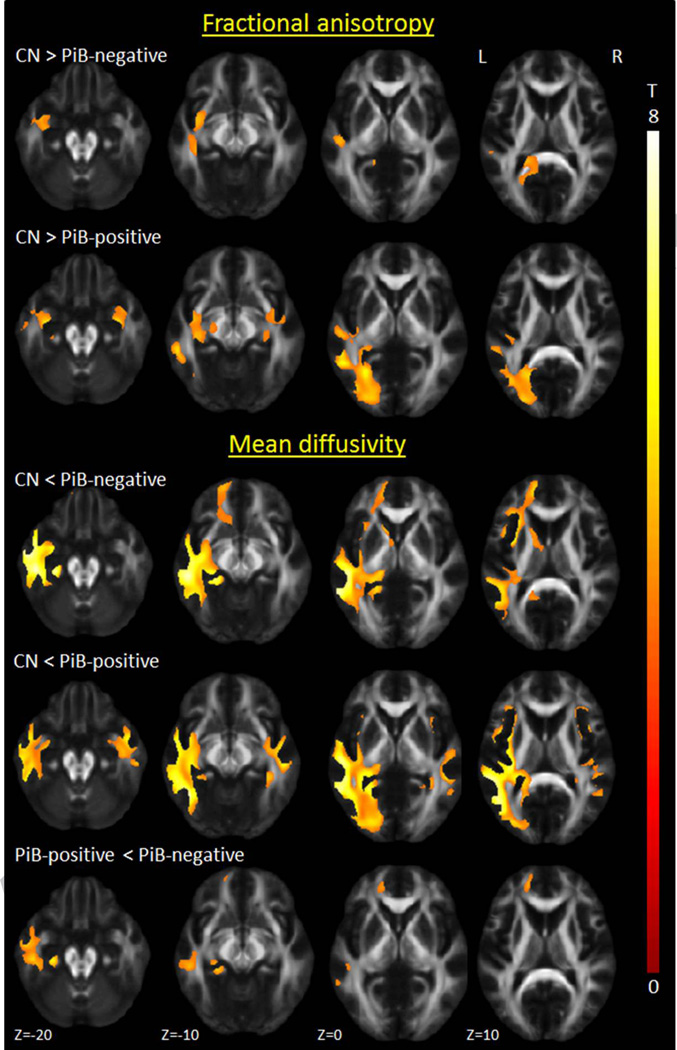
The PiB-negative lvPPA group did not show any reduced FA compared to controls after correction for multiple comparisons, therefore DTI results were assessed at p<0.001 with cluster-level correction (Figure 4). At this threshold, both groups showed reduced FA in uncinate fasciculus, inferior longitudinal fasciculus, and inferior parietal superior longitudinal fasciculus, compared to controls, although findings were more left-sided and asymmetric in the PiB-negative lvPPA group. More widespread increases in MD were observed in both groups with additional involvement of the fronto-occipital fasciculus, although findings in the PiB-negative lvPPA group were still limited to the left hemisphere. The PiB-negative patients showed increased MD in left anterior inferior longitudinal fasciculus, uncinate fasciculus, and forceps minor, compared to PiB-positive lvPPA patients (Figure 4). No other group differences between PiB-positive and PiB-negative lvPPA were observed. Significantly greater asymmetry scores were observed in the inferior fronto-occipital fasciculus in the PiB-negative lvPPA group compared to the PiB-positive lvPPA group (Supplemental Table 1).
Figure 4. Voxel-level maps of DTI fractional anisotropy and mean diffusivity.
Results are shown on axial slices of a mean fractional anisotropy image at p<0.001 corrected for multiple comparisons at the cluster-level.
3.4. Classification accuracy
Data on the classification accuracy of the significant clinical and imaging variables are shown in Table 2. The two clinical variables, action fluency and VOSP Cube Analysis, showed high AUROC values of 0.76 and 0.77 respectively, but poor PPV for PiB-negative lvPPA. Classification was generally improved with the MRI and FDG-PET imaging variables, with AUROC values ranging between 0.78 and 0.93. Excellent PPV, specificity and sensitivity for PiB-negative lvPPA were observed for right lateral temporal volume (100%:100%:83%) and hippocampal asymmetry score on both MRI (100%:100%:83%) and FDG-PET (100%:100%:83%). The DTI asymmetry scores for MD in inferior fronto-occipital fasciculus also showed excellent PPV (100%), but showed low sensitivity (67%).
Table 2.
Area under the ROC curve analysis for the significant clinical and imaging variables
| AUROC | Sensitivity (%) | Specificity (%) | PPV (%) | |
|---|---|---|---|---|
| Clinical variables | ||||
| Action fluency | 0.76 | 83 | 60 | 38 |
| VOSP Cube Analysis | 0.77 | 67 | 80 | 50 |
| MRI volumes | ||||
| Right lateral temporal | 0.92 | 83 | 100 | 100 |
| Right lateral parietal | 0.88 | 83 | 90 | 71 |
| Right lateral frontal | 0.88 | 100 | 65 | 46 |
| FDG uptake values | ||||
| Left hippocampus | 0.83 | 83 | 95 | 83 |
| Left temporal pole | 0.86 | 83 | 95 | 83 |
| Left medial frontal | 0.80 | 100 | 50 | 38 |
| Right lateral temporal | 0.90 | 67 | 100 | 100 |
| Right lateral parietal | 0.82 | 67 | 90 | 67 |
| Right lateral frontal | 0.78 | 67 | 95 | 80 |
| MRI asymmetry scores | ||||
| Hippocampus | 0.84 | 83 | 100 | 100 |
| Lateral temporal | 0.86 | 83 | 90 | 71 |
| Temporal pole | 0.88 | 83 | 90 | 71 |
| FDG asymmetry scores | ||||
| Hippocampus | 0.84 | 83 | 100 | 100 |
| Lateral temporal | 0.82 | 83 | 90 | 71 |
| Temporal pole | 0.89 | 83 | 95 | 83 |
| Medial frontal | 0.84 | 67 | 100 | 100 |
| DTI asymmetry scores | ||||
| IFO FA | 0.79 | 83 | 79 | 56 |
| IFO MD | 0.78 | 67 | 100 | 100 |
IFO = inferior fronto-occipital fasciculus; FA= fractional anisotropy; MD = mean diffusivity Values shown to optimize accuracy to diagnose PiB-negative patients (optimum defined as the greatest sens – (1-spec)).
4. Discussion
This study demonstrates that clinical and neuroimaging features do differ according to the presence or absence of Aβ deposition in patients with lvPPA, and hence that it may be possible to predict the patients that do not have AD pathology.
The most striking difference observed across PiB-negative and PiB-positive lvPPA patients was in the degree of hemispheric asymmetry, as noted both in the voxel-level maps and asymmetry scores. While both groups showed asymmetric patterns of temporoparietal atrophy and hypometabolism in the voxel-level maps, with greater involvement of the left hemisphere consistent with the prominent language impairment, the PiB-negative patients showed greater asymmetry scores and sparing of the right hemisphere. This asymmetry was evident on the single-subject MRI and FDG-PET, as we have previously shown (Josephs et al., 2014). As a result of this difference, the PiB-positive patients showed greater involvement of the right hemisphere, including temporal, parietal and frontal regions, compared to the PiB-negative patients. Importantly, these differences were observed in cohorts that were matched for disease duration and hence the greater involvement of the right hemisphere in PiB-positive patients was not due to longer disease. The groups also showed similar aphasia severity, and hence the differences were also not due to more severe disease in the PiB-positive group. The asymmetry scores had excellent sensitivity and specificity for differentiating PiB-positive and negative patients and, hence, highly asymmetric findings may prove to have some clinical utility for predicting PiB-negative lvPPA, particularly within the first few years of the disease. Longitudinal studies will be needed to determine whether the right hemisphere remains relatively spared in PiB-negative lvPPA with disease progression. The striking asymmetry observed in the PiB-negative lvPPA patients may reflect underlying FTLD-TDP type A pathology which is also associated with asymmetric patterns of atrophy (Rohrer, Geser, et al., 2010; Whitwell, Jack, Parisi, et al., 2010). We also found that half of our PiB-negative cohort had progranulin gene mutations (Josephs et al., 2014) which are typically associated with FTLD-TDP type A (Josephs et al., 2007; Whitwell et al., 2012) and have also been shown to result in highly asymmetric patterns of atrophy and hypometabolism (Beck et al., 2008; Whitwell et al., 2012). One previous study similarly found that one in two lvPPA patients with FTLD pathology had a mutation in progranulin (Hu et al., 2010). Importantly, we did not observe a difference in asymmetry scores between the PiB-negative subjects with and without progranulin gene mutations, suggesting the findings are not driven solely by the progranulin cases. The three PiB-negative patients without progranulin mutations may be sporadic forms of FTLD-TDP. Indeed, these patients did not have any family history of dementia. However, it is still possible that they may have another yet undiscovered mutation or may have larger progranulin deletions which can be missed using conventional sequencing analysis.
The PiB-negative lvPPA patients also showed greater involvement of left anteromedial temporal and medial frontal regions than the PiB-positive lvPPA patients, again perhaps reflecting the presence of FTLD pathology (Hu et al., 2010; Rohrer, Geser, et al., 2010; Whitwell, Jack, Parisi, et al., 2010). Anterior temporal atrophy has similarly been observed in two patients that had lvPPA with progranulin mutations (Rohrer, Ridgway, et al., 2010). The anteromedial temporal lobe is usually associated with the semantic variant of PPA (svPPA) (Chan et al., 2001). Importantly, however, no differences in performance on the Boston Naming Test, Pyramids and Palm Trees test, or repetition, were observed across the PiB-negative and PiB-positive lvPPA groups. We have previously reported language data for each of the six PiB-negative patients demonstrating that they did not fulfill clinical criteria for svPPA (Gorno-Tempini et al., 2011; Josephs et al., 2014).
Both the PiB-positive and PiB-negative lvPPA patients showed white matter tract degeneration of temporal and parietal tracts, including uncinate fasciculus, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus and superior longitudinal fasciculus, with greatest involvement of the left hemisphere. The anatomical location of these findings concurs with previous studies of lvPPA (Agosta et al., 2012; Galantucci et al., 2011; Mahoney et al., 2013). However, concordant with the grey matter analysis, the voxel-level DTI findings were strikingly more asymmetric in the PiB-negative lvPPA patients. The PiB-negative lvPPA patients also showed greater degeneration of the left inferior longitudinal fasciculus, uncinate fasciculus and the forceps minor, compared to the PiB-positive patients. The inferior longitudinal fasciculus and uncinate fasciculus both project into the anterior temporal lobes, while the forceps minor connects the medial and lateral surfaces of the frontal lobe. Degeneration of these tracts is therefore likely associated with the hypometabolism we observed in the anterior temporal and medial frontal lobe of the PiB-negative patients. The only DTI measure with potential clinical utility was the MD asymmetry score from the inferior fronto-occipital fasciculus which showed excellent specificity and positive predictive value for PiB-negative lvPPA, although low sensitivity. The findings with MD were generally more striking than the findings with FA, as others have previously reported in lvPPA (Galantucci et al., 2011). The poor performance of the DTI measures to separate PiB-negative and PiB-positive lvPPA is at odds with studies that have suggested that white matter damage is greater in subjects with FTLD pathology compared to AD (McMillan et al., 2012). However, severe white matter damage may be a particular feature of tau-positive FTLD (McMillan et al., 2013; Whitwell et al., 2014), rather than FTLD-TDP which is most likely to underlie PiB-negative lvPPA.
Given the striking differences in neuroimaging across groups, it was surprising that there were so few clinical differences. The two groups showed comparable performance on all speech and language tests, including those measuring aphasia severity, anomia, repetition and phonological errors, and showed a similar degree of cognitive impairment. Some of the patients in both the PiB-positive and PiB-negative groups showed mild memory deficits, however this is consistent with lvPPA cohorts from other centers (Lehmann et al., 2013; Leyton, Hsieh, Mioshi, & Hodges, 2013; Sajjadi, Patterson, Arnold, Watson, & Nestor, 2012). In fact, it has been recognized that memory deficits can develop in PPA (M. M. Mesulam, Rogalski, et al., 2014). The only differences observed across groups were that the PiB-negative lvPPA patients performed worse on action fluency and better on the VOSP Cube Analysis. Poor performance on action fluency could reflect the greater involvement of the left anterior temporal and frontal lobes (Damasio & Tranel, 1993) and better performance on the VOSP Cube Analysis could reflect the relative sparing of the right hemisphere in the PiB-negative patients. Indeed, action fluency scores did correlate with Z scores from the left temporal pole (pairwise correlations, MRI: R=0.34, p=0.09 and FDG: R=0.40, p=0.04) and medial frontal lobe (FDG: R=0.56, p=0.003), and VOSP cube correlated with Z scores from right lateral temporal (MRI: R=0.43, p=0.03) and parietal (MRI: R=0.41, p=0.04) lobes. However, the differences observed across groups in these clinical measures were weak and would not have survived correction for multiple comparisons. Moreover, both measures showed poor positive predictive value, and hence are unlikely to be useful in helping to predict PiB-negative lvPPA. It is possible, however, that neuropsychological differences between groups may exist that were not assessed in our battery. Larger independent cohorts of lvPPA subjects that ideally assess a more comprehensive neuropsychological battery will be needed to determine whether clinical measures have any diagnostic utility, and also to validate the observed imaging differences. Our findings do not support one previous small study that used CSF biomarkers to divide lvPPA patients into those with AD (n=8) versus those without AD (n=5), and found that those without AD performed better on tests of single word comprehension, category fluency and phonology, and had less severe left temporoparietal hypoperfusion on SPECT (Teichmann et al., 2013). Explanations for the discrepancy could be that we utilized PiB-PET rather than CSF biomarkers to categorize patients, or the fact that we had a larger comparison group of PiB-positive lvPPA patients. Our results would generalize to other amyloid imaging studies in lvPPA patients.
A potential limitation to our study is that we matched the cohorts on disease duration, rather than measures of clinical severity, since disease duration does not correlate well with disease severity in lvPPA (Machulda et al., 2013). However, both groups were also well matched on the WAB AQ, a well-recognized and validated measure of aphasia severity. The number of subjects in the PiB-negative group was also relatively small which could have limited statistical power in the SPM comparisons to controls. Nevertheless, differences were still identified on the direct comparisons performed between the PiB-negative and PiB-positive groups, supporting our conclusions.
5. Conclusions
Although our findings will need to be confirmed in larger, independent cohorts, the findings from this study suggest that neuroimaging features will be more useful than clinical findings in helping to predict which lvPPA patients do not have Aβ deposition. The presence of highly asymmetric imaging findings with sparing of the right hemisphere and involvement of the left anteromedial temporal lobes strongly suggests that AD pathology is absent in lvPPA. In such instances, screening for progranulin gene mutations should be considered.
Supplementary Material
Highlights.
Logopenic aphasia is usually, although not always, associated with Alzheimer disease
Neuroimaging features differed between lvPPA patients with and without Aβ deposition
Striking asymmetry and sparing of the right hemisphere suggested PiB-negative lvPPA
Acknowledgements
This study was funded by NIH grant R01 DC010367 (PI Josephs) and Alzheimer’s Association grant NIRG-12-242215 (PI Whitwell). The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosta F, Scola E, Canu E, Marcone A, Magnani G, Sarro L, et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex. 2012;22(12):2705–2714. doi: 10.1093/cercor/bhr288. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008;131(Pt 3):706–720. doi: 10.1093/brain/awm320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Annals of neurology. 2001;49(4):433–442. [PubMed] [Google Scholar]
- Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-306948. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci U S A. 1993;90(11):4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011 doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System (DKEFS): Examiner’s manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- DeRenzi E, Vignolo LA. The token test: A sensitive measure to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134(Pt 10):3011–3029. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen H, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Picture. Thames Valley Test Company; 1992. [Google Scholar]
- Hu WT, McMillan C, Libon D, Leight S, Forman M, Lee VM, et al. Multimodal predictors for Alzheimer disease in nonfluent primary progressive aphasia. Neurology. 2010;75(7):595–602. doi: 10.1212/WNL.0b013e3181ed9c52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec J, Smith GE, Tangalos EG, Petersen RC, Kokmen E. Mayo's Older American Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56–97. The Clincial Neuropsychologist. 1992;6(supplement):1–104. [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knopman DS, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol. 2007;66(2):142–151. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain : a journal of neurology. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Vemuri P, Senjem ML, et al. Progranulin-associated PiB-negative logopenic primary progressive aphasia. J Neurol. 2014;261:604–614. doi: 10.1007/s00415-014-7243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Tsuboi Y, Cookson N, Watt H, Dickson DW. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch Neurol. 2004;61(10):1579–1584. doi: 10.1001/archneur.61.10.1579. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Boeve BF, Knopman DS, Petersen RC, Hu WT, et al. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer's disease. Mov Disord. 2010;25(9):1246–1252. doi: 10.1002/mds.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70(1):25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery (Revised) San Antonio, Tx: PsychCorp; 2007. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 1999;14(6):481–487. [PubMed] [Google Scholar]
- Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Jr, Corbetta-Rastelli C, Weiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain. 2013;136(Pt 3):844–858. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Rohrer JD, Clarkson MJ, Ridgway GR, Scahill RI, Modat M, et al. Reduced cortical thickness in the posterior cingulate gyrus is characteristic of both typical and atypical Alzheimer's disease. J Alzheimers Dis. 2010;20(2):587–598. doi: 10.3233/JAD-2010-1401. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Hsieh S, Mioshi E, Hodges JR. Cognitive decline in logopenic aphasia: more than losing words. Neurology. 2013;80(10):897–903. doi: 10.1212/WNL.0b013e318285c15b. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, et al. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain : a journal of neurology. 2011;134(Pt 10):3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, et al. Identification of an atypical variant of logopenic progressive aphasia. Brain Lang. 2013;127(2):139–144. doi: 10.1016/j.bandl.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer's type. PLoS One. 2013;8(4):e62471. doi: 10.1371/journal.pone.0062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Malone IB, Ridgway GR, Buckley AH, Downey LE, Golden HL, et al. White matter tract signatures of the progressive aphasias. Neurobiol Aging. 2013;34(6):1687–1699. doi: 10.1016/j.neurobiolaging.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Brun C, Siddiqui S, Churgin M, Libon D, Yushkevich P, et al. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology. 2012;78(22):1761–1768. doi: 10.1212/WNL.0b013e31825830bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Irwin DJ, Avants BB, Powers J, Cook PA, Toledo JB, et al. White matter imaging helps dissociate tau from TDP-43 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2013;84(9):949–955. doi: 10.1136/jnnp-2012-304418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of neurology. 2008;63(6):709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Rogalski EJ, Wieneke C, Hurley RS, Geula C, Bigio EH, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554–569. doi: 10.1038/nrneurol.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137(Pt 4):1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson's disease with and without dementia. J Clin Exp Neuropsychol. 1999;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of neurology. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76(21):1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Crutch SJ, Warrington EK, Warren JD. Progranulin-associated primary progressive aphasia: a distinct phenotype? Neuropsychologia. 2010;48(1):288–297. doi: 10.1016/j.neuropsychologia.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75(24):2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. NeuroImage. 2010;49(1):984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, et al. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008;4(9):e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78(21):1670–1677. doi: 10.1212/WNL.0b013e3182574f79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, et al. Improved DTI registration allows voxel-based analysis that outperforms Tract-Based Spatial Statistics. Neuroimage. 2014;94:65–78. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. Compendium of Neuropsychological tests, second edition: administration, norms and commentary. New York: Oxford University Press; 1998. [Google Scholar]
- Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain. 2013;136(11):3474–3488. doi: 10.1093/brain/awt266. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Warrington E, James M. The visual object and space perception battery. Bury St Edmonds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler Memory Scale Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- Wertz RT, Keith RL, Custer DD. Normal and aphasic behavior on a measure of auditory input and a measure of verbal output. Paper presented at the Annual Convention of the American Speech and Hearing Association; Chicago. 1971. Nov, [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010;75(21):1879–1887. doi: 10.1212/WNL.0b013e3181feb2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Parisi JE, Senjem ML, Knopman DS, Boeve B, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology. 2010;75(24):2212–2220. doi: 10.1212/WNL.0b013e31820203c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Przybelski SA, Parisi JE, Senjem ML, Boeve BF, et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2011;32(9):1531–1541. doi: 10.1016/j.neurobiolaging.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Schwarz CG, Reid RI, Kantarci K, Jack CR, Jr, Josephs KA. Diffusion tensor imaging comparison of progressive supranuclear palsy and corticobasal syndromes. Parkinsonism Relat Disord. 2014;20(5):493–498. doi: 10.1016/j.parkreldis.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72 tau, progranulin and sporadics. Brain. 2012;135(Pt 3):794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.